Adenovirus infections occur in 5% to 21% of patients following stem cell transplantation (SCT), with an associated mortality of up to 50%. However, a lack of prospective studies has hampered further developments in the understanding and management of this infection in the posttransplantation setting. We prospectively studied the incidence and outcome of adenovirus infections after SCT using preemptive screening and a policy of reduction or withdrawal of immunosuppressive therapy if the virus was isolated. The incidence of adenovirus infection was 19.7% (15 of 76), and the virus was isolated exclusively in recipients of T-cell–depleted grafts. Patients receiving 50 or 100 mg alemtuzumab in vivo were at the greatest risk of adenovirus infection (45% probability) regardless of donor type, and this was related to the slower lymphocyte recovery. Six (40%) of the 15 adenovirus-infected patients developed adenovirus disease. Severe lymphocytopenia (less than 300/μL) at the time of first detection of adenovirus was a major risk factor for development of adenovirus disease (P = .001). In addition, failure to reduce immunosuppression (P = .04) and a positive result of adenovirus polymerase chain reaction (PCR) in blood at diagnosis (P = .01) were both associated with fatal adenovirus disease. On the basis of this study, we recommend active surveillance for adenovirus infection in T-cell–depleted SCT and withdrawal or reduction of immunosuppressive treatment, if possible, in patients with adenovirus infection. Preemptive antiviral therapy is warranted for patients with severe lymphocytopenia or positive blood PCR, and in those in whom immunosuppressive therapy cannot be reduced.
Introduction
The last decade has seen some major developments in the field of hemopoietic stem cell transplantation (SCT). Notable among them is a better understanding of the biology and immunology of the 2 main infectious agents, cytomegalovirus (CMV) and Epstein-Barr virus (EBV).1-3 However, other viruses have now emerged as important pathogens in the posttransplantation period, of which adenovirus is most significant. Adenovirus infections have been increasingly recognized as an important cause of morbidity and mortality in allogeneic stem cell transplant recipients, with an incidence of up to 21%.1-11 Moderate to severe graft-versus-host disease (GVHD) and the isolation of the virus from several sites have been reported as significant risk factors for disseminated infection in 2 large studies.4,5,8 9
The outcome of disseminated or fulminant infections has remained dismal despite antiviral therapy.8,9,12 Although large retrospective studies have been performed, it remains unclear why some patients develop severe disease and succumb to it, whereas others have a more benign course. Because of the retrospective nature of these studies, the natural history of infections in the posttransplantation period remains largely unexplored. A role for immunosuppression as a major factor in the development of these infections has been suggested. If this is the case, then there may be a place for immunotherapy in the treatment of adenoviral infections. However, evidence of any correlation between adenovirus infections and posttransplantation immune recovery to justify such an approach has been lacking to date. Hromas et al13 first reported on the use of donor lymphocyte infusion (DLI) in the treatment of recalcitrant adenovirus infections, and we have previously reported on the possible role of immunotherapy in the control of posttransplantation adenovirus infections.14
A viral surveillance study was carried out in our institution over the last 3 years, examining stool, urine, and throat specimens from bone marrow transplantation (BMT) recipients collected weekly for 6 months after transplantation. We now report our observations on the incidence and outcome of posttransplantation adenovirus infections in relation to graft manipulation with alemtuzumab (CAMPATH) in vivo or in vitro, immunosuppression and its manipulation, and immune recovery.
Patients and methods
We evaluated 76 allograft recipients who were treated in the BMT unit at Birmingham Heartlands Hospital between May 1997 and April 2001. All of the patients were nursed in single rooms with high-efficiency particulate air filters.
Conditioning treatment and T-cell depletion
All related and unrelated donors were fully matched at both human leukocyte antigen (HLA) class I and II alleles. Conventional conditioning consisted of cyclophosphamide alone (n = 2), and cyclophosphamide or etoposide and total-body irradiation (n = 46) or busulfan (n = 3). The nonmyeloablative conditioning schedule (n = 25) consisted of fludarabine and melphalan. Fourteen patients received an unmanipulated graft. T-cell depletion was carried out with alemtuzumab (anti-CD52) antibodies (Therapeutic Antibody Centre, Oxford, United Kingdom) in the remainder. Twenty-seven patients receiving conventional and 4 receiving nonmyeloablative conditioning underwent transplantation from matched family (n = 26) or unrelated donors (UDs; n = 5) T-cell depleted by 10 or 20 mg alemtuzumab in vitro, as previously described.15 Others (n = 31) received alemtuzumab in vivo. Recipients of UD grafts following myeloablative conditioning were treated with alemtuzumab 10 mg/d for 10 days from day −5 to day +4 (n = 9) or for 5 days from day −5 to day −1 (n = 3). alemtuzumab at 20 mg/d from day −8 to day −4 was used in 20 patients receiving nonmyeloablative conditioning,16including 6 patients receiving UD grafts. All of these patients also received cyclosporin A as GVHD prophylaxis. For the remainder, GVHD prophylaxis consisted of cyclosporin A with short-course methotrexate.
Supportive care
Antimicrobial prophylaxis consisted of oral fluconazole, ciprofloxacin, and oral acyclovir from the beginning of conditioning treatment until engraftment. Oral cotrimoxazole was initiated when the neutrophil count was greater than 1.0 × 109/L. All patients from 1998 onward received metronidazole 400 mg, 3 times a day from the day of transplantation to engraftment for anaerobic decontamination as a part of GVHD prophylaxis.8
Patients at risk of CMV disease (recipient or donor positive for CMV IgG) received preemptive therapy with ganciclovir with or without foscarnet based on a polymerase chain reaction (PCR) assay.9 CMV-seronegative patients received blood products screened for CMV infection.
Study design
For viral surveillance, stool, urine, and throat samples were examined from all patients prior to transplantation and weekly to fortnightly thereafter to 180 days after transplantation. The specimens were cultured in Hep2, rhesus monkey kidney cells, and primary liver carcinoma cell lines (Alexander cells). The cell cultures were examined for cytopathic effect for 14 days. In addition, the stool and urine samples were examined by electron microscopy (EM). One hundred eighty days after transplantation, only relevant samples from symptomatic patients were examined. The tissue samples such as liver, lungs, or gut obtained antemortem or postmortem were examined by EM and culture. The virus isolates were confirmed by direct immunofluorescence using species-specific fluorescein-conjugated monoclonal antibodies (Dako Diagnostics, Cambridgeshire, United Kingdom). Adenovirus isolates were serotyped using conventional neutralization assay with sera provided by Central Public Health Laboratory Services (Colindale, United Kingdom).
Specimens for bacteriologic and fungal investigations were processed using standard methods of microscopy, culture, and sensitivity testing.
Polymerase chain reaction
A blood sample was collected from patients when an adenovirus was detected in a surveillance sample. DNA was extracted from 50 μL whole blood as described by Casas et al.17 The PCR assay was carried out as described by Cooper et al.18 In brief, the reaction mixture for the PCR comprised 10 mM Tris-hydrochloric acid (pH 8.3), 50 nM potassium chloride, 200 μM each deoxyneucleoside triphosphate, 1.5 mM MgCl2, 0.2 μM adenovirus primers ADRJC1 and ADRJC2,18 1.25 U Taq DNA polymerase (Advanced Biotechnologies, Epsom, United Kingdom), and 5 μL appropriate DNA sample or sterile water to make up a final volume of 50 μL. The assay was performed on a Programmable Dri-Block Gene Ataq thermal cycler (Pharmacia LKB, Uppsala, Sweden) using one cycle each of 94°C for 7 minutes, 55°C for 1 minute, and 72°C for 1.5 minutes, followed by 40 cycles each of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1.5 minutes. The amplification products were analyzed by electrophoresis in 6% polyacrylamide gels.
The adenovirus primers (ADRJC1 and ADRJC2)18 were derived from the highly conserved DNA region coding for the carboxyl end of the monomeric protein II that forms the trimeric pseudohexagonal base of the adenovirus hexon. These primers yielded a product size of 140 bp. Mismatches were deliberately introduced into the primer sequence such that each primer had a maximum of 2 nonterminal mismatches when compared with known DNA sequences of the hexon proteins of adenovirus types 2 through 5, 7, and 16, and a maximum of 4 mismatches compared with the sequences of the hexon gene of the other serotypes. These mismatches did not involve the 3′ termini of the other primers, and the specificity of the test for detection of representative serotypes was not compromised. The sensitivity was determined by PCR amplification of purified adenovirus type 2 DNA. The detection limit was 40 copies of adenovirus type 2 genomic DNA per reaction mixture.
Pretransplantation serology
The pretransplantation serum samples from both donors and recipients were stored at −20°C. The sera were tested for the presence of adenovirus-specific antibodies by an enzyme-linked immunosorbent assay (ELISA) using heat-inactivated adenovirus 2 and 5 virions as antigens, as previously described.19 All patients with adenovirus infection and their respective donors were examined, and also 10 such paired sera from patients without adenovirus infections and their donors were similarly examined.
Definitions
In this study, we defined adenovirus infection as the identification of adenovirus from a surveillance sample on culture or EM. The presence of the virus together with appropriate symptoms in the absence of any other recognizable cause was termed adenovirus disease. The isolation of adenovirus from a tissue site with or without histologic evidence of involvement was compatible with the definition of definite disease. In the absence of tissue diagnosis, the term probable adenovirus disease was used. In addition, if other pathogens were isolated from the same site that could explain the symptoms, this was termed probable adenovirus disease, even in the presence of tissue diagnosis of adenovirus. However, for upper respiratory infections, we did not depend on histologic detection of adenoviruses in tissue biopsy specimens as evidence of adenovirus disease because the virus could establish persistent and latent infection in the tonsils and adenoids. We considered isolation of adenoviruses from respiratory samples in the absence of copathogens in patients with appropriate symptoms as evidence of definite adenovirus disease.
All of the patients were monitored for gastrointestinal, respiratory, and urinary symptoms. Documented infections at other sites were also noted. GVHD was graded according to standard criteria.
Immune reconstitution
The absolute lymphocyte count (ALC) was noted at least once every 4 weeks following discharge for the first 6 months. ALC was also recorded at the first detection of an adenovirus isolate. The CD4+ and CD8+ T-cell counts (since August 1999) were measured every 6 to 8 weeks after transplantation. For correlation with adenovirus infection, the count nearest to the time of detection of the virus was considered. Blood samples were collected in ethylenediamine tetra-acetic acid, and the red cells were lysed using IX fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson, Oxford, United Kingdom) following the manufacturer's protocol. CD3+CD4+ and CD3+CD8+ T-lymphocyte subsets were detected by flow cytometry using Leu-4 fluorescein isothiocyanate (FITC) + Leu-3a phycoerythrin (PE) and Leu-4 FITC + Leu-2a PE on a FACScan with SimulSET software (Becton Dickinson). The normal ranges for ALC and T-cell subsets as determined from healthy adult donors were as follows: ALC, 1500 to 4500/μL; and CD4+ T cells, 700 to 1100/μL.
Interventions
An active attempt was made to wean the patients off the immunosuppression at the first detection of an adenovirus isolate. DLIs were not administered specifically for treatment of adenovirus, but if DLI coincided with an adenovirus infection, the outcome of the infection was closely followed without introducing any other specific treatment, if possible. Intravenous immunoglobulin was not routinely administered in the posttransplantation period. Antiviral therapy was instituted only in patients who had definite or probable adenovirus disease and if the immunosuppressive therapy could not be reduced. Therapy was not guided by blood PCR results. Intravenous ribavirin (ICN Pharmaceuticals, Basingstoke, United Kingdom; 15 mg · kg−1 · d−1) was considered as the first line of treatment. Cidofovir (Pharmacia, Milton Keynes, United Kingdom; 5 mg · kg−1 · wk−1) was considered in patients not suitable to receive ribavirin.
Statistical methods
Univariate P values and odds ratios were calculated from 2 × 2 contingency tables using Epi info version 6 (Centers for Disease Control and Prevention, Atlanta, GA). The continuous variables were compared using the Mann-Whitney nonparametric method. A multiple logistic regression model was fitted to the data with a stepwise approach using the statistical program of SPSS (SPSS version 9 for Windows, Woking, United Kingdom) for risk-factor analysis. The probability of various events was examined by the Kaplan-Meier method, and the groups were compared using the log-rank test.
Results
Incidence and pattern of adenovirus infection
Adenovirus was isolated from 15 (19.7%) of 76 allograft recipients. Stool was the most frequent source of isolation (n = 15), followed by throat (n = 3) and urine (n = 2). The virus was isolated from more than one site in 5 patients. The median time to isolation was 90 days (range, 15-202 days). Ten of the 15 infections were diagnosed between 88 and 135 days. Three infections occurred within the first 30 days, and 2 others were identified beyond 180 days. The infections occurred at all times throughout the year, with no seasonal clustering of infections. The neutralization assay showed that all of the isolates were serotyped as adenovirus type 2 (subgenus C).
The patient characteristics are shown in Tables1 and 2. There was no difference in age, sex, or the underlying disease in terms of adenovirus infection. CMV seropositivity, CMV reactivation, or CMV disease did not influence the incidence of adenovirus infections. The time to neutrophil engraftment was also similar in the 2 groups.
Characteristics of patients with and without adenovirus infections
| . | Patients with adenovirus (n = 15) . | Patients without adenovirus (n = 61) . | P (odds ratio/95% CI) . |
|---|---|---|---|
| Median age (range) | 39 (18-52) | 38 (18-53) | 1.0 |
| Sex, male/female | 10/5 | 37/24 | .89 |
| Diagnosis | .9 | ||
| Acute/chronic leukemia | 6/7 | 31/14 | |
| Lymphoma/myeloma | 2/0 | 12/2 | |
| Severe aplastic anemia | 0 | 2 | |
| Donor, family/unrelated | 8/7 | 45/16 | .2 |
| Conditioning, conventional/nonmyeloablative | 5/10 | 46/15 | .004 (6.13/1.5-26) |
| T-cell depletion, yes/no | 15/0 | 47/14 | .05 (undefined) |
| CMV seropositive, yes/no | 6/9 | 35/26 | .3 |
| CMV reactivation/disease | 4/1 | 13/4 | .4 |
| GVHD | .6 | ||
| None | 10 | 38 | |
| Grades 1-2 | 3 | 18 | |
| Grades 3-4 (or extensive chronic) | 2 | 5 | |
| Neutrophils > 0.5 × 109/L, days after transplantation | 14 | 13.5 | |
| Non-CMV infection | 10 | 19 | .02 (4.4/1.1-18.4) |
| . | Patients with adenovirus (n = 15) . | Patients without adenovirus (n = 61) . | P (odds ratio/95% CI) . |
|---|---|---|---|
| Median age (range) | 39 (18-52) | 38 (18-53) | 1.0 |
| Sex, male/female | 10/5 | 37/24 | .89 |
| Diagnosis | .9 | ||
| Acute/chronic leukemia | 6/7 | 31/14 | |
| Lymphoma/myeloma | 2/0 | 12/2 | |
| Severe aplastic anemia | 0 | 2 | |
| Donor, family/unrelated | 8/7 | 45/16 | .2 |
| Conditioning, conventional/nonmyeloablative | 5/10 | 46/15 | .004 (6.13/1.5-26) |
| T-cell depletion, yes/no | 15/0 | 47/14 | .05 (undefined) |
| CMV seropositive, yes/no | 6/9 | 35/26 | .3 |
| CMV reactivation/disease | 4/1 | 13/4 | .4 |
| GVHD | .6 | ||
| None | 10 | 38 | |
| Grades 1-2 | 3 | 18 | |
| Grades 3-4 (or extensive chronic) | 2 | 5 | |
| Neutrophils > 0.5 × 109/L, days after transplantation | 14 | 13.5 | |
| Non-CMV infection | 10 | 19 | .02 (4.4/1.1-18.4) |
Characteristics and outcomes of patients with adenovirus infections
| UPN . | Age/ sex . | Type of transplantation . | Alemtuzumab dose, mg . | Onset of infection, d . | Sites . | Lymphocytes/ CD4+ T cells/mm3 . | GVHD grade . | Blood PCR . | CyA/ steroid . | Adenovirus disease . | Antiviral . | Other virus infection, non-CMV . | Outcome of adenovirus infection . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43/M | UD CMT | 100 | 202 | Stool, throat | 100/nd | 3 | Pos | C/C | Liver (def) | Ribavirin | HSV | Died; adeno hepatitis |
| 2 | 50/F | MSD NMT | 100 | 105 | Stool | 290/nd | 0 | Neg | W/− | Gut (prob) | No | HSV | Improved |
| 3 | 38/F | MSD NMT | 100 | 15 | Stool, urine | 480/nd | 0 | Neg | W/Ü+ DLI) | None | No | Polyoma | Improved |
| 4 | 33/M | UD CMT | 100 | 93 | Stool | 100/nd | 0 | nd | C/− | Pneumonia/ MOF (prob) | Ribavirin | RSV, Infl A | Died; viral pneumonia/ MOF |
| 5 | 18/M | UD NMT | 100 | 90 | Stool | 460/180 | 0 | nd | W/− | None | No | None | Improved |
| 6 | 36/M | MSD NMT | 100 | 126 | Stool, throat | 800/160 | 0 | Neg | −/Ü+ DLI) | None | No | Polyoma, RSV, PIV | Improved |
| 7 | 26/M | UD CMT | 20 | 120 | Stool | 900/230 | 0 | Neg | −/− | None | No | PIV | Improved |
| 8 | 22/F | MSD NMT | 100 | 19 | Stool, urine | 0/0 | 0 | Pos | C/− | Liver (def) | Ribavirin | None | Died; adeno hepatitis |
| 9 | 46/M | MSD CMT | 20 | 20 | Stool | 120/50 | 0 | Neg | W/− | None | No | None | Improved |
| 10 | 37/M | MSD NMT | 100 | 135 | Stool | 480/150 | 0 | Neg | −/− | None | No | Polyoma | Improved |
| 11 | 50/F | MSD NMT | 100 | 113 | Stool | 410/90 | 0 | Neg | −/− | None | No | HZV, PIV, Infl B | Improved |
| 12 | 47/M | MSD NMT | 20 | 90 | Stool | 330/160 | 0 | Neg | W/− | None | No | None | Improved |
| 13 | 52/M | UD NMT | 100 | 90 | Stool, throat | 210/30 | 0 | Neg | W/− | Upper respiratory (def) | No | HZV | Improved |
| 14 | 22/F | UD CMT | 50 | 187 | Stool | 250/90 | 3 | Neg | R/W | Gut (prob) | Cidofovir IVIg | HSV | Cleared adeno, died; CMV pneumonia |
| 15 | 39/M | UD NMT | 100 | 88 | Stool | 420/100 | 2 | Neg | R/W | None | No | HSV | Improved |
| UPN . | Age/ sex . | Type of transplantation . | Alemtuzumab dose, mg . | Onset of infection, d . | Sites . | Lymphocytes/ CD4+ T cells/mm3 . | GVHD grade . | Blood PCR . | CyA/ steroid . | Adenovirus disease . | Antiviral . | Other virus infection, non-CMV . | Outcome of adenovirus infection . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43/M | UD CMT | 100 | 202 | Stool, throat | 100/nd | 3 | Pos | C/C | Liver (def) | Ribavirin | HSV | Died; adeno hepatitis |
| 2 | 50/F | MSD NMT | 100 | 105 | Stool | 290/nd | 0 | Neg | W/− | Gut (prob) | No | HSV | Improved |
| 3 | 38/F | MSD NMT | 100 | 15 | Stool, urine | 480/nd | 0 | Neg | W/Ü+ DLI) | None | No | Polyoma | Improved |
| 4 | 33/M | UD CMT | 100 | 93 | Stool | 100/nd | 0 | nd | C/− | Pneumonia/ MOF (prob) | Ribavirin | RSV, Infl A | Died; viral pneumonia/ MOF |
| 5 | 18/M | UD NMT | 100 | 90 | Stool | 460/180 | 0 | nd | W/− | None | No | None | Improved |
| 6 | 36/M | MSD NMT | 100 | 126 | Stool, throat | 800/160 | 0 | Neg | −/Ü+ DLI) | None | No | Polyoma, RSV, PIV | Improved |
| 7 | 26/M | UD CMT | 20 | 120 | Stool | 900/230 | 0 | Neg | −/− | None | No | PIV | Improved |
| 8 | 22/F | MSD NMT | 100 | 19 | Stool, urine | 0/0 | 0 | Pos | C/− | Liver (def) | Ribavirin | None | Died; adeno hepatitis |
| 9 | 46/M | MSD CMT | 20 | 20 | Stool | 120/50 | 0 | Neg | W/− | None | No | None | Improved |
| 10 | 37/M | MSD NMT | 100 | 135 | Stool | 480/150 | 0 | Neg | −/− | None | No | Polyoma | Improved |
| 11 | 50/F | MSD NMT | 100 | 113 | Stool | 410/90 | 0 | Neg | −/− | None | No | HZV, PIV, Infl B | Improved |
| 12 | 47/M | MSD NMT | 20 | 90 | Stool | 330/160 | 0 | Neg | W/− | None | No | None | Improved |
| 13 | 52/M | UD NMT | 100 | 90 | Stool, throat | 210/30 | 0 | Neg | W/− | Upper respiratory (def) | No | HZV | Improved |
| 14 | 22/F | UD CMT | 50 | 187 | Stool | 250/90 | 3 | Neg | R/W | Gut (prob) | Cidofovir IVIg | HSV | Cleared adeno, died; CMV pneumonia |
| 15 | 39/M | UD NMT | 100 | 88 | Stool | 420/100 | 2 | Neg | R/W | None | No | HSV | Improved |
UPN indicates unique patient number; CyA, cyclosporin A; M, male; UD, unrelated donor; CMT, conventional myeloablative transplantation; nd, not done; C, continued; def, definite; HSV, herpes simplex virus; F, female; MSD, matched sibling donor; NMT, nonmyeloablative transplantation; W, withdrawn; prob, probable; MOF, multiorgan failure; RSV, respiratory syncytial virus; Infl, influenza; PIV, parainfluenza virus; HZV, herpes zoster virus; R, reduced; and IVIg, intravenous immunoglobulin.
Pretransplantation serology
All of the pretransplantation serum samples from the adenovirus-infected patients and their respective donors revealed antiadenovirus antibodies when tested with adenovirus types 2 and 5 as antigens. The same was demonstrated in the 10 donor–recipient samples from patients without evidence of posttransplantation adenovirus infection. There was no difference in the degree of antibody response between the groups.
Graft manipulation and adenovirus infection
Adenovirus infection occurred in 15 of 62 recipients of T-cell–depleted grafts, compared with none of 14 patients receiving an unmanipulated graft (P = .05). There was a clear relationship between the incidence of adenovirus infection and the mode and the amount of alemtuzumab antibodies used (Figure1). Among the patients receiving alemtuzumab in vitro, none of the 9 patients receiving 10 mg alemtuzumab antibody and only 3 of 22 patients receiving 20 mg of the antibody developed an adenovirus infection. In contrast, 11 of 28 patients receiving alemtuzumab 100 mg in vivo and 1 of 3 patients receiving 50 mg in vivo had an adenovirus infection (P = .007).
Adenovirus infection and disease in relation to T-cell depletion.
The solid line represents patients receiving alemtuzumab in vivo (50/100 mg). (A) Adenovirus infection: events in 12 of 31, probability 45%; 95% CI, 28-62.9. (B) Disease: events in 6 of 31, probability 27%; 95% CI, 8-46. The broken line represents patients receiving alemtuzumab in vitro (10 mg/20 mg). (A) Adenovirus infection: events in 3 of 31, probability 11%; 95% CI, 1-21.9. (B) Disease: events in 0 of 31. The dotted line represents patients not receiving alemtuzumab antibodies (A and B: events in 0 of 14). The difference was significant with log rank: P = .001 for adenovirus infection (A) and P = .005 for adenovirus disease (B). The Δ indicate censured patients in alemtuzumab in vitro group; ○, censured patients in no alemtuzumab group.
Adenovirus infection and disease in relation to T-cell depletion.
The solid line represents patients receiving alemtuzumab in vivo (50/100 mg). (A) Adenovirus infection: events in 12 of 31, probability 45%; 95% CI, 28-62.9. (B) Disease: events in 6 of 31, probability 27%; 95% CI, 8-46. The broken line represents patients receiving alemtuzumab in vitro (10 mg/20 mg). (A) Adenovirus infection: events in 3 of 31, probability 11%; 95% CI, 1-21.9. (B) Disease: events in 0 of 31. The dotted line represents patients not receiving alemtuzumab antibodies (A and B: events in 0 of 14). The difference was significant with log rank: P = .001 for adenovirus infection (A) and P = .005 for adenovirus disease (B). The Δ indicate censured patients in alemtuzumab in vitro group; ○, censured patients in no alemtuzumab group.
All but one recipient of nonmyeloablative conditioning received alemtuzumab antibodies in vivo or in vitro, and this was associated with a higher incidence of adenovirus infections (10 of 25, compared with 5 of 51 receiving conventional conditioning). The relationships to unrelated donor grafts (7 of 23 UD grafts versus 8 of 53 family grafts;P = .2) and GVHD of any grade were not statistically significant (Table 1). A higher incidence of non-CMV infections (Table2) was noted among patients with adenovirus infections (10 of 15, versus 19 of 61 patients without adenovirus infections,P = .02).
Immune reconstitution and adenovirus infection
Adenovirus infection was further correlated with the ALC and CD4+ and CD8+ T-cell counts (Figure2A-B). Because the median time of adenovirus isolation was 100 days, we compared the immune recovery between 90 and 120 days in various subgroups (excluding patients who had a relapse of the underlying disease before 100 days). The ALC of 14 patients who had adenovirus infection and were evaluable at 100 days was 400 ± 228/μL, compared with 838 ± 455/μL among 48 patients without adenovirus infection (P = .001). Thirteen of the 15 adenovirus-infected patients at the first detection and 12 of 14 evaluable at 100 days (unique patient number [UPN] 8 died of adenovirus hepatitis 45 days after transplantation) had an ALC less than 500/μL, compared with 15 of 48 patients without adenovirus infection (P = .0001). The trend was similar with CD4+ T-cell recovery at 100 days (112 ± 69/μL in 11 infected patients, versus 173 ± 85/μL in 23 uninfected patients, P = .04).
Adenovirus infection in relation to absolute lymphocyte count and CD4+ T-cell counts.
Scatter diagrams showing the absolute lymphocyte count (A) or CD4+ T-cell count (B) at 100 days on the y-axis and the different doses of alemtuzumab on the x-axis (10 mg/20 mg in vitro and 50 mg/100 mg in vivo). The black circles (●) represent patients with adenovirus infection (n = 14 in A and n = 11 in B), and the open circles (○) represent those without adenovirus infection (n = 48 in A and n = 23 in B).
Adenovirus infection in relation to absolute lymphocyte count and CD4+ T-cell counts.
Scatter diagrams showing the absolute lymphocyte count (A) or CD4+ T-cell count (B) at 100 days on the y-axis and the different doses of alemtuzumab on the x-axis (10 mg/20 mg in vitro and 50 mg/100 mg in vivo). The black circles (●) represent patients with adenovirus infection (n = 14 in A and n = 11 in B), and the open circles (○) represent those without adenovirus infection (n = 48 in A and n = 23 in B).
Both ALC and CD4+ T-cell counts between 90 and 120 days were significantly lower in patients receiving alemtuzumab in vivo than in those receiving alemtuzumab in vitro (ALC, 484 ± 254/μL versus 843 ± 484/μL, P = .001; CD4+ T-cell count, 107 ± 51/μL versus 200 ± 86/μL, P = .001) (Figure 2A-B). The CD4+ T-cell count did not differ significantly based on donor type (unrelated or family donor) among recipients of T-cell–depleted grafts, but the ALC did. There was no difference in the CD8+ T-cell count among the different subgroups.
A multiple logistic regression analysis was carried out with the pretransplantation variables, namely, mode of alemtuzumab administration (in vivo, in vitro, or none), type of conditioning (conventional or nonmyeloablative), and the type of donor (unrelated or family) in the model. The use of 50 to 100 mg alemtuzumab in vivo emerged as the only predictor of adenovirus infection (odds ratio, 8.8; 95% confidence interval [CI], 2.2-35). However, when the ALC at 90 to 120 days was added to this model, this had the strongest impact, with an inverse correlation between the ALC and adenovirus infection (odds ratio, 0.994; 95% CI, 0.991-0.997), and the alemtuzumab dose was no longer significant in the model.
Adenovirus disease
Six (40%) of the 15 infected patients developed probable (n = 3) or definite (n = 3) adenovirus disease (Table 2). Adenovirus disease occurred exclusively in patients receiving alemtuzumab in vivo (P = .005, Figure 1B). Four of them were recipients of UD grafts (4 of 7 versus 2 of 8;P = .3). There was no relationship to GVHD (3 of 6, versus 2 of 9 without adenovirus disease), although both patients with GVHD higher than grade 2 developed adenovirus disease.
Two patients died of fulminant hepatic failure with definite evidence of adenovirus disease on autopsy. Adenovirus was detected in the stool specimens of another patient (UPN 4) before he developed upper respiratory infection with respiratory syncytial virus and influenza virus. There was progressive worsening despite clearance of the respiratory viruses with aerosolized and intravenous ribavirin. This was followed by rapid deterioration with multiorgan failure, including of the liver. Adenovirus was persistently detected in the stool samples as the only detectable pathogen until death. Tissue diagnosis or blood PCR could not be obtained in this patient, and hence this was termed probable adenovirus disease. Two patients had gut involvement. Both had biopsies done, which showed evidence of adenovirus colitis without GVHD. However, because of the concomitant presence of copathogens (Clostridium difficile in UPN 2 and CMV in UPN 14), these cases were termed probable adenovirus disease. UPN 13 developed severe upper respiratory symptoms with clinical signs of lower respiratory involvement with wheezing. However, there was no hypoxia and there were no new radiologic changes in the lungs, and hence this was qualified as upper respiratory illness only. Adenovirus was detected in throat samples, and no other respiratory virus was detected on direct immunofluorescence and culture of upper respiratory secretions, and hence this was termed definite adenovirus disease. Adenovirus was also isolated from stool samples in this patient.
Adenovirus disease and blood PCR
A PCR for adenovirus was performed on whole blood at the time of first detection of adenovirus in a surveillance specimen in 13 patients. The PCR was positive in 2 patients, and both died of adenovirus disease. None of the other 11 patients died of adenovirus, although 2 of them had adenovirus disease of the gut and the upper respiratory tract at the time of the negative PCR (P = .01). One of the patients, who had a positive stool isolate over 14 weeks and cleared the virus following DLI, never had a positive PCR from a blood sample despite the prolonged duration of infection.
Adenovirus isolation from multiple sites
The isolation of adenovirus from multiple sites was not a significant predictor of invasive disease in our surveillance. Three of the 5 patients with virus isolation from multiple sites developed adenovirus disease, compared with 3 of the 10 patients with adenovirus isolation from a single site (P = .3). In addition, the virus was detected initially in stool specimens from all patients. Isolation from further sites was not simultaneous, but occurred at a median of 2 weeks (range, 1-3 weeks). In both patients with a fatal outcome, the blood PCR was positive before the virus was detected from a second site.
Adenovirus disease and immune reconstitution
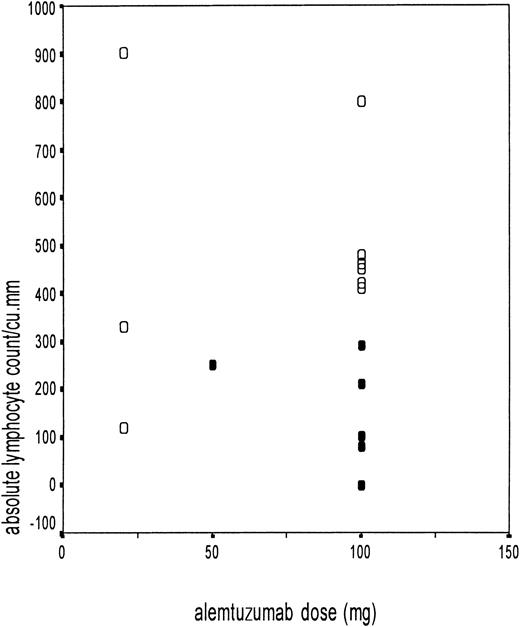
The ALC at the time of adenovirus isolation was noted in all patients. The relationship between the ALC and the development of adenovirus disease is shown in Figure 3. The ALC for patients with adenovirus disease was 158 ± 109/μL, compared with 485 ± 234/μL in patients not developing adenovirus disease (P = .003). All of the patients who developed adenovirus disease had an ALC of less than 300/μL. Six of the 7 patients with an ALC of less than 300/μL developed adenovirus disease, compared with none who had an ALC above this value (P = .001). This segregation was more marked for the patients receiving alemtuzumab in vivo (Figure 3). The probability of adenovirus-related death in adenovirus-infected patients with low ALC was 46.5%. The CD4+ T-cell count was available in 11 patients with adenovirus infection. The median CD4+ T-cell count was 30/μL (range, 0-90/μL) in the 3 patients who went on to develop adenovirus disease, compared with 155/μL (range, 50-230/μL) in the 8 patients who remained asymptomatic and cleared the virus.
Adenovirus disease in relation to absolute lymphocyte count.
Scatter diagram showing the absolute lymphocyte count at the time of first adenovirus isolation on the y-axis and the different doses of alemtuzumab on the x-axis (10 mg/20 mg in vitro and 50 mg/100 mg in vivo). The black circles (●) represent patients with adenovirus disease (n = 6), and the open circles (○) represent adenovirus-infected patients without adenovirus disease (n = 9).
Adenovirus disease in relation to absolute lymphocyte count.
Scatter diagram showing the absolute lymphocyte count at the time of first adenovirus isolation on the y-axis and the different doses of alemtuzumab on the x-axis (10 mg/20 mg in vitro and 50 mg/100 mg in vivo). The black circles (●) represent patients with adenovirus disease (n = 6), and the open circles (○) represent adenovirus-infected patients without adenovirus disease (n = 9).
Adenovirus disease and its relation to immunosuppression
A preemptive approach of withdrawing immunosuppression when possible at the first detection of the virus was employed in this study (Figure 4). In addition, 2 of these patients had received DLIs for treatment of their underlying disease. None of the patients who were off immunosuppression (n = 4) at the time of first detection of the virus developed adenovirus disease. Six patients were taking cyclosporine alone, and this was withdrawn within 2 weeks in 5 patients and later for another patient (UPN 3) because she was in the early posttransplantation period. Two of them had adenovirus disease within 2 weeks of the first detection, but both cleared the virus with symptomatic improvement without the addition of antiviral drugs. Both patients (UPN 3 and UPN 6) receiving DLI cleared the virus 4 weeks after the infusion.11 Two additional patients were taking tapering doses of prednisolone, which were stopped, and the dose of cyclosporine was reduced. One of them developed adenovirus colitis, and the other cleared the virus without developing symptoms. The former also received cidofovir and intravenous immunoglobulin for concomitant CMV disease and cleared the adenovirus. All 3 patients in whom the immunosuppression could not be reduced developed definite (n = 2) or probable (n = 1) adenovirus disease and succumbed to it, despite treatment with intravenous ribavirin. Thus, all 3 patients with continued immunosuppression developed adenovirus disease and all died, whereas only 3 of the 12 patients in whom immunosuppression was withdrawn or reduced developed adenovirus disease. All of these patients except one improved without antiviral agents, and none of them died of adenovirus disease (P = .002).
Outcome of adenovirus infection in relation to manipulation of immunosuppression.
Outcome of adenovirus infection in relation to manipulation of immunosuppression.
Survival
The actuarial probability of overall survival of patients infected with adenovirus was 56.8% (95% CI, 31.8-81.8) at 3 years, compared with 59.5% (95% CI, 47.8-71.2) in uninfected patients (P = .78). The probability of survival for patients with adenovirus disease was 16.6% (95% CI, 1.4-32) at 12 months, compared with 63.1% (95% CI, 51.4-74.8) for patients without adenovirus disease (P = .004) (Figure5). Among the adenovirus-infected patients, the median survival of the patients with a low ALC (less than 300/μL) was 222 days and the probability of nonrelapse mortality at 18 months was 57.2% (95% CI, 24.1-61.5), compared with 0% in patients with a higher ALC (P = .02).
Overall survival in patients with and without adenovirus disease.
The broken line shows the probability of survival among patients with adenovirus disease (surviving 1 of 6, probability 16.6%; 95% CI, 1.4-32). The solid line shows those without adenovirus disease (surviving 47 of 70, probability 63.1%; 95% CI, 51.4-74.8). The difference was significant with log rank,P = .004.
Overall survival in patients with and without adenovirus disease.
The broken line shows the probability of survival among patients with adenovirus disease (surviving 1 of 6, probability 16.6%; 95% CI, 1.4-32). The solid line shows those without adenovirus disease (surviving 47 of 70, probability 63.1%; 95% CI, 51.4-74.8). The difference was significant with log rank,P = .004.
Discussion
A number of retrospective studies have highlighted the increasing recognition of adenovirus as a serious pathogen in SCT recipients. The incidence of adenovirus infections varied from 4.9% to 20.9% in these studies,1-11 and this was higher among pediatric transplant recipients.5,9 However, the incidence of adenovirus disease or invasive infections varied between 20% and 89% among the infected patients.1-11 This variability in overall incidence and progression is partly due to the retrospective nature of the studies, which failed to evaluate the natural history of these infections. No effective management strategy, therapeutic or preemptive, has evolved from these studies even though a large number of infected patients were studied.
Our study, although based on a relatively smaller cohort, is the first attempt to prospectively address this issue. We have shown a relationship between graft manipulation and lymphocyte recovery and adenovirus isolation. Because T-cell depletion was carried out using alemtuzumab antibodies alone, we could demonstrate a correlation between the dose and mode of alemtuzumab and the incidence of adenovirus infection. Pharmacokinetic studies have demonstrated that this antibody could be detected at a greater amount and for a longer duration after transplantation when used in vivo.20 Thus, the difference we noted between the patients receiving 50 to 100 mg in vivo and those having transplantation with grafts treated with 10 to 20 mg alemtuzumab in vitro reflected not only the extent of T-cell depletion of the graft, but also the kinetics of T-cell recovery. The low lymphocyte count was the most significant predictor of adenovirus infection, and its recovery in turn was significantly delayed in patients receiving alemtuzumab in vivo. This explains the association between the mode and dose of alemtuzumab antibody and adenovirus infections in relation to immune reconstitution. This is the first time an association between posttransplantation lymphocyte recovery and adenovirus infection has been demonstrated. The increased incidence of non-CMV infections is probably also a reflection of the poor immune recovery of these patients.
The other important question to answer was why some patients went on to develop adenovirus disease, whereas others remained asymptomatic. Isolation of the virus from multiple sites has been identified as a risk factor in 3 studies.5,9 10 With our approach of surveillance and preemptive immunomodulation, this finding was not predictive of invasive disease. We have shown that lymphocyte recovery at the time of the first virus isolation was the most important predictor of invasive disease, even though the patient may have been asymptomatic at that stage. The CD4+ T-cell count followed a similar pattern, although the data were not available in all patients. It has always been speculated that the more immunosuppressed the patient, the greater the likelihood of dissemination of adenovirus, but this is the first time that an association with the severity of lymphocytopenia has been demonstrated.
Our data establish a definite case for immunologic manipulation in cases of adenoviral infection, along the lines of that used for herpes viruses such as CMV or EBV.1-3 We made an active effort to reduce or withdraw immunosuppression in all of the patients. It is worth noting that the patients who remained on immunosuppression succumbed, whereas the outcome was improved if immunosuppression could be reduced or withdrawn, even though 2 of the patients had established adenovirus disease. Interestingly, in 2 patients, DLI was temporally associated with the clearance of adenovirus. Again, this is the first time that immunomodulation has been effectively used as a management strategy for adenovirus infections. The corollary of our findings was the identification of the intensity of immunosuppression as a risk factor for dissemination in one of the large retrospective studies.8 GVHD has been identified as a risk factor in some studies, and this probably indirectly links to the immunosuppressive therapy used for its treatment. It is worth noting that such early manipulation of immunosuppression could be feasible only following T-cell–depleted transplantations. Our data suggest that the patients receiving non-T–depleted grafts are not at risk of adenovirus disease, but could be at risk when they receive additional immunosuppression for GVHD or other indications. In the latter situation, withdrawal or reduction of immunosuppression might not be feasible, warranting preemptive antiviral therapy.
A positive PCR from serum or peripheral blood mononuclear cells has been associated with an adverse outcome in 2 retrospective studies.21 22 Detection of the virus in blood by PCR was directly related to a fatal outcome in our study, and for the first time, such an association has been demonstrated prospectively. Our study suggests that the isolation of adenovirus from multiple sites is a sequential event, and this is preceded by viremia, as suggested by a positive blood PCR. Thus, rather than isolation of the virus from multiple sites, detection of viremia by more sensitive techniques such as PCR might be an easier and more sensitive predictor of outcome. The data on PCR were unfortunately not available for all patients, and the total cohort of adenovirus-infected patients was not large enough to allow a meaningful multivariate analysis. Although with our preemptive strategy of manipulation of immunosuppressive therapy, the overall survival of adenovirus-infected patients was similar to that of uninfected patients, the outcome of patients with adenovirus disease was not improved with antiviral therapy. It should also be borne in mind that it might be difficult to estimate the exact contribution of adenovirus to the severity of the symptoms in the presence of copathogens, compounded by the difficulty of obtaining tissue diagnosis in seriously ill patients. However, the 3 factors—severe lymphocytopenia, continued immunosuppression, and a positive blood PCR—might identify the patients at risk of dissemination. If these factors are considered, we may be able to identify a window of opportunity to intervene clinically in all patients before the onset of fulminant disease. Antiviral therapies currently available are ineffective for disseminated or fulminant adenovirus disease, but they might be effective as preemptive therapy if introduced early based on this model.
Based on these findings, our current protocol in asymptomatic patients with adenovirus infection is as follows: (1) If the patient is not receiving any immunosuppression and has an ALC greater than 300/μL, observe without intervention with clinical and virologic monitoring; (2) if the patient is asymptomatic and receiving immunosuppression, attempt to withdraw or reduce immunosuppression to the minimum; and (3) initiate antiviral therapy if the ALC is less than 300/μL, if blood PCR is positive, or if immunosuppression cannot be withdrawn because of GVHD or because the patient is in the early posttransplantation period.
These data are limited to adult patients, and it is likely that the adenovirus infections were caused by reactivation of endogenous virus. There are several findings in favor of a “reactivation” theory as opposed to exogenous infection. First, all the isolates were serotyped as type 2 (subgenus C), which is the most common single serotype in the United Kingdom population (personal communication, Nicola Goddard, Communicable Diseases Surveillance Centre). Subgenus C serotypes have a greater propensity to establish latency or persistent infection among the various serotypes infecting in childhood.23 Serotype data were not available in most studies, but 2 large reviews suggested that the isolates identified in North America are mostly from subgenus B (serotypes 11, 34, 35).4,5 We have demonstrated antiadenovirus antibodies recognizing types 2 and 5 antigens in the pretransplantation sera of both patients and donors, suggesting reactivation. Second, although it is suspected that the adenovirus can establish latent or persistent infection, the site and nature of latent infection are largely unknown.21 All of the patients in our study had a positive stool isolate regardless of the other sites of isolation, suggesting reactivation from gut-associated lymphoid tissue. Third, the infections were not clustered and there were no outbreaks. Fourth, there seemed to be a trend for the adenovirus infections to occur between 80 and 130 days, which is a temporal pattern seen with reactivation, as in herpes viruses.
What is not clear from our study is whether the infection can be transmitted from donor cells. A recent study24 suggested that a positive donor serology was a risk factor for adenovirus infection. We have studied a large sample of normal population and adult BMT donors, and all tested positive for adenovirus antibodies in an ELISA (Chakrabarti, unpublished data, December 2002). Thus, the donor or recipient serostatus is not predictive of adenovirus infection in adult patients, and PCR screening on whole-blood samples should possibly be restricted to adenovirus-infected patients or patients at high risk of adenovirus disease (heavy immunosuppression or poor immune reconstitution). The donor serology might be significant in pediatric transplantations, where the recipient might not have been exposed to the virus, but this was clearly not the case in the adult population. Our treatment algorithm may not be effective in children. Depending on the age of the donor and recipient, adenovirus infections may be primary and occur early, and there will be little scope of generating antiviral immunity from the donor graft that has not been previously exposed to the virus.
Our study has laid the foundation for further research into adenovirus-specific cellular immunity. It is possible that the total lymphocyte or CD4+ T-cell recovery is a surrogate marker for adenovirus-specific CD4+ or CD8+ T-cell recovery, and that this may be accelerated by withdrawal of the immunosuppression. A better understanding of this process would enable us to select candidates for adoptive transfer of adenovirus-specific cytotoxic T cells (CTLs). Flomenberg et al demonstrated adenovirus-specific cellular immunity in healthy donors,25,26 and others have also reported the generation of adenovirus-specific CTLs.27,28 Unlike neutralizing antibodies, adenovirus CTLs may not be serotype restricted, suggesting that serotype variation might not be a stumbling block for developing adoptive cellular therapy.29
We are currently investigating the kinetics of adenovirus-specific T-cell recovery following an allograft and whether this is predictive of the acquisition and outcome of adenovirus infections. Until this stage of precision is reached, our study suggests a preemptive strategy of active withdrawal of immunosuppression for all adenovirus-infected patients and early antiviral therapy for those at risk of invasive disease, as determined by the severity of lymphocytopenia, positive blood PCR, or continued immunosuppressive therapy.
We thank Prof G. Hale, Prof H. Waldmann, and the staff of the Therapeutic Antibody Centre, University of Oxford, for their contributions to the production of alemtuzumab antibodies.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-02-0377.
Supported by the United Kingdom Medical Research Council, Leukosite Inc, and the E. P. Abraham's Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Suparno Chakrabarti, Bone Marrow Transplant Unit, Bristol Royal Hospital for Sick Children, Bristol, BS2 8BJ, United Kingdom; e-mail: suparno@doctors.org.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal