Systemic sclerosis (SSc) is a multisystem disease of presumed autoimmune pathogenesis for which no proven effective treatment exists. High-dose immunosuppressive therapy (HDIT) has been proposed as an investigational treatment for severe autoimmune diseases. Nineteen patients with poor-prognosis SSc underwent HDIT. The median age was 40 years (range, 23-61 years), the median modified Rodnan skin score (a measure of dermal sclerosis) was 31, and the median DLCO was 57%. Conditioning therapy involved 800 cGy total body irradiation (TBI) (± lung shielding to approximately 200 cGy), 120 mg/kg cyclophosphamide, and 90 mg/kg equine antithymocyte globulin. CD34-selected granulocyte–colony-stimulating factor–mobilized autologous blood stem cells provided hematopoietic rescue. With median follow-up at 14.7 months, the Kaplan-Meier estimated 2-year survival rate was 79%. Three patients died of treatment complications and one of disease progression. Two of the first 8 patients had fatal regimen-related pulmonary injury, a complication not found among 11 subsequent patients who received lung shielding for TBI. Overall, internal organ functions were stable to slightly worse after HDIT, and 4 patients had progressive or nonresponsive disease. As measured by modified Rodnan skin scores and modified health assessment questionnaire disability index (mHAQ-DI) scores, significant disease responses occurred in 12 of 12 patients evaluated at 1 year after HDIT. In conclusion, though important treatment-related toxicities occurred after HDIT for SSc, modifications of initial approaches appear to reduce treatment risks. Responses in skin and mHAQ-DI scores exceed those reported with other therapies, suggesting that HDIT is a promising new therapy for SSc that should be evaluated in prospective randomized studies.
Introduction
Systemic sclerosis (SSc) is an uncommon multisystem disease characterized by diffuse inflammation and subsequent fibrosis of skin together with varying degrees of internal organ involvement. Internal organ involvement entails poor prognosis, and deaths result primarily from pulmonary failure and cardiac events. Although the pathogenesis of SSc remains unclear, early disease is thought to be primarily immunologic in nature with secondary involvement of tissue fibroblasts.1 Because of this immunosuppressive agents have been used to treat SSc. Although some reports have suggested that immunosuppressive agents are effective in SSc, controlled trials have not confirmed this.2 Given the lack of effective treatment together with known prognostic factors for early mortality,3 patients with SSc were considered appropriate candidates for investigation of high-dose immunosuppressive therapy (HDIT).4 5
Previous experience with allogeneic and autologous stem cell transplantation together with experimental rodent studies formed the basis for our initial HDIT protocols.6,7 An underlying hypothesis for this study was that HDIT followed by the infusion of lymphocyte-depleted (CD34-selected) autologous peripheral blood stem cells (PBSCs) would allow near or complete immune ablation leading to disease control. This would be followed by regeneration of a self-tolerant immune system from multipotential hematopoietic progenitors. Results of this study and similar studies in other autoimmune diseases also could potentially provide important insights into the pathogenesis and mechanisms of autoimmune diseases. We have previously reviewed issues pertaining to protocol development6 and the selection of patients with SSc8 for trials of HDIT followed by autologous hematopoietic cell transplantation. Here we report initial results investigating HDIT for severe SSc.
Patients and methods
Study design and eligibility
This pilot study was designed to evaluate the safety and potential efficacy of HDIT using total body irradiation (TBI), cyclophosphamide (Cy), and equine antithymocyte globulin for severe SSc. Primary end points were grades 3-4 regimen-related toxicity and engraftment. Secondary end points included disease response, safety of granulocyte–colony-stimulating factor (G-CSF) mobilization, and immunologic recovery. Limited pooled data from 8 patients in this study have previously been reported.9
This report describes 19 consecutive patients enrolled between January 1997 and August 1980 at the Fred Hutchinson Cancer Research Center (FCHRC) and Virginia Mason Medical Center in Seattle (n = 13), Wayne State University/Karmanos Cancer Institute (n = 3), University of Michigan (n = 2), and Loma Linda University (n = 1). Written institutional review board–approved consent for treatment was obtained from all patients. Figure 1 shows patient eligibility criteria that predict 5-year mortality risks of approximately 50% with conventional treatment.8
Treatment and supportive care
Patients underwent PBSC mobilization as outpatients using G-CSF at 16 μg/kg per day subcutaneously, with the first apheresis on day 4. G-CSF–mobilized PBSC products were CD34-selected10using an Isolex 300I (Nexell, Irvine, CA) with targets greater than 3.5 × 106 CD34+ cells/kg, though lower doses were used in 2 patients. Unmodified PBSC (more than 3.0 × 106 CD34+ cells/kg) were also stored for treating engraftment failure or severe immunodeficiency after HDIT. Cells were cryopreserved using standard techniques. Grafts were evaluated for content of CD34+ cells and T cells (CD3+) by flow cytometry. HDIT consisted of fractionated total body irradiation (TBI) delivered as 200 cGy per fraction, 2 fractions per day, on day −5 and day −4, for a total dose of 800 cGy TBI, 60 mg/kg Cy intravenously on each of days −3 and −2, and 15 mg/kg equine antithymocyte globulin (ATGAM; Pharmacia, Peapack, NJ) intravenously on days −5, −3, −1, +1, +3, and +5. Methylprednisolone (1 mg/kg) was given intravenously with each dose of ATG. Individual TBI treatments were delivered with a minimum of 5 hours between fractions. TBI was administered from dual-opposed cobalt Co 60 sources or linear accelerators at dose rates of 7 cGy to 15 cGy per minute. TBI without lung shielding was used for the first 8 patients, and the subsequent 11 patients were treated with TBI modified to shield the lungs with 5 half-value layers of lead (97% shielding) after the first 200 cGy fraction. As protection against lung toxicity and to standardize steroid therapy prednisone, 0.5 mg/kg prednisone per day was given from the start of conditioning until day +30 and was tapered over 1 month in patients 5 to 19. This also had the potential benefit of protecting against ATG-induced serum sickness. CD34-selected PBSCs were infused on day 0. G-CSF (5 μg/kg per day) was given intravenously from day 0 until the absolute neutrophil count was greater than 500/μL for 3 days. Patients were hospitalized from the start of conditioning therapy until neutrophil engraftment and resolution of major toxicities. Infection prophylaxis included trimethoprim-sulfamethoxazole for Pneumocystis carinii,11 fluconazole forCandida,12 and ganciclovir for cytomegalovirus (CMV) reactivation.13
Evaluation of outcomes
Neutrophil engraftment was defined as occurring on the first of 3 consecutive postnadir days with more than 500 neutrophils per microliter, and platelet engraftment was defined as occurring on the first of 3 consecutive days with more than 20 000 platelets per microliter without transfusion. Regimen-related toxicities were defined according to the Bearman scale as those occurring within 28 days of transplantation.14
Formal disease status evaluations were performed before growth factor mobilization and at 3, 12, 24, and 36 months after HDIT. These included a modified Rodnan skin score (mRSS),15 pulmonary function testing, echocardiogram, serum creatinine, 24-hour urinary creatinine clearance and protein, autoantibodies, and modified Health Assessment Questionnaire Disability Index (mHAQ-DI).16 Briefly, the mRSS uses physical examination to measure dermal thickening. Seventeen anatomic sites are scored 0 to 3 (0 indicates normal) resulting in a composite score of 0 to 51. The mHAQ-DI is a validated tool for assessing activities of daily living in SSc.3 17-19 It is a functional index that involves a self-assessment questionnaire of daily living activities and is reported as a composite score on a scale of 0 to 3, with 0 indicating normal.
Immune recovery
Peripheral blood (PB) T cells, including CD4+ and CD8+ subsets, natural killer (NK) cells, and B cells, were measured in a single laboratory before PBSC mobilization, then at approximately 7, 30, 90, 180, 365, and 730 days after transplantation using 3-color flow cytometry.20-22 B cells were defined as mononuclear cells (MNCs) expressing CD19 or CD20 and not expressing T, NK, monocyte, or progenitor cell markers CD10 and CD34. CD4+ cells were CD3+ CD4+ CD8-MNC. CD8+ cells were CD3+ CD8+ CD4-MNC. NK cells were CD16+ or CD56+ MNC not expressing CD3 or CD14.21 22
Statistics
Outcomes are reported as of April 2001. Medians and ranges are reported unless otherwise specified. Survival was estimated using the method of Kaplan and Meier,23 and associated confidence intervals were estimated using the Greenwood formula. Post-HDIT values of parameters were compared with pre-HDIT values using the signed-rank test. All reported P values are 2-sided without adjustments for multiple comparisons.
Results
Patient characteristics
Table 1 summarizes patient characteristics before transplantation. The median age was 40 years (range, 23-61 years), and median disease duration was 20 months (range, 4-41 months). Sixteen of 19 patients were female. The median pretransplantation DLCO was 57%, and the median mRSS was 31.
Pretransplantation characteristics of patients
| Pt. . | Study UPN . | Sex . | Age, y . | Group* . | Primary organ† . | DLCO/FVC‡ . | Serum creatinine . | mRSS . | Disease duration, mo . | Scl-70 +ve . | Prior therapy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FH11956 | F | 47 | 1 | R | 76/74 | 1.4 | 47 | 38 | No | Hcq, St, D-pen, CSA |
| 2 | FH12946 | M | 38 | 1 | P | 48/72 | 1 | 29 | 30 | Yes | Hcq, CSA, St |
| 3 | FH13316 | F | 23 | 1 | P | 57/62 | 0.7 | 30 | 28 | Yes | Mtx, D-pen, Ctx, St |
| 4 | FH13483 | F | 40 | 1 | P | 47/75 | 0.5 | 32 | 22 | Yes | IVIG, St |
| 5 | FH13833 | F | 39 | 1 | P | 46/77 | 0.5 | 30 | 35 | Yes | CSA, D-pen |
| 6 | FH14767 | F | 61 | 1 | P | 67/83 | 0.8 | 35 | 7 | No | St, Cy |
| 7 | FH14709 | F | 56 | 1 | P | 52/76 | 0.5 | 50 | 9 | No | D-pen |
| 8 | UM 1 | F | 40 | 1 | P | 62/69 | 0.8 | 39 | 15 | Yes | Mtx |
| 9 | FH15570 | F | 49 | 2 | P | 45/75 | 0.8 | 14 | 13 | Yes | St, Aza, Mtx |
| 10 | KCI-1 | F | 27 | 2 | P | 66/78 | 0.9 | 3 | 18 | No | Mtx, St |
| 11 | FH16089 | F | 37 | 1 | P | 57/87 | 0.6 | 40 | 11 | No | St, Mtx, Rl |
| 12 | FH15782 | F | 55 | 1 | P | 57/64 | 0.4 | 27 | 29 | No | St, Cy |
| 13 | KCI-2 | M | 48 | 1 | P | 47/65 | 0.9 | 24 | 20 | Yes | Cy, St |
| 14 | UM-2 | M | 23 | 1 | P | 66/103 | 1 | 18 | 4 | Yes | None |
| 15 | FH15735 | F | 23 | 1 | P | 84/65 | 0.6 | 31 | 34 | ND | D-pen, St, Cy |
| 16 | KCI-3 | F | 42 | 1 | P | 56/67 | 1.3 | 36 | 41 | No | Mtx |
| 17 | FH16891 | F | 28 | 1 | P | 47/54 | 0.6 | 43 | 19 | Yes | St, D-pen |
| 18 | FH16855 | F | 40 | 1 | P | 69/66 | 0.6 | 19 | 28 | Yes | Enb |
| 19 | LLU-1 | F | 34 | 1 | P | 72/61 | 0.4 | 32 | 16 | No | Hcq, Mtx |
| Median | 40 | 57/72 | 0.7 | 31 | 20 |
| Pt. . | Study UPN . | Sex . | Age, y . | Group* . | Primary organ† . | DLCO/FVC‡ . | Serum creatinine . | mRSS . | Disease duration, mo . | Scl-70 +ve . | Prior therapy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FH11956 | F | 47 | 1 | R | 76/74 | 1.4 | 47 | 38 | No | Hcq, St, D-pen, CSA |
| 2 | FH12946 | M | 38 | 1 | P | 48/72 | 1 | 29 | 30 | Yes | Hcq, CSA, St |
| 3 | FH13316 | F | 23 | 1 | P | 57/62 | 0.7 | 30 | 28 | Yes | Mtx, D-pen, Ctx, St |
| 4 | FH13483 | F | 40 | 1 | P | 47/75 | 0.5 | 32 | 22 | Yes | IVIG, St |
| 5 | FH13833 | F | 39 | 1 | P | 46/77 | 0.5 | 30 | 35 | Yes | CSA, D-pen |
| 6 | FH14767 | F | 61 | 1 | P | 67/83 | 0.8 | 35 | 7 | No | St, Cy |
| 7 | FH14709 | F | 56 | 1 | P | 52/76 | 0.5 | 50 | 9 | No | D-pen |
| 8 | UM 1 | F | 40 | 1 | P | 62/69 | 0.8 | 39 | 15 | Yes | Mtx |
| 9 | FH15570 | F | 49 | 2 | P | 45/75 | 0.8 | 14 | 13 | Yes | St, Aza, Mtx |
| 10 | KCI-1 | F | 27 | 2 | P | 66/78 | 0.9 | 3 | 18 | No | Mtx, St |
| 11 | FH16089 | F | 37 | 1 | P | 57/87 | 0.6 | 40 | 11 | No | St, Mtx, Rl |
| 12 | FH15782 | F | 55 | 1 | P | 57/64 | 0.4 | 27 | 29 | No | St, Cy |
| 13 | KCI-2 | M | 48 | 1 | P | 47/65 | 0.9 | 24 | 20 | Yes | Cy, St |
| 14 | UM-2 | M | 23 | 1 | P | 66/103 | 1 | 18 | 4 | Yes | None |
| 15 | FH15735 | F | 23 | 1 | P | 84/65 | 0.6 | 31 | 34 | ND | D-pen, St, Cy |
| 16 | KCI-3 | F | 42 | 1 | P | 56/67 | 1.3 | 36 | 41 | No | Mtx |
| 17 | FH16891 | F | 28 | 1 | P | 47/54 | 0.6 | 43 | 19 | Yes | St, D-pen |
| 18 | FH16855 | F | 40 | 1 | P | 69/66 | 0.6 | 19 | 28 | Yes | Enb |
| 19 | LLU-1 | F | 34 | 1 | P | 72/61 | 0.4 | 32 | 16 | No | Hcq, Mtx |
| Median | 40 | 57/72 | 0.7 | 31 | 20 |
Eligibility group as per Table 1.
Primary organ dysfunction for which HDIT was performed. P indicates pulmonary; R, renal.
DLCO corrected for hemoglobin expressed as %; FVC, forced vital capacity expressed as %. FH indicates Fred Hutchinson Cancer Research Center; KCI, Karmanos Cancer Institute; UM, University of Michigan; LLU, Loma Linda University Medical Center; Aza, azathioprine; CSA, cyclosporine; Cy, cyclophosphamide; D-Pen, D-penicillamine; Enb, Enbrel; F, female; Hcq, Hydroxychloroquine; IVIG, intravenous immunoglobulin; M, male; mRSS, modified Rodnan skin score; Mtx, methotrexate; Rl, relaxin; St, corticosteroids.
PBSC collection and engraftment
G-CSF was tolerated without major complications, and all grafts were collected with a single course of G-CSF. Four patients had what appeared to be low-grade disease flares. These were manifested by mild skin inflammation including edema (n = 2), skin tightness (n = 1), erythema (n = 1), and arthralgias (n = 2) that resolved without specific therapy after G-CSF was stopped. Data from PBSC mobilizations and the CD34 selections of the PBSC products are summarized in Table2. Engraftment occurred promptly (Table2) with full platelet and neutrophil recovery in all patients. Patient 1 had longstanding erythropoietin-responsive anemia because of scleroderma-induced renal insufficiency that persisted after HDIT.
PBSC mobilization, grafts, and engraftment
| . | Median . | Range . | No. patients . |
|---|---|---|---|
| Mobilization and grafts | |||
| Number of aphereses* | 3 | 2 -7 | 19 |
| CD34+ cells × 106/kg | 4.2 | 2.7 -7.4 | 19 |
| Purity (%) | 91.5 | 55 -96 | 19 |
| T cells × 104/kg† | 3.6 | 0 -25.2 | 17 |
| Engraftment | |||
| Days to ANC > 500/μL‡ | 9 | 7 -13 | 19 |
| Days to platelets > 20 000/μL‡ | 10 | 9 -25 | 19 |
| . | Median . | Range . | No. patients . |
|---|---|---|---|
| Mobilization and grafts | |||
| Number of aphereses* | 3 | 2 -7 | 19 |
| CD34+ cells × 106/kg | 4.2 | 2.7 -7.4 | 19 |
| Purity (%) | 91.5 | 55 -96 | 19 |
| T cells × 104/kg† | 3.6 | 0 -25.2 | 17 |
| Engraftment | |||
| Days to ANC > 500/μL‡ | 9 | 7 -13 | 19 |
| Days to platelets > 20 000/μL‡ | 10 | 9 -25 | 19 |
To collect CD34-selected autografts and backup.
T-cell data were incomplete for 2 patients.
Day 1 of 3 consecutive days.
Infections
Bacteremia developed in 3 patients, venous catheter infection in 2 patients, urinary tract infection in 1 patient, and thumb cellulitis in 1 patient (7 patients overall). These conditions were readily managed with antibacterial agents. No fungal infections developed. CMV reactivations (antigenemia) occurred in 3 of 10 CMV-seropositive patients and were treated effectively with ganciclovir. Epstein-Barr virus (EBV)–related posttransplantation lymphoproliferative disorder (PTLD) and recurrent herpes simplex virus (HSV) infections occurred in one patient.
Regimen-related toxicities
Conditioning therapy was well tolerated by most patients. Mucositis was mild (grades 0 to 2) and other early regimen toxicities were grade 2 or less except for grade 4 pulmonary toxicity in patient 4. Three patients had fatal treatment-related complications. Patient 4 (baseline DLCO of 46%) acquired pneumonitis at 14 days. Patient 7 (baseline DLCO of 52%) had pneumonitis at 2 months. Both had gradual progressive respiratory failure and died after transient stabilization with high-dose corticosteroids. Bronchoalveolar fluid analyses from both patients showed no evidence of bacterial, fungal, or viral infection. Histologic analysis of an open lung biopsy specimen taken from patient 4 at the time of presentation showed acute bronchiolitis and alveolitis together with background fibrosis related to the underlying SSc. No evidence of infection was found on histopathologic examination. Biopsy was not performed on patient 7 for safety reasons, and autopsy permission was denied. Because both of these pulmonary toxicities appeared to be regimen-related, lung shielding was adopted for subsequent patients.
Patient 10 had fatal PTLD. During conditioning therapy, rabbit ATG (2.5 mg/kg 6 times) was substituted for horse ATG because of a hypersensitivity reaction. Recurrent acyclovir-resistant HSV infections occurred early after HDIT. In contrast to all but one other patient, PB T cells were undetectable at 1 month after HDIT. Two months after HDIT, cardiorespiratory arrest occurred requiring mechanical ventilation. Elevated serum troponin levels suggested possible myocardial injury. After the patient remained stable for 2 weeks, rapidly increasing PB lymphocytosis developed, and liver, spleen, and lymph nodes became enlarged. EBV-related PTLD was diagnosed on lymph node biopsy. Death from multiorgan failure occurred shortly after rituximab treatment was initiated.24 Autopsy tissues from lungs, liver, and bone marrow showed extensive invasion with lymphocytes.
Survival
At a median follow-up of 15 months (range, 8-45 months), 15 of 19 (79%) patients were alive with a projected 2-year survival rate of 78.9% (95% CI, 60.6%-97.3%; Figure2). Table 3summarizes survival and status.
Survival after HDIT.
Kaplan-Meier survival estimate as of April 1, 2001.
Summary of outcomes
| Pt. . | Baseline mRSS . | Baseline DLCO . | Survival, mo . | Status . | Last disease evaluation . | |||
|---|---|---|---|---|---|---|---|---|
| Months after HDIT . | mRSS . | DLCO, % . | mHAQ-DI . | |||||
| 1 | 47 | 76 | 44.8 | Alive, Cy for PD (lung disease) | 36 | 10 | 55 | 1.0 |
| 2 | 29 | 48 | 39.6 | Alive, FK-506 for PD (lung disease) | 36 | 12 | 42 | 0 |
| 3 | 30 | 57 | 36.1 | Alive, no therapy for SSc | 36 | 8 | 69 | 0 |
| 4 | 32 | 47 | 3-150 | Died on + 58 pulmonary toxicity | — | — | — | — |
| 5 | 30 | 46 | 31.9 | Alive, no therapy for SSc | 24 | 20 | 42 | 0.375 |
| 6 | 35 | 67 | 24.8 | Alive, no therapy for SSc | 24 | 3 | 60 | 0.125 |
| 7 | 50 | 52 | 3-150 | Died on + 79 pulmonary toxicity | — | — | — | — |
| 8 | 39 | 62 | 22.9 | Alive, no therapy for SSc | 12 | 26 | 57 | 0.2 |
| 9 | 14 | 45 | 17.1 | Alive, no therapy for SSc | 12 | 1 | 52 | 0 |
| 10 | 3 | 66 | 3-150 | Died on + 64 EBV-PTLD | — | — | — | — |
| 11 | 40 | 57 | 14.7 | Alive, no therapy for SSc | 12 | 24 | 60 | 0.75 |
| 12 | 27 | 57 | 14.5 | Alive, no therapy for SSc | 12 | 18 | 41 | 0.25 |
| 13 | 24 | 47 | 12.8 | Alive, no therapy for SSc | 12 | 6 | 54 | 0.5 |
| 14 | 18 | 66 | 12.8 | Alive, no therapy for SSc | 12 | 10 | 66 | 0 |
| 15 | 31 | 84 | 12.8 | Alive, prednisone 8 mg/d | 12 | 21 | 60 | 1.25 |
| 16 | 36 | 56 | 3-150 | Died + 123 of PD (renal failure) | — | — | — | — |
| 17 | 43 | 47 | 10.8 | Alive, no therapy for SSc | 6 | 46 | 28 | 2.25 (3) |
| 18 | 19 | 69 | 9.6 | Alive, no therapy for SSc | 3 | 15 | 49 | 2.125 |
| 19 | 32 | 72 | 7.5 | Alive, no therapy for SSc | 3 | 7 | 52 | 0.450 |
| Pt. . | Baseline mRSS . | Baseline DLCO . | Survival, mo . | Status . | Last disease evaluation . | |||
|---|---|---|---|---|---|---|---|---|
| Months after HDIT . | mRSS . | DLCO, % . | mHAQ-DI . | |||||
| 1 | 47 | 76 | 44.8 | Alive, Cy for PD (lung disease) | 36 | 10 | 55 | 1.0 |
| 2 | 29 | 48 | 39.6 | Alive, FK-506 for PD (lung disease) | 36 | 12 | 42 | 0 |
| 3 | 30 | 57 | 36.1 | Alive, no therapy for SSc | 36 | 8 | 69 | 0 |
| 4 | 32 | 47 | 3-150 | Died on + 58 pulmonary toxicity | — | — | — | — |
| 5 | 30 | 46 | 31.9 | Alive, no therapy for SSc | 24 | 20 | 42 | 0.375 |
| 6 | 35 | 67 | 24.8 | Alive, no therapy for SSc | 24 | 3 | 60 | 0.125 |
| 7 | 50 | 52 | 3-150 | Died on + 79 pulmonary toxicity | — | — | — | — |
| 8 | 39 | 62 | 22.9 | Alive, no therapy for SSc | 12 | 26 | 57 | 0.2 |
| 9 | 14 | 45 | 17.1 | Alive, no therapy for SSc | 12 | 1 | 52 | 0 |
| 10 | 3 | 66 | 3-150 | Died on + 64 EBV-PTLD | — | — | — | — |
| 11 | 40 | 57 | 14.7 | Alive, no therapy for SSc | 12 | 24 | 60 | 0.75 |
| 12 | 27 | 57 | 14.5 | Alive, no therapy for SSc | 12 | 18 | 41 | 0.25 |
| 13 | 24 | 47 | 12.8 | Alive, no therapy for SSc | 12 | 6 | 54 | 0.5 |
| 14 | 18 | 66 | 12.8 | Alive, no therapy for SSc | 12 | 10 | 66 | 0 |
| 15 | 31 | 84 | 12.8 | Alive, prednisone 8 mg/d | 12 | 21 | 60 | 1.25 |
| 16 | 36 | 56 | 3-150 | Died + 123 of PD (renal failure) | — | — | — | — |
| 17 | 43 | 47 | 10.8 | Alive, no therapy for SSc | 6 | 46 | 28 | 2.25 (3) |
| 18 | 19 | 69 | 9.6 | Alive, no therapy for SSc | 3 | 15 | 49 | 2.125 |
| 19 | 32 | 72 | 7.5 | Alive, no therapy for SSc | 3 | 7 | 52 | 0.450 |
PD indicates progressive disease; mRSS, modified Rodnan skin score, mHAQ-DI, modified Health Assessment Questionnaire Disability Index.
Patient died.
Disease evaluations
Table 4 summarizes various parameters at baseline and at 3, 12, 24, and 36 months after HDIT. Thirteen of 15 (86.7%) patients at 3-month follow-up and 12 of 12 (100%) patients at 12-month follow-up had disease response as determined by mRSS (more than 25% improvement) or mHAQ-DI (more than 0.4-point improvement). No patient had progression of mRSS (more than 25% increase) or mHAQ-DI (more than 0.4-point increase). Three patients had disease progression including 2 with pulmonary and 1 with renal disease.
Changes in organ function, mRSS, and mHAQ-DI after HDIT
| Parameter . | Months after HDIT . | No. patients . | Median difference from baseline . | P . |
|---|---|---|---|---|
| mRSS | 3 | 15 | − 7 (1 to − 25) | .002 |
| 12 | 12 | − 13 (− 8 to − 19) | .0005 | |
| 24 | 5 | − 19 (− 10 to − 32) | .06 | |
| 36 | 3 | − 21 (− 17 to − 37) | .25 | |
| mHAQ-DI | 3 | 14 | − 1.0 (0 to − 2) | .001 |
| 12 | 11 | − 1.675 (0 to − 2.26) | .002 | |
| 24 | 5 | − 1.875 (− .125 to − 2.01) | .06 | |
| 36 | 3 | − 2.01 (− 2 to − 2.125) | .25 | |
| Serum creatinine | 3 | 15 | 0.1 (− 0.1 to 0.6) | .02 |
| 12 | 11 | 0.1 (− 0.1 to 0.8) | .02 | |
| 24 | 5 | 0.3 (0.2 to 0.7) | .06 | |
| 36 | 2 | 0.5 (0.2 to 0.8) | .50 | |
| DLCO | 3 | 15 | − 11 (− 32 to 3) | .001 |
| 12 | 12 | − 5 (− 26 to 7) | .11 | |
| 24 | 5 | − 4 (− 22 to 10) | .88 | |
| 36 | 3 | 4 (− 28 to 12) | 1.0 | |
| FVC | 3 | 16 | 1 (− 16 to 7) | .87 |
| 12 | 12 | 4 (− 12 to 13) | .10 | |
| 24 | 5 | 3 (− 2 to 16) | .19 | |
| 36 | 3 | − 2 (− 17 to 24) | 1.0 | |
| LVEF | 3 | 12 | − 2 (− 17 to 5) | .02 |
| 12 | 8 | − 0.5 (− 12 to 5) | .81 | |
| 24 | 2 | − 2.5 (− 6 to 1) | 1.0 | |
| 36 | 2 | 0 (− 3 to 3) | 1.0 |
| Parameter . | Months after HDIT . | No. patients . | Median difference from baseline . | P . |
|---|---|---|---|---|
| mRSS | 3 | 15 | − 7 (1 to − 25) | .002 |
| 12 | 12 | − 13 (− 8 to − 19) | .0005 | |
| 24 | 5 | − 19 (− 10 to − 32) | .06 | |
| 36 | 3 | − 21 (− 17 to − 37) | .25 | |
| mHAQ-DI | 3 | 14 | − 1.0 (0 to − 2) | .001 |
| 12 | 11 | − 1.675 (0 to − 2.26) | .002 | |
| 24 | 5 | − 1.875 (− .125 to − 2.01) | .06 | |
| 36 | 3 | − 2.01 (− 2 to − 2.125) | .25 | |
| Serum creatinine | 3 | 15 | 0.1 (− 0.1 to 0.6) | .02 |
| 12 | 11 | 0.1 (− 0.1 to 0.8) | .02 | |
| 24 | 5 | 0.3 (0.2 to 0.7) | .06 | |
| 36 | 2 | 0.5 (0.2 to 0.8) | .50 | |
| DLCO | 3 | 15 | − 11 (− 32 to 3) | .001 |
| 12 | 12 | − 5 (− 26 to 7) | .11 | |
| 24 | 5 | − 4 (− 22 to 10) | .88 | |
| 36 | 3 | 4 (− 28 to 12) | 1.0 | |
| FVC | 3 | 16 | 1 (− 16 to 7) | .87 |
| 12 | 12 | 4 (− 12 to 13) | .10 | |
| 24 | 5 | 3 (− 2 to 16) | .19 | |
| 36 | 3 | − 2 (− 17 to 24) | 1.0 | |
| LVEF | 3 | 12 | − 2 (− 17 to 5) | .02 |
| 12 | 8 | − 0.5 (− 12 to 5) | .81 | |
| 24 | 2 | − 2.5 (− 6 to 1) | 1.0 | |
| 36 | 2 | 0 (− 3 to 3) | 1.0 |
mRSS indicated modified Rodnan skin score expressed as points on a scale of 0-51. mHAQ-DI expressed as points on a scale of 0-3. A baseline mHAQ-DI score was not obtained on patient 13. Serum creatinine was expressed in μg/dL. Carbon monoxide diffusing capacity was corrected for hemoglobin; percentage predicted. Forced vital capacity, percentage of predicted. Left ventricular ejection fraction, percentage.
Compared with baseline, the median decrease in mRSS values was 21% (range, −3%-86%) at 3 months (P = .002) and 39% (range, 32%-93%) at 12 months (P = .0005). These reductions began early after HDIT and continued for 3 years (Figure3A).
Disease evaluation after HDIT.
(A) mRSS after HDIT. (B) DLCO percentage of predicted values corrected for hemoglobin. (C) mHAQ-DI values after HDIT.
Disease evaluation after HDIT.
(A) mRSS after HDIT. (B) DLCO percentage of predicted values corrected for hemoglobin. (C) mHAQ-DI values after HDIT.
Among survivors the median decrease in DLCO values was statistically significant at 3 months (P = .001) but not at 12 months (P = .11) (Table 4; Figure 3B). Among 6 patients with lung shielding, the median DLCO change was −3% (−24%-7%), not significantly different than −5% (−26%-4%) among 6 patients without lung shielding. Progressive pulmonary disease requiring therapy developed in 2 patients. Patient 1 acquired pneumonitis at 3 years and commenced treatment with oral Cy. Patient 2 had progressive pneumonitis 12 months after HDIT that responded to FK-506 therapy. Neither patient had progression of skin disease.
Overall renal function deteriorated after HDIT, but the magnitude of the changes (median creatinine increase, 0.1) was not clinically meaningful. Patient 1, who had a prior scleroderma renal crisis, contracted mild renal failure possibly related to the discontinuation of ACE inhibitors during transplantation. Patient 16 had rapidly progressive renal failure from SSc at 3 months and died suddenly of multiorgan failure 123 days after transplantation. Left ventricular ejection fractions were overall slightly reduced at 3 months but were no different from baseline at 12 months after HDIT, and no patient had evidence of progressive cardiac disease. Patient 15 had severe gastrointestinal disease that remained stable with 12-month follow-up.
Quality-of-life assessments using mHAQ-DI showed marked improvements early after HDIT (Table 4; Figure 3C). Median reductions were 1.0 (0-2.0) at 3 months (P = .001) and 1.675 (0-2.26) at 12 months (P = .002). At 3 months 9 of 13 (69%) and at 12 months 10 of 10 (100%) evaluable patients with baseline scores of 0.5 or greater had mHAQ-DI reductions of more than 0.4 points.
Ten of 19 patients had detectable autoantibodies to topoisomerase I (anti-Scl-70) before transplantation. Of 9 evaluable patients, one (patient 9) became anti–Scl-70 negative for 12 months after HDIT, whereas the others remained persistently positive.
Immune reconstitution
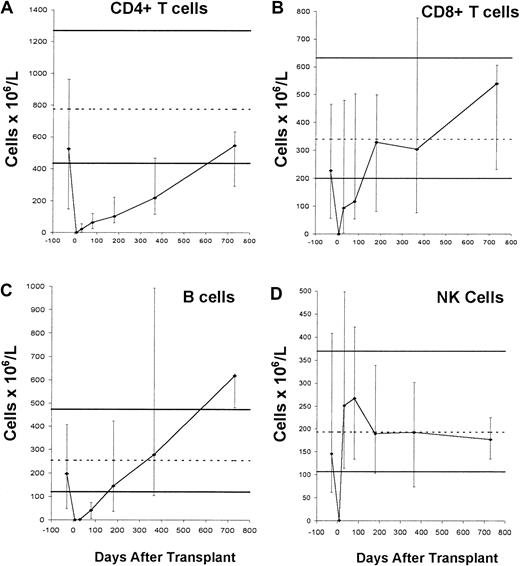
HDIT induced profound lymphocytopenia. Recovery to normal values was the fastest for NK cells, intermediate for B cells and CD8 T cells, and slowest for CD4 T cells (Figure 4). Whereas the tempos of recoveries of NK cells and B cells were similar to those reported after autografting using unmodified PBSCs, recoveries of CD4 and CD8 T cells appeared to be slower.25-27 Most T cells were of memory/effector phenotype (data not shown).
Immune recovery after HDIT.
Median lymphocyte subset counts in the transplant recipients before transplantation (before G-CSF mobilization, arbitrarily displayed as day −30) and approximately on days 7, 30, 80, 180, 365, and 730. Error bars denote the 10th to 90th percentiles. Solid thick horizontal lines that represent the 10th to 90th percentiles and the broken thin horizontal lines that represent the medians of 104 healthy adults indicate reference ranges. Numbers of patients studied were 13 before transplantation, 11 on day 7, 17 on day 30, 11 on day 80, 11 on day 180, 9 on day 365, and 3 on day 730. Lymphocyte subset counts were determined using 3-color flow cytometry as previously described.20
Immune recovery after HDIT.
Median lymphocyte subset counts in the transplant recipients before transplantation (before G-CSF mobilization, arbitrarily displayed as day −30) and approximately on days 7, 30, 80, 180, 365, and 730. Error bars denote the 10th to 90th percentiles. Solid thick horizontal lines that represent the 10th to 90th percentiles and the broken thin horizontal lines that represent the medians of 104 healthy adults indicate reference ranges. Numbers of patients studied were 13 before transplantation, 11 on day 7, 17 on day 30, 11 on day 80, 11 on day 180, 9 on day 365, and 3 on day 730. Lymphocyte subset counts were determined using 3-color flow cytometry as previously described.20
Discussion
Although there is an overall 15% 8-year mortality rate for patients with SSc, subsets of patients with much poorer prognoses can be identified by skin scores and involvement of lungs, heart, or kidneys.8,28,29 High skin scores and significant pulmonary involvement predict an approximately 50% mortality rate within 5 years.30 The lack of effective therapies and likely autoimmune pathogenesis for SSc, together with preclinical and clinical transplantation data in autoimmunity, prompted our investigation of HDIT. Several case reports31,32 and retrospective registry reports9,33 concerning HDIT for SSc have been published. Limitations of the SSc-specific registry report9 included the wide heterogeneity of patient entry criteria of multiple small studies, the multiple different transplantation regimens and graft manipulation approaches that were used, the limited data that were reported, and the lack of standardized measures of disease response. Here we provide detailed outcome descriptions from the largest single study of HDIT for SSc.
We hypothesized that intensive immunosuppression would ablate immune responses driving disease activity, thus leading to disease stabilization or disease responses. The infused CD34-selected hematopoietic progenitors, depleted in vitro of disease-causing mature lymphoid elements, might then be able to generate a new nonautoreactive immune system. To deliver intensive and potentially immunoablative conditioning therapy, we used agents (TBI, Cy, and ATG) with potent immunosuppressive properties. In preclinical rodent studies, TBI was more immunosuppressive than chemotherapy conditioning.34TBI has been an integral part of conditioning regimens aimed at suppressing immunity before allogeneic hematopoietic cell transplantation to prevent graft rejection. Although we selected an attenuated TBI dose two thirds of that usually used in transplantation for malignancy (1200 cGy or more), 800 cGy TBI is myeloablative35 and requires progenitor cell support.
PBSCs were mobilized with G-CSF but without Cy to avert unnecessary neutropenia and cardiotoxicity,9 shorten treatment, and minimize costs. Adequate progenitor cell grafts were collected without serious disease activation. Limited prior exposure to marrow toxic agents among these patients might have accounted for the efficacy of G-CSF mobilization. Several patients had what appeared to be mild disease exacerbation that occurred during G-CSF treatment and resolved when drug treatment stopped. Disease flares, in some instances severe, have been reported with G-CSF mobilization in patients with other autoimmune diseases,36-38 and this has encouraged the use of Cy-based PBSC mobilization by some investigators. In multiple sclerosis, severe and even life-threatening neurologic changes were reported when G-CSF alone was used to mobilize PBSCs.36 In patients with rheumatoid arthritis G-CSF, induced flares of RA; however, as in this study, they were not life threatening and generally resolved with discontinuation of the drug.37 Although potentially useful suppression of autoimmunity may occur with Cy-based PBSC mobilization,38 fatal toxicities have been reported in SSc patients.9 38 Results of this study showed that G-CSF mobilization without Cy was safe and effective and that it may be the preferred approach for SSc patients.
Because severe immunosuppression was anticipated, unmodified PBSCs were stored as a precaution against immunodeficiency, and infection prophylaxis was based on current recommendations for allogeneic transplantation. HDIT produced profound reductions of PB T cells, NK cells, and B cells, but it did not produce complete immune ablation because T cells were regenerated presumably from residual T cells. CD4+ cell recovery appeared delayed compared to that previously reported after unmodified autografts.25-27Because adults have reduced thymic function,39-41 slow recovery of naive T cells from hematopoietic progenitors was expected in these patients. Infection risks may therefore be prolonged compared with the use of unmodified autografts that contain much larger numbers of mature lymphocytes. Further follow-up is needed to determine the long-term effects of HDIT on immunity in these patients.
Fatal PTLD in patient 16 most likely resulted from profound T-cell suppression leading to uncontrolled growth of EBV-infected B cells. PTLD is rare after autologous transplantation, typically occurring after allogeneic hematopoietic cell transplantation using T-cell depletion techniques or T-cell–directed antibodies.42,43In this case, rabbit ATG (used because of demonstrated hypersensitivity to horse ATG), which is likely a more potent immunosuppressive than horse ATG, might have contributed to PTLD when a highly immunosuppressive TBI-based regimen was used.44-46Observations from this case indicate that there are limits to the degree of immunosuppression that can be safely achieved after HDIT. Possible future approaches to dealing with the problem of PTLD in these studies could include the use of rituximab or autologous expanded EBV-specific T cells47 48 given either preemptively or in response to increasing levels of plasma EBV DNA levels.
Two patients died of what appeared to be regimen-induced pulmonary toxicity. Both had high mRSS and compromised pulmonary function that otherwise predicted poor survival. There was no firm histologic, cytologic, or clinical evidence that progressive SSc after HDIT contributed to these deaths. However, though BAL findings excluded infection as a cause for the pulmonary changes, BAL results and biopsy findings in patient 4 were not specific for regimen-related lung injury. Our conclusions that regimen-related effects were primarily responsible for lung injury were also based on the timing of these events in relation to HDIT, their rapid onset and progression, the lack of other manifestations of progressive SSc, and the subsequent finding that lung shielding appeared to protect against pulmonary toxicity in later patients. As seen after transplantation for malignancy, DLCO reductions occurred early after conditioning therapy. These usually recovered to approximately baseline over the first year. Although our results may suggest an increased sensitivity of scleroderma lungs to high-dose conditioning therapy, pre-existing pulmonary compromise is known to predispose to transplantation complications.49Because of de-escalation of the regimen through the introduction of lung shielding, none of 11 subsequent patients had similar serious lung injury.
In other studies of SSc, baseline mRSS and mHAQ-DI have been surrogates for survival.8,15,50 In retrospective analyses of prospective treatment trials in SSc, the skin scores were stable over 12 months and were improved at 2 and 3 years after enrollment,50,51 whereas mHAQ-DI scores were stable at 12 months and minimally changed at 24 months.52 In this study, at 1 year after HDIT, 12 of 12 patients had major improvements in mRSS or mHAQ-DI. Initial responses, particularly mHAQ-DI, frequently occurred by 3 months after HDIT. Although it is possible that the prednisone given after transplantation might have contributed to initial responses, the short duration of this therapy, together with the fact that 12 of 19 patients had received steroids before HDIT, argues against an important role for corticosteroids in producing the sustained skin responses observed in this study. These impressive and rapid responses are important findings in that they appear to substantially exceed responses observed in controlled studies of other therapies.51,52 For example, a 25% or greater improvement in the mRSS is significant and outside the range of intraobserver error.15 50 The mHAQ-DI changes are less variable, and results here were even more impressive. No previously reported therapy has produced similar improvements in the mRSS and mHAQ-DI in patients with severe SSc. However, pulmonary disease did not appear to respond to HDIT, possibly because irreversible damage might have already occurred. It is possible that the stable internal organ disease observed after HDIT in these patients represents a positive effect given the poor underlying prognosis for SSc and the likelihood for further progressive pulmonary compromise. Any benefit in this regard, together with the significance of responses observed in the mRSS and mHAQ-DI, cannot be adequately assessed without a concurrent control group.
Autoantibodies to Scl-70 (anti–topoisomerase I) were present in 10 patients before HDIT. Nine of these patients were survivors who could be evaluated after HDIT, and 8 remained positive for antibodies to topoisomerase I. Thus, with limited follow-up, anti–Scl-70 was not eliminated, and it remains to be determined whether autoantibody levels will be useful for evaluating disease responses after HDIT for SSc.
Of concern for this study were the potential toxicities of the preparative regimen. High-dose, involved-field radiation (eg, 5000 cGy) given for the treatment of malignancy in SSc patients may cause accelerated fibrosis within the radiation fields.53,54 In our study, however, rapid skin improvements were typical, suggesting that TBI doses were too low to elicit fibrosis or that control of systemic autoimmunity contributed to these responses. Although secondary cancers may result from TBI, lower dose-fractionated TBI delivered at low dose rates, as used here, is less oncogenic.55 An International Bone Marrow Transplant Registry report on 19 229 patients showed that with less than 1000 cGy TBI, late cancers were not increased compared with non-TBI–containing regimens.55 Because TBI-based regimens are more lymphoablative than chemotherapy-only regimens and given the promising results observed with respect to skin and mHAQ-DI scores, we have continued to investigate the use of TBI/Cy/ATG conditioning. Investigators in Europe are testing chemotherapy-only regimens. Ultimately, short-term safety and long-term outcomes may determine which regimens are superior.
Based on this and other reports,9,33,56-58 HDIT for SSc may be associated with increased risk for severe complications compared with HDIT for other autoimmune diseases, most likely because of the nature of pre-existing advanced disease-related organ damage. Modifications introduced into our protocol after initial unfavorable outcomes, including lung shielding for TBI, have resulted in no further episodes of pulmonary toxicity. Although the disease responses reported here are encouraging with respect to the potential for prolonging survival and improving function, disease progression in some initial patients treated suggests that the potential for HDIT producing definitive disease cures could be limited. However, this cannot be adequately evaluated from this study. Importantly, our early experience and the subsequent protocol modifications have set the stage for further studies to formally evaluate the efficacy of HDIT for SSc. The role of HDIT as a therapy for SSc will be more clearly defined by planned prospective randomized studies comparing this approach against conventional dose treatment using Cy, which in uncontrolled studies has shown some evidence of efficacy.59-65
We thank the medical, nursing, and support staff at the listed institutions for their important contributions to this study through their careful and dedicated care of the patients.
Supported in part by the National Institute of Allergy and Infectious Diseases (grant N01-A1-05410), the National Institutes of Health (grants HL36444, AI46108, HL03701, CA15704, and M01-RR00042), the Department of Health and Human Services, the University of Michigan Venture Investment Fund (grant VIF.097), and Amgen, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter A. McSweeney, Bone Marrow Transplant Program, University of Colorado Health Sciences Center, B-190, 4200 East 9th Ave, Denver, CO 80262; e-mail: peter.mcsweeney@uchsc.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal