Abstract
Idiopathic hypereosinophilic syndrome (HES) is a myeloproliferative disorder characterized by persistent eosinophilia and organ involvement. Different treatments have been investigated in HES with modest success. It has been suggested that imatinib is active in HES. We treated 9 patients with HES with 100 mg imatinib daily. Doses for patients without response after 4 weeks were increased to 400 mg daily. Prior therapy had failed for 7 patients. Five patients responded: 4 achieved sustained complete remission lasting a median of 12+ weeks (range, 9+ to 36+ weeks), and 1 had a transient response. One patient died in complete remission. Responses occurred within 4 weeks of therapy; only 1 responder required an increase in dose to 400 mg daily. Three of 4 nonresponders failed to respond to an increase in dose. Toxicity was minimal. We conclude that imatinib therapy is effective for HES.
Introduction
Hypereosinophilic syndrome (HES) is a myeloproliferative disorder characterized by the overproduction of eosinophils in the absence of other causes of eosinophilia.1,2 Tissue infiltration frequently involves the heart, skin, central and peripheral nervous systems, lungs, spleen, liver, eyes, and gastrointestinal tract. The disease is more common among men (9:1 ratio) and is usually seen between the ages of 20 and 50 years. The long-term outcome varies, with reported median survivals of 15% to 40% at 10 years.1-3 There is no effective therapy for HES. Current therapy consists of corticosteroids; interferon-α; chemotherapeutic agents such as hydroxyurea, vincristine, etoposide, and alkylating agents; and T-cell–suppressing agents such as cyclosporin A and 2-chlorodeoxyadenosine. Responses to therapy are frequently transient, and most patients require multiple therapies.2,4
Imatinib mesylate (Gleevec) is a selective tyrosine kinase inhibitor with potent inhibiting activity against c-abl, Bcr-Abl, c-kit, and platelet-derived growth factor receptor (PDGF-R).5,6 Imatinib is highly effective in patients with Philadelphia (Ph) chromosome-positive chronic myeloid leukemia (CML).7 We investigated the efficacy of imatinib in patients with HES.
Study design
Patients with HES, defined according to established criteria,8 were included in this phase 2, open-label study regardless of their treatment history. Other eligibility criteria were (1) Eastern Cooperative Oncology Group (ECOG) performance status less than or equal to 2; (2) life expectancy longer than 12 weeks; (3) total bilirubin level less than 2 mg/dL; (4) creatinine level less than 2 mg/dL; and (5) New York Heart Association cardiac class less than or equal to 2. The protocol was approved by the Institutional Review Board, and patients gave signed, informed consent. Results of the first patient treated have been previously reported.9
The starting dose of imatinib for all patients was 100 mg orally, daily. Patients showing no objective response after 4 weeks of therapy, or progression of disease at any time during therapy, could have their dose escalated to 400 mg daily. Guidelines for administration, interruption of therapy, and dose adjustments were identical to those used for patients with Ph-positive CML, as recently reported.7 The next lower dose level for patients requiring dose reduction was 100 mg every other day. Patients were followed up with monthly physical examinations and weekly complete blood counts and differential and with monthly renal and liver function tests. Bone marrow aspiration was performed every 3 to 4 months after the initiation of therapy. Therapy was continued for at least 8 weeks unless patients experienced unmanageable toxicity. For the purpose of this report, complete remission (CR) was defined as the normalization of peripheral blood counts, including normal eosinophil counts, and the complete disappearance of all signs and symptoms of HES. Patients responding to treatment continued therapy as long as they showed evidence of clinical benefit without undue toxicity. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0.
Results and discussion
From November 2001 to September 2002, 9 patients with HES were referred to MD Anderson Cancer Center, and all were included in this study. Their clinical characteristics are shown in Table 1. Their median age was 50 years (range, 25-73 years); 6 patients were men. Seven patients underwent prior therapy with a median of 3 (range, 1-7) different agents, including steroids (n = 5), hydroxyurea (n = 5), interferon (n = 4), chemotherapy (busulfan, cladribine, and cytarabine; n = 1 each), and cyclosporin A (n = 1). All patients were symptomatic at the start of therapy. The most common symptoms were fatigue (n = 5), skin rash, pruritus, or both (n = 4), bone or muscle pain (n = 3), dyspnea (n = 2), and weight loss (n = 2). Eight patients had current evidence or history of tissue involvement, the most frequent of which were neurologic (transient ischemic attack, n = 2; peripheral neuropathy, n = 2; stroke, n = 1), cardiovascular (myocardial infiltration, n = 2; ventricular thrombosis, n = 1), and skin (skin lesions, n = 4). Two patients did not have peripheral eosinophilia at the time imatinib therapy was started; both were receiving steroids and one was receiving hydroxyurea up to this time. All patients had elevated bone marrow eosinophil counts. One patient had anemia and thrombocytopenia in addition to eosinophilia. All patients had diploid cytogenetics, and none of the 6 patients investigated by polymerase chain reaction (PCR) had the Bcr/Abl rearrangement.
Characteristics of patients with HES treated with imatinib
. | Median . | Range . |
|---|---|---|
| Age, y | 50 | 25-73 |
| Time from diagnosis, mo | 35 | 8-142 |
| No. prior therapies | 2 | 0-7 |
| WBC count, × 109/L | 9.4 | 6.2-17.5 |
| Peripheral eosinophils, % | 21 | 2-68 |
| Peripheral absolute eosinophil count, × 109/L | 1.8 | 0.3-6.6 |
| Marrow eosinophils, % | 18 | 10-44 |
. | Median . | Range . |
|---|---|---|
| Age, y | 50 | 25-73 |
| Time from diagnosis, mo | 35 | 8-142 |
| No. prior therapies | 2 | 0-7 |
| WBC count, × 109/L | 9.4 | 6.2-17.5 |
| Peripheral eosinophils, % | 21 | 2-68 |
| Peripheral absolute eosinophil count, × 109/L | 1.8 | 0.3-6.6 |
| Marrow eosinophils, % | 18 | 10-44 |
The male-to-female ratio of patients was 6:3.
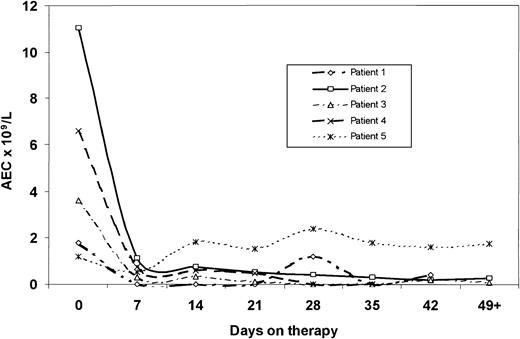
Four patients (44%; 95% confidence interval [95% CI], 0.14-0.79) achieved CR. All 4 responders were men. In 3 patients hematologic response was evident within the first 2 weeks of therapy and was accompanied by symptomatic improvement (Figure 1). One patient (Figure 1, patient 1) required a dose increase to 400 mg on day 28 before sustained normalization of the peripheral blood eosinophil count was confirmed, though symptomatic improvement was evident within 2 weeks from the start of therapy. The patient with anemia and thrombocytopenia at the start of therapy (Figure 1, patient 4) experienced normalization of these parameters after 4 weeks of therapy. Normalization of eosinophilia was accompanied by the resolution of symptoms in all patients. Follow-up bone marrow aspiration was obtained in 2 patients achieving CR, confirming normalization of the eosinophils. Among the other 2 patients, 1 died in CR before aspiration was due, and the other refused bone marrow aspiration. In addition, 1 female patient (Figure 1, patient 5) experienced transient normalization of peripheral blood eosinophil counts within 1 week of the start of therapy. This was accompanied by the complete but transient resolution of symptoms (fatigue and pruritus). Two weeks later, the symptoms recurred and the eosinophil count increased without further improvement, even after the dose was increased to 400 mg; she was eventually taken off therapy. One male patient discontinued therapy after 10 days because of the exacerbation of psoriasis. The other 3 nonresponders (1 man, 2 women) had dose increases to 400 mg daily and received therapy for a median of 12 weeks (range, 8-13 weeks) before imatinib was discontinued for lack of response.
Response to imatinib therapy in patients with hypereosinophilic syndrome. Median follow-up for all patients was 13 weeks (range, 6 to 36+ weeks). One of the patients who achieved CR underwent splenectomy before starting treatment with imatinib; he died in CR of pneumococcal sepsis after 9 weeks of therapy. The other 3 patients who achieved CR continue on therapy and are in remission 13+,25+, and 36+ weeks from the start of therapy. None of these patients has had any objective evidence of residual or recurrent tissue infiltration.
Response to imatinib therapy in patients with hypereosinophilic syndrome. Median follow-up for all patients was 13 weeks (range, 6 to 36+ weeks). One of the patients who achieved CR underwent splenectomy before starting treatment with imatinib; he died in CR of pneumococcal sepsis after 9 weeks of therapy. The other 3 patients who achieved CR continue on therapy and are in remission 13+,25+, and 36+ weeks from the start of therapy. None of these patients has had any objective evidence of residual or recurrent tissue infiltration.
Overall treatment was well tolerated. One patient had a rash consistent with the exacerbation of preexisting psoriasis and was taken off therapy after 10 days. Abdominal cramps and nausea developed in 1 patient who achieved CR, necessitating a dose reduction of imatinib to 100 mg every other day. This patient has sustained his response at the lower dose. All other adverse events were grade 1 and were consistent with previously reported toxicity with imatinib, including fluid retention (n = 2), diarrhea (n = 1), muscle cramps (n = 1), and nausea (n = 1).
Results in this larger series of patients confirm previous, mostly anecdotal observations of the efficacy of imatinib in the treatment of HES. The report by Schaller et al10 first documented the response with low-dose imatinib in one patient with HES. Two subsequent reports have confirmed this observation. Gleich et al11 reported 4 of 5 patients with HES responding to imatinib. Three of the responders were receiving interferon-α when imatinib was started, but all were eventually able to discontinue interferon therapy and to sustain the response with imatinib. We reported a patient with a similar response to imatinib9 ; this report confirms the activity of imatinib in patients with HES. Responses occur rapidly, with the normalization of eosinophil counts and symptom improvement usually occurring within 1 to 2 weeks. All patients in this series were treated with low-dose imatinib (100 mg daily) as a single agent, though in 1 patient the dose was increased to 400 mg before the response could be confirmed. However, responses in 1 of our patients (Figure 1, patient 2) and 4 from Gleich et al11 could be frequently sustained with even lower doses of imatinib. In contrast, doses lower than 300 mg daily have resulted in few complete hematologic responses and no cytogenetic responses in CML.12 This could suggest that a tyrosine kinase with increased sensitivity to imatinib may be involved in HES. In addition, responses are sustained with no evidence of recurrence, albeit with a short follow-up. An interesting feature of the response to imatinib in patients with HES is that all responders were men. Nine of 11 male patients (81%; 95% CI, 0.48-0.98) reported in previous studies10,11 and in the present study achieved CR. In contrast, none of the 4 female patients (0%; 95% CI, 0.0-0.60) achieved CR, although 1 female patient in our study had a transient response. If confirmed in a series of larger scale, this observation might suggest a different pathophysiology of HES in men (response to imatinib) than in women.
The mechanism of action of imatinib in HES is unknown. Overproduction of eosinophilic cytokines and abnormal signal transduction through cytokine receptors have been proposed as part of the etiology of HES.1 Increased levels of interleukin-5 (IL-5) are found in some patients.13 It has been suggested that those with high levels may be less likely to respond to imatinib.11 Several tyrosine kinases are important for eosinophil survival: IL-5 activates Lyn, Syk, and Janus kinase 2 (JAK2),14-16 and these in turn activate the Ras–mitogen-activated protein kinase (Ras-MAPK) and the JAK-signal transducer and activator of transcription (JAK-Stat) pathways. This process may be required for prolonged eosinophil survival induced by IL-5.14 However, imatinib does not inhibit the activity of any of these kinases.17 Eosinophils express mRNA and secrete stem cell factor (SCF), the ligand for c-kit,18 and they express a functional c-kit receptor.19 Activation of c-kit induces eosinophil activation and degranulation,20 proliferation that may be synergistic with IL-3, granulocyte macrophage–colony-stimulating factor (GM-CSF), and IL-5,21 and increased adhesion that could contribute to tissue localization.19 In addition, platelet-derived growth factor activates eosinophils.22 Although this suggests the presence of PDGF-R in eosinophils, it has not been identified.
We conclude that imatinib at low doses is active in patients with HES. The mechanism of action of imatinib in HES is under investigation, and findings could lead to better understanding of the pathophysiology of HES.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2003-01-0081.
One of the authors (L.L.) is employed by a company (Novartis Pharmaceuticals) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
J.C. is a Clinical Research Scholar for the Leukemia and Lymphoma Society.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal