The potential benefits of unrelated donor marrow transplantation are offset by the immunologic complications of graft-versus-host disease (GVHD) and infection. Therefore, we used cryopreserved umbilical cord blood (UCB) as a strategy to reduce the risks of GVHD and treatment-related mortality (TRM) and improve survival. Data on 102 patients (median age 7.4 years) who received transplants between 1994 and 2001 for the treatment of malignant (n = 65; 68% were high-risk patients) and nonmalignant (n = 37) diseases were evaluated. Log-rank tests and Cox regression analyses were used to determine the effects of various demographic, graft-related, and treatment factors on engraftment, GVHD, TRM, relapse, and survival. As of October 15, 2001, the median follow-up was 2.7 years (range, 0.3-7.2). Incidences of neutrophil and platelet engraftment were 0.88 (CI, 0.81-0.95) and 0.65 (CI, 0.53-0.77), respectively. Notably, incidences of severe acute and chronic GVHD were 0.11 (CI, 0.05-0.17) and 0.10 (CI, 0.04-0.16), respectively. At 1 year after transplantation, proportions of TRM and survival were 0.30 (CI, 0.21-0.39) and 0.58 (CI, 0.48-0.68), respectively. In Cox regression analyses, CD34 cell dose was the one factor consistently identified as significantly associated with rate of engraftment, TRM, and survival. Despite the low incidence of GVHD, the proportion of patients with leukemia relapse at 2 years was 0.17 (CI, 0.00-0.38) and 0.45 (CI, 0.28-0.61) for patients with standard and high-risk disease, respectively. There is a high probability of survival in recipients of UCB grafts that are disparate in no more than 2 human leukocyte antigens (HLAs) when the grafts contain at least 1.7 × 105 CD34+ cells per kilogram of recipient's body weight. Therefore, graft selection should be based principally on CD34 cell dose when multiple UCB units exist with an HLA disparity of 2 or less.
Introduction
Transplantation of hematopoietic stem cells (HSCs) derived from bone marrow and umbilical cord blood (UCB) of sibling and unrelated donors has been used successfully to treat patients with high-risk or recurrent hematological malignancies, bone marrow failure syndromes, selected hereditary immunodeficiency states, and metabolic disorders.1-10 Successful use of HSC transplant therapy, however, has been limited by a lack of human leukocyte antigen (HLA)–matched donors and the high risk of graft-versus-host disease (GVHD) after transplantation. Although there are currently 7 million donors typed for HLA-A, HLA-B, and HLA-DR registered in marrow donor registries around the world, more than 30% of patients requiring transplant therapy are still unable to find a donor disparate in no more than one HLA,11 and the proportion of unsuccessful searches is even greater among patients who are not of Northern European descent. For patients receiving unrelated donor marrow, a single HLA disparity has clearly been shown to increase the risk of treatment-related mortality (TRM) and adversely affect survival, attributable in part to high risks of GVHD and opportunistic infection in every age group.12-16
To potentially alleviate the shortage of suitable donors and reduce the length of the search process for unrelated marrow donors, repositories of banked, HLA-typed UCB have been developed since 1993.17,18 In 1996, Kurtzberg et al6 and Wagner et al7 reported preliminary clinical results of unrelated donor UCB transplantation suggesting that banked unrelated donor UCB contained sufficient numbers of HSCs to achieve engraftment in the majority of cases with lower than anticipated risk of severe acute GVHD in children and smaller adults.
Since these initial reports, more than 40 000 UCB units have been stored and more than 2000 UCB transplantations have been performed worldwide.8 However, there have been few reports on clinical outcomes after UCB transplantation, especially from single centers or consortiums that use uniform treatment plans and methods of assessment.6,7,9 19 Therefore, we report the results of UCB transplantation at the University of Minnesota with analysis of the influence of various demographic, treatment, and graft characteristics—particularly the effect of HLA disparity and CD34 cell dose—on rate of hematopoietic recovery and probabilities of engraftment, GVHD, TRM, relapse, and survival.
Patients and methods
Patients with acute leukemia, other malignancies, bone marrow failure syndromes, immunodeficiency states, or metabolic disorders were eligible for unrelated donor UCB transplantation if (1) a related or unrelated donor of bone marrow with no more than one HLA disparity was unavailable and (2) the patient or the patient's parent consented to the transplantation procedure. Protocols for myeloablative therapy and the use of banked unrelated donor UCB for transplant were reviewed and approved by the institutional review board at the University of Minnesota.
Patients
Between July 7, 1994, and September 30, 2001, 152 patients received transplants of banked unrelated donor UCB (37 patients in 1994-1997, 115 patients in 1998-2001). Patients were excluded from this analysis if transplantation occurred after June 30, 2001 (n = 18), if prior allogeneic HSC transplantation was performed (n = 17), if a nonmyeloablative cytoreductive regimen was administered (n = 10), or if the UCB graft was composed of more than one unit (n = 5). After these exclusions, 102 patients treated for various malignant and nonmalignant disorders were evaluable. Patient and treatment characteristics are shown in Table1. Median age of the patients was 7.4 years (22 were aged 18 years or older) and median weight was 25.9 kg. Six patients with malignancy who had relapsed after prior autologous transplantation were included in the analysis.
Patient and treatment characteristics
| Patient characteristics . | No. . |
|---|---|
| Age, median, y (range) | 7.4 (0.2-56.9) |
| Weight, median, kg (range) | 25.9 (5.0-107.5) |
| Sex, no. (%) | |
| Male | 60 (59) |
| Female | 42 (41) |
| Race, no. (%) | |
| White | 80 (78) |
| Other | 22 (22) |
| CMV serostatus, no. (%) | |
| Negative | 61 (60) |
| Positive | 41 (40) |
| Diagnosis, no. (%) | |
| Malignant disease | |
| Acute lymphocytic leukemia | 28* (27) |
| Acute myelocytic leukemia | 26† (25) |
| Chronic myelogenous leukemia | 6‡ (6) |
| Juvenile myelomonocytic leukemia | 3 (3) |
| Non-Hodgkin lymphoma | 1 (1) |
| Hodgkin disease | 1 (1) |
| Nonmalignant disease | |
| Severe aplastic anemia | 2 (2) |
| Fanconi anemia | 4 (4) |
| Diamond-Blackfan syndrome | 1 (1) |
| Osteopetrosis | 1 (1) |
| Myelodysplastic syndrome (refractory anemia) | 3 (3) |
| Immune deficiency | 5 (5) |
| Metabolic disorders | 21 (21) |
| Degree of HLA-A, B, DRB1 match, no. (%)1-153 | |
| 6/6 antigens | 14 (14) |
| 5/6 antigens | 44 (43) |
| 4/6 antigens | 42 (41) |
| 3/6 antigens | 2 (2) |
| ABO blood type match, no. (%) | |
| Match | 32 (31) |
| Minor mismatch | 36 (35) |
| Major mismatch | 34 (33) |
| Treatment, no. (%) | |
| TBI | 87 (85) |
| No TBI | 15 (15) |
| G-CSF | 80 (78) |
| No G-CSF | 22 (22) |
| Patient characteristics . | No. . |
|---|---|
| Age, median, y (range) | 7.4 (0.2-56.9) |
| Weight, median, kg (range) | 25.9 (5.0-107.5) |
| Sex, no. (%) | |
| Male | 60 (59) |
| Female | 42 (41) |
| Race, no. (%) | |
| White | 80 (78) |
| Other | 22 (22) |
| CMV serostatus, no. (%) | |
| Negative | 61 (60) |
| Positive | 41 (40) |
| Diagnosis, no. (%) | |
| Malignant disease | |
| Acute lymphocytic leukemia | 28* (27) |
| Acute myelocytic leukemia | 26† (25) |
| Chronic myelogenous leukemia | 6‡ (6) |
| Juvenile myelomonocytic leukemia | 3 (3) |
| Non-Hodgkin lymphoma | 1 (1) |
| Hodgkin disease | 1 (1) |
| Nonmalignant disease | |
| Severe aplastic anemia | 2 (2) |
| Fanconi anemia | 4 (4) |
| Diamond-Blackfan syndrome | 1 (1) |
| Osteopetrosis | 1 (1) |
| Myelodysplastic syndrome (refractory anemia) | 3 (3) |
| Immune deficiency | 5 (5) |
| Metabolic disorders | 21 (21) |
| Degree of HLA-A, B, DRB1 match, no. (%)1-153 | |
| 6/6 antigens | 14 (14) |
| 5/6 antigens | 44 (43) |
| 4/6 antigens | 42 (41) |
| 3/6 antigens | 2 (2) |
| ABO blood type match, no. (%) | |
| Match | 32 (31) |
| Minor mismatch | 36 (35) |
| Major mismatch | 34 (33) |
| Treatment, no. (%) | |
| TBI | 87 (85) |
| No TBI | 15 (15) |
| G-CSF | 80 (78) |
| No G-CSF | 22 (22) |
TBI indicates total body irradiation; G-CSF, prophylactic granulocyte colony-stimulating factor.
Of these patients, 8 had complete remission (CR) 1, 10 had CR 2, 6 had CR 3 or higher, and 4 had early relapse.
Of these patients, 1 had CR 1, 14 had CR 2, 1 had CR 3 or higher, and 10 had relapse.
Of these patients, 3 had chronic phase and 3 had accelerated phase disease.
Maximal degree of HLA disparity (rejection/GVHD vector).
HLA typing and unrelated donor selection
Units were obtained from 6 UCB banks: New York Blood Center Placental Blood Program (n = 67); St Louis Cord Blood Bank (n = 26); and Netcord (n = 9) in Barcelona, Milano, Dusseldorf, and Firenze. All unrelated-donor UCB units were HLA-typed by the UCB bank. Prior to transplantation, confirmatory HLA typing of the selected UCB unit and recipient was performed at the transplantation center and/or the UCB bank. HLA-A and HLA-B were typed by means of the standard 2-stage complement-dependent microcytotoxicity assay, and antigens were assigned as defined by the World Health Organization (WHO) HLA nomenclature committee.20 HLA-DRB1 type was determined by hybridization of polymerase chain reaction (PCR)–amplified DNA with sequence-specific oligonucleotide probes (SSOPs),21 with sequencing if needed. Prior to March 1999, HLA-A, HLA-B, and HLA-DRB1 typing was used to select the most closely matched donor unit–recipient pair, with preference given to HLA-DRB1–matched units; subsequently, priority was given to the unit (0, 1, or 2 HLA disparities) with the largest nucleated cell dose.
Myeloablative regimen and GVHD prophylaxis
Pretransplantation conditioning varied according to the patient's disease, disease status, and history of prior radiotherapy. All but 15 patients received a cyclophosphamide (CY) and TBI-containing regimen; all patients received antithymocyte globulin (ATGAM; Pharmacia, Kalamazoo, MI) prior to unrelated donor UCB transplantation. All patients with malignant disease received CY (120 mg/kg) and TBI (1320-1375 cGy).
Prophylaxis for acute GVHD consisted of cyclosporine A and methotrexate (CsA/MTX, n = 2) or cyclosporine A and methylprednisolone (CsA/MP, n = 100). CsA was initiated on day −3 and continued at least 6 months before a 10% per week taper was initiated. Methylprednisolone (1 mg/kg intravenously every 12 hours) was administered on days 5 to 19 with a taper thereafter (25% decrease every other day). For the 2 patients receiving CsA/MTX, MTX was administered intravenously on day 1 (15 mg/m2) and days 3, 6, and 11 (10 mg/m2).
Transplantation of UCB
UCB grafts used for transplantation in this study contained a median of 3.1 × 107 nucleated cells (range, 0.7-57.9 cells), 2.8 × 105 CD34 cells (range, 0.4-39.1 cells), and 8.0 × 106 CD3 cells (range, 0.003-80 cells) per kilogram recipient body weight after thawing. Cryopreserved units of UCB were transported to the transplantation center via overnight delivery in a dry shipper previously cooled by liquid nitrogen (temperature < −150°C) before initiation of the preparative regimen and then maintained in the vapor phase of liquid nitrogen at the transplantation center until the day of transplantation. Except for the first 2 patients, for whom the unit of UCB was thawed at the bedside and infused intravenously over a 10- to 15-minute period without manipulation, units were thawed in a 37°C water bath with gentle agitation, using the method described by Rubinstein et al.22 After thawing, an equal volume of dextran/albumin solution was added over 10 minutes, centrifuged at 250g for 5 to 10 minutes at 10°C, and the supernatant removed. The cell pellet was resuspended in dextran/albumin and immediately infused into the patient over 1 to 2 hours.
Supportive care
Patients were hospitalized in single rooms ventilated with high-efficiency particulate air filtration systems. Patients at high risk for recurrence of herpes simplex (ie, IgG titer ≥ 1:8) received prophylactic intravenous acyclovir. Patients at high risk for recurrence of cytomegalovirus (CMV; IgG titer ≥ 1:8) received prophylactic high-dose acyclovir or ganciclovir during cytoreduction, followed by high-dose acyclovir until neutrophil recovery, followed by ganciclovir or acyclovir until day 100. Documented CMV reactivation or infection demonstrated by antigenemia testing after transplantation was treated with therapeutic doses of ganciclovir with or without intravenous immunoglobulin.23 Broad-spectrum antibiotics were administered for fever during aplasia, and amphotericin B (0.3-1.2 mg/kg/d) was added for persistent fever unresponsive to antibiotic therapy. All patients received fluconazole for prophylaxis of yeast infections for 100 days, trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis carinii after engraftment for 12 months after transplantation, and penicillin for prophylaxis of Gram-positive organisms during treatment of GVHD. GG-CSF (5 μg/kg/d) was administered from day 0 to all patients since March 1997 (n = 80).
Hematopoietic recovery and engraftment
Hematologic recovery was defined as time to absolute neutrophil count (ANC) equal to or greater than 5 × 108/L (first of 3 consecutive laboratory measurements on different days) and platelet count equal to or greater than 5 × 1010/L (first of 7 days without transfusion support). Donor cell engraftment and remission status in the marrow were assessed on days 21, 60, and 100 and at 6 months, 1 year, and 2 years after transplantation, with chimerism status determined by quantitative PCR analysis of informative polymorphic variable number tandem repeat (VNTR) regions. Complete chimerism was defined as the presence of donor hematopoietic cells only; mixed chimerism was defined as the presence of both donor and host (> 10%) hematopoietic cells simultaneously. Autologous recovery was defined as the presence of host hematopoietic cells (> 90%).
Graft-versus-host disease
Patients were evaluated for acute GVHD daily during initial hospitalization, at least once weekly after initial discharge during the first 100 days, and at routine follow-up evaluations at the transplantation center at 6 months, 1 year, and at least yearly thereafter. Diagnosis of acute GVHD was based on clinical criteria, with histopathologic confirmation when possible. Overall staging was based on published criteria24 and was retrospectively assigned by an independent review team. Patients with clinical stage II or later disease were treated initially with methylprednisolone (≥ 48 mg/m2 intravenously or oral equivalent) daily for a minimum of 2 weeks prior to a 10% taper per week.
Statistical analysis
Data on transplant patient characteristics, posttransplantation complications, and outcomes were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized collection procedures. Cumulative incidence rates and their 95% confidence intervals (CIs) were estimated for engraftment, grade II-IV and grade III-IV acute GVHD, chronic GVHD, TRM, and relapse (for patients with malignant diseases).25 Kaplan-Meier methods were used to estimate survival.26 Event times for neutrophil engraftment were measured from the date of transplantation to the date of neutrophil recovery, with censoring for early death (ie, death before day 28 without neutrophil recovery; n = 5) or evidence of persistent malignant disease (n = 3). Patients who had very slow engraftment (ie, those who achieved an ANC ≥ 5 × 108/L after day 42) or failed to have marrow reconstitution of donor origin (< 5%) were scored as having primary graft failure. Those with severe neutropenia of more than 1 week's duration or autologous recovery after primary engraftment were scored as having secondary graft failure. The Spearman rank correlation coefficient was used to estimate a correlation between nucleated cell dose and CD34 cell dose.27
Cox proportional hazard models and log-rank test statistics were used to evaluate the univariate and multiple effects of risk factors on outcome. The Tarone test for trend was applied when the alternative hypothesis was a ranked trend rather than differences between unordered categories.28 The outcome variables were engraftment, GVHD, treatment-related mortality, relapse, and survival. The following factors were considered potential predictors of outcomes: recipient's age, weight, sex, race, CMV serostatus, diagnosis (malignant vs nonmalignant disease), and malignancy risk category; donor-recipient ABO match; donor-recipient HLA match; presence of class I vs class II disparity; use of G-CSF; conditioning regimen (TBI + chemotherapy vs chemotherapy alone); graft cell dose (nucleated cells, CD34 cells, CD3 cells); development of acute GVHD; and history of prior autologous transplantation. All factors were tested for the proportional hazards assumption with HLA disparity included in every model.29Event times were analyzed as of October 15, 2001.
For purposes of these analyses, patients with malignancy were categorized as having disease at standard risk or high risk for relapse after transplantation. Patients were considered to have high-risk disease if they had (1) acute leukemia in second remission after a short first remission (< 3 years for patients with acute lymphocytic leukemia [ALL] (n = 14) and < 1 year for patients with acute myelocytic leukemia [AML]) or in third remission and beyond or in relapse (n = 22); (2) chronic myelogenous leukemia (CML) in accelerated phase; (3) juvenile myelomonocytic leukemia; or (4) lymphoma in relapse or refractory to therapy. All other patients, regardless of cytogenetic abnormality, were considered to have standard-risk disease. Effect of HLA disparity on probabilities of engraftment and acute GVHD took into account graft rejection and GVHD vectors (ie, mismatch in the rejection or GVHD direction15), respectively. When both donor and recipient were heterozygous at the mismatched locus, the disparity was present for both the graft rejection and GVHD vectors. When the donor was heterozygous and the recipient was homozygous or displayed a blank at the mismatched locus, HLA was considered mismatched only in the graft rejection vector and not in the GVHD vector. Conversely, when the recipient was heterozygous and the donor was homozygous at the mismatched locus, the disparity was considered only in the GVHD vector and not the graft rejection vector. Any mismatch, regardless of vector, was considered in the analyses of survival, treatment-related mortality, and relapse.
Results
Hematopoietic recovery and engraftment
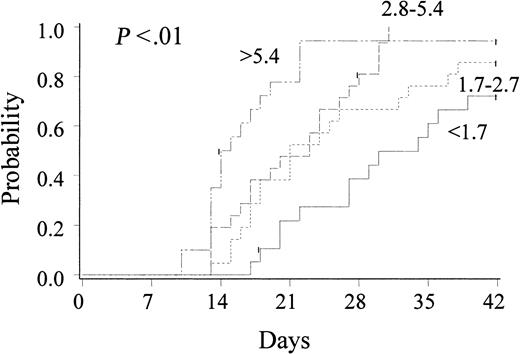
The incidence of neutrophil recovery by day 42 was 0.88 (95% CI, 0.81-0.95), with 4 patients engrafting after day 42 (2 of 4 alive at 298 days and 1830 days after transplantation). Median time to achieve an ANC equal to or greater than 5 × 108/L was 23 days (range, 9-54 days). In univariate analyses, rate of recovery and engraftment of neutrophils were strongly associated with CD34 cell dose (P < .01) (Figure 1). Notably, with patients grouped according to CD34 cell dose quartiles, rate and likelihood of engraftment were markedly inferior in 29 pediatric and adult patients (with 53% in this quartile older than 18 years) receiving a cell dose of less than 1.7 × 105 CD34 cells/kg (0.72 [CI, 0.51-0.93]) at a median of 34 days (range, 17-54 days) as compared with other cell doses (P < .01). In univariate analysis, there was a trend toward more rapid and more frequent engraftment in recipients of G-CSF (P = .09). For those patients receiving G-CSF (n = 80), the incidence of neutrophil engraftment by day 42 was 0.90 (CI, 0.84-0.97) at a median of 21 days (range, 9-54 days), as compared with 0.80 (CI, 0.60-1.00) at a median of 31 days (range, 17-45 days) in those not receiving G-CSF.
Cumulative incidence of neutrophil engraftment after unrelated donor UCB transplantation (n = 102): effect of CD34 cell dose (× 105/kilogram recipient body weight).
Cumulative incidence of neutrophil engraftment after unrelated donor UCB transplantation (n = 102): effect of CD34 cell dose (× 105/kilogram recipient body weight).
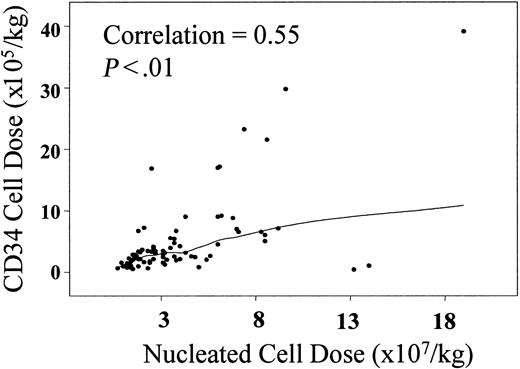
While neutrophil recovery was strongly associated with thawed CD34 cell dose, the association with infused nucleated cell dose was not statistically significant (P = .06). However, there is a positive linear correlation between CD34 and nucleated cell doses (R = 0.55, P < .01), as shown in Figure2. With patients grouped according to nucleated cell dose quartiles, engraftment occurred in fewer recipients of less than 1.7 × 107 nucleated cells/kg (0.80 [CI, 0.62-0.97]) at a median of 28 days (range, 15-39 days) as compared with other cell doses (P = .07).
Correlation between infused nucleated cell dose (× 107/kilogram recipient body weight) and infused CD34 cell dose (× 105/kilogram recipient body weight).
Correlation between infused nucleated cell dose (× 107/kilogram recipient body weight) and infused CD34 cell dose (× 105/kilogram recipient body weight).
The incidence of platelet recovery by 6 months was 0.65 (CI, 0.53-0.77). Median time required to achieve a platelet count equal to or greater than 5 × 1010/L was 86 days (range, 29-276 days). In univariate analyses, platelet engraftment by 6 months was associated with younger recipient age (P < .01), higher CD34 cell dose (P < .01), and lack of grade III-IV acute GVHD (P = .04) (data not shown).
The incidence of secondary graft failure, as manifested by autologous recovery after primary engraftment, was 0.05 (CI, 0.00-0.10). Autologous recovery was restricted to patients with nonmalignant diagnoses, specifically those with Hurler syndrome (n = 2), Chediak Higashi syndrome (n = 1), and metachromatic leukodystrophy (n = 1), and occurred 21 to 80 days after UCB transplantation. Chimerism was complete in 100% of patients with malignancy who achieved primary engraftment and had no evidence of recurrent disease (ie, stable mixed chimerism has not been observed).
In Cox regression analysis, both neutrophil (Table2) and platelet engraftment were associated with CD34 cell dose. Development of severe GVHD was associated with delayed platelet engraftment (data not shown). HLA match and use of G-CSF were not associated with either neutrophil or platelet engraftment.
Cox regression analyses of factors potentially associated with neutrophil engraftment, treatment-related mortality, and survival after unrelated donor UCB transplantation
| Variable . | Relative risk (95% CI) . | P . |
|---|---|---|
| Neutrophil recovery | Model χ2= 25.6, P < .01 | |
| CD34 dose (× 105/kg) | ||
| Less than 1.7 | 1.0 | |
| 1.7-2.7 | 1.7 (0.8-3.6) | .14 |
| 2.8-5.4 | 2.6 (1.3-5.4) | < .01 |
| More than 5.4 | 4.7 (2.2-9.8) | < .01 |
| Growth factor | ||
| No | 1.0 | .09 |
| Yes | 3.4 (0.8-2.1) | |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | NS |
| 4/6 | 0.9 (0.6-1.6) | |
| Acute GVHD | Model χ2 = 0.9, NS | |
| CD3 dose (× 106/kg) | ||
| Less than 8 | 1.0 | |
| 8 or more | 0.7 (0.2-1.9) | NS |
| Age (by decade) | 1.0 (0.98-1.03) | NS |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | |
| 4/6 | 1.2 (0.5-2.7) | NS |
| Treatment-related mortality | Model (χ2 = 29.2, P < .01) | |
| CD34 dose (× 105/kg) | ||
| 1.7 or less | 1.0 | |
| More than 1.7 | 0.2 (0.1-0.5) | < .01 |
| Age (by decade) | 1.3 (1.0-1.6) | .03 |
| Grade III-IV acute GVHD | ||
| No | 1.0 | |
| Yes | 4.4 (1.7-11.4) | < .01 |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | |
| 4/6 | 1.6 (0.6-3.9) | .34 |
| Survival | Model χ2 = 22.8, P< .01) | |
| CD34 dose (× 105/kg) | ||
| 1.7 or less | 1.0 | |
| More than 1.7 | 0.3 (0.1-0.5) | < .01 |
| Grade III-IV acute GVHD | ||
| No | 1.0 | |
| Yes | 3.5 (1.5-7.9) | < .01 |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | |
| 4/6 | 2.4 (1.2-4.7) | .01 |
| Variable . | Relative risk (95% CI) . | P . |
|---|---|---|
| Neutrophil recovery | Model χ2= 25.6, P < .01 | |
| CD34 dose (× 105/kg) | ||
| Less than 1.7 | 1.0 | |
| 1.7-2.7 | 1.7 (0.8-3.6) | .14 |
| 2.8-5.4 | 2.6 (1.3-5.4) | < .01 |
| More than 5.4 | 4.7 (2.2-9.8) | < .01 |
| Growth factor | ||
| No | 1.0 | .09 |
| Yes | 3.4 (0.8-2.1) | |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | NS |
| 4/6 | 0.9 (0.6-1.6) | |
| Acute GVHD | Model χ2 = 0.9, NS | |
| CD3 dose (× 106/kg) | ||
| Less than 8 | 1.0 | |
| 8 or more | 0.7 (0.2-1.9) | NS |
| Age (by decade) | 1.0 (0.98-1.03) | NS |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | |
| 4/6 | 1.2 (0.5-2.7) | NS |
| Treatment-related mortality | Model (χ2 = 29.2, P < .01) | |
| CD34 dose (× 105/kg) | ||
| 1.7 or less | 1.0 | |
| More than 1.7 | 0.2 (0.1-0.5) | < .01 |
| Age (by decade) | 1.3 (1.0-1.6) | .03 |
| Grade III-IV acute GVHD | ||
| No | 1.0 | |
| Yes | 4.4 (1.7-11.4) | < .01 |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | |
| 4/6 | 1.6 (0.6-3.9) | .34 |
| Survival | Model χ2 = 22.8, P< .01) | |
| CD34 dose (× 105/kg) | ||
| 1.7 or less | 1.0 | |
| More than 1.7 | 0.3 (0.1-0.5) | < .01 |
| Grade III-IV acute GVHD | ||
| No | 1.0 | |
| Yes | 3.5 (1.5-7.9) | < .01 |
| HLA match | ||
| 6/6 and 5/6 | 1.0 | |
| 4/6 | 2.4 (1.2-4.7) | .01 |
Acute and chronic graft-versus-host disease
Acute GVHD occurred in 63 patients and was scored as grade I (n = 24), grade II (n = 28), grade III (n = 8), or grade IV (n = 3) disease (Table 3). By day 100 after transplantation, incidences of grade II-IV and grade III-IV acute GVHD were 0.39 (CI, 0.29-0.49) and 0.11 (CI, 0.05-0.17), respectively. The median time to acute GVHD was 35 days (range, 8-86 days). Skin and lower gastrointestinal tract were the organs most likely to be affected, with mild disease in the majority of patients (Table 3). Of the 39 patients who developed grade II-IV acute GVHD, 24 had a complete response to primary therapy with methylprednisolone. Of those requiring secondary therapy with antithymocyte globulin, methylprednisolone (250 mg/m2/d), or other investigational agents (n = 15), 8 had a complete response, but only 2 remain alive. In Cox regression analysis, acute GVHD was not associated with any predictor, including CD3 cell dose, HLA match, and class of HLA disparity (Table 2).
Clinical grade of acute GVHD by organ system
| Severity . | Skin . | Upper GI . | Lower GI . | Liver . | Overall . |
|---|---|---|---|---|---|
| Grade I | 24 | 0 | 0 | 0 | 24 |
| Grade II | 23 | 7 | 3 | 0 | 28 |
| Grade III | 7 | 3 | 8 | 2 | 8 |
| Grade IV | 3 | 1 | 3 | 3 | 3 |
| Total | 57 | 11 | 14 | 5 | 63 |
| Severity . | Skin . | Upper GI . | Lower GI . | Liver . | Overall . |
|---|---|---|---|---|---|
| Grade I | 24 | 0 | 0 | 0 | 24 |
| Grade II | 23 | 7 | 3 | 0 | 28 |
| Grade III | 7 | 3 | 8 | 2 | 8 |
| Grade IV | 3 | 1 | 3 | 3 | 3 |
| Total | 57 | 11 | 14 | 5 | 63 |
Of the 102 patients, 39 had no GVHD.
GI indicates gastrointestinal tract.
The incidence of chronic GVHD was 0.09 (CI, 0.04-0.14) at 1 year after transplantation. All patients with chronic GVHD had extensive disease diagnosed at a median of 5 months (range, 2-7 months) after transplantation. Disease involved the skin (n = 5), liver (n = 1), mucous membranes (n = 2), lung (n = 1), and joints (n = 1).
Treatment-related mortality
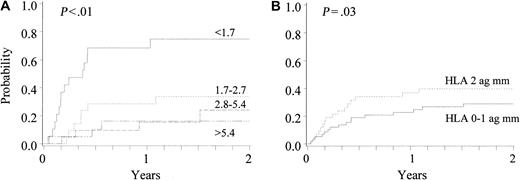
The incidence of TRM at 1 year and 2 years after unrelated donor UCB transplantation was 0.30 (CI, 0.21-0.39) and 0.35 (CI, 0.25-0.45), respectively. In univariate analyses, TRM was associated with CD34 cell dose, with a 1-year TRM of 0.20 (0.10-0.30) for patients receiving more than 1.7 × 105 CD34 cells per kilogram body weight (Figure 3). In addition, recipient age (P < .01), nucleated cell dose (P = .042), and development of grade III-IV acute GVHD (P = .05) were factors also associated with TRM, with favorable outcomes occurring in younger recipients, those receiving a higher cell dose, and those without a history of severe GVHD. TRM was lower in CMV-negative recipients (0.26 vs 0.37 at 1 year; P = .09) and recipients without a history of prior autologous transplantation (0.29 vs 0.50 at 1 year; P = .06). TRM was not associated with recipient's weight, sex, race, HLA match (P = .14), ABO match, diagnosis (malignancy vs nonmalignancy), malignancy risk group, preparative therapy (regimen containing TBI vs no TBI), or use of G-CSF. In Cox regression analyses (Table 2), CD34 cell dose, development of grade III-IV acute GVHD, and age were the only factors associated with TRM; there was no association with HLA match.
Cumulative incidence of treatment-related mortality after unrelated donor UCB transplantation (n = 102).
(A) Effect of CD34 cell dose (× 105/kilogram recipient body weight). (B) Effect of HLA disparity (mm = mismatch).
Cumulative incidence of treatment-related mortality after unrelated donor UCB transplantation (n = 102).
(A) Effect of CD34 cell dose (× 105/kilogram recipient body weight). (B) Effect of HLA disparity (mm = mismatch).
Relapse
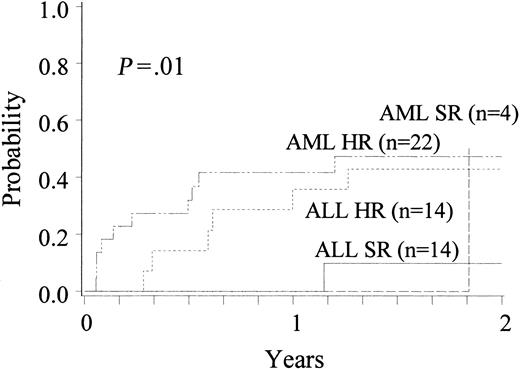
Hematological relapse was detected between 21 and 672 days (median, 196 days) after transplantation in 21 of 65 patients treated for malignant disease. The cumulative incidence of relapse was 0.37 (CI, 0.24-0.50) at 2 years. For patients with ALL, the incidence of relapse at 2 years was 0.10 (CI, 0.00-0.29) for patients with standard-risk disease (n = 14) and 0.43 (CI, 0.17-0.61) for patients with high-risk disease (n = 14). For patients with high-risk AML (n = 22), the incidence of relapse was 0.47 (CI, 0.25-0.69) at 2 years; 1 of 4 patients with standard-risk disease relapsed (Figure 4). For the entire group, relapse was not associated with cell dose, HLA match, or history of prior acute or chronic GVHD. In Cox regression analysis, relapse was associated only with recipient's age (P = .01) and malignancy risk group (P = .05).
Cumulative incidence of relapse in recipients with ALL and AML, stratified by disease risk.
HR indicates high risk; SR, standard risk.
Cumulative incidence of relapse in recipients with ALL and AML, stratified by disease risk.
HR indicates high risk; SR, standard risk.
Survival
With a median follow-up of 2.7 years, the cumulative proportions surviving at 1 year and 2 years after unrelated donor UCB transplantation were 0.58 (CI, 0.49-0.70) and 0.47 (CI, 0.36-0.57), respectively, with a survival of 0.70 (CI, 0.49-0.90) at 1 year in patients with UCB grafts containing more than 1.7 × 105CD34 cells per kilogram. In transplant recipients with nonmalignant and malignant disease, the proportions surviving at 2 years were 0.60 (CI, 0.44-0.77) and 0.38 (CI, 0.25-0.51), respectively. In the 28 patients with ALL, the survival rate was 0.55 (CI, 0.23-0.87) for standard-risk and 0.32 (CI, 0.06-0.59) for high-risk patients. Similarly, of 26 patients with AML, 2 of 4 survived with standard-risk disease; the survival rate was 0.33 (CI, 0.12-0.54) for high-risk patients. In univariate analyses, survival was associated with recipient's age (P < .01), disease (P = .04), CD34 cell dose (P < .01; Figure 5), CMV serostatus (P = .05), and history of severe acute GVHD (P < .01), with favorable outcomes in younger recipients, those with nonmalignant disease, those receiving higher cell doses, and those without history of severe GVHD. Notably, potential risk factors such as HLA match (P = .08), recipient's CMV serostatus (P = .09), malignancy risk group (P = .10), and history of prior autologous transplantation (P = .07) did not reach statistical significance in the univariate analysis. Further, survival was not associated with recipient's weight, sex, race, ABO match, class of HLA disparity, malignancy risk group, preparative therapy (regimen containing TBI vs no TBI), or use of G-CSF. In Cox regression analyses, however, HLA match, CD34 cell dose, and development of grade III-IV acute GVHD were associated with survival (Table 2).
Cumulative proportion surviving after unrelated donor UCB transplantation (n = 102).
(A) Effect of CD34 cell dose (× 105/kilogram recipient body weight). (B) Effect of HLA disparity (mm = mismatch).
Cumulative proportion surviving after unrelated donor UCB transplantation (n = 102).
(A) Effect of CD34 cell dose (× 105/kilogram recipient body weight). (B) Effect of HLA disparity (mm = mismatch).
In this series, 54 patients died. Death was most frequently associated with relapse of malignant disease (n = 26) and opportunistic infection (n = 15). Other primary causes of death were GVHD (n = 7), graft failure (n = 3), interstitial pneumonitis (n = 2), and hepatic veno-occlusive disease (n = 1). Epstein-Barr virus lymphoproliferative syndrome was diagnosed in 2 patients30 but was not listed as the primary cause of death for any patient.
Discussion
It has been established that a single UCB unit contains sufficient numbers of HSCs for durable engraftment in most patients.2-5,31-33 Importantly, the results presented here indicate that the proportion achieving neutrophil engraftment by day 42 and platelet engraftment by 6 months after unrelated donor UCB transplantation is similar to that observed after unrelated donor bone marrow transplantation (BMT)34; neutrophil engraftment occurred in 88% of UCB patients and 90% of BMT patients, and platelet engraftment occurred in 50% of UCB patients and 55% of BMT patients. It is noteworthy that UCB units engraft successfully, considering the low numbers of CD34 cells infused. In contrast to the typical bone marrow allograft, which contains a median of 3 × 106 CD34 cells per kilogram recipient body weight, recipients of UCB receive a CD34 cell dose more than 1 log lower (median, 2.7 × 105 CD34 cells per kilogram). In fact, the incidence of engraftment is similar to that observed after unrelated donor BMT until the CD34 cell dose approaches 1.7 × 105/kg, below which the rate of recovery and incidence of engraftment are unsatisfactory. These data, therefore, support the contention that a threshold dose exists, defining which UCB units are acceptable for each transplantation candidate. As a result of these findings, a dose of 1.7 × 105 CD34/kg (when available) has now been established as the threshold dose for patients at the University of Minnesota. Arguably, differences in preparative and supportive care therapies as well as methods of CD34 analysis may prevent the establishment of a universally applicable threshold value. Importantly, Thompson et al9 and Laughlin et al10 did not observe an association between CD34 cell dose and engraftment and survival, possibly due to differences in treatment and methods of CD34 analysis or smaller patient numbers. Nonetheless, these data suggest a need for the quantification of CD34 cells in each UCB unit, using a standardized method of CD34 analysis, by all cord blood banks so that a threshold limit can be defined.
An advantage of UCB is its apparent reduced alloreactive response as compared with bone marrow.35-37 The data would suggest that UCB, despite HLA mismatching, is associated with low GVHD risk. Davies et al13 previously reported a probability of grade III-IV acute GVHD of 32% and 49% in recipients of bone marrow disparate in 0 and 1 HLAs, respectively, at the University of Minnesota, whereas the data presented here demonstrated an incidence of 11% in recipients of UCB disparate in 0, 1, or 2 HLAs, according to the same grading criteria. In addition, the infrequent development of severe acute GVHD and extensive chronic GVHD after unrelated donor UCB transplantation as compared with unrelated BMT is striking.38 Although it may be related to treatment factors such as the use of pretransplantation ATGAM, the explanation for such a low incidence of GVHD in recipients of unrelated donor UCB is unclear. Although the cytotoxic T-cell precursor frequency has been found to be similar in UCB and adult peripheral blood,39the median CD3 cell dose of 8 × 106/kg in UCB units makes it similar to a marrow graft after modest T-cell depletion. However, neither such a level of T-cell depletion nor the choice of immunoprophylaxis fully explains the low incidence of GVHD observed by us and others.40-42 It is more likely that functional differences, such as a defective cytotoxic response reported with UCB lymphocytes,43 an altered cytokine profile,31 44 or other differences may explain the decreased incidence of severe GVHD.
TRM is the principal obstacle to successful transplantation outcome in recipients of unrelated donor BMT and is the major reason for evaluating UCB as an alternate source of HSCs. Of the 3 risk factors associated with risk of TRM in Cox regression analysis (ie, recipient's age, development of severe acute GVHD, and CD34 cell dose), CD34 cell dose is the only pretransplantation variable that can be manipulated. Half the patients in this study received a CD34 cell dose of more than 2.7 × 105/kg, and these patients had a TRM of only 15% at 1 year. This compares favorably with a TRM of 24% to 51% reported for pediatric recipients of unrelated BMT.45-47 However, patients receiving UCB grafts that contained 1.7 to 2.7 × 105 CD34/kg and less than 1.7 × 105 CD34/kg had higher rates of TRM (29% and 68%, respectively). Although an interaction between low cell dose and age older than 18 years may exist, the small number of patients in the adult age range prevents further analyses. These results underscore the argument that UCB units containing less than 1.7 × 105CD34/kg should be considered inadequate for routine use.
Survival in this study was somewhat higher than that reported by registries.3,4,36 However, direct comparisons with registry data are difficult because differences are multifactorial, reflecting differences in eligibility criteria, treatment and supportive care plans, and definitions of end points. Further investigations are under way as part of a national study sponsored by the National Heart, Lung, and Blood Institute.48Regardless, pediatric patients in this study had a high survival rate (71% and 63% for patients aged 0-1 and 2-17 years, respectively), with poorer results for adults (30%) at 1 year. As might be expected on the basis of results with unrelated donor BMT, HLA mismatch, lower cell doses, history of severe GVHD, and history of prior autologous transplantation were all factors associated with poorer survival in univariate analysis. With HLA disparity and CD34 cell dose both being independent risk factors for survival, the major question is how to weigh the relative risks of HLA disparity and CD34 cell dose. The answer will not only aid the clinician in the instruction of prospective patients on the relative importance of UCB CD34 cell dose and HLA disparity but will also aid in the development of a UCB graft selection algorithm. As previously suggested by Rubinstein et al,49 our results indicate that a higher CD34 cell dose partially overcomes the negative impact of HLA for each level of HLA disparity. For example, in recipients of UCB grafts disparate in 2 HLAs, patients who received transplants of more than 1.7 × 105 CD34 cells/kg had a higher survival (0.61 [CI, 0.43-0.79], n = 30) than those receiving a lower cell dose (0.11 [CI, 0.00-0.32], n = 9). For each degree of HLA disparity, the data presented here indicate that there is a critical infused cell dose below which survival is significantly impaired, particularly in recipients of UCB units with 2-HLA disparity. Resolution of this issue will require larger patient numbers.
As with unrelated donor BMT, it is important to note that the most common causes of death after UCB transplantation were infection and relapse. Owing to the profound influence of CD34 cell dose on rate of neutrophil engraftment, TRM, and survival, risk of infection is likely related in part to the prolonged length of neutropenia in recipients of lower cell doses rather than to an impairment of neutrophil function, as has been suggested.31 Although defects in neutrophil function could play a role, most recipients of UCB with an adequate cell dose do not die of infection. Relapse, however, was the major limiting factor. Although decreased GVHD raised the concern that the graft-vs-leukemia ratio might be decreased, relapse after unrelated donor UCB transplantation is similar to that previously reported for unrelated BMT patients.46,47 Although only a randomized trial with larger numbers of patients will answer the question of relative risk of relapse after UCB transplantation as compared with BMT, these results are similar to those reported by Locatelli et al,50 who observed a 40% incidence of relapse in recipients with acute leukemia, with disease status being the only risk factor.
Together, these results provide clear justification for the further development of UCB banks worldwide. Banks should focus not only on the collection of larger units with greater numbers of CD34+cells but also on collecting UCB units from ethnic and racial minorities. Greater HLA disparity between donor and recipient adversely affects survival, which has particular relevance for patients of ethnic minority descent. Targeting collection centers with large minority populations may help reduce the degree of HLA disparity for minority patients and serve as an important adjunct to marrow donor registries worldwide. In addition, these results suggest that there should be routine quantitation of CD34 cells, preferably by UCB banks using a standardized procedure, so that rapid decisions can be made as to the optimal unit for a specific patient. Although small units should not be routinely prescribed, such units might be made available for phase 1 clinical trials of ex vivo expansion, for multiunit UCB transplantation, or when an alternative hematopoietic stem cell source is not available.
In summary, we have demonstrated the importance of graft CD34 cell dose in determining outcome after unrelated donor UCB transplantation. Even in recipients of grafts disparate in 2 HLAs, the data suggest that higher CD34 cell dose can partially reduce the negative impact of HLA disparity on survival. Therefore, these data suggest that the choice of UCB graft should be based primarily on CD34 cell dose and secondarily on degree of HLA disparity. The tolerability of 2-HLA disparate grafts will likely increase the availability of HSC transplantation, particularly for patients with infrequent HLA haplotypes. The importance of cell dose for transplantation outcomes provides the most compelling argument for focusing on the collection of larger UCB grafts and for investigating ex vivo HSC expansion and transplantation of multiple UCB units in future clinical trials.
We gratefully thank the many faculty, nurses, house staff, and clinical postdoctoral fellows of the Adult and Pediatric Blood and Marrow Transplant Programs who care for the patients and families treated at the Fairview University Medical Center, Minneapolis, MN. We also acknowledge Dr Kathryn Chaloner, codirector of the Biostatistical Support Group, for her careful review of the manuscript.
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI 10.1182/blood-2002-01-0294.
Supported by grants from the Children's Cancer Research Fund (J.E.W., J.N.B.) and the National Institutes of Health (N01-HB-67139 [J.E.W., D.J.W., S.M.D.] and P01-CA21737 [J.E.W.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John E. Wagner, Mayo Mail Stop 366, University of Minnesota, 420 Delaware St, SE, Minneapolis, MN 55455; e-mail:wagner002@tc.umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal