Essential thrombocythemia (ET) is a heterogeneous disorder in which the clonality of hematopoiesis varies. The clinical significance of clonality status in ET remains to be determined. We used the human androgen receptor gene (HUMARA)–polymerase chain reaction assay to investigate X-chromosome inactivation patterns (XCIPs) and their value in predicting vascular complications in 89 female patients with ET. Fifty-four (68.4%) patients had a clonal pattern of XCIP, and 15 (19.0%) had a polyclonal pattern. The remaining 20 patients had either an ambiguous or a homozygous pattern of XCIP and were therefore excluded from further analysis. Patients with clonal XCIPs were older (P = .029) and were at greater risk for thrombosis (P = .007) than were those with polyclonal XCIPs. We did not find a correlation between the occurrence of hemorrhage and XCIP (P = .492). Advanced age was predictive of thrombosis and hemorrhage. Platelet count did not influence the risk for vascular complications. Hypertension was significantly correlated with thrombotic events (P = .002), whereas diabetes mellitus and hypercholesterolemia were of no predictive value. In a multivariate analysis, age was the significant predictor of thrombosis (P = .030); however, XCIPs (P = .083) and hypertension (P = .073) tended to predict thrombosis. Our results suggest that older patients who have clonal XCIPs or hypertension are at increased risk for thrombosis and should be monitored closely for this complication.
Introduction
Essential thrombocythemia (ET) has traditionally been considered a clonal disorder.1 However, no specific markers have been established, and the diagnosis of ET is still based on exclusion of other possible diagnoses.2 The X-chromosome inactivation patterns (XCIPs) assay provides a means of assessing clonality without the use of tumor-specific genetic or cytogenetic markers, and this assay is potentially applicable to clonality analysis of myeloid stem cell disorders in female patients.3 The human androgen receptor (HUMARA) locus is especially useful in the clonality assay because it is amenable to polymerase chain reaction (PCR) analysis, and it is informative for its high heterozygosity rate.4 ET, as diagnosed by standard clinical and laboratory criteria, is a heterogeneous disorder—19% to 37.5% of patients demonstrate polyclonal hematopoiesis, and 33% to 67% demonstrate clonal hematopoiesis.5-8 Using the HUMARA-PCR assay, we have shown that the presence of clonal XCIPs in young patients with ET is a positive marker of clonal hematopoiesis and that the presence of polyclonal XCIPs in elderly patients indicates that ET is an unlikely diagnosis.7 Few studies on the correlation of clinical features with XCIPs have been published.5,6 8 Furthermore, the numbers of patients in those studies were relatively small, and the results were conflicting. To resolve these conflicting findings and to increase our understanding of the natural course of ET and the clinical significance of clonality status, we undertook an investigation of a large number of patients.
The present study of female patients with ET addresses the clinical relevance of their clonality status, especially with regard to its predictive value on the risk for vascular complications. XCIPs were determined by HUMARA-PCR assay, and the results were analyzed to determine whether XCIPs were correlated with clinical features and hematologic measures of patients with ET who had a long follow-up duration. We also sought to determine other risk factors associated with vascular complications in patients with ET.
Patients and methods
Patient characteristics
Between January 1989 and September 2000, ET was diagnosed in 89 consecutive female patients by using the criteria of the Polycythemia Vera Study Group.2 Diagnoses were made by the staff members in the Hematology Division, Chang Gung Memorial Hospital, which is a tertiary care facility with 3000 beds. Informed consent was obtained from all patients before their entry into the study, which was approved by the Institutional Review Board of Chang Gung Memorial Hospital. Nearly all patients in the study were Chinese (Han ethnic group); one was Filipino. Patients were referred by other departments or other hospitals for evaluation and treatment of thrombocytosis. At the time of diagnosis, 16 patients had thrombosis, 7 with hemorrhagic episodes; thrombocytosis was incidentally diagnosed in 41 patients treated for other medical problems, and thrombocytosis was detected during a routine health examination of 25 patients who were asymptomatic.
Before they entered the study, all patients had platelet counts that were higher than 700 × 109/L on at least 2 occasions. Platelet counts ranged from 706 × 109/L to 3666 × 109/L (median, 1176 × 109/L). At the time of initial evaluation, none of the patients had erythrocytosis, Philadelphia chromosome or BCR-ABL fusion gene, collagen fibrosis, or cytogenetic or morphologic evidence of a myelodysplastic syndrome. In addition, none showed any evidence of other causes of reactive thrombocytosis, including inflammatory disease, chronic infection, iron deficiency, malignancy, or splenectomy. The duration of follow-up ranged from 13 months to 156 months (median, 45 months), and no causes of secondary thrombocytosis were identified during that time. Patient ages ranged between 15 and 92 years (median, 57 years). Nineteen patients had hypertension (blood pressure higher than 150/90 mm Hg) and received antihypertensive agents; 7 patients had diabetes mellitus, for which 6 were treated with oral hypoglycemic agents and 1 with insulin; 11 patients had hypercholesterolemia (serum total cholesterol concentration more than 200 mg/dL), for which most were treated with dietary control alone; and only 2 patients smoked cigarettes. Thirty-five patients with thrombotic episodes, advanced age, or both were treated with myelosuppressive agents, most with hydroxyurea, and 38 patients received aspirin (100 mg/d).
Cell separation and DNA extraction
Granulocytes and T cells were separated from heparinized bone marrow or peripheral blood as previously described.7Genomic DNA was extracted from granulocytes and T cells by using a Puregene DNA Purification Kit (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions or by using a standard phenol-chloroform extraction procedure.9
HUMARA-PCR assay
The state of activation of the X-chromosome was determined by using the methylation-sensitive restrictive enzyme HpaII.HpaII cleaves the unmethylated (active) X-chromosome and precludes amplification by PCR with primers flanking bothHpaII sites and the CAG repeat in the first exon of the HUMARA locus.4
The HUMARA-PCR assays of samples from the first 48 patients were performed according to the method of Gale et al10 with minor modification, which has been previously described in detail.7 Briefly, 1 μg genomic DNA was digested with 20 U RsaI (New England BioLabs, Beverly, MA) for 3 hours at 37°C and then with 10 U HpaII (New England BioLabs) overnight at 37°C. PCR was carried out in 25 μL reaction mixture that contained 0.1 μg digested DNA mixture, 0.3 μL each HUMARA primer (0.25 μg/μL), 2.5 μL of 10× PCR buffer, 1 μL of 2.5 mM dNTP, 1 μL dimethyl sulfoxide (Sigma, St Louis, MO), 0.1 μLTaq DNA polymerase (5 U/μL) (HT Biotechnology, Cambridge, United Kingdom), and 16.5 μL H2O. The upstream primer sequence was 5'-TCCAGAATCTGTTTCCAGAGC-3', and the downstream primer sequence was 5'-TGGGGAGAACCATCCTCACC-3'. The downstream primer was labeled with 32P. PCR was performed in a 9600 thermal cycler (Perkin-Elmer-Cetus, Norwalk, CT). The program consisted of an initial denaturation step at 94°C for 3 minutes, which was followed by 28 cycles of the following steps: 94°C for 45 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. After the last cycle, a final extension step was performed at 72°C for 7 minutes. PCR products were subjected to electrophoresis on a 5% denaturing polyacrylamide gel, and the gel was then dried and exposed to x-ray film (Eastman Kodak, Rochester, NY). Autoradiographic signals were quantified by using a Fluorescent Image Analyzer FLA2000 with SW-Mac BAS Ver-2E software (FujiFilm, Tokyo, Japan).
For the subsequent 41 patients, HUMARA-PCR assays were performed as described above except that the AR1 primer (CAGGCACCCAGAGGCCGCGAG) and the AR2 primer (CCAGGACCAGGTAGCCTGTGGGGC) were used. The AR2 primer was labeled with fluorescein, as described by El-Kassar et al.5 Samples (4 μL) of PCR products were mixed with 5 μL solution of formamide (95%) and loading buffer (5% blue dextran, 25 mM EDTA) that contained 0.55 μL Rox-500. A 1.5-μL sample of this mixture was loaded on a 5% Long Ranger–6 M urea gel with 1× TBE (0.089 M Tris, 0.089 M borate, 0.002 M EDTA) buffer. Electrophoresis was performed at 200 W for 2.25 hours, and the data were analyzed by an automated ABI 377 DNA sequencer (Applied Biosystems, Foster City, CA) and quantified by Genescan 3.1 software (Applied Biosystems).
Each sample was analyzed at least in duplicate, and the results were expressed as a mean of the values. To compare the different methods, we analyzed duplicate samples from 20 patients by performing PCR analysis with 32P-labeled primers and fluorescein-labeled primers; this analysis yielded identical results for samples from the same patient.
Interpretation of XCIPs
A proportion of granulocytes derived from the dominant clone was analyzed by using the method of Asimakopoulos et al.11After correcting for the preferential amplification of alleles afterRsaI digestion, we derived the granulocyte allele ratios (RG). After HpaII digestion, we divided the signal of the less intense granulocyte allele by that of the other granulocyte allele. T-cell allele ratios (RT) were similarly derived. The percentage of clonal granulocytes (GC) was calculated from the RT and RG as follows: if RT was greater than RG, then GC = [(RT − RG)/RT(RG + 1)] × 100%; if RT was less than RG, then GC = [(RG − RT)/(RG + 1)] × 100%.
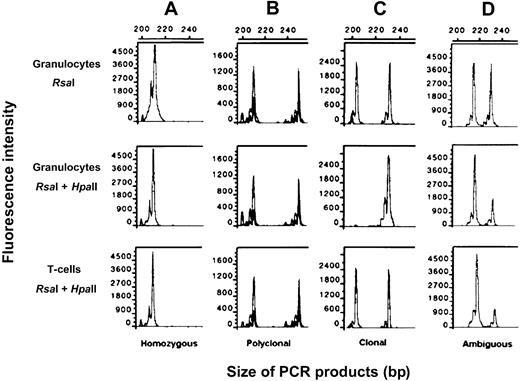
XCIPs were divided into 3 categories (Figure1): (1) clonal XCIP, in which the GC was greater than 50% (this category corresponds to an RG less than 0.33 in the presence of an RT that equals 1.0.); (2) polyclonal XCIP, in which the GC was less than 50% and the RT was greater than 0.33; and (3) ambiguous XCIP, in which the GC was less than 50% and the RT was less than 0.33.
Clonality analysis using the HUMARA-PCR assay with a fluorescein-labeled primer.
Top row: DNA from granulocytes digested with RsaI. Middle row: DNA from granulocytes digested with RsaI andHpaII. Bottom row: DNA from T cells digested withRsaI and HpaII. After digestion of DNA with the restriction enzymes, the area under the curve was calculated for each allele by using Genescan software. (A) Homozygous XCIP. (B) Polyclonal XCIP. (C) Monoclonal XCIP. (D) Ambiguous XCIP.
Clonality analysis using the HUMARA-PCR assay with a fluorescein-labeled primer.
Top row: DNA from granulocytes digested with RsaI. Middle row: DNA from granulocytes digested with RsaI andHpaII. Bottom row: DNA from T cells digested withRsaI and HpaII. After digestion of DNA with the restriction enzymes, the area under the curve was calculated for each allele by using Genescan software. (A) Homozygous XCIP. (B) Polyclonal XCIP. (C) Monoclonal XCIP. (D) Ambiguous XCIP.
Statistical analysis
Fisher exact test, χ2 analysis, unpairedt test, or Wilcoxon rank sum test was used, as appropriate, to compare data between groups. Multiple logistic regression analysis was used to identify the independent predictors of thrombosis or hemorrhage. P = .05 was required for retention in the model. All P values were calculated by using 2-sided tests.
Results
Correlation between clinicohematologic features and XCIPs
Of the 89 female patients with ET, 10 were homozygous at the HUMARA locus; 54 had clonal patterns, 15 had polyclonal patterns, and 10 had ambiguous patterns. Patients with homozygous or ambiguous patterns that could not be interpreted were excluded from further analysis. Thus, 69 patients formed the basis of the present study. Table 1 shows the comparisons of clinicohematologic features between the clonal and polyclonal XCIP categories. Patients with clonal XCIPs were significantly older than those with polyclonal XCIPs (P = .029). Between the 2 groups, no differences were observed in hematocrit (Ht), WBC count, platelet count, leukocyte alkaline phosphatase (LAP) score, duration of follow-up, or incidence of splenomegaly or myelofibrosis.
Correlation between XCIPs and clinicohematologic features in female patients with essential thrombocythemia
| Feature . | Clonal XCIP, n = 54 . | Polyclonal XCIP, n = 15 . | P . |
|---|---|---|---|
| Age, y | 57.7 ± 19.9 | 45.3 ± 15.5 | .029 |
| Hematocrit, % | 36.2 ± 5.4 | 37.0 ± 3.2 | .457 |
| WBC count, × 109/L | 15.3 ± 7.7 | 13.3 ± 9.3 | .403 |
| Platelet count, × 109/L | 1309.1 ± 424.8 | 1176.7 ± 276.7 | .259 |
| LAP score | 136.1 ± 67.9 | 131.9 ± 53.5 | .824 |
| Follow-up, mo | 53.0 ± 36.4 | 49.0 ± 23.8 | .614 |
| Splenomegaly, no. patients | 14 | 2 | .492 |
| Myelofibrosis, no. patients | 19 | 6 | 1.000 |
| Feature . | Clonal XCIP, n = 54 . | Polyclonal XCIP, n = 15 . | P . |
|---|---|---|---|
| Age, y | 57.7 ± 19.9 | 45.3 ± 15.5 | .029 |
| Hematocrit, % | 36.2 ± 5.4 | 37.0 ± 3.2 | .457 |
| WBC count, × 109/L | 15.3 ± 7.7 | 13.3 ± 9.3 | .403 |
| Platelet count, × 109/L | 1309.1 ± 424.8 | 1176.7 ± 276.7 | .259 |
| LAP score | 136.1 ± 67.9 | 131.9 ± 53.5 | .824 |
| Follow-up, mo | 53.0 ± 36.4 | 49.0 ± 23.8 | .614 |
| Splenomegaly, no. patients | 14 | 2 | .492 |
| Myelofibrosis, no. patients | 19 | 6 | 1.000 |
Values represent mean ± SD.
Correlation between vascular complications and XCIPs or other risk factors
Twenty-five of the 69 patients with ET experienced thrombotic complications during the course of ET—8 patients had a history of thrombosis, 9 experienced thrombotic events at the time of diagnosis, 1 experienced thrombotic events before and at the time of diagnosis of ET, and 7 experienced thrombotic complications during follow-up. Three patients had recurrent thrombotic events, and 1 had 2 recurrent episodes. The thrombotic complications included 10 episodes of cerebrovascular occlusion, 6 of peripheral arterial disease, 4 of transient ischemic attack, 2 of ischemic cardiovascular disease, 2 of deep venous thrombosis, 2 of erythromelalgia, 1 of splenic infarction, and 1 of pulmonary embolism. Bleeding occurred in 16 patients (gastrointestinal tract bleeding in 14, hematoma in 1, and subarachnoid hemorrhage in 1). Bleeding occurred before or at diagnosis in 8 patients and during follow-up in the other 8 patients. Eight patients had thrombotic and hemorrhagic complications. Two of the 29 patients who received aspirin experienced gastrointestinal bleeding after aspirin therapy. At a median follow-up time of 43 months, 6 patients had died; 1 death was caused by a thrombotic complication, 1 by acute myeloid leukemia, 1 by myelodysplastic syndrome, and the remaining 3 patients, who were older than 80 years, died of pneumonia or sepsis. Thirty-seven (53.6%) patients remained asymptomatic throughout follow-up.
Table 2 shows the results of univariate analyses of potential risk factors for vascular complications in 69 female patients with ET. Clonal XCIPs were associated with a significantly higher risk for thrombosis (P = .007) but not with hemorrhagic events (P = .492). Older age was strongly associated with a significantly higher risk for thrombosis (P = .014) or hemorrhage (P < .0001), and an elevated WBC count was also a predictor of hemorrhage (P = .010). We found that platelet count did not influence the risk for thrombosis (P = .402) or hemorrhage (P = .414). There was a trend for patients with splenomegaly (P = .057) or elevated LAP score (P = .073) to be at increased risk for thrombosis. Neither splenomegaly nor elevated LAP score was associated with an increased risk for hemorrhage. There was no difference in Ht, follow-up time, or presence of myelofibrosis between patients with vascular complications and those without. Among the known cardiovascular risk factors evaluated, hypertension was significantly associated with thrombotic complications (P = .002) but not with hemorrhage (P = .287). Neither diabetes mellitus nor hypercholesterolemia was linked to the risk for thrombosis or hemorrhage. Only 2 patients smoked; therefore, an analysis of the effect of cigarette smoking was precluded.
Univariate analysis of risk factors of vascular complications in 69 female patients with essential thrombocythemia and clonal or polyclonal XCIPs
| Features . | Occurrence of thrombosis . | Occurrence of hemorrhage . | ||||
|---|---|---|---|---|---|---|
| Yes, n = 25 . | No, n = 44 . | P . | Yes, n = 16 . | No, n = 53 . | P . | |
| Age, y* | 66.4 ± 16.2 | 48.5 ± 18.5 | <.001 | 70.1 ± 10.2 | 50.4 ± 19.5 | <.001 |
| 40 or younger† | 1 | 15 | — | 0 | 16 | — |
| 41 to 55† | 5 | 12 | — | 0 | 17 | — |
| 56 to 65† | 6 | 8 | — | 6 | 8 | — |
| 66 to 75† | 5 | 5 | — | 5 | 5 | — |
| 76 or older | 8 | 4 | — | 5 | 7 | — |
| Hematocrit, %*,† | 37.3 ± 4.5 | 35.8 ± 5.3 | .245 | 35.7 ± 6.3 | 36.6 ± 4.6 | .531 |
| WBC count, × 109/L* | 16.3 ± 7.5 | 14.1 ± 8.4 | .272 | 19.7 ± 11.4 | 13.5 ± 6.4 | .010 |
| Less than or equal to 12† | 7 | 21 | — | 3 | 25 | — |
| Greater than 12† | 18 | 23 | — | 13 | 28 | — |
| Platelet count, × 109/L* | 1226.4 ± 395.9 | 1311.0 ± 402.4 | .402 | 1208.2 ± 369.3 | 1302.1 ± 408.7 | .414 |
| Less than or equal to 1200† | 16 | 21 | — | 10 | 27 | — |
| Greater than 1200† | 9 | 23 | — | 6 | 26 | — |
| Follow-up, mo* | 50.0 ± 33.6 | 53.3 ± 34.4 | .701 | 50.3 ± 32.4 | 52.7 ± 34.6 | .801 |
| Clonal XCIP† | 24 | 30 | .007 | 14 | 40 | .492 |
| Elevated LAP score† | 21 | 28 | .073 | 14 | 35 | .124 |
| Splenomegaly† | 9 | 7 | .057 | 6 | 10 | .175 |
| Myelofibrosis† | 8 | 20 | .274 | 7 | 21 | .768 |
| Hypertension† | 10 | 4 | .002 | 5 | 9 | .287 |
| Diabetes mellitus† | 3 | 3 | .463 | 2 | 4 | .617 |
| Hypercholesterolemia† | 4 | 4 | .448 | 3 | 5 | .376 |
| Features . | Occurrence of thrombosis . | Occurrence of hemorrhage . | ||||
|---|---|---|---|---|---|---|
| Yes, n = 25 . | No, n = 44 . | P . | Yes, n = 16 . | No, n = 53 . | P . | |
| Age, y* | 66.4 ± 16.2 | 48.5 ± 18.5 | <.001 | 70.1 ± 10.2 | 50.4 ± 19.5 | <.001 |
| 40 or younger† | 1 | 15 | — | 0 | 16 | — |
| 41 to 55† | 5 | 12 | — | 0 | 17 | — |
| 56 to 65† | 6 | 8 | — | 6 | 8 | — |
| 66 to 75† | 5 | 5 | — | 5 | 5 | — |
| 76 or older | 8 | 4 | — | 5 | 7 | — |
| Hematocrit, %*,† | 37.3 ± 4.5 | 35.8 ± 5.3 | .245 | 35.7 ± 6.3 | 36.6 ± 4.6 | .531 |
| WBC count, × 109/L* | 16.3 ± 7.5 | 14.1 ± 8.4 | .272 | 19.7 ± 11.4 | 13.5 ± 6.4 | .010 |
| Less than or equal to 12† | 7 | 21 | — | 3 | 25 | — |
| Greater than 12† | 18 | 23 | — | 13 | 28 | — |
| Platelet count, × 109/L* | 1226.4 ± 395.9 | 1311.0 ± 402.4 | .402 | 1208.2 ± 369.3 | 1302.1 ± 408.7 | .414 |
| Less than or equal to 1200† | 16 | 21 | — | 10 | 27 | — |
| Greater than 1200† | 9 | 23 | — | 6 | 26 | — |
| Follow-up, mo* | 50.0 ± 33.6 | 53.3 ± 34.4 | .701 | 50.3 ± 32.4 | 52.7 ± 34.6 | .801 |
| Clonal XCIP† | 24 | 30 | .007 | 14 | 40 | .492 |
| Elevated LAP score† | 21 | 28 | .073 | 14 | 35 | .124 |
| Splenomegaly† | 9 | 7 | .057 | 6 | 10 | .175 |
| Myelofibrosis† | 8 | 20 | .274 | 7 | 21 | .768 |
| Hypertension† | 10 | 4 | .002 | 5 | 9 | .287 |
| Diabetes mellitus† | 3 | 3 | .463 | 2 | 4 | .617 |
| Hypercholesterolemia† | 4 | 4 | .448 | 3 | 5 | .376 |
Values represent mean ± SD.P was computed using unpaired t test.
Values represent number of patients. P was computed using χ2 test or Fisher exact test.
Multivariate analysis (Table 3) indicated that age was the most important risk factor for thrombosis (P = .030) or hemorrhage (P = .003). XCIPs and hypertension were also associated with the development of thrombosis. After the data were adjusted for age and presence of hypertension, we found that patients with clonal XCIPs had a 6.9-fold higher risk (95% confidence interval, 0.78-60.66) for thrombosis than did those with polyclonal XCIPs. The type of XCIP had no impact on the occurrence of hemorrhage.
Multivariate analysis using multiple logistic regression of risk factors of vascular complications in 69 female patients with essential thrombocythemia and clonal or polyclonal XCIPs
| Features . | Thrombosis . | Hemorrhage . | ||||
|---|---|---|---|---|---|---|
| Odds ratio . | 95% CI . | P . | Odds ratio . | 95% CI . | P . | |
| XCIP | .083 | .990 | ||||
| Clonal | 6.87 | 0.78-60.66 | — | 1.01 | 0.16-6.58 | — |
| Polyclonal | 1.00 | Reference | — | 1.00 | Reference | — |
| Age, y | 1.64 | 1.05-2.56 | .030 | 2.24 | 1.31-3.81 | .003 |
| WBC count, × 109/L | .231 | |||||
| Less than or equal to 12 | — | — | — | 1.00 | Reference | — |
| Greater than 12 | — | — | — | 2.48 | 0.56-10.99 | — |
| Hypertension | .073 | |||||
| No | 1.00 | Reference | — | — | — | — |
| Yes | 3.69 | 0.83-15.34 | — | — | — | — |
| Features . | Thrombosis . | Hemorrhage . | ||||
|---|---|---|---|---|---|---|
| Odds ratio . | 95% CI . | P . | Odds ratio . | 95% CI . | P . | |
| XCIP | .083 | .990 | ||||
| Clonal | 6.87 | 0.78-60.66 | — | 1.01 | 0.16-6.58 | — |
| Polyclonal | 1.00 | Reference | — | 1.00 | Reference | — |
| Age, y | 1.64 | 1.05-2.56 | .030 | 2.24 | 1.31-3.81 | .003 |
| WBC count, × 109/L | .231 | |||||
| Less than or equal to 12 | — | — | — | 1.00 | Reference | — |
| Greater than 12 | — | — | — | 2.48 | 0.56-10.99 | — |
| Hypertension | .073 | |||||
| No | 1.00 | Reference | — | — | — | — |
| Yes | 3.69 | 0.83-15.34 | — | — | — | — |
Age was treated as an ordinal (40 or younger, 41-55, 56-65, 66-75, 76 or older).
Discussion
Analysis of XCIPs is a useful tool in the diagnosis of clonal myelopoiesis in female patients with myeloid stem cell disorders. In the present study, clonality analysis of granulocytes was carried out in patients with ET; analysis of platelets or megakaryocytes was not performed because all these cell types belong to the myeloid lineage. However, during the course of ET, granulocytes can be polyclonal, whereas platelets and megakaryocytes are clonal. Indeed, evidence of this type of clonal evolution has been found in patients with polycythemia vera. Gilliland et al12 found that 1 of 3 patients with polycythemia vera had clonal erythropoiesis but not clonal granulopoiesis. Similarly, El-Kassar et al5demonstrated that 3 of 10 patients with ET exhibited polyclonal XCIPs in their granulocytes but clonal XCIPs in their platelets. In contrast, Harrison et al6 did not demonstrate restriction of clonality to the megakaryocytic lineage in their 8 patients who had ET with polyclonal granulopoiesis. Chiusolo et al8 reported that none of their 15 patients with polyclonal granulopoiesis had clonal patterns in their platelets.
Distinguishing extreme lyonization from acquired clonal dominance affecting granulocytes and T cells is impossible in patients with ambiguous XCIPs13; therefore, our evaluation of the clinical significance of XCIPs was restricted to patients with clonal or polyclonal hematopoiesis. When we compared the clinical and hematologic features of patients with clonal XCIPs and those of patients with polyclonal XCIPs, we found that older age was significantly associated with clonal XCIPs, whereas there was no correlation between XCIPs and platelet counts or other hematologic features.
The incidence of vascular complications in ET varied considerably among the different studies.14 Our results showed that the incidence of thrombosis (36.2%) was more frequent than that of hemorrhage (23.2%). Most (72%) of the thrombotic events occurred before or at the time of diagnosis. In a randomized study, hydroxyurea effectively prevented thrombosis in high-risk patients with ET.15 In the current study, all patients who had thrombosis were treated with myelosuppressive agents immediately following the initial diagnosis; this treatment probably resulted in a low rate of recurrence of thrombosis in the subsequent course of disease.
Patients with clonal XCIPs were more likely to experience thrombotic complications (24 of 54) than were those with polyclonal XCIPs (1 of 15). We did not observe a correlation between bleeding episodes and XCIP categories; however, we did evaluate other clinicohematologic features that might be predictive of vascular complications. Older age was strongly associated with thrombosis and hemorrhage. Elevated WBC count was also a predictor for hemorrhage. In contrast, no associations were detected between vascular complications and platelet count, Ht level, elevated LAP score, duration of follow-up, presence of splenomegaly, or presence of myelofibrosis.
Findings from different studies of risk factors of vascular complications in patients with ET are conflicting.15-23Most investigators observed a lack of correlation between platelet count and risk for thrombosis.15-21 On the other hand, degree of thrombocytosis was identified as a risk factor for bleeding manifestations in some studies16,22,23 but not in others, including ours.18,19 Besses et al20 found that cardiovascular risk factors such as hypertension, diabetes mellitus, and hypercholesterolemia were linked to thrombotic events, but others did not detect this association.15 21 When we analyzed these cardiovascular risk factors in our patients, we observed that hypertensive patients had a significantly higher incidence of thrombosis than normotensive patients. No predictive value was observed for diabetes mellitus or hypercholesterolemia. In addition, no correlation was found between cardiovascular risk factors and hemorrhagic complications.
We and other investigators7,13 24 have shown that excessive skewing is more frequent in granulocytes than in T cells in healthy women, especially in elderly women. Excessive skewing that affects granulocytes more than T cells would result in a pseudoclonal pattern in elderly patients. In addition, the replacement of normal polyclonal hematopoiesis with clonal hematopoiesis may be a slowly progressive process in ET, if it happens at all. Thus, patients with clonal granulopoiesis might represent those whose disease has progressed from polyclonal to clonal hematopoiesis by clonal evolution and expansion with time. Older patients with ET might have had the disease longer; hence, their cells might have had more time to evolve and acquire a more detectable clonal pattern.
To adjust for the acquired skewing toward clonal XCIPs occurring with increasing age, we used multivariate analysis to evaluate the risk factors. In this analysis, older age was the most predictive risk factor. Because of the strong correlation between the XCIP category and age, XCIP and hypertension were only marginally related to the development of thrombosis.
Clonal progression in patients who have ET remains an important process that requires further elucidation. If patients demonstrated clonal granulopoiesis at the time of initial diagnosis, there were no earlier samples available to clarify whether clonal evolution in those patients might have lasted longer than in their polyclonal counterparts. On the other hand, serial clonality assays of patients with polyclonal XCIPs may be beneficial. However, many of our patients received myelosuppressive agents after their initial examination, and these agents might have induced a shift in the clonality of hematopoiesis toward normal.
Before the current study, only 3 smaller studies that investigated the clinical relevance of XCIPs in ET had been performed (Table4). In the earlier studies, only univariate analysis of data from patients with polyclonal XCIPs and from younger patients (younger than 65 years) with monoclonal disease was performed. The incidence of thrombosis in our patients who were younger than 65 years and had clonal XCIPs (33.3%) tended to be higher than that of patients with polyclonal XCIPs (6.7%;P = .073). Harrison et al6 and Chiusolo et al8 observed that thrombosis occurred significantly more frequently in the monoclonal group. In contrast, El-Kassar et al5 found that there was no correlation between the prevalence of ischemic or hemorrhagic episodes and XCIPs. These discrepant results were probably caused by the small sample size in the studies. The inclusion of a larger number of patients and the use of multivariate analysis allowed us to firmly establish the relation between older age and the development of thrombosis. To determine whether monoclonal XCIPs and hypertension are associated with increased risk for thrombosis will require additional studies.
Comparison of the clinicohematologic features and XCIPs in studies of patients with essential thrombocythemia
| Reference . | No. patients . | Age, y . | Platelet count, × 109/L . | Splenomegaly . | Follow-up, mo . | Vascular complications . | P . | |
|---|---|---|---|---|---|---|---|---|
| Hemorrhage (%) . | Thrombosis (%) . | |||||||
| El-Kassar et al5 (n = 33) | .330 | |||||||
| Monoclonal | 19 | 48 (16-65) | 1025 (631-2160) | NA | 42 (1-123) | NA | 6 (31.6)4-151 | — |
| Polyclonal | 14 (30.4%)4-150 | 37 (13-76) | 766 (600-1400) | NA | 54.5 (1-336) | NA | 3 (21.4)4-151 | — |
| Harrison et al6 (n = 23) | .039 | |||||||
| Monoclonal | 10 | 53 (11-64) | 722 (600-1457) | 5 | 83 (18-260) | 1 (10) | 6 (60) | — |
| Polyclonal | 13 (28.3%)4-150 | 48 (15-84) | 778 (600-1917) | 2 | 32 (6-116) | 0 (0) | 2 (15) | — |
| Chiusolo et al8 (n = 32) | .040 | |||||||
| Monoclonal | 17 | 37 (22-59) | 779 (391-1300) | NA | 24 (5-180) | NA | 7 (41.7) | — |
| Polyclonal | 15 (37.5%)4-150 | 37 (24-64) | 656 (309-1000) | NA | 36 (9-120) | NA | 1 (6.7) | — |
| Current study (n = 48) | .073 | |||||||
| Monoclonal | 33 | 50 (16-65) | 1212 (753-2400) | 8 | 46 (14-156) | 5 (15.2) | 11 (33.3) | — |
| Polyclonal | 15 (19%)4-150 | 43 (21-76) | 1176 (706-1752) | 2 | 49 (18-102) | 2 (13.3) | 1 (6.7) | — |
| Reference . | No. patients . | Age, y . | Platelet count, × 109/L . | Splenomegaly . | Follow-up, mo . | Vascular complications . | P . | |
|---|---|---|---|---|---|---|---|---|
| Hemorrhage (%) . | Thrombosis (%) . | |||||||
| El-Kassar et al5 (n = 33) | .330 | |||||||
| Monoclonal | 19 | 48 (16-65) | 1025 (631-2160) | NA | 42 (1-123) | NA | 6 (31.6)4-151 | — |
| Polyclonal | 14 (30.4%)4-150 | 37 (13-76) | 766 (600-1400) | NA | 54.5 (1-336) | NA | 3 (21.4)4-151 | — |
| Harrison et al6 (n = 23) | .039 | |||||||
| Monoclonal | 10 | 53 (11-64) | 722 (600-1457) | 5 | 83 (18-260) | 1 (10) | 6 (60) | — |
| Polyclonal | 13 (28.3%)4-150 | 48 (15-84) | 778 (600-1917) | 2 | 32 (6-116) | 0 (0) | 2 (15) | — |
| Chiusolo et al8 (n = 32) | .040 | |||||||
| Monoclonal | 17 | 37 (22-59) | 779 (391-1300) | NA | 24 (5-180) | NA | 7 (41.7) | — |
| Polyclonal | 15 (37.5%)4-150 | 37 (24-64) | 656 (309-1000) | NA | 36 (9-120) | NA | 1 (6.7) | — |
| Current study (n = 48) | .073 | |||||||
| Monoclonal | 33 | 50 (16-65) | 1212 (753-2400) | 8 | 46 (14-156) | 5 (15.2) | 11 (33.3) | — |
| Polyclonal | 15 (19%)4-150 | 43 (21-76) | 1176 (706-1752) | 2 | 49 (18-102) | 2 (13.3) | 1 (6.7) | — |
Except for number of patients, values in parentheses are ranges, preceded by medians. Each P value represents the difference in the risk for thrombosis between monoclonal and polyclonal XCIPs in each series.
NA indicates not available.
Indicates the frequency of polyclonal XCIPs in patients with informative human androgen receptor gene polymorphism.
Numbers of all vascular complications (also including hemorrhage) that occurred.
ET can evolve into acute myeloid leukemia (AML) or myelofibrosis.25 26 Disease progression was assessed in our patients, and only one experienced AML transformation. This patient had clonal hematopoiesis, was treated with busulfan and long-term hydroxyurea therapy, and experienced AML transformation 9.5 years after the initial diagnosis of ET. Two other patients with clonal XCIPs demonstrated increasing myelofibrosis during follow-up. None of the patients with polyclonal XCIPs had myelofibrosis progression. The small number of patients with AML or myelofibrosis progression precluded any analysis of the difference in disease progression between clonal and polyclonal hematopoiesis.
We thank Ching-Ping Tseng, PhD, for technical counseling, Dr Tzung-Chih Tang for help in sample collection, Dr Ching-Hon Pui and Dr Angela McArthur for critical review, and Ms Yu-Feng Wang for secretarial assistance.
Supported by grants NSC87-2314-B-182-018 and NSC88-2314-B-182-013 from the National Science Council, Taiwan and grant CMRP859 from Chang Gung Memorial Hospital, Taiwan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lee-Yung Shih, Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, 199 Tung Hwa North Rd, Taipei 105, Taiwan; e-mail: sly7012@adm.cgmh.org.tw.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal