Abstract
We report the results of unrelated cord blood transplantation (CBT) for 13 adult patients with advanced myelodysplastic syndrome (MDS). The median age was 40 years, the median weight was 51 kg, and the median number of infused nucleated cells was 2.43 × 107/kg. Twelve patients had myeloid reconstitution, and the median time to more than 0.5 × 109/L (5 × 108/L) absolute neutrophil count was 22.5 days. A self-sustained platelet count more than 50 × 109/L was achieved in 11 patients at a median time of 49 days. Acute graft versus host disease (GVHD) occurred in 9 of 12 evaluable patients and chronic GVHD in 8 of 11 evaluable patients. Ten patients are alive and free of disease at between 171 and 1558 days after transplantation. The probability of disease-free survival at 2 years was 76.2%. These results suggest that adult advanced MDS patients without suitable related or unrelated bone marrow donors should be considered as candidates for CBT.
Introduction
The prognosis of advanced myelodysplastic syndrome (MDS) is poor. Although some patients with advanced MDS achieve remission with standard intensive chemotherapy, the duration is usually limited.1 Therefore, allogeneic stem cell transplantation is considered to be the only curative therapy for advanced MDS patients. Recently, alternative donor sources other than human leukocyte antigen (HLA)–identical siblings have been used as allogeneic stem cell sources.2-4 We have previously reported the results of a group of 7 adult patients with MDS-related secondary acute myeloid leukemia (AML) who underwent unrelated cord blood transplantation (CBT).5 Here, we report our clinical results for a larger group of 13 adult patients with advanced MDS treated with unrelated CBT.
Study design
Between August 1998 and June 2002, 13 adult patients with advanced MDS were treated with unrelated CBT at The Institute of Medical Science, University of Tokyo. MDS was defined by the French-American-British (FAB) Cooperative Group criteria.6 MDS-related secondary AML was defined as AML that developed during the follow-up period of MDS. Analyses of data were performed on December 1, 2002. All patients received 3 to 4 fractionated 12 Gy total body irradiation (TBI) on days –9, –8, and –7 or days –9 and –8. Cytosine arabinoside (Ara-C) was administered intravenously over 2 hours at a dose of 3 g/m2 every 12 hours on days –6 and –5 or days –5 and –4 (total dose, 12 g/m2). Recombinant human granulocyte colony-stimulating factor (G-CSF) was administered by continuous infusion at a dose of 5 μg/kg/d. Infusion of G-CSF was started 12 hours before the first dose of Ara-C and stopped at the completion of the last dose. Cyclophosphamide (CY) was administered intravenously over 2 hours at a dose of 60 mg/kg once daily on days –4 and –3 or days –3 and –2 (total dose, 120 mg/kg). Two days or 3 days after the completion of conditioning, patients received a cord blood transplant. All patients received standard cyclosporine (CyA) and methotrexate (MTX) as a graft-versus-host disease (GVHD) prophylaxis. CyA was given every day starting on day –1 at a dose of 3 mg/kg/d. MTX (15 mg/m2 intravenously) was given on day 1, and 10 mg/m2 MTX was given on days 3 and 6. Both acute and chronic GVHD were graded according to the previously published criteria.7-9 All patients received G-CSF (5 μg/kg/d) by intravenous infusion starting on day 1 until durable granulocyte recovery was achieved. Cord blood unit was selected according to the number of nucleated cells per recipient's weight and HLA compatibility (HLA-A and B by serology and HLA-DRB1 by high-resolution DNA typing). The chimerism status after CBT was determined either by fluorescence in situ hybridization with a Y chromosome probe for sex-mismatched CBT or by polymerase chain reaction DNA typing of HLA antigen for HLA-mismatched CBT. Seven patients with MDS-related secondary AML include in our previous study were also included (cases 1 to 7).5 No patient had a related or unrelated bone marrow donor available at the time of transplantation. Approval was obtained from the institutional review board at the Institute of Medical Science, University of Tokyo, for this study. Informed consent was provided according to the Declaration of Helsinki. Written informed consent for treatment was obtained from all patients. The probability of disease-free survival (DFS) was estimated by the Kaplan-Meier method.
Results and discussion
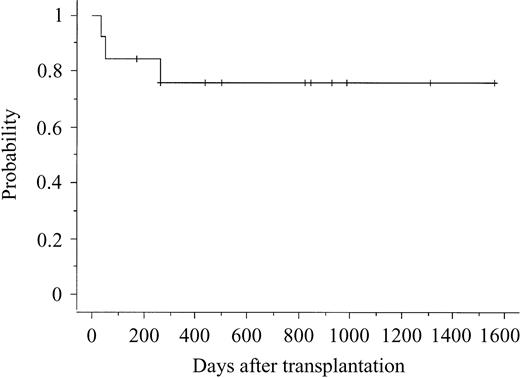
The characteristics of the 13 patients and cord blood units are shown in Table 1. Among the 13 patients, 8 did not receive any induction therapy before transplantation. Among the other 5 patients who received induction therapy, 4 could not achieve complete remission. Therefore, all but 1 received CBT as an up-front treatment—not a postremission consolidation. Among the patients, the median age was 40 years (range, 20-51 years), the median weight was 51 kg (range, 43-68 kg), and the median number of infused nucleated cells, measured before freezing, was 2.43 × 107/kg (range, 2.09 × 107 to 4.06 × 107/kg). All but 1 patient had myeloid reconstitution, and median time to more than 0.5 × 109/L (5 × 108/L) absolute neutrophil count was 22.5 days (range, 19-35 days). A self-sustained hemoglobin more than 85 g/L (8.5 g/dL) was achieved in 11 patients at a median time of 54 days (range, 34-224 days). A self-sustained platelet count more than 50 × 109/L was achieved in 11 patients at a median time of 49 days (range, 30-164 days). All but 1 patient with myeloid reconstitution showed full donor chimerism at the time of first bone marrow examination after CBT. Although 1 patient showed a mixed chimera, the patient had a full donor chimera at the time of writing. Acute GVHD occurred in 9 of 12 evaluable patients and chronic GVHD in 8 of 11 evaluable patients. Three patients died of relapse on days 107, 307, and 368, respectively. Ten patients are alive and free of disease at between 171 and 1558 days after transplantation (Table 2). The probability of DFS 2 years was 76.2% (Figure 1). Among the 10 patients who are alive and free of disease, 8 had not received any induction therapy before transplantation.
Characteristics of patients and cord blood units
Case . | Stage . | Age, y/sex . | Body weight, kg . | No. of HLA-A, B, DRB1 mismatches . | Cord blood cell dose, × 107/kg . | Recipient CMV status . | Mo. from diagnosis to transplantation . | Blasts in bone marrow before transplantation, % . | Cytogenetics* . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | sAML | 27/F | 43 | 2 (B, DRB1) | 4.06 | Positive | 101 | 69.8 | Poor |
| 2 | sAML | 37/F | 51 | 2 (DRB1, DRB1) | 2.3 | Positive | 7 | 78.8 | Good |
| 3 | sAML | 49/F | 53 | 1 (A) | 2.13 | Negative | 4 | 34.2 | Good |
| 4 | sAML | 47/F | 44 | 1 (B) | 2.09 | Positive | 37 | 61.6 | Intermediate |
| 5 | sAML | 50/M | 63 | 1 (A) | 2.5 | Positive | 9 | 41 | Intermediate |
| 6 | sAML | 38/F | 45 | 1 (DRB1) | 2.09 | Negative | 5 | 86 | Good |
| 7 | sAML | 20/M | 57 | 2 (B, DRB1) | 2.18 | Positive | 38 | 34 | Poor |
| 8 | RAEB | 41/F | 44 | 2 (B, DRB1) | 3.1 | Positive | 60 | 12 | Intermediate |
| 9 | sAML | 51/M | 59 | 2 (A, B) | 2.43 | Positive | 28 | 11.5 | Poor |
| 10 | RAEB | 36/F | 45 | 2 (A, B) | 2.54 | Positive | 15 | 8.7 | Intermediate |
| 11 | sAML | 34/M | 56 | 3 (A, DRB1, DRB1) | 2.59 | Positive | 6 | 18 | Good |
| 12 | sAML | 43/M | 48 | 3 (A, B, DRB1) | 2.54 | Negative | 24 | 3.3 | Good |
| 13 | sAML | 40/M | 68 | 2 (A, DRB1) | 2.37 | Positive | 7 | 50.7 | Poor |
Case . | Stage . | Age, y/sex . | Body weight, kg . | No. of HLA-A, B, DRB1 mismatches . | Cord blood cell dose, × 107/kg . | Recipient CMV status . | Mo. from diagnosis to transplantation . | Blasts in bone marrow before transplantation, % . | Cytogenetics* . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | sAML | 27/F | 43 | 2 (B, DRB1) | 4.06 | Positive | 101 | 69.8 | Poor |
| 2 | sAML | 37/F | 51 | 2 (DRB1, DRB1) | 2.3 | Positive | 7 | 78.8 | Good |
| 3 | sAML | 49/F | 53 | 1 (A) | 2.13 | Negative | 4 | 34.2 | Good |
| 4 | sAML | 47/F | 44 | 1 (B) | 2.09 | Positive | 37 | 61.6 | Intermediate |
| 5 | sAML | 50/M | 63 | 1 (A) | 2.5 | Positive | 9 | 41 | Intermediate |
| 6 | sAML | 38/F | 45 | 1 (DRB1) | 2.09 | Negative | 5 | 86 | Good |
| 7 | sAML | 20/M | 57 | 2 (B, DRB1) | 2.18 | Positive | 38 | 34 | Poor |
| 8 | RAEB | 41/F | 44 | 2 (B, DRB1) | 3.1 | Positive | 60 | 12 | Intermediate |
| 9 | sAML | 51/M | 59 | 2 (A, B) | 2.43 | Positive | 28 | 11.5 | Poor |
| 10 | RAEB | 36/F | 45 | 2 (A, B) | 2.54 | Positive | 15 | 8.7 | Intermediate |
| 11 | sAML | 34/M | 56 | 3 (A, DRB1, DRB1) | 2.59 | Positive | 6 | 18 | Good |
| 12 | sAML | 43/M | 48 | 3 (A, B, DRB1) | 2.54 | Negative | 24 | 3.3 | Good |
| 13 | sAML | 40/M | 68 | 2 (A, DRB1) | 2.37 | Positive | 7 | 50.7 | Poor |
CMV indicates cytomegalovirus; sAML, MDS-related secondary acute myeloid leukemia; RAEB, refractory anemia with excess blasts.
Cytogenetic analyses were performed immediately before transplantation and according to the International Prognostic Scoring System (IPSS) classification.10
Outcome
Case . | Neutrophils more than 5 × 108/L, d . | Reticulocytes more than 1%, d . | Hemoglobin level more than 8.5 g/dL, d . | Platelets more than 50 × 109/L, d . | Acute GVHD grade (organ involvement and stage) . | Chronic GVHD (organ involvement and type) . | Hospitalization, d . | Survival, d* . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 25 | 46 | 50 | II (skin 1, liver 0, gut 1) | Limited (skin, quiescent) | 89 | 1558+ | — |
| 2 | 35 | 50 | 224 | 164 | III (skin 3, liver 2, gut 2) | Limited (skin, liver, progressive) | 472 | 307 | Relapse |
| 3 | 26 | 35 | 54 | 49 | 0 | None | 96 | 1312+ | — |
| 4 | 24 | NE | NE | NE | 0 | NE | 169 | 107 | Relapse |
| 5 | 25 | 33 | 88 | 88 | I (skin 1, liver 0, gut 0) | Extensive (skin, lung, progressive) | 243 | 990+ | — |
| 6 | 19 | 28 | 91 | 35 | I (skin 2, liver 0, gut 0) | None | 234 | 928+ | — |
| 7 | 22 | 32 | 46 | 40 | I (skin 1, liver 0, gut 0) | Limited (skin, liver, progressive) | 131 | 828+ | — |
| 8 | 23 | 26 | 46 | 52 | I (skin 1, liver 0, gut 0) | Limited (skin, liver, quiescent) | 172 | 849+ | — |
| 9 | NE | NE | NE | NE | NE | NE | 533 | 368 | Relapse |
| 10 | 26 | 36 | 160 | 85 | I (skin 1, liver 0, gut 0) | None | 240 | 500+ | — |
| 11 | 20 | 26 | 34 | 30 | IV (skin 4, liver 0, gut 2) | Extensive (skin, eye, lung, progressive) | 267 | 437+ | — |
| 12 | 21 | 28 | 34 | 37 | 0 | Limited (skin, liver, de novo) | 124 | 266+ | — |
| 13 | 21 | 28 | 62 | 33 | I (skin 2, liver 0, gut 0) | Extensive (skin, progressive) | 199 | 171+ | — |
Case . | Neutrophils more than 5 × 108/L, d . | Reticulocytes more than 1%, d . | Hemoglobin level more than 8.5 g/dL, d . | Platelets more than 50 × 109/L, d . | Acute GVHD grade (organ involvement and stage) . | Chronic GVHD (organ involvement and type) . | Hospitalization, d . | Survival, d* . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 25 | 46 | 50 | II (skin 1, liver 0, gut 1) | Limited (skin, quiescent) | 89 | 1558+ | — |
| 2 | 35 | 50 | 224 | 164 | III (skin 3, liver 2, gut 2) | Limited (skin, liver, progressive) | 472 | 307 | Relapse |
| 3 | 26 | 35 | 54 | 49 | 0 | None | 96 | 1312+ | — |
| 4 | 24 | NE | NE | NE | 0 | NE | 169 | 107 | Relapse |
| 5 | 25 | 33 | 88 | 88 | I (skin 1, liver 0, gut 0) | Extensive (skin, lung, progressive) | 243 | 990+ | — |
| 6 | 19 | 28 | 91 | 35 | I (skin 2, liver 0, gut 0) | None | 234 | 928+ | — |
| 7 | 22 | 32 | 46 | 40 | I (skin 1, liver 0, gut 0) | Limited (skin, liver, progressive) | 131 | 828+ | — |
| 8 | 23 | 26 | 46 | 52 | I (skin 1, liver 0, gut 0) | Limited (skin, liver, quiescent) | 172 | 849+ | — |
| 9 | NE | NE | NE | NE | NE | NE | 533 | 368 | Relapse |
| 10 | 26 | 36 | 160 | 85 | I (skin 1, liver 0, gut 0) | None | 240 | 500+ | — |
| 11 | 20 | 26 | 34 | 30 | IV (skin 4, liver 0, gut 2) | Extensive (skin, eye, lung, progressive) | 267 | 437+ | — |
| 12 | 21 | 28 | 34 | 37 | 0 | Limited (skin, liver, de novo) | 124 | 266+ | — |
| 13 | 21 | 28 | 62 | 33 | I (skin 2, liver 0, gut 0) | Extensive (skin, progressive) | 199 | 171+ | — |
GVHD indicates graft-versus-host disease; NE, not evaluable; —, not applicable.
Ten patients are alive in complete remission at the time of writing. Cases 2, 4, and 9 relapsed on day 266, day 33, and day 53, respectively.
Probability of disease-free survival after cord blood transplantation.
Although allogeneic stem cell transplantation from an HLA-identical related donor offers a potential cure for advanced MDS patients, a suitably matched related donor is unavailable for approximately two thirds of patients. Recently, results of transplantation from alternative donors other than HLA-identical siblings have been reported.2,4,11 Because nonrelapse mortality was higher in patients who received transplants from HLA-identical unrelated bone marrow donors and HLA-nonidentical related donors than from HLA-identical related donors, Anderson et al12 suggested that the outcome after transplantation for advanced MDS may be improved by transplanting as soon as possible after the diagnosis of advanced MDS in an attempt to reduce transplantation-related toxicity. Although the patients required early transplantation, none of our patients had any related or unrelated bone marrow donors. Therefore, unrelated cord blood, which has the advantage of rapid availability, was used as an alternative stem cell source. The long-term DFS rate for advanced MDS patients receiving allogeneic stem cell transplantation is approximately 30%,3,12,13 and high relapse rates may result in an unfavorable rate of DFS. The relatively higher incidence of chronic GVHD and the use of G-CSF–combined preparative regimen, which was capable of reducing the posttransplantation relapse rate in refractory myeloid malignancies,14,15 may be associated with a high DFS rate (76.2% at 2 years) in our study. Also, all patients in our study received more than 2 × 107 nucleated cells per weight, perhaps due to the smaller size of our patients. This may be one of the possible reasons for our favorable result. Although several studies have suggested the promising results of unrelated CBT for adult patients,16-18 the role of unrelated cord blood as an alternative stem cell source is not well defined in adult MDS patients. Therefore, we updated the results of unrelated CBT for adult MDS patients. These results suggest that adult advanced MDS patients without suitable related or unrelated bone marrow donors should be considered as candidates for CBT and provide the rationale for a larger clinical study of CBT.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-12-3917.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal