Abstract
The effects of combined exposure to the checkpoint abrogator UCN-01 and pharmacologic MEK1/2 inhibitors were examined in human multiple myeloma (MM) cell lines. Treatment of RPMI8226, NCI-H929, and U266 MM cells with a minimally toxic concentration of UCN-01 (150 nM) for 24 hours resulted in mitogen-activated protein (MAP) kinase activation, an effect that was blocked by coadministration of the MEK1/2 inhibitor PD184352. These events were accompanied by enhanced activation of p34cdc2 and a marked increase in mitochondrial damage (loss of ΔΨm; cytochrome c and Smac/DIABLO (direct IAP binding protein with low pI) release), poly(ADP-ribose) polymerase (PARP) cleavage, and apoptosis. PD184352/UCN-01 also dramatically reduced clonogenic survival in each of the MM cell lines. In contrast to As203, apoptosis induced by PD184352/UCN-01 was not blocked by the free-radical scavenger n-acetyl-l-cysteine. Whereas exogenous interleukin 6 substantially prevented dexamethasone-induced lethality in MM cells, it was unable to protect them from PD184352/UCN-01–induced apoptosis despite enhancing Akt activation. Insulinlike growth factor 1 (IGF-1) also failed to diminish apoptosis induced by this drug regimen. MM cell lines selected for a high degree of resistance to doxorubicin, melphalan, or dexamethasone, or displaying resistance secondary to fibronectin-mediated adherence, remained fully sensitive to PD184352/UCN-01–induced cell death. Finally, primary CD138+ MM cells were also susceptible to UCN-01/MEK inhibitor-mediated apoptosis. Together, these findings suggest that simultaneous disruption of cell cycle and MEK/MAP kinase signaling pathways provides a potent stimulus for mitochondrial damage and apoptosis in MM cells, and also indicate that this strategy bypasses the block to cell death conferred by several other well-described resistance mechanisms.
Introduction
Multiple myeloma (MM), the most common of the plasma cell dyscrasias, is a progressive and generally incurable disorder of mature B lymphocytes.1 The mainstay of treatment for myeloma involves chemotherapy, using agents such as steroids (eg, dexamethasone), alkylating agents (eg, melphalan), and topoisomerase inhibitors (eg, doxorubicin).2 Although most myeloma patients respond to such approaches, at least initially, the pre-existence or emergence of drug-resistant cells3represents a formidable barrier to cure.
Recently, attempts to understand the pathophysiology of MM have focused on apoptosis, a genetically regulated program of cell suicide that is particularly involved in hematopoietic cell homeostasis.4For example, there is accumulating evidence that interleukin 6 (IL-6), a growth and survival factor for myeloma cells, acts, at least in part, by blocking apoptosis.5 While the downstream signaling cascades responsible for the antiapoptotic actions of IL-6 remain to be fully elucidated, JNK/SAPK-, Stat-, ΝFκΒ-, PI3K/Akt-, Mcl-1–, and Bcl-xL–related pathways have been implicated.5-9 Recently, attention focused on the p42/44 mitogen-activated protein kinase (MAP kinase) cascade as a key regulator of neoplastic cell survival.10 MAP kinase represents one of a family of parallel signaling modules that includes the c-Jun N-terminal kinase (JNK) and the p38 MAP kinase.11 Although exceptions exist, activation of JNK and p38 MAPK is generally associated with proapoptotic actions,10,12 whereas MAP kinase exerts cytoprotective functions.10,13 The observation that in MM cells, IL-6– and vascular endothelial growth factor (VEGF)–related proliferative actions involve MAP kinase activation14,15 argues that the latter signaling pathway may play an important role in MM cell survival. The potential clinical implications of such findings are highlighted by the recent development of MEK1/2 inhibitors (eg, PD184352) that display activity in vivo,16 and the introduction of PD184352 into phase I trials.17
UCN-01 (7-hydroxystaurosporine) is a staurosporine derivative with in vivo activity that was originally developed as a selective protein kinase C (PKC) inhibitor.18 Subsequently, UCN-01 has been reported to act as an inhibitor of Chk1 and an abrogator of the G2M checkpoint.19 In human leukemia cells, UCN-01 interacts synergistically with antimetabolites such as ara-C20 and gemcitabine21 and induces apoptosis in human leukemia cells, including those of lymphoid origin.22 Phase I/II trials of UCN-01 are currently under way,23 and preliminary evidence suggests that it may have activity, particularly when combined with other agents, in B-cell malignancies.24 The potential activity of UCN-01 against MM cells remains largely unexplored.
In a recent communication, we reported that exposure of multiple human myeloid leukemia cell lines to subtoxic, pharmacologically achievable UCN-01 concentrations (ie, ∼150 nM) induced activation of MAP kinase, and that interference with the latter process (eg, by coadministration of MEK1/2 inhibitors) resulted in a dramatic potentiation of mitochondrial damage and apoptosis.25 In view of evidence that MAP kinase activation plays an important role in MM cell proliferation/survival,14 the notion that combined exposure of myeloma cells to UCN-01 and MEK1/2 inhibitors might lead to enhanced apoptosis appeared plausible. To test this possibility, the effects of cotreatment with UCN-01 and MEK1/2 inhibitors on survival have been examined in a variety of MM cell lines. Our results indicate that concurrent exposure of myeloma cells to UCN-01 and MEK1/2 inhibitors synergistically induces mitochondrial damage, caspase activation, and apoptosis, and that these lethal effects are undiminished by administration of exogenous IL-6 or insulinlike growth factor (IGF-1). Moreover, myeloma cells selected for resistance to dexamethasone, melphalan, or doxorubicin, or displaying cell adherence–related drug resistance, retain full susceptibility to UCN-01/MEK1/2 inhibitor-induced lethality. Taken together, these findings suggest that combined treatment with a checkpoint abrogator and MEK1/2 inhibitor may warrant further investigation as a therapeutic strategy in MM.
Materials and methods
Cells and reagents
Human MM cell lines RPMI8226, NCI-H929, and U266 were purchased from American Type Culture Collection (Rockille, MD). The dexamethasone-sensitive (MM.1S) and -resistant (MM.1R) human MM cell lines26 were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 200 units/mL penicillin, 200 μg/mL streptomycin, minimal essential vitamins, sodium pyruvate, and glutamine. Doxorubicin (Dox40)– and melphalan (LR5)–resistant sublines27 28 of 8226 cells were maintained in RPMI 1640 medium as described above containing 400 nM doxorubicin and 5 μM melphalan, respectively.
The selective MEK inhibitor PD184352 was purchased from Upstate Biotechnology (Lake Placid, NY), and PD98059 and UO126 were supplied by Calbiochem (San Diego, CA) as powders. The inhibitors were dissolved in sterile dimethyl sulfoxide (DMSO), and stored frozen under light-protected conditions at −20°C. UCN-01 was kindly provided by Dr Edward Sausville (Developmental Therapeutics Program, National Cancer Institute [NCI]), dissolved in DMSO at a stock concentration of 1 mM and stored at −20°C, and subsequently diluted with serum-free RPMI medium prior to use. Arsenic trioxide (As2O3) was supplied by Sigma (St Louis, MO), dissolved in 1.65M NaOH at 50 mM as a stock solution.n-acetyl-l-cysteine (L-NAC, Calbiochem) was prepared in sterile water immediately before use. Recombinant human IL-6 and IGF-1 were purchased from Sigma and R&D Systems (Minneapolis, MN), rehydrated in phosphate buffered saline (PBS) and 10 mM acetic acid, respectively, both of which contained 0.1% bovine serum albumin (BSA), aliquoted, and stored at −80°C. The PI3 kinase inhibitor LY294002, dexamethasone, and doxorubicin were purchased from Sigma, dissolved in DMSO, aliquoted, and stored at −20°C. Melphalan (Sigma) was dissolved in HCl-ethanol (47:1000) aliquoted, and stored at −80°C. In all experiments, the final concentration of DMSO did not exceed 0.1%.
Experimental format
All experiments were performed using logarithmically growing cells (4-6 × 105 cells/mL). Cell suspensions were placed in sterile 25 cm2 T-flasks (Corning, Corning, NY) and incubated with PD184352 for 30 minutes at 37°C. At the end of this period, UCN-01 was added to the suspension, and the flasks placed in 37°C/5% CO2 incubator for various intervals, generally 24 hours. In some studies, IL-6 or IGF-1 was added concurrently with PD184352. After drug treatment, cells were harvested and subjected to further analysis as described below.
Assessment of apoptosis
The extent of apoptosis was evaluated by assessing Wright-Giemsa–stained cytospin slides under light microscopy and scoring the number of cells exhibiting classic morphological features of apoptosis. For each condition, 5 to 10 randomly selected fields per slide were evaluated, encompassing at least 800 cells. To confirm the results of morphologic analysis, in some cases cells were also evaluated by TdT-mediated dUTP nick end labeling (TUNEL) staining and annexin V–fluorescein isothiocyanate (FITC) staining.
For TUNEL staining,29 cytospin slides were fixed with 4% formaldehyde/PBS for 10 minutes, treated with acetic acid/ethanol (1:2) for 5 minutes, and incubated with terminal transferase reaction mixture containing 1 × terminal transferase reaction buffer, 0.25U/L terminal transferase, 2.5 mM CoCl2, and 2 pmol fluorescein-12-dUTP (Boehringer Mannheim, Indianapolis, IN) at 37°C for 1 hour. The slides were mounted with Vectashield with propidium iodide (Vector Laboratories, Burlingame, CA) and visualized using fluorescence microscopy.
For annexin V–FITC staining, 1 × 106 cells were washed twice with cold PBS and then resuspended in 1 × binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]/NaOH, pH7.4, 140 mM NaOH, 2.5 mM CaCl2). The cells were incubated with annexin V–FITC (BD PharMingen, San Diego, CA) and 5 μg/mL propidium iodide (PI), and incubated for 15 minutes at room temperature in the dark per the manufacturer's instructions. The samples were analyzed by flow cytometry within 1 hour to determine the percentage of cells displaying annexin V staining (early apoptosis) or both annexin V and PI staining (late apoptosis).
Mitochondrial membrane potential (ΔΨm) assay
After drug treatment, 2 × 105 cells were incubated with 40 nM 3,3-dihexyloxacarbocyanine (DiOC6, Molecular Probes, Eugene, OR) in PBS at 37°C for 20 minutes and then analyzed by flow cytometry.30 The percentage of cells exhibiting a low level of DiOC6 uptake, which reflects loss of mitochondrial membrane potential, was determined using Becton Dickinson FACScan (Becton Dickinson, San Jose, CA).
Western blot assay
After drug treatment, whole-cell pellets were lysed by sonication in 1 × sample buffer (62.5 mM Tris base, pH6.8, 2% SDS, 50 mM dithiothreitol [DTT], 10% glycerol, 0.1% bromophenol blue, and 5 μg/mL each chymostatin, leupeptin, aprotinin, pepstatin, and soybean trypsin inhibitor) and boiled for 5 minutes. For analysis of protein phosphorylation, 1 mM each Na vanadate and Na pyrophosphate was added to 1 × sample buffer. Protein samples were harvested as the supernatant following centrifugation of the samples at 12 800g for 5 minutes and amount of protein quantified using Coomassie Protein Assay Reagent (Pierce, Rockford, IL). Equal amounts of protein (30 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto nitrocellulose membrane. For blotting phosphoproteins, no SDS was included in the transfer buffer. The blots were blocked with 5% milk in PBS-Tween 20 (0.1%) at room temperature for 1 hour and probed with the appropriate dilution of primary antibody in 5% BSA/PBS-Tween 20 overnight at 4°C. The membranes were washed twice in PBS-Tween 20 for 30 minutes and then incubated with a 1:2000 dilution of horseradish peroxidase (HRP)–conjugated secondary antibody (Kirkegaard & Perry, Gaithersburg, MD) in 5% milk/PBS-Tween 20 at room temperature for 1 hour. After washing twice in PBS-Tween 20 for 30 minutes, the blots were visualized by Western Blot Chemiluminescence Reagent (NEN Life Science Products, Boston, MA). For blots of phosphoproteins, Tris-buffered saline (TBS) was used instead of PBS throughout. Where indicated, the blots were reprobed with antibodies against β-actin (BD PharMingen) to ensure equal loading and transfer of proteins. The following antibodies were used as primary antibodies: phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody (1:1000, rabbit polyclonal, Cell Signaling Technology, Beverly, MA), p44/42 MAP kinase antibody (1:1000, rabbit polyclonal, Cell Signaling Technology), phospho-cdc2 (Tyr15) antibody (1:1000, rabbit polyclonal, Cell Signaling Technology), cdc2 antibody (1:1000, rabbit polyclonal, Cell Signaling Technology), phospho-cdk2 (Thr160) antibody (1:500, rabbit polyclonal, Cell Signaling Technology), cdk2 antibody (1:1000, mouse monoclonal, Transduction Laboratories, Lexington, KY), PARP antibody (1:2500, mouse monoclonal, Biomol Research Laboratories, Plymouth Meeting, PA), phospho-Akt (Ser473) antibody (1:1000, rabbit polyclonal, Cell Signaling Technology), and Akt antibody (1:1000, rabbit polyclonal, Cell Signaling Technology).
Analysis of cytosolic cytochrome c and Smac/DIABLO
After drug treatment, 4 × 106 cells were washed in PBS and lysed by incubating for 30 seconds in lysis buffer (75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA, and 350 μg/mL digitonin). The lysates were centrifuged at 12 000g for 1 minute, and the supernatant was collected in an equal volume of 2 × sample buffer.31 The proteins were quantified, separated by 15% SDS-PAGE, and subjected to Western blot as described above. Cytochrome c antibody (1:500, mouse monoclonal, Pharmingen) and Smac/DIABLO antibody (1:500, rabbit polyclonal, Upstate Biotechnology) were used as primary antibody.
Cyclin-dependent kinase assay
Following drug treatment, 1 × 107 cells were disrupted in radioimmunoprecipitation assay (RIPA) buffer by repeated aspiration through a 21-gauge needle. Cell extracts were collected by centrifugation at 10 000g for 10 minutes and protein levels quantified. For each condition, 200 μg protein was immunoprecipitated with 20 μL of cdc2 p34 (mouse monoclonal, Santa Cruz Biotechnology, Santa Cruz, CA) or cdk2 agarose conjugate (rabbit polyclonal, Santa Cruz Biotechnology) at 4°C overnight. The agarose was washed 4 times with RIPA buffer and incubated in Assay Dilution Buffer containing 400 μg/mL histone H1 (Upstate Biotechnology), 100 μM adenosine triphosphate (ATP), 15 mM MgCl2, and 2 μCi (0.074 MBq) γ-[32P]ATP at 30°C for 20 minutes. An equal volume of 2 × sample buffer was added in the reaction mixture and boiled for 3 minutes. γ-[32P]-histone H1 was separated by 12% SDS-PAGE and visualized by exposure of the dried gels to x-ray film (KODAK) at −80°C for 1 hour.
Cell survival and clonogenic assays
For cell viability assays, CellTiter 96 AQueous One Solution (Promega, Madison, WI) was used per the manufacturer's instructions, and the absorbance at 490 nm was recorded using a 96-well plate reader (Molecular Devices, Sunnyvale, CA).
Colony-forming ability following drug treatment was evaluated using a soft agar cloning assay as described previously.30Briefly, cells were washed 3 times with serum-free RPMI medium. Subsequently, 500 cells/well were mixed with RPMI medium containing 20% FBS and 0.3% agar and plated on 12-well plates (3 wells per condition). The plates were then maintained in a 37°C/5% CO2, fully humidified incubator. After 10 days' incubation, colonies consisting of more than 50 cells were scored using an Olympus Model CK inverted microscope (Olympus Optical, Miami, FL), and colony formation for each condition calculated in relation to values obtained for untreated control cells.
Assessment of effects of fibronectin adherence on drug-induced apoptosis
96-well plates were coated with 50 μg/mL human cellular fibronectin (FN, Sigma) overnight, and the wells were washed twice with serum-free RPMI 1640 media. 4 × 104 cells/well were added to FN-coated plates and incubated at 37°C/5% CO2for 1 hour in serum-free media, and nonadherent cells were removed by washing the wells twice with serum-free media as previously described.32 FN-adhered cells were then treated with the drugs for 24 hours. The percentage of apoptotic cells was determined by assessing Wright-Giemsa–stained cytospin slides as described above.
Isolation of CD138+ myeloma cells
Bone marrow mononuclear cells were obtained with informed consent from patients with MM undergoing routine diagnostic aspirations. Approval was obtained from the institutional review board of Virginia Commonwealth University for these studies. Informed consent was provided according to the Declaration of Helsinki. Cells were collected in syringes containing preservative-free heparin and diluted 1:4 in RPMI 1640 medium. The mononuclear cell fraction was isolated by centrifugation at 400g for 38 minutes over Histopaque-1077 (Sigma Diagnostics). The interface layer was extracted with a Pasteur pipette, and the cells washed twice in buffer (PBS containing 2 mM EDTA and 0.5% BSA). The cells were incubated with MACS CD138 Microbeads (Miltenyi Biotec, Auburn, CA) at 4°C for 15 minutes. CD138+ and CD138− cells were separated using an MS+/LS+ column and a magnetic separator according to the manufacturer's instructions (Miltenyi Biotec).33 The purity of isolated CD138+ cells was assessed by CD138-PE staining and flow cytometry and was determined to be greater than 90% positive. Viability of the cells was regularly greater than 95% by trypan blue exclusion. CD138+ cells were cultured in RPMI 1640 medium containing 10% FCS in 96-well plates under the same condition described above. In addition, parallel studies were performed using the CD138− cell population. Following drug treatment, the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin slides under light microscopy.
Statistical analysis
For morphological assessment of apoptotic cells, analysis of ΔΨm and clonogenic and cell survival assays, experiments were repeated at least 3 times. Values represent the mean ± SD for at least 3 separate experiments performed in triplicate. The significance of differences between experimental variables was determined using the Student t test.
Results
UCN-01 and PD184352 interact synergistically to promote mitochondrial damage and apoptosis in MM cells
To assess the effects of combined exposure to UCN-01 and MEK inhibitors on MM cell survival, 3 MM cell lines (8226, H929, and U266) were exposed to 150 nM UCN-01 ± 10 μM PD184352 for 24 hours, after which apoptosis was evaluated by morphologic criteria and loss of ΔΨm determined by monitoring DiOC6 uptake (Table1).
Effects of combined exposure to UCN-01 and PD184352 on apoptosis and loss of Δψm in MM cells
| . | Apoptotic cells (%) . | “Low” Δψm (% cells) . |
|---|---|---|
| 8226 | ||
| Control | 5.9 ± 2.7 | 2.8 ± 1.0 |
| PD184352 | 12.7 ± 2.6 | 12.6 ± 1.6 |
| UCN-01 | 9.1 ± 5.1 | 8.4 ± 1.3 |
| PD + UCN | 61.9 ± 5.9 | 39.6 ± 2.7 |
| H929 | ||
| Control | 3.6 ± 2.6 | 3.5 ± 1.4 |
| PD184352 | 10.0 ± 4.5 | 8.7 ± 0.1 |
| UCN-01 | 5.9 ± 1.9 | 6.8 ± 0.2 |
| PD + UCN | 73.8 ± 2.9 | 44.8 ± 0.9 |
| U266 | ||
| Control | 3.5 ± 0.8 | 3.2 ± 0.4 |
| PD184352 | 10.5 ± 1.7 | 8.1 ± 0.3 |
| UCN-01 | 7.5 ± 2.2 | 8.6 ± 0.1 |
| PD + UCN | 56.5 ± 11.6 | 37.8 ± 8.0 |
| . | Apoptotic cells (%) . | “Low” Δψm (% cells) . |
|---|---|---|
| 8226 | ||
| Control | 5.9 ± 2.7 | 2.8 ± 1.0 |
| PD184352 | 12.7 ± 2.6 | 12.6 ± 1.6 |
| UCN-01 | 9.1 ± 5.1 | 8.4 ± 1.3 |
| PD + UCN | 61.9 ± 5.9 | 39.6 ± 2.7 |
| H929 | ||
| Control | 3.6 ± 2.6 | 3.5 ± 1.4 |
| PD184352 | 10.0 ± 4.5 | 8.7 ± 0.1 |
| UCN-01 | 5.9 ± 1.9 | 6.8 ± 0.2 |
| PD + UCN | 73.8 ± 2.9 | 44.8 ± 0.9 |
| U266 | ||
| Control | 3.5 ± 0.8 | 3.2 ± 0.4 |
| PD184352 | 10.5 ± 1.7 | 8.1 ± 0.3 |
| UCN-01 | 7.5 ± 2.2 | 8.6 ± 0.1 |
| PD + UCN | 56.5 ± 11.6 | 37.8 ± 8.0 |
8226, H929, and U266 MM cells were exposed for 24 hours to 10 μM PD184352 + 150 nM UCN-01, after which the percentage of morphologically apoptotic cells was determined by evaluating Wright-Giemsa–stained cytospin preparations (left column) as described in “Materials and methods”. Alternatively, cells were treated as above, after which loss of mitochondrial membrane potential (Δψm) was monitored by analyzing DiOC6-treated cells by flow cytometry (right column) as described in “Materials and methods”. Values correspond to the percentage of cells displaying “low” DiOC6 uptake. In each case, values represent the mean ± SD for 3 separate experiments performed in triplicate.
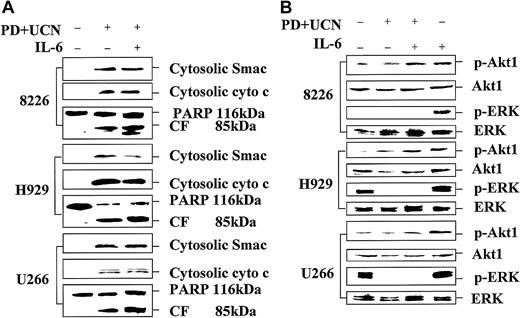
In each cell line, drugs administered individually were minimally toxic, whereas combined treatment resulted in a substantial increase in cell death and mitochondrial damage. Concordant results were obtained when apoptosis was monitored by annexin V/PI staining (data not shown) or the TUNEL assay (Figure 1). In separate studies, sequential exposure to these agents also resulted in an increase in cell death, but simultaneous exposure yielded optimal lethality (data not shown). Similarly, when MM.1S myeloma cells were exposed to other MEK1/2 inhibitors (eg, 20 μM UO126 or 50 μM PD98059) or to 150 nM UCN-01 individually for 24 hours, the percentage of annexin V/PI+ cells was less than 10%. However, combined treatment of cells with UCN-01 + PD98059 or UO126 resulted in an increase in the percentage of annexin V–stained cells to 62% and 77%, respectively (data not shown). Consistent with these findings, combined (but not individual) treatment of MM cells with PD184352 and UCN-01 resulted in PARP degradation into an 85-kDa cleavage fragment and release of the proapoptotic proteins cytochrome c and Smac/DIABLO into the cytosolic S-100 fraction (Figure 2A). Together, these findings indicate that combined treatment with UCN-01 and MEK1/2 inhibitors represents a potent stimulus for mitochondrial damage and apoptosis in MM cells.
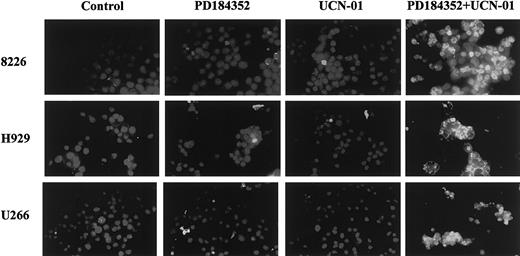
UCN-01 and PD184352 interact synergistically to induce apoptosis in MM cells.
Logarithmically growing 8226, H929, and U266 MM cells were exposed to 10 μM PD184352 ± 150 nM UCN-01 for 24 hours, after which cytospin preparations were obtained and apoptosis assessed by TUNEL assay as described in “Materials and methods.” Slides were viewed under fluorescence microscopy under × 60 magnification. An additional experiment yielded equivalent results.
UCN-01 and PD184352 interact synergistically to induce apoptosis in MM cells.
Logarithmically growing 8226, H929, and U266 MM cells were exposed to 10 μM PD184352 ± 150 nM UCN-01 for 24 hours, after which cytospin preparations were obtained and apoptosis assessed by TUNEL assay as described in “Materials and methods.” Slides were viewed under fluorescence microscopy under × 60 magnification. An additional experiment yielded equivalent results.
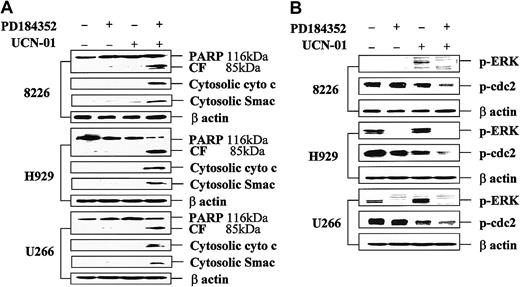
Coadministration of PD184352 and UCN-01 in MM cells results in enhanced cytochrome c and Smac/DIABLO release, PARP degradation, and p34cdc2 dephosphorylation, but diminished ERK activation.
Logarithmically growing 8226, H929, and U266 MM cells were exposed to 10 μM PD184352 ± 150 nM UCN-01 for 24 hours, after which cells were lysed, the proteins separated by SDS-PAGE, and Western blot analysis performed to monitor expression of PARP (A), phospho-ERK (B; p-ERK), or phospho-p34cdc2 (B; p-cdc2) as described in “Materials and methods.” Alternatively, S-100 cytosolic fractions were obtained as described in “Materials and methods,” and expression of cytochrome c (A; cyto c) and Smac/DIABLO (A; Smac) assessed by Western blot analysis. For each condition, lanes were loaded with 30 μg of protein; blots were subsequently stripped and reprobed for expression of β actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results. CF indicates PARP cleavage fragment.
Coadministration of PD184352 and UCN-01 in MM cells results in enhanced cytochrome c and Smac/DIABLO release, PARP degradation, and p34cdc2 dephosphorylation, but diminished ERK activation.
Logarithmically growing 8226, H929, and U266 MM cells were exposed to 10 μM PD184352 ± 150 nM UCN-01 for 24 hours, after which cells were lysed, the proteins separated by SDS-PAGE, and Western blot analysis performed to monitor expression of PARP (A), phospho-ERK (B; p-ERK), or phospho-p34cdc2 (B; p-cdc2) as described in “Materials and methods.” Alternatively, S-100 cytosolic fractions were obtained as described in “Materials and methods,” and expression of cytochrome c (A; cyto c) and Smac/DIABLO (A; Smac) assessed by Western blot analysis. For each condition, lanes were loaded with 30 μg of protein; blots were subsequently stripped and reprobed for expression of β actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results. CF indicates PARP cleavage fragment.
MEK1/2 inhibition blocks UCN-01–mediated ERK phosphorylation while promoting p34cdc2 activation in MM cells
Exposure of 8226 cells to 150 nM UCN-01 for 24 hours resulted in a striking increase in extracellular signal-regulated kinase (ERK) activation, whereas in H929 and U266 cells, which displayed constitutive ERK phosphorylation, the increase was more modest (Figure2B). However, coexposure to PD184352 abrogated ERK activation in each cell line, comparable to results initially observed in human myeloid leukemia cells.25 UCN-01 also induced dephosphorylation of p34cdc2, consistent with its role as an inhibitor of Chk1.19 Moreover, in each MM cell line, coadministration of PD184352 resulted in a pronounced increase in p34cdc2dephosphorylation. Similar findings were obtained when other MEK1/2 inhibitors (eg, UO126 and PD98059) were employed (data not shown). These findings indicate that MEK1/2 inhibition blocks UCN-01–mediated ERK activation and markedly potentiates the capacity of UCN-01 to induce dephosphorylation of p34cdc2 in MM cells.
To confirm that the diminished phosphorylation of p34cdc2was specific for this CDK and corresponded to increased activity, p34cdc2 and CDK2 activities were assessed in drug-treated U266 and MM.1S cells (Figure 3). Consistent with the previous results in which PD184352 was employed, coadministration of UO126 and UCN-01 resulted in a marked decrease in p34cdc2 phosphorylation, but no change in total p34cdc2 expression. Moreover, immune kinase assays demonstrated a clear increase in p34cdc2 activity in cells exposed to both drugs. In contrast, combined exposure to UO126 and UCN-01 did not modify CDK2 phosphorylation status or activity.
Coadministration of UO126 and UCN-01 in MM cells results in dephosphorylation and increased activation of p34cdc2but not CDK2.
U266 and MM.1S cells were exposed to 20 μM UO126 ± 150 nM UCN-01 for 24 hours, after which cells were lysed, the proteins separated by SDS-PAGE, and total/phosphorylated p34cdc2(p-cdc2) and CDK2 (cdk2) were monitored by Western blot analysis. For each condition, lanes were loaded with 30 μg of protein. Alternatively, kinase assays were performed after immunoprecipitation with p34cdc2- and CDK2-specific antibodies as described in “Materials and methods.” The activity of p34cdc2 and CDK2 was determined by monitoring incorporation of γ-[32P] into histone H1, indicated by32P-histone (IP: cdc2). The results of a representative experiment are shown; an additional study yielded equivalent results.
Coadministration of UO126 and UCN-01 in MM cells results in dephosphorylation and increased activation of p34cdc2but not CDK2.
U266 and MM.1S cells were exposed to 20 μM UO126 ± 150 nM UCN-01 for 24 hours, after which cells were lysed, the proteins separated by SDS-PAGE, and total/phosphorylated p34cdc2(p-cdc2) and CDK2 (cdk2) were monitored by Western blot analysis. For each condition, lanes were loaded with 30 μg of protein. Alternatively, kinase assays were performed after immunoprecipitation with p34cdc2- and CDK2-specific antibodies as described in “Materials and methods.” The activity of p34cdc2 and CDK2 was determined by monitoring incorporation of γ-[32P] into histone H1, indicated by32P-histone (IP: cdc2). The results of a representative experiment are shown; an additional study yielded equivalent results.
Combined exposure to UCN-01/PD184352 potently inhibits the self-renewal capacity of MM cells
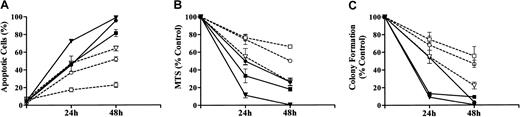
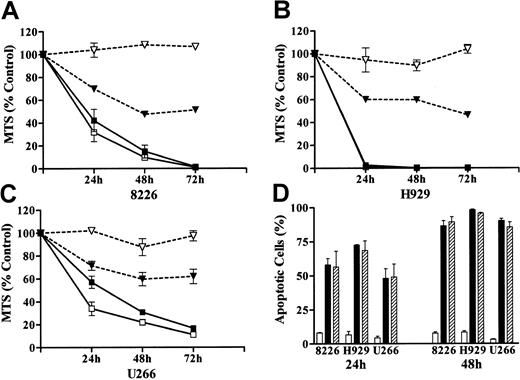
To gain insights into the combined effects of UCN-01 and MEK1/2 inhibitors on the survival and self-renewal capacity of MM cells, parallel studies were performed using a pharmacologically relevant concentration of As203 (ie, 1 μM), an agent that has shown promising activity against MM cells34 (Figure 4). For each of the endpoints examined (ie, induction of apoptosis, MTS dye reduction, loss of clonogenicity), the MM cell lines displayed a differential sensitivity to a 48-hour exposure to As203, with H929 cells the most sensitive, 8226 cells the least sensitive, and U266 cells displaying an intermediate susceptibility (Figure 4). In particular, 8226 cells were quite resistant to As203, in that apoptosis was induced in only ∼20% of cells after 48 hours' exposure, and clonogenicity was reduced by only ∼40%. This general sensitivity pattern was also observed in cells exposed to UCN-01/PD184352. However, in each of the lines, the UCN-01/PD184352 combination was highly toxic, inducing apoptosis in the large majority of cells, and essentially abrogating clonogenic survival.
Coadministration of PD184352 and UCN-01 potently induces loss of viability and clonogenic survival in MM cells
. 8226, H929, and U266 MM cells were exposed to either 1 μM As2O3 or 10 μM PD184352 + 150 nM UCN-01 for 24 or 48 hours, after which the extent of morphological apoptosis (A) or loss of viability, reflected by MTS dye reduction (B), was determined as described in “Materials and methods.” Alternatively, cells were washed free of drug and clonogenic assays performed as described in “Materials and methods” (C). Values represent the means ± SD for 3 separate experiments performed in triplicate. For all panels: ▪ indicates 8226: PD + UCN; ■, 8226: As2O3; ▾, H929: PD + UCN; ▿, H929: As2O3; ●, U266: PD + UCN; ○, U266: As2O3.
Coadministration of PD184352 and UCN-01 potently induces loss of viability and clonogenic survival in MM cells
. 8226, H929, and U266 MM cells were exposed to either 1 μM As2O3 or 10 μM PD184352 + 150 nM UCN-01 for 24 or 48 hours, after which the extent of morphological apoptosis (A) or loss of viability, reflected by MTS dye reduction (B), was determined as described in “Materials and methods.” Alternatively, cells were washed free of drug and clonogenic assays performed as described in “Materials and methods” (C). Values represent the means ± SD for 3 separate experiments performed in triplicate. For all panels: ▪ indicates 8226: PD + UCN; ■, 8226: As2O3; ▾, H929: PD + UCN; ▿, H929: As2O3; ●, U266: PD + UCN; ○, U266: As2O3.
UCN-01/MEK1/2 inhibitor-mediated apoptosis in MM cells, in contrast to that induced by As203, is not antagonized by a free radical scavenger
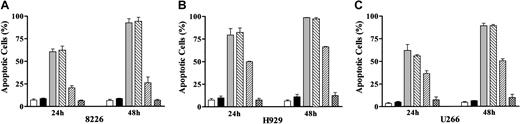
The results of previous studies suggested that As203 induced apoptosis in MM cells through generation of reactive oxygen species (ROS).35 To determine whether a similar mechanism might be responsible for UCN-01/MEK1/2 inhibitor-induced apoptosis, MM cells were exposed to these agents for 48 hours in the presence or absence of the free-radical scavenger L-NAC,36 after which apoptosis was assessed (Figure 5). Coadministration of L-NAC essentially abrogated the lethal effects of As203 in each of the MM cell lines, consistent with the results of earlier studies.35 In marked contrast, L-NAC exerted no effect on UCN-01/MEK1/2 inhibitor-mediated lethality. These findings suggest that the lethal actions of UCN-01/MEK1/2 inhibitors in MM cells involve factors other than or in addition to generation of reactive oxygen species.
The free-radical scavenger L-NAC blocks As203- but not PD184352/UCN-01–induced apoptosis in MM cells.
8226 (A), H929 (B), and U266 (C) MM cells were exposed to either 1 μM As2O3 or 10 μM PD184352 + 150 nM UCN-01 for 24 or 48 hours after 2 hours pretreatment of 10 mM L-NAC, after which the percentage of apoptotic cells was determined by evaluating Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” For all panels: ■ indicates control; ▪, LNAC; ░, PD + UCN; ▧, PD + UCN + LNAC; ▨, As2O3; ▩, As2O3 + LNAC. Values represent the means ± SD for 3 separate experiments performed in triplicate.
The free-radical scavenger L-NAC blocks As203- but not PD184352/UCN-01–induced apoptosis in MM cells.
8226 (A), H929 (B), and U266 (C) MM cells were exposed to either 1 μM As2O3 or 10 μM PD184352 + 150 nM UCN-01 for 24 or 48 hours after 2 hours pretreatment of 10 mM L-NAC, after which the percentage of apoptotic cells was determined by evaluating Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” For all panels: ■ indicates control; ▪, LNAC; ░, PD + UCN; ▧, PD + UCN + LNAC; ▨, As2O3; ▩, As2O3 + LNAC. Values represent the means ± SD for 3 separate experiments performed in triplicate.
UCN-01/MEK1/2 inhibitor-induced apoptosis proceeds through IL-6– and IGF-1–independent pathways
In addition to its role in promoting MM cell survival, IL-6 also has been shown to protect MM cells from the lethal actions of cytotoxic agents, including dexamethasone.37 To determine whether and to what extent IL-6 might exert a similar function in cells exposed to UCN-01/MEK1/2 inhibitors, MM cells were exposed to these agents for 72 hours in the presence or absence of 100 ng/mL IL-6 (Figure6). Consistent with earlier results,37 exogenous IL-6 essentially abrogated the lethal effects of 10 μM dexamethasone in each of the MM cell lines, reflected by loss of viability as determined by the MTS assay. Similar results were obtained when apoptosis was monitored (data not shown). In marked contrast, IL-6 failed to attenuate UCN-01/PD184352–mediated lethality in any of the MM cell lines. Similarly, IGF-1 (400 ng/mL), which as also been shown to act as survival factor in MM,37 was unable to block apoptosis in UCN-01/MEK1/2 inhibitor–treated cells (Figure 6D). These findings indicate that the lethal actions of the UCN-01/MEK1/2 inhibitor combination in MM cells operate downstream of IL-6 and IGF-1 cytoprotective pathways.
PD184352/UCN-01–induced apoptosis proceeds via IL-6– and IGF-1–independent pathways in MM cells.
8226 (A), H929 (B), and U266 (C) MM cells were exposed to 10 μM PD184352 + 150 nM UCN-01 or 10 μM dexamethasone for 24 to 72 hours in the presence or absence of 100 ng/mL IL-6, after which the loss of viability, reflected by MTS dye reduction, was determined as described in “Materials and methods.” Alternatively, cells were exposed to PD184352 + UCN-01 for 24 or 48 hours in the presence or absence of 400 ng/mL IGF-1, after which the percentage of apoptotic cells was determined (D) by examining Wright-Giemsa–stained specimens as described in “Materials and methods.” For panels A, B, and C: ▪ indicates PD + UCN; ■, PD + UCN + IL-6; ▾, Dex; ▿, DEX + IL-6. For panel D: ■ indicates control; ▪, PD + UCN; ▨, PD + UCN + IGF-1. In all cases, values represent the means ± SD for 3 separate experiments performed in triplicate.
PD184352/UCN-01–induced apoptosis proceeds via IL-6– and IGF-1–independent pathways in MM cells.
8226 (A), H929 (B), and U266 (C) MM cells were exposed to 10 μM PD184352 + 150 nM UCN-01 or 10 μM dexamethasone for 24 to 72 hours in the presence or absence of 100 ng/mL IL-6, after which the loss of viability, reflected by MTS dye reduction, was determined as described in “Materials and methods.” Alternatively, cells were exposed to PD184352 + UCN-01 for 24 or 48 hours in the presence or absence of 400 ng/mL IGF-1, after which the percentage of apoptotic cells was determined (D) by examining Wright-Giemsa–stained specimens as described in “Materials and methods.” For panels A, B, and C: ▪ indicates PD + UCN; ■, PD + UCN + IL-6; ▾, Dex; ▿, DEX + IL-6. For panel D: ■ indicates control; ▪, PD + UCN; ▨, PD + UCN + IGF-1. In all cases, values represent the means ± SD for 3 separate experiments performed in triplicate.
IL-6 fails to block UCN-01/MEK1/2 inhibitor-induced mitochondrial damage in MM cells
To determine whether the failure of IL-6 to attenuate UCN-01/PD184352–induced apoptosis in MM cells originated at the mitochondrial level, cytochrome c and Smac/DIABLO release were monitored in cells exposed to these agents (Figure7A). In each of the MM cell lines, coadministration of IL-6 with UCN-01/PD184352 failed to attenuate cytochrome c and Smac/DIABLO release or PARP degradation. These findings suggest that UCN-01/MEK1/2 inhibitor-induced mitochondrial damage occurs independently of IL-6–related cytoprotective signaling pathways.
IL-6 fails to block PD184352/UCN-01–mediated mitochondrial damage despite enhancing Akt phosphorylation.
(A) 8226, H929, and U266 MM cells were exposed to 10 μM PD184352 + 150 nM UCN-01 in the presence or absence of 100 ng/mL IL-6 for 24 hours, after which cells were lysed and Western blot analysis performed to monitor PARP cleavage (whole cell lysates) or cytochrome c and Smac/DIABLO release (S-100 fractions) as described in “Materials and methods.” (B) Alternatively, Western blot analysis was performed to assess the effects of these agents on phosphorylation of Akt and ERK as well as total ERK expression. In each case, lanes were loaded with 30 μg of protein. The results of a representative experiment are shown; a second study yielded equivalent results.
IL-6 fails to block PD184352/UCN-01–mediated mitochondrial damage despite enhancing Akt phosphorylation.
(A) 8226, H929, and U266 MM cells were exposed to 10 μM PD184352 + 150 nM UCN-01 in the presence or absence of 100 ng/mL IL-6 for 24 hours, after which cells were lysed and Western blot analysis performed to monitor PARP cleavage (whole cell lysates) or cytochrome c and Smac/DIABLO release (S-100 fractions) as described in “Materials and methods.” (B) Alternatively, Western blot analysis was performed to assess the effects of these agents on phosphorylation of Akt and ERK as well as total ERK expression. In each case, lanes were loaded with 30 μg of protein. The results of a representative experiment are shown; a second study yielded equivalent results.
UCN-01/MEK1/2 inhibitor-induced apoptosis occurs in IL-6–treated MM cells despite Akt activation
Recent studies suggest that in MM cells, IL-6–related cytoprotective effects involve activation of the PI3K/Akt cascade.9 Consistent with these results, exposure of each of the MM lines to IL-6 resulted in Akt phosphorylation (Figure 7B). However, despite the failure of IL-6 to attenuate UCN-01/MEK1/2 inhibitor-induced apoptosis in MM cells (Figure 6), IL-6 administration continued to increase Akt phosphorylation over basal levels in UCN-01/PD184352–treated cells. IL-6 also increased ERK activation in each MM cell line, but this effect was abrogated in cells exposed to UCN-01/PD184352 (Figure 7B). Lastly, coadministration of the PI3K inhibitor LY294002 (10 μM) failed to modify UCN-01/PD184352–mediated lethality in any of the MM cell lines, although slight increases in UCN-01–induced apoptosis were noted (data not shown). Together, these findings argue that factors other than or in addition to perturbations in the PI3K/Akt pathway are responsible for MM cell death following exposure to UCN-01/MEK1/2 inhibitors.
UCN-01/PD184352 effectively induces apoptosis in MM cells resistant to doxorubicin or melphalan
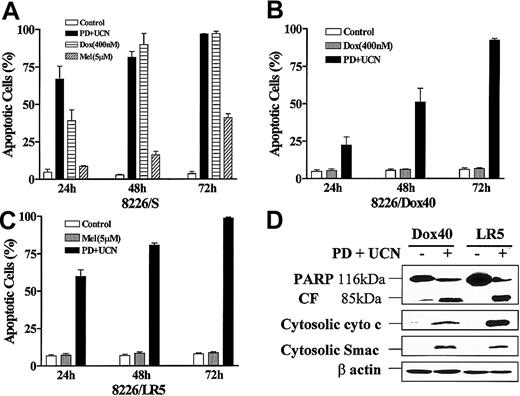
To determine whether MM cells resistance to standard chemotherapeutic drugs would extend to the UCN-01/MEK1/2 inhibitor combination, doxorubicin-resistant (8226/Dox40)27 and melphalan-resistant (8226/LR5)28 cells were treated with 10 μM PD184352 ± 150 nM UCN-01 for 24 to 72 hours, after which the extent of apoptosis was assessed (Figure8). Whereas sensitive cells (8226/S) were highly susceptible to 400 nM doxorubicin and 5 μM melphalan (Figure8A), the corresponding resistant lines were essentially immune to these agents (Figure 8B,C). Equivalent results were obtained when loss of ΔΨm was monitored (data not shown). In marked contrast, both 8226/Dox40 and 8226/LR5 cells were as sensitive to the PD184352/UCN-01 combination as parental cells, with approximately 100% of cells undergoing apoptosis after 72 hours. Western analysis confirmed that induction of cytochrome c/Smac/DIABLO release and PARP degradation by the combination of UCN-01 and PD184352 in 8226/Dox40 and 8226/LR5 cells (Figure 8D) was equivalent to effects observed in parental cells (Figure 2A). These findings indicate that MM cells highly resistant to doxorubicin or melphalan remain fully sensitive to mitochondrial injury and apoptosis induced by UCN-01/PD184352.
MM cells resistant to doxorubicin or melphalan retain full sensitivity to UCN-01/PD184352.
Logarithmically growing parental 8226 cells (8226/S; A), a doxorubicin-resistant cell line (8226/Dox40; B), and a melphalan-resistant line (8226/LR5; C) were exposed to 10 μM PD184352 + 150 nM UCN-01, 400 nM doxorubicin (Dox), or 5 μM melphalan (Mel) for 24 to 72 hours, after which the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” Values represent the means ± SD for 3 separate experiments performed in triplicate. (D) 8226/Dox40 and 8226/LR5 cells were exposed to PD184352 + UCN-01 as above for 48 hours and 24 hours, respectively, after which Western blot analysis was employed to monitor degradation of PARP into an 85-kDa fragment (CF) or release of cytochrome c and Smac/DIABLO into the cytosolic S-100 fraction as described above. Lanes were loaded with 30 μg of protein; blots were subsequently stripped and reprobed for expression of β actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results.
MM cells resistant to doxorubicin or melphalan retain full sensitivity to UCN-01/PD184352.
Logarithmically growing parental 8226 cells (8226/S; A), a doxorubicin-resistant cell line (8226/Dox40; B), and a melphalan-resistant line (8226/LR5; C) were exposed to 10 μM PD184352 + 150 nM UCN-01, 400 nM doxorubicin (Dox), or 5 μM melphalan (Mel) for 24 to 72 hours, after which the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” Values represent the means ± SD for 3 separate experiments performed in triplicate. (D) 8226/Dox40 and 8226/LR5 cells were exposed to PD184352 + UCN-01 as above for 48 hours and 24 hours, respectively, after which Western blot analysis was employed to monitor degradation of PARP into an 85-kDa fragment (CF) or release of cytochrome c and Smac/DIABLO into the cytosolic S-100 fraction as described above. Lanes were loaded with 30 μg of protein; blots were subsequently stripped and reprobed for expression of β actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results.
MM cells highly resistant to dexamethasone retain their sensitivity to combined treatment with UCN-01 and PD184352
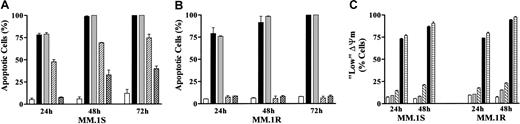
Using a previously described dexamethasone-resistant myeloma cell line (MM.1R),26 attempts were made to determine whether cross-resistance to the lethal effects of UCN-01/PD184352 would occur. As shown in Figure 9A,B, MM.1R cells were highly resistant to dexamethasone-induced apoptosis but retained full sensitivity to apoptosis induced by 150 nM UCN-01 + 10 μM PD184352. Furthermore, while IL-6 significantly attenuated dexamethasone-induced apoptosis, it had no effect on PD184352/UCN-01–induced cell death in either cell line. Similar results were obtained when UO126 was employed instead of PD184352 (data not shown). Concordant results were also obtained when the reduction in ΔΨm was monitored (Figure9C).
IL-6 fails to protect dexamethasone-sensitive and -resistant MM cells from the lethal actions of UCN-01/PD184352.
Logarithmically growing dexamethasone-sensitive (MM.1S; Figure 9A) and -resistant MM cells (MM.1R; Figure 9B) were exposed to 10 μM PD184352 + 150 nM UCN-01 or 10 μM dexamethasone in the presence or absence of 100 ng/mL IL-6 for 24 to 72 hours, after which the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin preparations. Alternatively, MM.1S and MM.1R cells were treated as above for 24 or 48 hours, after which the percentage of cells displaying a reduction in ΔΨm was determined (panel C) as described in “Materials and methods.” For panels A and B: ■ indicates Control; ▪, PD + UCN; ░, PD + U + IL-6; ▨, Dex; ▩, Dex + IL-6. For panel C: ■ indicates Control; ░, PD184352; ▧, UCN-01; ▪, PD + UCN; ▤, PD + UCN + IL-6. For all panels, values represent the means ± SD for 3 separate experiments performed in triplicate.
IL-6 fails to protect dexamethasone-sensitive and -resistant MM cells from the lethal actions of UCN-01/PD184352.
Logarithmically growing dexamethasone-sensitive (MM.1S; Figure 9A) and -resistant MM cells (MM.1R; Figure 9B) were exposed to 10 μM PD184352 + 150 nM UCN-01 or 10 μM dexamethasone in the presence or absence of 100 ng/mL IL-6 for 24 to 72 hours, after which the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin preparations. Alternatively, MM.1S and MM.1R cells were treated as above for 24 or 48 hours, after which the percentage of cells displaying a reduction in ΔΨm was determined (panel C) as described in “Materials and methods.” For panels A and B: ■ indicates Control; ▪, PD + UCN; ░, PD + U + IL-6; ▨, Dex; ▩, Dex + IL-6. For panel C: ■ indicates Control; ░, PD184352; ▧, UCN-01; ▪, PD + UCN; ▤, PD + UCN + IL-6. For all panels, values represent the means ± SD for 3 separate experiments performed in triplicate.
Finally, consistent with results obtained in other MM cell lines (Figure 2B), both MM.1S and MM.1R cells displayed activation of MAP kinase following UCN-01 exposure, an effect that was abrogated by PD184352. Moreover, the combination of UCN-01 and PD184352, but not drugs administered alone, induced an equivalent increase in cytosolic release of cytochrome c and Smac/DIABLO as well as PARP degradation in dexamethasone-sensitive and -resistant MM cells (data not shown). These results indicate that a high level of resistance to dexamethasone provides MM cells essentially no protection from the mitochondrial injury and lethality of the UCN-01/MEK1/2 inhibitor combination.
The UCN-01/PD184352 regimen is active against MM cells exhibiting cell adherence–associated drug resistance
Based upon recent evidence that induction of MM cell adherence (eg, by fibronectin) confers resistance to multiple classes of cytotoxic agents,32 attempts were made to determine whether such treatment would protect MM cells from exposure to UCN-01/PD184352. As shown in Figure 10, treatment of MM.1S and MM.1R with fibronectin significantly reduced the toxicity of both doxorubicin and melphalan, consistent with results previously reported by Damiano et al in 8226 MM cells.32However, in marked contrast to these findings, fibronectin-adhered cells remained fully sensitive to PD184352/UCN-01–mediated apoptosis. These observations suggest that the lethal effects of checkpoint abrogation/MEK1/2 inhibition, unlike conventional cytotoxic agents, are minimally attenuated by cytoprotective signaling events associated with the adherent phenotype.
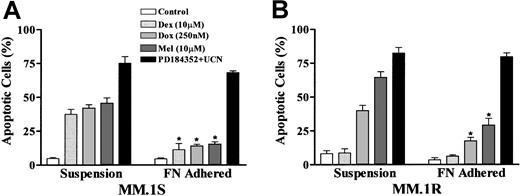
Fibronectin-adhered MM cells remain sensitive to UCN-01/PD184352–induced apoptosis.
MM.1S and MM.1R cells were seeded into 96–well plates coated with fibronectin, and cells remaining in suspension removed as described in “Materials and methods.” Adherent cells and cells in suspension were separately exposed to 250 nM doxorubicin (Dox), 10 μM melphalan (Mel), 10 μM dexamethasone (Dex), or 10 μM PD184352 + 150 nM UCN-01 for 24 hours, after which the extent of apoptosis was determined by evaluating Wright-Giemsa–stained cytospin preparations as described previously. Values represent the means ± SD for 3 separate experiments performed in triplicate. * indicates data values are significantly less than values for cells in suspension; P < .01.
Fibronectin-adhered MM cells remain sensitive to UCN-01/PD184352–induced apoptosis.
MM.1S and MM.1R cells were seeded into 96–well plates coated with fibronectin, and cells remaining in suspension removed as described in “Materials and methods.” Adherent cells and cells in suspension were separately exposed to 250 nM doxorubicin (Dox), 10 μM melphalan (Mel), 10 μM dexamethasone (Dex), or 10 μM PD184352 + 150 nM UCN-01 for 24 hours, after which the extent of apoptosis was determined by evaluating Wright-Giemsa–stained cytospin preparations as described previously. Values represent the means ± SD for 3 separate experiments performed in triplicate. * indicates data values are significantly less than values for cells in suspension; P < .01.
Coadministration of UCN-01 with a MEK1/2 inhibitor displays selective lethality toward primary bone marrow CD138+MM cells
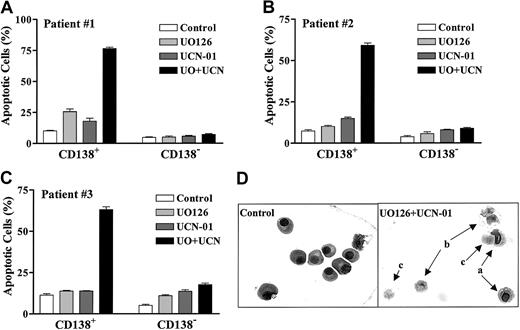
To determine whether the UCN-01/MEK inhibitor strategy would be lethal toward primary MM specimens, CD138+ MM cells were isolated from the bone marrow of MM patients as previously described.33 Samples #1 and #2 were obtained from patients who had progressed following therapy, whereas sample #3 was obtained from a newly diagnosed patient. The cells were then exposed to UO126 ± UCN-01 for 24 hours, after which the extent of apoptosis was determined by morphological analysis of Wright-Giemsa–stained cytospin preparations. As shown in Figure11A-C, coadministration of UO126 + UCN-01 resulted in a clear increase in apoptosis in each of the 3 primary specimens evaluated. Interestingly, UO126 + UCN-01 exerted relatively minimal toxicity toward the CD138− cell population, raising the possibility that primary MM cells may exhibit selective sensitivity to this regimen. Representative photomicrographs of pretreatment and posttreatment MM specimens (obtained from patient #2; Figure 11D) illustrate the striking increase in MM cell death following ex vivo UCN-01/UO126 exposure.
Primary CD138+ MM cells display selective sensitivity to UCN-01/UO126–induced apoptosis.
CD138+ and CD138− cells were isolated from the bone marrow of MM patients as described in “Materials and methods.” The cells were exposed individually or in combination to drugs as follows: UO126 (A: 25 μM; B and C: 20 μM); UCN-01 (A: 150 nM; B: 250 nM; C: 100 nM). After 24 hours' treatment, the extent of apoptosis was determined by evaluating Wright-Giemsa–stained cytospin preparations as described previously. Values represent the means ± SD for more than 20 randomly selected fields encompassing more than 300 cells. Representative microphotographs of cells obtained from patient #2 are shown in D, a: early apoptotic cells; b: late apoptotic cells; c: postapoptotic (ghost) cells.
Primary CD138+ MM cells display selective sensitivity to UCN-01/UO126–induced apoptosis.
CD138+ and CD138− cells were isolated from the bone marrow of MM patients as described in “Materials and methods.” The cells were exposed individually or in combination to drugs as follows: UO126 (A: 25 μM; B and C: 20 μM); UCN-01 (A: 150 nM; B: 250 nM; C: 100 nM). After 24 hours' treatment, the extent of apoptosis was determined by evaluating Wright-Giemsa–stained cytospin preparations as described previously. Values represent the means ± SD for more than 20 randomly selected fields encompassing more than 300 cells. Representative microphotographs of cells obtained from patient #2 are shown in D, a: early apoptotic cells; b: late apoptotic cells; c: postapoptotic (ghost) cells.
Discussion
The present results indicate that combined exposure to a clinically relevant concentration of UCN-01 in conjunction with pharmacologic MEK1/2 inhibitors potently induces programmed cell death in MM cells, including those selected for resistance to a variety of agents useful in the treatment of this disease, including melphalan, doxorubicin, and dexamethasone. Despite recent advances in our understanding of the molecular pathogenesis of MM, survival of patients with this disorder has not changed appreciably over the last several decades.1,2 Consequently, attention has begun to focus on the development of novel agents that target specific MM survival pathways. These have included thalidomide, an inhibitor of the VEGF signaling cascade38; proteasome inhibitors such as PS341, which interfere with NFκB cytoprotective functions39; and As2O3, which promotes the formation of lethal free-radical species in MM cells.35 However, the concept of combining novel signal transduction and cell cycle inhibitors in MM or other hematologic malignancies remains relatively unexplored. The data presented here indicate that combined treatment with UCN-01, a checkpoint abrogator in clinical evaluation23 and pharmacologic MEK1/2 inhibitors, including PD184352, which is now in phase I trials,17represents an extremely potent stimulus for mitochondrial injury and apoptosis in MM cells. Taken in conjunction with observations involving myeloid leukemia cells,25 these findings raise the possibility that malignant hematopoietic cells may be particularly susceptible to a strategy in which cell cycle regulatory and cytoprotective signaling pathways are simultaneously interrupted.
While the mechanism(s) by which MEK1/2 inhibition potentiates UCN-01–induced apoptosis in MM cells remains to be defined, it is tempting to speculate that inappropriate activation of p34cdc2 may be involved. For example, consistent with its role as an inhibitor of Chk1,19 UCN-01 induced dephosphorylation (activation) of p34cdc2 in each of the MM cell lines (Figure 2). Moreover, this event was enhanced by coadministration of PD184352. As unscheduled activation of p34cdc2 in hematopoietic cells represents a potent stimulus for apoptosis,40 the cytoprotective actions of MAP kinase activation may serve to limit this process. Such a concept could help to explain the observed potentiation of p34cdc2dephosphorylation and activation in UCN-01/PD184352–treated MM cells. In this context, a requirement for MAP kinase activation in G2M progression, an event that is regularly accompanied by p34cdc2 activation,41 would be fully consistent with this model.
Although activation of the MAP kinase pathway is generally known to exert antiapoptotic actions,10 in MM cells it appears to play a specific role in IL-6–related cytoprotection. Moreover, the ability of UCN-01/MEK1/2 inhibitors to induce MM cell death in an IL-6–independent manner is entirely compatible with this notion. For example, the finding that the MEK1/2 inhibitor PD98059 prevents IL-6 from protecting OPM-6 MM cells from dexamethasone-mediated cell death37 implicates the MAP kinase pathway in IL-6 survival functions. It also suggests that activation of the latter pathway represents a downstream consequence of IL-6 actions. However, while administration of IL-6 resulted in MAP kinase activation in each of the MM cell lines examined here (Figure 7), this effect was abrogated in UCN-01/PD184352–treated cells. Thus, administration of MEK1/2 inhibitors blocked MAP kinase activation by exogenous IL-6, UCN-01, as well as the combination of these agents. It should be noted that recently histone deacetylase inhibitors (HDIs) have been shown to trigger apoptosis in MM cells in an IL-6–independent manner.42 The relationship between this phenomenon and effects on MAP kinase activation remains to be defined. Finally, the cytoprotective effects of IL-6 and IGF-1 in MM cells have recently been related to the PI3K/Akt pathway.9 The ability of UCN-01/PD184352 to trigger MM cell apoptosis in the presence of IL-6 and despite Akt activation suggests that these agents act independently, or at a point downstream, of Akt actions.
The inability of the free-radical scavenger L-NAC to block UCN-01/PD184352–induced apoptosis in MM cells argues that this process proceeds through an ROS-independent mechanism. Although ROS generation has been implicated in the induction of apoptosis by various cytotoxic agents,43 in some cases it may represent a secondary event stemming from mitochondrial injury and disruption of the mitochondrial respiratory chain.44 It is noteworthy that As2O3, which also potently induces apoptosis in MM cells, including those resistant to conventional cytotoxic agents, appears to trigger cell death through a ROS-dependent process.35 The finding that UCN-01/PD184352 also effectively induced cell death in drug-refractory MM cell lines indicates circumvention of conventional drug resistance in MM cells can involve apoptotic pathways operating independently of ROS.
Previous studies have demonstrated that in MM cells, cell death induced by different stimuli can elicit distinct forms of mitochondrial injury. For example, in MM.1S cells, ionizing radiation primarily induced cytochrome c release, whereas dexamethasone triggered release of Smac/DIABLO.45 In contrast, UCN-01/PD184352 induced, although to varying extents, cytosolic distribution of both cytochrome c and Smac/DIABLO in each of the MM cell lines. The relative contributions of these proteins to UCN-01/PD184352–induced lethality is unclear. In this context, recent studies in prostate cancer cells suggest that Smac/DIABLO release following PI3K/Akt inhibitor LY294002 exposure is necessary for cytochrome c–mediated activation of the apoptotic cascade.46 Whether similar events occur in MM cells remains to be determined.
Resistance of 8226/Dox40 cells to doxorubicin has been attributed to increased expression of P-glycoprotein (Pgp), leading to reduced intracellular drug accumulation.27 Although enhanced expression of Pgp has been shown to reduce, albeit weakly, uptake of small molecule inhibitors such as flavopiridol,47 it has been found to be somewhat more effective in diminishing the toxicity of UCN-01 in MCF-7 breast cancer cells.48 However, the finding that Dox40 MM cells were fully sensitive to UCN-01/PD184352 toxicity argues that Pgp-related mechanisms do not play a major role in determining the response of MM cells to either of these agents. Resistance of MM.1R cells to dexamethasone has been attributed to a mutation in the steroid receptor.26 In view of the presumed distal site of action for the UCN-01/PD184352 combination (ie, downstream of IL-6/MEK/MAP kinase signaling), it seems likely that this regimen simply bypasses the block to steroid action. The basis for alkylating-agent resistance in MM or other neoplastic cells may be multifactorial, including diminished drug uptake, decreased DNA binding, increased glutathione (GSH) levels, or enhanced repair mechanisms, among others.49 The unimpaired ability of UCN-01/PD184352 to induce apoptosis in 8226/LR5 cells suggests that none of these mechanisms is involved in conferring resistance to this drug combination. Finally, the phenomenon of cell adhesion–mediated drug resistance (CAM-DR) has recently been described in MM cells, suggesting that integrins can raise the threshold for drug-induced apoptosis.32 Although the mechanism underlying this phenomenon remains to be elucidated, the present findings indicate that the lethal effects of the UCN-01/PD184352 combination operate independently of adhesion-related survival pathways.
In summary, the present findings indicate that combined treatment with the checkpoint abrogator UCN-01 and the MEK1/2 inhibitor PD184352, both of which are currently in clinical trials, represents a highly potent trigger for mitochondrial damage and apoptosis in MM cells. These events are associated with inhibition of UCN-01–mediated activation of MAP kinase and enhanced activation of p34cdc2. They also indicate that UCN-01/PD184352-mediated cell death proceeds in an IL-6–independent manner, and in contrast to As2O3, is not inhibitable by free-radical scavengers. In addition, this drug combination effectively induces apoptosis in MM cells that are resistant to 3 cytotoxic agents widely used in the MM treatment, ie, dexamethasone, melphalan, and doxorubicin, as well as in cells displaying adhesion-related drug resistance. Finally, this strategy is active against at least some primary MM cell samples ex vivo. A theoretical model that might provide a framework for understanding the present findings is illustrated in Figure 12. UCN-01, by inhibiting Chk1, spares cdc25C from proteasomal degradation, thereby activating p34cdc2, which is known to be a potent apoptotic stimulus.40 The compensatory activation of MEK1/2 and ERK1/2, which lie downstream of IL-6, IGF-1, and integrin-stimulated PKC/Ras/Raf-related pathways, may permit cells to survive the inappropriate activation of p34cdc2 in UCN-01–treated cells. Conversely, abrogation of the compensatory ERK1/2 response might render cells particularly sensitive to UCN-01–induced mitochondrial injury and apoptosis. Such a model could also account for the failure of effectors such as IL-6, IFG-1, and integrins, which signal upstream of the Ras/Raf/MEK/MAP kinase cascade, to circumvent UCN-01/MEK1/2 inhibitor-associated lethality. However, the possibility that MEK inhibitors may induce perturbations in NFκB, which has been implicated in MM cell survival decision,39 cannot be excluded, nor can a direct effect of MEK inhibitors on cdc25C activity be ruled out. In any case, the present findings suggest that MM cells may be highly sensitive to a therapeutic strategy in which cell cycle regulatory and survival signaling pathways are simultaneously disrupted. However, the issue of therapeutic selectivity will clearly play an important role in determining the ultimate use of this approach. In this regard, we have recently observed that UCN-01/MEK1/2 inhibitor regimens exhibit relatively modest toxicity toward normal human peripheral blood mononuclear cells, bone marrow CD34+cells, primary rodent hepatocytes, and in this study, CD138− bone marrow cells.50 In view of these considerations, efforts to develop this strategy in MM and possibly in other B-cell malignancies appear warranted.
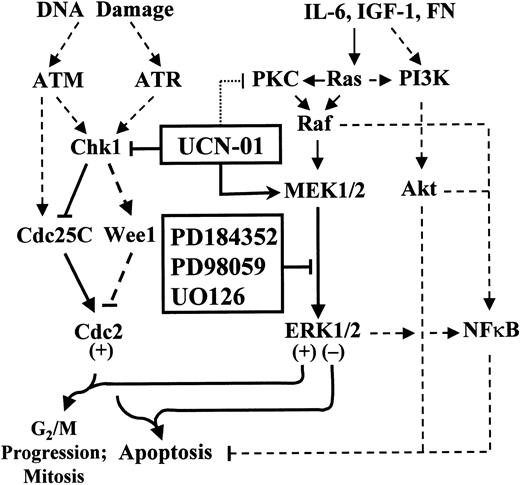
Model of UCN-01 and MEK1/2 inhibitor interactions in MM cells.
The DNA damage response genes ATM andATR activate Chk1, which phosphorylates the Cdc25C phosphatase, leading to its proteasomal degradation. Inhibition of Chk1 phosphorylation results in activation of p34cdc2, which, if unscheduled, leads to apoptosis. UCN-01, by inhibiting Chk1 phosphorylation, spares Cdc25C, which in turn promotes activation (dephosphorylation) of p34cdc2. The putative proapoptotic actions of activated p34cdc2 may be opposed by a compensatory activation of the cytoprotective Raf/MEK/MAP kinase cascade, which is also stimulated by several MM survival factors, including IL-6, IGF-1, and integrins. Blocking the MEK/MAP kinase cascade (eg, by pharmacologic MEK1/2 inhibitors) downstream of IL-6–, IGF-1–, and integrin-related actions (eg, fibronectin, FN) may thus render MM cells particularly vulnerable to the lethal actions of UCN-01. The contribution of the NFκB axis, which is linked to both ERK1/2 and PI3K/Akt (dashed lines), to these events remains to be fully elucidated. Finally, the possibility that MEK1/2 inhibitors act directly on cdc2 regulatory molecules (ie, cdc25C, or Wee1) cannot be excluded.
Model of UCN-01 and MEK1/2 inhibitor interactions in MM cells.
The DNA damage response genes ATM andATR activate Chk1, which phosphorylates the Cdc25C phosphatase, leading to its proteasomal degradation. Inhibition of Chk1 phosphorylation results in activation of p34cdc2, which, if unscheduled, leads to apoptosis. UCN-01, by inhibiting Chk1 phosphorylation, spares Cdc25C, which in turn promotes activation (dephosphorylation) of p34cdc2. The putative proapoptotic actions of activated p34cdc2 may be opposed by a compensatory activation of the cytoprotective Raf/MEK/MAP kinase cascade, which is also stimulated by several MM survival factors, including IL-6, IGF-1, and integrins. Blocking the MEK/MAP kinase cascade (eg, by pharmacologic MEK1/2 inhibitors) downstream of IL-6–, IGF-1–, and integrin-related actions (eg, fibronectin, FN) may thus render MM cells particularly vulnerable to the lethal actions of UCN-01. The contribution of the NFκB axis, which is linked to both ERK1/2 and PI3K/Akt (dashed lines), to these events remains to be fully elucidated. Finally, the possibility that MEK1/2 inhibitors act directly on cdc2 regulatory molecules (ie, cdc25C, or Wee1) cannot be excluded.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-03-0940.
Supported by awards CA 63753, CA 83705, DK 52855, and CA 88906 from the National Institutes of Health, and an award from the Multiple Myeloma Research Foundation.
Portions of this work were presented in preliminary form at the meeting of the American Society of Hematology, Orlando, FL, December 7-11, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven Grant, Division of Hematology/Oncology, Medical College of Virginia, MCV Station, Box 230, Richmond, VA 23298; e-mail: stgrant@hsc.vcu.edu.

![Fig. 3. Coadministration of UO126 and UCN-01 in MM cells results in dephosphorylation and increased activation of p34cdc2but not CDK2. / U266 and MM.1S cells were exposed to 20 μM UO126 ± 150 nM UCN-01 for 24 hours, after which cells were lysed, the proteins separated by SDS-PAGE, and total/phosphorylated p34cdc2(p-cdc2) and CDK2 (cdk2) were monitored by Western blot analysis. For each condition, lanes were loaded with 30 μg of protein. Alternatively, kinase assays were performed after immunoprecipitation with p34cdc2- and CDK2-specific antibodies as described in “Materials and methods.” The activity of p34cdc2 and CDK2 was determined by monitoring incorporation of γ-[32P] into histone H1, indicated by32P-histone (IP: cdc2). The results of a representative experiment are shown; an additional study yielded equivalent results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood-2002-03-0940/4/m_h82123341003.jpeg?Expires=1770911999&Signature=AvG52hluBTmAhv8fQ1yEOh3wi5sI8qXYom3~UBcxILCLg51AT2JJN6KA7aDAZK36U~aepRj3wKRX~wMm0sdbmKY9gCpWN0UR~fQpY3oKUNiC8-5r4ziB8lKUMezpvRrvMBccsat9Ktei-0i9fX1c9Au4XT1S0nX93kFtLgs0cRLT42Tp90IoR4b2C5Ko2vJmuRNU9leEJHpyZaYuCw0anGBndX4a9X~H07vJmDg~-VleC5-FEg63wyjG6VLAN77d6FjaQMEZH2FWNRFRC6Uq7YnipDSs-BpPNU28ErgG8cWnzr1zKFRA74o3pqP4dx3xovw69nSrKqH9J2BgnhDU2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal