RAS mutations are one of the most frequent molecular abnormalities associated with myeloid leukemia and preleukemia, yet there is a poor understanding of how they contribute to the pathogenesis of these conditions. Here, we describe the consequences of ectopic mutant N-Ras (N-Ras*) expression on normal human erythropoiesis. We show that during early (erythropoietin [EPO]–independent) erythropoiesis, N-Ras* promoted the amplification of a phenotypically primitive but functionally defective subpopulation of CD34+ erythroblasts. N-Ras* also up-regulated the expression of megakaryocyte antigens on human erythroblasts. Although early erythroblasts expressing N-Ras* were able to respond to erythropoietin and generate mature progeny, this occurred with greatly reduced efficiency, probably explaining the poor colony growth characteristics of these cells. We further report that this oncogene promoted the expression and activation of protein kinase C (PKC) and that the effects of N-Ras* on erythropoiesis could be abrogated or attenuated by inhibition of PKC. Similarly, the effects of this oncogene could be partially mimicked by treatment with PKC agonist. Together, these data suggest that expression of N-Ras* is able to subvert the normal developmental cues that regulate erythropoiesis by activating PKC. This gives rise to phenotypic and functional abnormalities commonly observed in preleukemia, suggesting a direct link between RAS mutations and the pathogenesis of preleukemia.
Introduction
Ras proteins (H-, K-, and N-Ras) are membrane-bound GTPases that convey the status of the extracellular environment by integrating signals from membrane-associated molecules (such as growth factor receptors) to a variety of effector molecules that, in turn, have been shown to modulate diverse cellular processes (reviewed by Rebollo et al1 and Crespo and Leon2). In acute myeloid leukemia patients, point mutations ofRAS genes (most commonly N-RAS) resulting in a constitutively active protein are found in about 30% of patients.3 Inappropriate activation of Ras can also arise as a result of other common molecular abnormalities associated with leukemia, such as activating mutations of the FLT3 gene, the BCR-ABL translocation, and loss of the negative regulator of Ras, neurofibromin.4-6 Together, these data suggest that deregulation of Ras signaling is a common feature of myeloid malignancy; however, there is limited understanding of its role in the pathogenesis of leukemia or of the mechanism by which it may induce the developmental irregularities associated with this disease. Study of the pathogenesis of acute myeloid leukemia has been hampered by the fact that no preleukemic phase generally manifests itself, making it difficult to establish how individual abnormalities bring about malignant change. Some insight into the processes that give rise to leukemia may, however, be found in myelodysplastic syndrome (MDS). These patients display disorders of development of one or more hematopoietic lineages and frequently progress to acute myeloid leukemia.7 MDS arises from a defective clone that exhibits many of same molecular abnormalities found in myeloid leukemia, including RAS oncogenes (30%-40% incidence8). The presence of RAS mutations in both leukemia and preleukemia suggests that this oncogene may play a role early in leukemogenesis, and this is also supported by the high frequency of myeloid malignancies in individuals with neurofibromatosis type I who lack neurofibromin expression.9,10 To investigate the role of mutant Ras in myeloid malignancy, we previously developed a model system to study the effect of this oncogene on the development of normal human CD34+ cells and showed that expression of mutant N-Ras (N-Ras*) resulted in impaired proliferative capacity of erythroid cells and also reduced erythroid but not myeloid colony formation.11 These data were of particular interest given that dyserythropoiesis and reduced red blood cell formation represent one of the most common manifestations of MDS (Chui and Clarke12 and Hoefsloot et al13 and references therein) supporting a role for this oncogene in the pathogenesis of preleukemia.
Erythropoietic development occurs in 2 distinct stages based on the requirement for erythropoietin (EPO). Early erythroblastic development takes place in the absence of this cytokine.14In vivo, the resulting committed progenitors form a reservoir that is able to quickly respond to increases in the availability of EPO, which is required to complete development.15 We show here that N-Ras* influences each of these developmental stages differently: promoting the expansion of an abnormal and functionally defective pool of primitive erythroblasts while inhibiting their subsequent proliferation in the presence of EPO. We further demonstrate that these abnormalities arise from aberrant activation of protein kinase C (PKC) by N-Ras*. PKC is a family of isoenzymes that function as lipid-dependent serine/threonine kinases.16 PKC isoenzymes are developmentally regulated during hematopoiesis,17-20and evidence suggests that they play a role during lineage commitment21-24 as well as during subsequent differentiation of hematopoietic lineages.25-27 The data presented here suggest that the capacity of constitutively active Ras to subvert normal hematopoietic development may be substantially dependent on its capacity to dysregulate the activity of PKC.
Materials and methods
Generation of retrovirus
N-RAS*–PINCO was created by blunt cloning human mutant N-RAS12ASP cDNA (GC61) into theBamHI site of the PINCO28 retroviral construct (gift from Pier Pelicci, European Institute of Oncology, Milan, Italy), which coexpresses green fluorescent protein (GFP) from an internal cytomegalovirus (CMV) promoter. Replication-defective retrovirus was generated by transient transfection of Phoenix packaging cells (gift from Garry Nolan, Stanford University School of Medicine, CA).
Cell culture
Human CD34+ cells (> 95% pure) were derived from neonatal cord blood using MiniMACS (Miltenyi Biotec, Camberley, Surrey, United Kingdom) according to the manufacturer's instructions. These cells were subsequently cultured at 1 × 105 cells per milliliter in Iscove modified Dulbecco medium (IMDM) containing 20% fetal calf serum (FCS) and the following growth factors (R&D Systems, Abingdon, United Kingdom): interleukin-3 (IL-3) (5 ng/mL), IL-6 (10 ng/mL), stem cell factor (SCF) (20 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF) (5 ng/mL), G-CSF (5 ng/mL), and Flt3 ligand (5 ng/mL). The following day, these cells were transferred to RetroNectin-coated dishes (Takara Shuzo, Shiga, Japan) that had been preadsorbed with retrovirus. On day 2 of culture, this infection procedure was repeated. Two cultures were derived from each CD34+ preparation: PINCO-infected (expressing GFP alone) and N-RAS*–PINCO infected. On day 3, each culture was harvested and stained with CD13-PE (phycoerythrin) (BD, Oxford, United Kingdom). To enrich for retrovirally transduced erythroid cells, GFP+, CD13− cells were isolated by fluorescence-activated cell sorting using a MoFlo cytometer (Cytomation, Fort Collins, CO). Sorted cells were more than 90% pure and were subsequently either reestablished into bulk liquid culture or used in colony assays (see below). Initially, liquid cultures were maintained in growth medium containing 5 ng/mL IL-3, IL-6, and SCF but without EPO. In some experiments, replicate cultures were grown in the presence of the PKC inhibitor, GF109203X,29 (LC Laboratories, Laufelfingen, Switzerland) or dimethyl sulfoxide (DMSO) vehicle. This inhibitor acts as competitor for adenosine triphosphate (ATP) binding by PKC and consequently is only effective at concentrations that exceed the intracellular ATP concentration. In these experiments we found 2 μM gave the maximum inhibitory effect with the minimum toxicity. In other experiments, cultures were grown in the presence of 12-O-tetradecanoylphorbol-13 acetate (TPA)/DMSO vehicle at the concentrations indicated. On day 10, a portion of each culture was supplemented with EPO (R&D Systems) at 1 U/mL or, in some experiments, thrombopoietin (100 U/mL) and cultured for a further 10 days.
Colony assays were performed by limiting dilution in 96-well plates in the same liquid medium containing IL-3, IL-6, SCF, and EPO. In each experiment, 100 cells were plated at a density of 0.3 cells per well. Individual colonies (> 50 cells) and clusters (> 5 cells) were scored after 7 or 14 days by microscopic analysis. Colonies were further analyzed by flow cytometry as below.
Flow cytometric analysis
Cultures were analyzed by 4-color immunophenotypic analysis. At the time points indicated, cells were stained with CD13-APC (allophycocyanin) (Leinco Technologies, St Louis, MO) in combination with CD36-biotin (Ancell, Bayport, MN) and one of the following PE-labeled antibodies: CD34, CD41, CD42b, glycophorin A (gly A) (Dako, Ely, United Kingdom), or CD61 (Serotec, Oxford, United Kingdom). Biotinylated CD36 was subsequently labeled with SA-PerCP (peridinin chlorophyll protein) (BD). Immunophenotypic analysis of CD34+ cells was performed by labeling with CD13-APC in combination with CD34–PerCP or CD34-PerCP-Cy5.5 (cyanine 5.5) (BD) and one of the following PE-labeled antibodies: CD71 (Cymbus Bioscience, Southampton, United Kingdom), CD38, CD45RO, CD11b (BD), AC133 (Miltenyi Biotec), or CD41. For analysis of individual colonies, 2 × 103 latex beads were added to each colony-containing well to control for recovery. Colonies were then carefully aspirated and stained with gly A–PE and CD13-APC simultaneously. All incubations were carried out at 4°C for 30 minutes in the presence of 0.5% human γ-globulin.
To stain for intracellular N-Ras expression, cells were fixed in 1% paraformaldehyde for 30 minutes at 20°C and then permeabilized with 0.1% Triton X-100 for 5 minutes. Cells were then incubated with an N-Ras–specific monoclonal antibody (F155-227) from Oncogene Research Products (Cambridge, MA) and subsequently with rat anti–mouse immunogloblulin G1 (IgG1)–PE monoclonal antibody (BD) for 30 minutes at 20°C.
Analysis of PKC
Subcellular fractions were prepared as follows: Cells were incubated in hypoosmotic buffer (15 mM KCl; 10 mM HEPES-KOH [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–potassium hydroxide], pH 7.2; 1.5 mM MgAc; 0.5 mM EDTA [ethylenediaminetetraacetic acid]; 0.5 mM EGTA [ethyleneglycoltetraacetic acid]; 10 mM 13-mercaptoethanol [BME]) for 4 minutes and then lysed in a cell cracker (HGM, Heidelberg, Germany). The lysate was then made iso-osmotic using 5 × buffer (375 mM KCl; 220 mM HEPES-KOH, pH 7.2; 22.5 mM MgAc). Following removal of nuclei (10 minutes at 1000g) the membrane fraction was pelleted (60 minutes at 20 000g). Both nuclear and membrane fractions were solubilized in iso-osmotic buffer containing 1% Triton X-100. These, together with the soluble (cytosolic) fraction, were then purified over diethylaminoethyl (DEAE) columns equilibrated in 25 mM Tris (tris(hydroxymethyl)aminomethane) HCl, pH 7.4; 0.5 mM EDTA; 0.5 mM EGTA; and 10 mM BME containing 0.02% Triton X-100. Bound PKC was eluted in the same buffer containing 300 mM NaCl. All processing was carried out in the presence of 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF) at 0°C to 4°C. The protein content of each fraction was determined using a modified Lowry method (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom).
PKC activity in each subcellular fraction was determined using the SignaTECT assay system according to the manufacturer's instructions:http://www.promega.com/tbs/tb242/tb242.pdf (Promega, Southampton, United Kingdom). In this assay, activity is measured as the transfer of the terminal phosphate group from [α-32P]ATP to the biotinylated peptide, neurogranin (28-43), in the presence of 320 ng/mL phosphatidylserine and 32 ng/mL diacylglycerol (DAG). Control reactions were also carried out in the absence of these activators. 32P-labeled substrate was captured on a streptavidin-coated membrane. Bound radioactivity following washing of the membrane was measured by scintillation counting.
Western blotting was performed using a NuPAGE BIS-Tris electrophoresis system (Invitrogen, Carlsbad, CA) using a precast 4% to 12% gradient gel at 50 μg per lane. Gels were electroblotted onto nitrocellulose membranes (Amersham, Buckinghamshire, United Kingdom) and incubated for 1 hour at 20°C in the presence of 1 μg/mL pan-specific PKC antibody (Oncogene Research), which binds conventional PKC isoforms or phospho-PKC (pan), which detects the α, β, δ, ε, and η isoforms only when phosphorylated at the C-terminal hydrophobic region (NEB, Hertfordshire, United Kingdom). Bound antibody was visualized by enhanced chemiluminescence (WesternBreeze, Invitrogen).
Statistical analysis
Significance of difference was tested using the pairedt test. The value “n” in the figure legends describes the number of independent experiments, each derived from a distinct preparation of CD34+ mononuclear cells.
Results
Expression of N-Ras* in human primary erythroid cells and its effect on colony formation
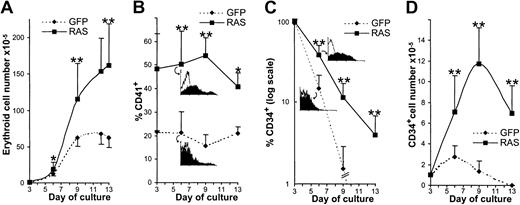
To ectopically express N-Ras*, we retrovirally infected CD34+ cells with recombinant retrovirus expressing this oncogene in combination with GFP. Following infection (day 3), 4-color analysis (Figure 1A) showed that these cultures consisted of predominantly CD34+ cells of which approximately 30% were of erythroid phenotype (CD13−, CD36+/−).31 Approximately 50% to 70% of these cells were GFP+ (Figure 1A). To facilitate analysis of the retrovirally transduced erythroid population, we enriched these day-3 cultures for GFP+, CD13−cells by flow sorting (thereby excluding myelomonocytic and nontransduced cells). Subsequent 4-color analysis showed that this resulted in cultures that were more than 90% GFP+ and predominantly CD34+, CD13−, CD36+equivalent to a late erythroid burst-forming unit (BFU-E) population32 (Figure 1B). As others have previously reported,28 we did not detect any effect of GFP expression on erythroid development compared with mock-transduced cells.
Enrichment and characterization of N-Ras*–transduced erythroid cultures.
(A) Representative dot plots showing the 4-color immunophenotypic status of N-Ras*–transduced cultures: (left) CD13-PE versus CD36-APC on whole population; (right) CD34-perCP-Cy5.5 versus GFP (gated on CD13− population). Numbers within plots represent percentage of cells within each quadrant; quadrants delimit background fluorescence of control-stained/mock-transduced cells. (B) Corresponding plots following enrichment of GFP+CD13− cells (each plot shows whole population). Similar data were generated from control cultures expressing GFP alone (not shown). (C) Representative density plots showing expression of N-Ras protein and GFP in control (GFP alone) and N-Ras*–transduced day 7 erythroid cultures. Similar results were obtained from day-14 EPO-replete cultures (not shown). Note that fixation/permeabilization conditions were optimized for detection of the N-Ras antigen; the fluorescence of GFP was reduced by approximately 10-fold compared with fresh cells.
Enrichment and characterization of N-Ras*–transduced erythroid cultures.
(A) Representative dot plots showing the 4-color immunophenotypic status of N-Ras*–transduced cultures: (left) CD13-PE versus CD36-APC on whole population; (right) CD34-perCP-Cy5.5 versus GFP (gated on CD13− population). Numbers within plots represent percentage of cells within each quadrant; quadrants delimit background fluorescence of control-stained/mock-transduced cells. (B) Corresponding plots following enrichment of GFP+CD13− cells (each plot shows whole population). Similar data were generated from control cultures expressing GFP alone (not shown). (C) Representative density plots showing expression of N-Ras protein and GFP in control (GFP alone) and N-Ras*–transduced day 7 erythroid cultures. Similar results were obtained from day-14 EPO-replete cultures (not shown). Note that fixation/permeabilization conditions were optimized for detection of the N-Ras antigen; the fluorescence of GFP was reduced by approximately 10-fold compared with fresh cells.
To confirm the coexpression of N-Ras oncoprotein and GFP in these cultures, we fixed and permeabilized retrovirally transduced cells and stained for the expression of N-Ras using an antibody specific to the C-terminus of this protein (Figure 1C). Control cells expressed generally low levels of N-Ras protein; expression of the N-Ras* retrovirus increased total N-Ras expression 2- to 5-fold (mean 3.2 ± 0.7).
Having generated a purified population of retrovirally transduced primitive erythroid cells, we examined their capacity for colony growth. This was carried out in liquid medium in 96-well plates by limiting dilution to facilitate subsequent flow cytometric analysis of colonies. On day 7 we individually labeled each colony with gly A and CD13 (to discriminate erythroid from any residual myeloid colonies) and added calibrating beads to allow us to estimate the number of cells constituting each colony. As expected, more than 90% of colonies were of erythroid phenotype, confirming the identity of the starting population. As we have previously reported, expression of N-Ras* suppressed erythroid colony formation (63% of control) with a corresponding increase in the number of clusters (Table1). This analysis additionally showed that N-Ras* also reduced colony size (55% of control). There was no indication of delayed colony formation because we observed no improved efficiency, relative to control cells, after 14 days (66% of control).
Effect of N-Ras* on erythroid colony formation
| . | GFP . | N-Ras* (%) . |
|---|---|---|
| Colonies per 100 cells | 36 ± 6.0 | 23 ± 7.4† (63) |
| Average cells per colony | 1319 ± 612 | 720 ± 425† (55) |
| Clusters per 100 cells | 16 ± 9.5 | 27 ± 10.4† (170) |
| . | GFP . | N-Ras* (%) . |
|---|---|---|
| Colonies per 100 cells | 36 ± 6.0 | 23 ± 7.4† (63) |
| Average cells per colony | 1319 ± 612 | 720 ± 425† (55) |
| Clusters per 100 cells | 16 ± 9.5 | 27 ± 10.4† (170) |
Colony/cluster formation by day-3 GFP+, CD13− N-Ras*/control cells in the presence of IL-3, IL-6, SCF, and EPO with corresponding SD (n = 9). Colonies were analyzed on day 10. Significant difference of N-Ras
from control cultures (GFP alone) is indicated as follows: †P < .01. Percentages indicate percentage of control values.
N-Ras* maintains expression of CD34 and up-regulates megakaryocyte antigens during the EPO-independent phase of growth
To determine the mechanism for the suppression of colony formation, we set out to determine the effects of N-Ras* on discrete stages of erythropoietic development. To this end, we first studied the effect of N-Ras* on early erythroid differentiation, which occurs independently of the requirement for EPO.14 Consequently, these cells were initially cultured in liquid medium containing IL-3, IL-6, and SCF only. Under these conditions, control cultures (expressing GFP alone) proliferated for a further 10 days (day 13) until they became growth-arrested at the EPO-dependent stage of development (Figure 2A). Surprisingly, we found that N-Ras* promoted EPO-independent expansion, giving rise to a 2.4-fold increase in the number of erythroid cells compared with control-infected cultures. To determine the basis for this increased expansion, we examined the immunophenotype of these cells using 4-color cytometric analysis. This analysis revealed some striking abnormalities. First, we found that N-Ras* strongly up-regulated the expression of the CD41 antigen, which was expressed on most N-Ras* CD13−, CD36+ cells, at high levels, throughout the EPO-independent phase (Figure 2B). CD41 (glycoprotein IIb) is expressed on the cell surface as a heterodimeric complex with CD61 (glycoprotein IIIa), which together constitute a major platelet integrin acting as a fibrinogen receptor. Concordantly, we found similar overexpression of CD61 on these cells (not shown). Although this platelet integrin is to some extent expressed on normal erythroid cells,33,34 it is considered to be predominantly a megakaryocyte antigen. This led us to investigate whether N-Ras* may be promoting megakaryocyte differentiation. Although we found that N-Ras* also up-regulated the expression of CD42b (a more definitive, though later marker of megakaryocyte development35), this was detected on only a minor fraction of the culture (4.5% ± 2% vs 1.6% ± 1% in controls, P = .05, at day 9) and correspondingly few cells of megakaryocyte morphology were observed (not shown). Even the addition of the megakaryocyte factor, thrombopoietin, failed to promote megakaryocyte development, suggesting that though N-Ras* up-regulated the expression of megakaryocyte antigens, it was unable to promote lineage conversion (see “Discussion”).
The effect of N-Ras* on EPO-independent erythropoiesis.
(A) Cumulative expansion of transduced erythroid cells (defined as GFP+, CD13− cells by flow cytometric analysis; see “Materials and methods”). (B) Frequency of CD41 expression. Insets show representative data at day 6 (open histograms show nonspecific fluorescence). (C) Frequency of CD34 expression on transduced erythroid cells. (D) Cumulative expansion of transduced erythroid CD34+ cells. Insets show representative data at day 6. Error bars represent SD (n > 5);P < .05, **P < .01.
The effect of N-Ras* on EPO-independent erythropoiesis.
(A) Cumulative expansion of transduced erythroid cells (defined as GFP+, CD13− cells by flow cytometric analysis; see “Materials and methods”). (B) Frequency of CD41 expression. Insets show representative data at day 6 (open histograms show nonspecific fluorescence). (C) Frequency of CD34 expression on transduced erythroid cells. (D) Cumulative expansion of transduced erythroid CD34+ cells. Insets show representative data at day 6. Error bars represent SD (n > 5);P < .05, **P < .01.
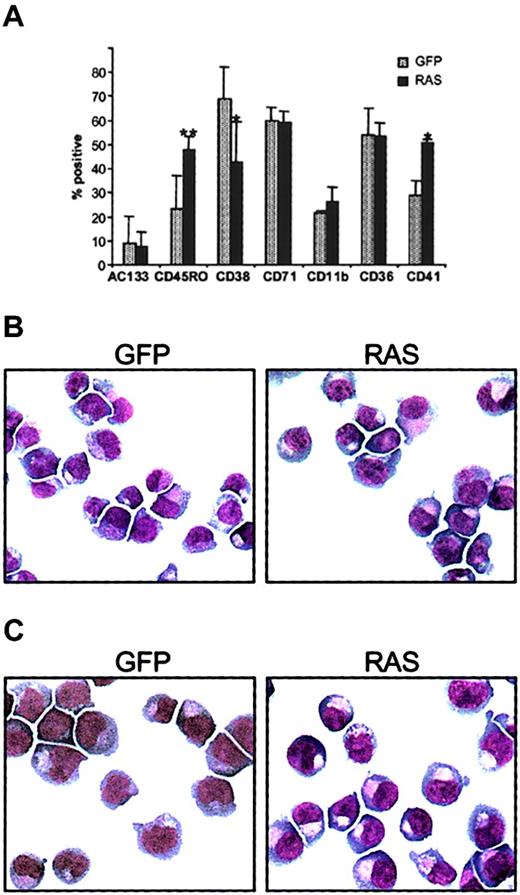
Phenotypic analysis also revealed abnormality in the expression of the CD34+ antigen. In control cultures, the frequency of erythroid cells retaining expression of CD34 declined rapidly. In contrast, N-Ras* cultures retained CD34+ erythroid cells throughout the period of EPO-independent growth (Figure 2C), giving rise to a 10-fold amplification of these cells (Figure 2D). To further evaluate the properties of these CD34+ cells, we first examined the immunophenotype of N-Ras*–expressing CD34+CD13− cells from day 8 cultures (Figure3A). Generally, the phenotype of these cells was consistent with early erythroblasts (AC133−, CD36+, CD11blo),31,36,37 though we again noted up-regulated expression of CD41 in N-Ras* CD34+cells. In addition, we noted a decreased frequency of CD38 expression and an increased frequency of CD45RO, which together imply that N-Ras*–expressing CD34+ cells constituted a more primitive population of erythroid cells.38 39 Morphologic assessment also showed that these cells were predominantly of erythroid appearance (Figure 3B-C).
Immunophenotypic and morphologic analysis.
(A) Immunophenotypic profile of GFP+, CD34+, CD13− cells expressing N-Ras* compared with corresponding controls; day-8 cultures were triple-labeled with antibodies to CD34, CD13, and to one of the determinates indicated. Error bars represent SD (n = 4); *P < .05, **P < .01. (B) Morphology of day-8 cultures and (C) morphology of CD34+cells from day-8 cultures. In each case, cytospin preparations were stained with Wright-Giemsa (original magnification × 400). Note: Vacuolization is a common feature of cultured human erythroblasts.
Immunophenotypic and morphologic analysis.
(A) Immunophenotypic profile of GFP+, CD34+, CD13− cells expressing N-Ras* compared with corresponding controls; day-8 cultures were triple-labeled with antibodies to CD34, CD13, and to one of the determinates indicated. Error bars represent SD (n = 4); *P < .05, **P < .01. (B) Morphology of day-8 cultures and (C) morphology of CD34+cells from day-8 cultures. In each case, cytospin preparations were stained with Wright-Giemsa (original magnification × 400). Note: Vacuolization is a common feature of cultured human erythroblasts.
Overall, these data suggest that N-Ras* promoted the amplification of early erythroblasts but that it also up-regulated the expression of antigens more closely associated with megakaryocyte development.
N-Ras* diminishes the proliferative response during the EPO-dependent phase of development
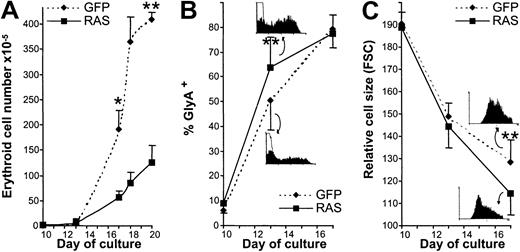
We next examined the effect of N-Ras* on late (EPO-dependent) development. Addition of EPO to day 10 control cultures immediately promoted a strong proliferative response. As expected, most of the growth potential of these cells lay in the late phase of erythropoiesis (410-fold expansion vs 67-fold expansion during the EPO-independent phase of growth) (Figure 4A). On the other hand, we found that erythroblasts expressing N-Ras* proliferated poorly in the presence of this cytokine, giving rise to fewer cells than in the EPO-independent phase of growth. These data therefore indicate that the overall reduction in proliferative capacity resulting from expression of this oncogene11,40 41 arises mainly from inhibition of proliferation during the EPO-dependent stage of development. The basis for this reduced proliferative potential did not appear to arise from increased frequency of apoptosis during EPO-dependent growth, as determined by annexin V positivity (< 10%, NS) and DNA content analysis (< 4% sub-G1, NS). Likewise, we found no overt developmental dysfunction associated with this phase of development: N-Ras*–expressing cells rapidly lost expression of both CD34 and CD41 (not shown) and demonstrated up-regulation of gly A and decrease in cell size consistent with erythroid maturation (Figure 4B-C). In fact, these maturation-associated changes occurred somewhat more rapidly in N-Ras* than in control cultures.
Influence of N-Ras* on EPO-dependent development.
(A) Cumulative expansion of GFP+, CD13− cells during the EPO-dependent phase of growth. (B) Frequency of gly A expression; insets show representative data at day 13 (open histograms show nonspecific fluorescence). (C) Cell size (forward scatter); insets show representative data at day 17. In each case, error bars represent SD (from at least 4 experiments); *P < .05, **P < .01.
Influence of N-Ras* on EPO-dependent development.
(A) Cumulative expansion of GFP+, CD13− cells during the EPO-dependent phase of growth. (B) Frequency of gly A expression; insets show representative data at day 13 (open histograms show nonspecific fluorescence). (C) Cell size (forward scatter); insets show representative data at day 17. In each case, error bars represent SD (from at least 4 experiments); *P < .05, **P < .01.
Although in agreement with the colony data, the poor proliferative response of N-Ras* cultures was unexpected given the higher content of cells with primitive phenotype prior to the addition of EPO. Correspondingly, we found that the persistence of CD34+cells in N-Ras* cultures did not correlate with colony-forming ability, which was lower in N-Ras* than in control cultures when reassayed on day 8 (Table 2). These data suggested that the CD34+ cells from N-Ras* cultures were defective in terms of colony formation. To confirm this, we isolated the CD34+ subpopulation from day-8 cultures and tested their colony-forming capacity in the presence of EPO. While control CD34+ cells retained their erythroid colony-forming potential, CD34+ cells from N-Ras* had only 5% of the colony-forming capacity of corresponding control cells (Table 2). Similarly, in bulk liquid culture, N-Ras* CD34+ cells were very poorly proliferative (20-fold ± 7-fold expansion over 10 days + EPO). These data suggest that, despite the ability of N-Ras* to amplify early erythroblasts, their proliferative response to EPO was greatly diminished; furthermore, this trait was exaggerated in the subpopulation retaining CD34 expression.
Effect of N-Ras* on erythroid colony formation
| . | GFP . | N-Ras* (%) . |
|---|---|---|
| Day 8 cultures, colonies per 100 cells | 3.1 ± 5.4 | 0.9 ± 1.6 (29) |
| CD34+ from day 8 cultures, colonies per 100 cells | 35.2 ± 11.0 | 1.9 ± 2.7† (5) |
| . | GFP . | N-Ras* (%) . |
|---|---|---|
| Day 8 cultures, colonies per 100 cells | 3.1 ± 5.4 | 0.9 ± 1.6 (29) |
| CD34+ from day 8 cultures, colonies per 100 cells | 35.2 ± 11.0 | 1.9 ± 2.7† (5) |
Colony formation by day 8 cultures or CD34+ cells from day 8 cultures in the presence of IL-3, IL-6, SCF, and EPO with corresponding SD (n = 3). Colonies were analyzed on day 15; all colonies formed were gly A+, CD13−. Significant difference of N-Ras
from control cultures (GFP alone) is indicated as follows: †P < .01. Percentages indicate percentage of control values.
N-Ras* modulates the expression of CD34 and CD41 by a PKC-dependent mechanism
Because it has been previously reported that activation of PKC can induce megakaryocyte features in erythroleukemia cells42as well as in primary erythroblasts,24,43 we investigated the possibility that N-Ras* was able to influence differentiation by activating PKC. First, we assessed the effect of this oncogene on the subcellular distribution of PKC (Figure5A). N-Ras* expression increased the amount of membrane-associated PKC 2-fold, suggesting that this oncogene promoted the activation of PKC. Further, N-Ras* increased the overall level of PKC in the cell by a similar degree. In support of this, we found that Western analysis indicated a similar increase in the total PKC content in N-Ras*–expressing cells using an antibody recognizing conventional PKC isoforms (α, β, and γ) (Figure 5B). We also found that N-Ras* promoted the phosphorylation of PKC (Figure 5C), a process that is associated with priming and activation of this group of kinases.44
Effect of N-Ras* on the distribution of PKC activity.
(A) Total PKC activity (106 cell equivalents) in cytosolic [C], membrane [M], and nuclear [N] fractions (day-7 cells). Error bars represent SD (n = 5); *P < .05. (B) Corresponding total PKC expression by Western blot: relative intensity of expression in N-Ras* versus control cells was 2.3-fold ± 0.28-fold (n = 3); P < .05. (C) Phospho-PKC expression by Western blot: Relative intensity of expression in N-Ras* versus control cells was 4.0-fold ± 1.4-fold.
Effect of N-Ras* on the distribution of PKC activity.
(A) Total PKC activity (106 cell equivalents) in cytosolic [C], membrane [M], and nuclear [N] fractions (day-7 cells). Error bars represent SD (n = 5); *P < .05. (B) Corresponding total PKC expression by Western blot: relative intensity of expression in N-Ras* versus control cells was 2.3-fold ± 0.28-fold (n = 3); P < .05. (C) Phospho-PKC expression by Western blot: Relative intensity of expression in N-Ras* versus control cells was 4.0-fold ± 1.4-fold.
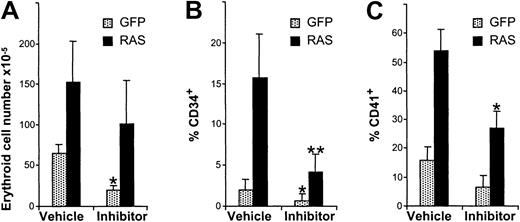
We next examined whether inhibition of PKC was able to antagonize the effects of N-Ras*. We therefore assessed the effect of the highly selective PKC inhibitor, GF109203X,29 (which preferentially inhibits conventional PKC isoforms) on growth and cell-surface antigen expression during EPO-independent development. The effect of GF109203X on control cells was growth inhibitory (Figure6A) though it did not promote apoptosis of these cells (annexin V positivity < 10%, NS vs vehicle alone). The proliferation of N-Ras* cells was, however, only slightly affected by the addition of inhibitor (P = .06). On the other hand, inhibition of PKC dramatically reduced the expression of the CD34 antigen on these cells to near control levels (Figure 6B). PKC inhibition also reduced the aberrant expression of CD41 on N-Ras* cells (Figure 6C). The presence of inhibitor did not affect CD13 or CD36 expression (not shown).
Inhibition of PKC activity antagonizes the effects of N-Ras*.
Data show the effect of PKC inhibitor addition (2 μM GF109203X on day 3) on growth and cell-surface antigen expression of GFP+, CD13− cells (analyzed on day 9). (A) Cumulative expansion. (B) Frequency of CD34 expression. (C) Frequency of CD41 expression. Error bars represent SD (n = 6). Significant effect of inhibitor compared with vehicle alone is indicated as follows: *P < .05, **P < .01.
Inhibition of PKC activity antagonizes the effects of N-Ras*.
Data show the effect of PKC inhibitor addition (2 μM GF109203X on day 3) on growth and cell-surface antigen expression of GFP+, CD13− cells (analyzed on day 9). (A) Cumulative expansion. (B) Frequency of CD34 expression. (C) Frequency of CD41 expression. Error bars represent SD (n = 6). Significant effect of inhibitor compared with vehicle alone is indicated as follows: *P < .05, **P < .01.
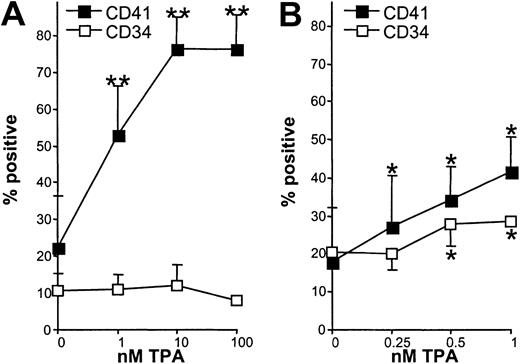
The above data indicated that PKC was necessary for the up-regulation of CD34 and CD41 mediated by N-Ras*. Previous reports have indicated that Ras may activate PKC through stimulation of 1,2-DAG production (Diaz-Laviada et al45 and references therein). To determine whether activation of PKC was sufficient to induce the expression of these antigens, we treated control cells with the DAG analog, TPA. This PKC agonist induced CD41 expression on control cells in a dose-dependent manner to levels similar to that observed on N-Ras* cells (Figure 7A). On the other hand, TPA was unable to stimulate de novo expression of the CD34 antigen at any dose tested. We further examined whether sustained treatment with low-dose TPA could prevent the down-regulation of CD34 expression on these cells. Under these conditions, TPA again up-regulated CD41 expression and also CD34 expression in a dose-dependent manner (Figure7B) though not to the same extent as that elicited by N-Ras*. Prolonged treatment with more than 1 nM TPA inhibited proliferation.
TPA partially mimics N-Ras*.
Data show the effect of TPA induction during EPO-independent culture. (A) Effect of TPA on CD41 and CD34 expression on day 7 control cells following 24-hour exposure to the indicated concentration of TPA (n = 3). (B) Effect of prolonged exposure to low-dose TPA on CD41 and CD34 expression on day 6 cells, following 72-hour exposure (n = 4). Error bars represent SD. Significant effect of TPA compared with vehicle alone is indicated as follows: *P < .05, **P < .01.
TPA partially mimics N-Ras*.
Data show the effect of TPA induction during EPO-independent culture. (A) Effect of TPA on CD41 and CD34 expression on day 7 control cells following 24-hour exposure to the indicated concentration of TPA (n = 3). (B) Effect of prolonged exposure to low-dose TPA on CD41 and CD34 expression on day 6 cells, following 72-hour exposure (n = 4). Error bars represent SD. Significant effect of TPA compared with vehicle alone is indicated as follows: *P < .05, **P < .01.
Inhibition of PKC abrogates the effect of N-Ras* on colony formation
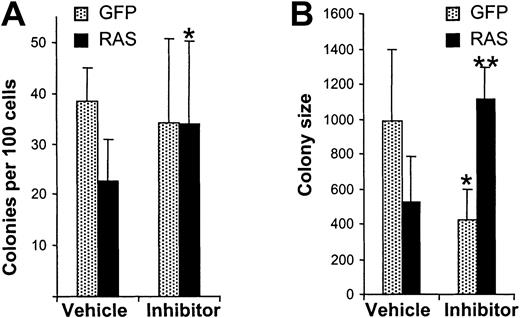
We next examined whether the presence of inhibitor was sufficient to restore normal colony growth to cells expressing N-Ras*. To this end, we repeated the colony-forming assays on N-Ras* and control cells but additionally compared the effect of PKC inhibitor. As expected, N-Ras* reduced both erythroid colony number and size compared with control cells, in the presence of vehicle alone (Figure8). The presence of inhibitor affected the performance of both N-Ras* and control cells. In the case of control cells, inhibition of PKC did not affect the number of colonies generated; however, it did suppress the size of the colonies produced, a result that is in accordance with the inhibition of proliferation observed in bulk liquid culture (Figure 6A). In contrast, for N-Ras* cells, inhibition of PKC promoted both colony formation and also increased the size of the colonies produced, restoring the performance of these cells to normal levels (uninhibited control cells).
PKC inhibition restores colony growth.
Data show erythroid colony formation from N-Ras* and control cells in the presence of vehicle or 2 μM GF109203X (conditions as in Table 1). (A) Effect on colony number. (B) Effect on colony size (average number of cells per colony). Error bars represent SD (n = 5). Significant effect of inhibitor compared with vehicle alone is indicated as follows: *P < .05, **P < .01.
PKC inhibition restores colony growth.
Data show erythroid colony formation from N-Ras* and control cells in the presence of vehicle or 2 μM GF109203X (conditions as in Table 1). (A) Effect on colony number. (B) Effect on colony size (average number of cells per colony). Error bars represent SD (n = 5). Significant effect of inhibitor compared with vehicle alone is indicated as follows: *P < .05, **P < .01.
Discussion
The suppression of erythropoiesis by mutant Ras has been demonstrated by both ourselves and others.11,40,41 Because impaired erythroid colony formation is a frequent manifestation of preleukemia12,13 and given the preponderance of RASmutations found in these conditions,8 these mutations may be causally linked to dyserythropoiesis in preleukemia. In this report we have identified a number of key abnormalities induced by N-Ras* in normal human erythroid cells and have also identified the likely mediator by which this oncogene subverts erythropoiesis.
By examining the effect of N-Ras* on discrete stages of erythropoiesis we found that this oncogene exerted distinct and opposing influences on each phase of development. During early (EPO-independent) erythropoiesis, N-Ras* promoted the amplification of erythroid cells, a surprising observation given its effect on colony formation. A possible explanation for this was that N-Ras* promoted the amplification of CD34+ erythroid cells, indicating that this oncogene was able to slow or inhibit differentiation of early erythroid cells without affecting their growth. This, in turn, may have extended the proliferative capacity of these cells during EPO-independent development. Because the overall influence of mutant Ras on erythropoiesis is antiproliferative,11,40,41 these observations suggested that the main proliferative defect associated with colony formation lay in the late (EPO-dependent) stage of erythropoiesis. This proved to be the case, because the proliferative capacity during this stage of development was found to be greatly reduced, giving rise to an overall reduction in proliferative capacity. These data also indicated that the cells of erythroid progenitor phenotype that were amplified within the N-Ras*–expressing population were functionally defective. By isolating these CD34+ cells from day-8 cultures we were able to show that these cells indeed had little colony-forming potential compared with equivalent control cells and proliferated very poorly in response to EPO. Interestingly, a similar population of cells has already been identified in MDS.46 This study showed that, in many cases, primitive erythroid cells from these patients were reluctant to differentiate. Furthermore, when these cells did undergo terminal differentiation, it occurred with greatly reduced efficiency, resulting in poor erythroid colony formation. Similar results have also been reported using an in vivo model in which mice were reconstituted with mutantN-ras–expressing hematopoietic cells.41 These mice demonstrated an amplification of erythroid progenitor cells but were at the same time anemic, suggesting that the development of these cells was impaired.
The basis for the reduced proliferative response to EPO is unclear, though this is also a common feature of myelodysplastic progenitors, where it has been linked with impaired activation of STAT5 by EPO.13 It does not appear to arise from ligand binding deficiency by EPO receptor47 and, in accordance with this, we have been unable to improve the performance of these cells with increased levels of EPO (R.L.D., unpublished data, 2002). We did observe that during EPO-dependent erythropoiesis differentiation occurred more rapidly under the influence of N-Ras*, suggesting that maturation-associated growth arrest may be partly responsible. Previously, we suggested that a block in differentiation may have been responsible for the proliferative deficiency of these cells11; however, this incorrect interpretation arose from fact that in these experiments EPO was present throughout the culture of these cells; consequently we observed a composite of the effects of N-Ras* on early and late erythropoiesis, and under these conditions an overall retardation of development is observed in N-Ras* cultures.
A further abnormality induced by N-Ras* during early erythropoiesis was the greatly augmented expression of a major platelet integrin (CD41/CD61) on the cell surface. Although this predominantly megakaryocyte antigen is also expressed on erythroid cells,33,34 its abundance on N-Ras*–expressing cells suggested that this oncogene may be inducing a partial megakaryocyte development program. The capacity of Ras to induce megakaryocyte development has previously been demonstrated in erythroleukemia cell lines.42 It is also interesting that overexpression of CD41 is very common in MDS,48 and myelodysplastic CD34+ cells also frequently coexpress megakaryocyte antigens.49 Overall, these data demonstrate that N-Ras* induces changes in the developmental programming of normal human erythroid cells that affected their proliferation, differentiation, and their cell-surface phenotype. Furthermore, these data indicate a close phenotypic and functional similarity between myelodysplastic and N-Ras*–expressing erythroblasts. One caveat to these data is that N-Ras* was overexpressed in these cells (3-fold on average), and this is a limitation of a model system relying on retroviral gene transfer. In this context, it is, however, interesting that overexpression of Ras is a common feature of leukemia and preleukemia.50 51
Surprisingly, our data also indicate that these abnormalities arose largely from changes in the activity of PKC isoenzymes. N-Ras* increased the abundance of PKC as well as the level of membrane-associated PKC and phospho-PKC, suggesting that this oncogene promoted the expression and the activation of this group of kinases. We also demonstrated that inhibition of PKC was able to abrogate or attenuate many of the Ras-induced changes in erythroid development and restored normal proliferation and colony formation as well as a near normal phenotype in terms of CD34 and CD41 expression. Because the inhibitor used is selective for the α, β, and γ PKC isoforms, it suggests that it is the conventional isoforms that are the principle targets of N-Ras* in these cells, and our provisional assessment indicates that of the predominant PKC isoforms in these cells (α, βI, βII, δ, and ζ) it is the α and β isoforms that are most affected in terms of their expression level and phosphorylated status (M.S., unpublished data, 2002). Interestingly, inhibition of conventional PKC isoforms was not toxic to the development of control cells though we did observe a 50% reduction in colony size consistent with the reduced amplification of these cells in bulk liquid culture (Figure 6A). These data, together with the increased proliferative activity of N-Ras* cultures during early erythropoiesis (Figure 2A), are consistent with a report that PKC may play a role in regulating proliferation during early erythropoiesis.52
One long-proposed mechanism by which Ras may activate PKC is via activation of phospholipase C (PLC) and induction of DAG.45 In support of this, we found that the DAG analog, TPA, was also able to potently induce expression of CD41 in human erythroid cells, as has been previously reported.24,43,53The same agonist has been reported to up-regulate CD34+expression on bone marrow cells, which already express this antigen,54 and we found that TPA also had the ability to preserve expression of CD34 on primary erythroid cells, though not to the same extent as that elicited by N-Ras*. Interestingly, cytokines such as IL-3, IL-5, and GM-CSF also induce PKC activation through a similar mechanism (reviewed by Mufson55). Because Ras is also known to be activated by these cytokines, it raises the possibility that they may, at least in part, activate PKC via Ras. The fact that PLC-ε has been recently identified as a direct downstream target of Ras-GTP (guanosine triphosphate) (reviewed by Cullen56) establishes a potential mechanism by which Ras activation may result in DAG production and PKC activation. However, in this respect, our preliminary data using effector mutants of Ras (which preferentially activate either Raf, phosphatidylinositol-3 kinase, or RalGDS/PLC-ε) indicate participation of all these targets of Ras in the activation of PKC, suggesting a complex interaction of these effector molecules in the generation of this phenotype (R.L.D., unpublished data).
All the above data strongly suggest that N-Ras* potentiates PKC activity in these cells and that this is largely responsible for the abnormalities induced by this oncogene. The role of PKC in regulating hematopoietic development is clearly established.21-23,25-27 With respect to erythroid and megakaryocyte development, the relative level of expression of PKC may form a developmental cue for these closely related lineages.57 Where comparisons have been made, erythroid development is associated with a general down-regulation of PKC, whereas PKC is up-regulated during megakaryocyte differentiation.19,20 In concordance with this, it has also been shown that treatment of normal human progenitors with TPA is able to switch the development of individual erythroid cells toward a megakaryocytic differentiation program.24,43 Thus, a possible explanation for these data is that, by promoting sustained PKC activity, mutant Ras subverts the normal developmental cues that are associated with erythroid development and, to a certain extent, reprograms the cell for megakaryocyte differentiation. While TPA may induce gross changes to erythroid development,24,43 a more physiologic stimulus emanating from Ras may give rise to confusion of developmental cues rather than lineage conversion and could be sufficient to stall normal differentiation, leading to amplification of the early progenitor pool. Subsequent addition of EPO may have had the effect of reinforcing the erythroid development program, allowing the maturation of these cells, though with greatly reduced efficiency. Because PKC activity is known to be required for EPO-dependent maturation,58 it is conceivable that aberrant activation of PKC by N-Ras* may also be affecting the late stages of erythropoiesis.
In conclusion, these results suggest that mutant Ras perturbs normal human erythropoietic development by dysregulating PKC. This, in turn, resulted in the amplification of primitive cells with similar functional and immunophenotypic characteristics to those found in preleukemia, providing further evidence of the direct role of this oncogene in leukemogenesis. Finally, these data have implications beyond the effect of N-Ras* on erythropoiesis. First, there is some evidence to suggest that, though activation of Ras does not suppress myeloid colony growth,11 it has the capacity to perturb myeloid development through activation of PKC (Gallagher et al30; R.L.D., unpublished data, 2002). Secondly, these data suggest that other common molecular abnormalities associated with leukemia may also in some part disrupt development by a similar mechanism. Both BCR-ABL and mutationally activated FLT3 receptor have been reported to activate Ras,4,5 which, in turn, may have the effect of dysregulating PKC. In support of this, BCR-ABL and v-ABL expression have been linked to alterations in PKC activity59,60 as well as giving rise to disturbed erythroid development.61
Prepublished online as Blood First Edition Paper, July 18, 2002; DOI 10.1182/blood-2002-05-1358.
Supported by the Leukaemia Research Fund of Great Britain and the International Association for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard L. Darley, Leukaemia Research Fund Differentiation Group, Department of Haematology, University of Wales College of Medicine, Cardiff, CF14 4XN, United Kingdom; e-mail:darley@cf.ac.uk.


![Fig. 5. Effect of N-Ras* on the distribution of PKC activity. / (A) Total PKC activity (106 cell equivalents) in cytosolic [C], membrane [M], and nuclear [N] fractions (day-7 cells). Error bars represent SD (n = 5); *P < .05. (B) Corresponding total PKC expression by Western blot: relative intensity of expression in N-Ras* versus control cells was 2.3-fold ± 0.28-fold (n = 3); P < .05. (C) Phospho-PKC expression by Western blot: Relative intensity of expression in N-Ras* versus control cells was 4.0-fold ± 1.4-fold.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-05-1358/5/m_h82323453005.jpeg?Expires=1763459260&Signature=VCVt5Z-sYZXbjrY39oOI~YKIdH2PLdF8L7rPAOoVB-QB6Z~19xOv5ZAzBGuX1VFvUT6lNZT9eTZpqlR-rW42HgCqU~Hx-zLEZfWAhzp8kK2nXmdm~1NtEU46binz2cV0D6mAjbpkrC6P0ix2eZAuIHqTGkVAXPxNL4R~0kQD3nuvoEAmacLgrGof6~f04uLsm5F0sPmpkuqSDvLLRDHXHGHRmWZMNbB7bCgI2fOEJJzNQ4jGzqZ4wMPzv9BVjtJT9wSPWb6TwWLwRCgoCJZrbmgbU2PktavlWvxUFk3Akgn7UzfsxNSw4Tyr1NYdHDnhZ0yycjCMJtn-ATK7uiogSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal