Severely impaired pulmonary microbial clearance was observed in granulocyte-macrophage colony-stimulating factor (GM-CSF)–deficient mice. To determine mechanisms by which GM-CSF mediates lung host defense, FcγR-mediated phagocytosis (opsonophagocytosis) by alveolar macrophages (AMs) was assessed in GM-CSF–sufficient (GM+/+) and –deficient (GM−/−) mice and in GM−/− mice expressing GM-CSF only in the lungs from a surfactant protein C (SPC) promoter (SPC-GM+/+/GM−/−). Opsonophagocytosis by GM−/− AMs was severely impaired and was restored by pulmonary GM-CSF expression in vivo or by PU.1 expression in vitro. Defective opsonophagocytosis by GM−/− AMs was associated with decreased FcγR expression. Because interferon-γ (IFN-γ) augments macrophage FcγR levels, the role of GM-CSF/PU.1 in the regulation of AM FcγR expression by IFN-γ was assessed during adenoviral lung infection. Adenoviral infection stimulated IFN-γ production and augmented FcγR levels on AMs in GM-CSF–expressing but not GM−/− mice. However, IFN-γ exposure ex vivo stimulated FcγR expression on GM−/− AMs. Because interleukin-18 (IL-18) and IL-12 stimulate IFN-γ production during adenoviral infection, their role in GM-CSF/PU.1 regulation of IFN-γ–augmented FcγR expression on AMs was assessed. Adenoviral infection stimulated IL-18 and IL-12 production in GM-CSF–expressing mice, but both were markedly reduced or absent in GM−/−mice. IL-18 expression by GM−/− AMs was severely impaired and was restored by pulmonary GM-CSF expression in vivo or by PU.1 expression in vitro. Pulmonary administration of IL-18 in GM−/− mice stimulated IFN-γ production and restored FcγR expression on AMs. These results show that GM-CSF, via PU.1, regulates constitutive AM FcγR expression and opsonophagocytosis and is required for the IFN-γ–dependent regulation of AM FcγR expression, enabling AMs to release IL-18/IL-12 during lung infection.
Introduction
The alveolar macrophage (AM) plays a central role in lung host defense through both effector and regulatory functions. As the resident professional phagocyte, AMs provide a first line of host defense by internalizing and degrading microbial pathogens encountered on the respiratory surface.1 Upon pathogen exposure, AMs express cytokines that influence recruitment and activation of inflammatory cells and modify adaptive immune responses in a pathogen-selective fashion.2 In this way, cytokines released from pathogen-exposed AMs provide important molecular “links” between innate and adaptive immunity in the lung. AMs also present processed antigens to lymphocytes, resulting in production of opsonizing, pathogen-specific immunoglobulins (Igs).
Opsonins such as IgG further enhance phagocytic pathogen clearance and modulate inflammatory responses through interaction with cell-surface receptors that recognize the Fc region of pathogen-bound IgG (FcγRs).3 Two general classes of FcγRs are currently recognized—activation receptors (eg, FcγRIA, FcγRIIIA) and inhibitory receptors (eg, FcγRIIB), both of which are present and functional in human and murine macrophages and other cells (reviewed by Ravetch and Bolland4). In macrophages, activating FcγRs initiate a complex intracellular signaling cascade culminating in enhanced phagocytosis and secretion of inflammatory cytokines.5-8 Inhibitory FcγRs, which are also present on macrophages and activated concomitantly, regulate the threshold of activation responses and ultimately terminate IgG-mediated effector stimulation.9 Phagocytosis mediated by FcγRs is morphologically and functionally distinct from uptake of unopsonized particles or that mediated by other receptors (eg, complement or mannose receptors).10 Phagocytic pathways are under differential regulatory control in macrophages as demonstrated by the observation that phagocytosis mediated by FcγRs (ie, IgG-coated beads), in contrast to nonspecific phagocytosis (unopsonized latex beads), can be blocked by inhibiting Src family kinases.11
Granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates growth and differentiation of cultured AMs12-14and plays a critical role in surfactant homeostasis and in lung host defense (reviewed by Trapnell and Whitsett15). Defects in AM functions in GM-CSF gene–targeted (GM−/−)16,17 mice result in impaired pulmonary clearance of and increased susceptibility to bacterial and fungal pathogens.18-20 For example, nonopsonic phagocytosis and bacterial killing are reduced in GM−/−AMs.20 GM-CSF stimulates FcγR levels on macrophages and enhances macrophage FcγR-mediated phagocytosis 21; however, the mechanism by which this occurs has not been defined. The ets family transcription factor, PU.1, is required for macrophage production,22,23 promotes the differentiation of myeloid progenitors,24 and stimulates the terminal maturation of AMs in the lung.20 PU.1 is deficient in AMs of GM−/− mice but is restored by local expression of GM-CSF in the lungs using the human 3.7-kb surfactant protein C (SPC) promoter (eg, SPC-GM+/+/GM−/− mice) rescuing defects in lung host defense.20 Because PU.1 stimulates FcγRI and FcγRIII transcription in monocyte/macrophage cell lines, we hypothesized that GM-CSF might regulate FcγR-mediated phagocytosis by mature AMs via PU.1.
Interferon-γ (IFN-γ) plays an important role in lung host defense by integrating pulmonary responses to microbial infection through pleiotropic effects on both innate and adaptive immunity. For example, IFN-γ stimulates macrophage activation25 and enhances FcγR-mediated phagocytosis by augmenting FcγR levels.26 IFN-γ also influences TH1/TH2 cell proliferation and function, enhancing humoral antibody production.27,28 Infection by microbial pathogens including adenovirus29 stimulates IFN-γ production by various cells, eg, natural killer (NK) and T helper-1 (TH1) cells.30 IFN-γ secretion by these cells is stimulated by either interleukin-18 (IL-18) or IL-12, both of which are released by AMs after exposure to pathogens such as adenovirus.30 Thus, IL-18/IL-12–stimulated IFN-γ secretion forms a feedback loop that enhances AM FcγR-mediated opsonophagocytosis during microbial lung infection. GM−/− mice have a blunted IFN-γ response to in vivo lipopolysaccharide exposure although GM−/− T cells have a normal IFN-γ response in vitro.31 These and other findings suggest that GM-CSF has an indirect effect on IFN-γ release from T cells in vivo, possibly through regulation of secretion of an “IFN-γ–releasing factor.”32 IL-18 and/or IL-12 are candidates for such a factor because (1) both are released by AMs after exposure to adenovirus, (2) both strongly stimulate IFN-γ production by T cells, and (3) blocking IL-18 and IL-12 signaling impairs IFN-γ production following pulmonary adenovirus infection.30
Together, these observations suggest that GM-CSF might regulate both FcγR-mediated phagocytosis by AMs and the IL-18/IFN-γ pathway that is required for augmentation of AM FcγR expression following pulmonary infection. To address this hypothesis, the role of GM-CSF and PU.1 in FcγR-mediated phagocytosis was assessed in primary AMs from GM+/+, GM−/−, or SPC-GM+/+/GM−/− mice and in cultured AM cell lines from GM+/+ and GM−/− mice.
Materials and methods
Mice
GM-CSF gene–targeted mice were previously created,16 bred into the C57BL/6 background, and maintained for several years (referred to as GM−/− mice hereafter).33 GM−/− mice in which GM-CSF was selectively expressed in the lung by the human 3.7-kb surfactant protein C promoter (SPC) were previously described34 and maintained in the C57BL/6 background (referred to as SPC-GM+/+/GM−/− mice hereafter). C57BL/6 mice (Charles River, Wilmington, MA) were used for comparison (referred to as GM+/+ hereafter). Mice were housed in a barrier facility and studied under procedures approved by the Institutional Animal Care and Use Committee of the Cincinnati Children's Hospital Research Foundation. Sentinel mice were tested periodically and were free of known viral and bacterial pathogens.
Alveolar macrophages and alveolar macrophage cell lines
Primary AMs were obtained from GM−/−, GM+/+, or SPC-GM+/+/GM−/− mice by bronchoalveolar lavage (BAL) as described.35 MH-S (American Type Culture Collection, CRL-2019, Mannasas, VA) is an AM cell line with morphologic features and functions of normal mature AMs, previously derived from GM+/+ BALB/cJ mice36; mAM is an AM cell line with an incompletely differentiated phenotype due to absence of expression of the transcription factor PU.1 previously derived from GM−/−mice without viral or other transformation.20 The mAM cells constitutively expressing murine PU.1 (referred to as mAM hereafter) were previously created by retroviral transduction.20 These cells also expressed green fluorescent protein (GFP), which is included in the vector as a selectable marker.37 As a transduction control, mAM cells expressing only the GFP marker were used (referred to as mAM hereafter).20 Retrovirally transduced alveolar macrophage cell lines (mAM, mAM) were maintained as unselected populations following transduction rather than as isolated clonal lines. Cultured AMs (MH-S, mAM, or mAM) were maintained as previously described.20 For clarity, AMs used in experiments after recovery from mice by BAL are referred to as primary AMs, while the various cultured AM cell lines are referred to as cultured AMs.
Adenovirus
The adenovirus used in this study is a replication-deficient derivative of human type 5 adenovirus whose structure has been previously reported.38 Methods for growth and purification of viruses in endotoxin-free media, media supplements, and solutions (supplied routinely or by special arrangement from BioWhittaker, Walkersville, MD) have been previously described.39,40 Virus concentration was determined from the optical density of the purified virions at 260 nm (OD260) using the formula 1 OD260 = 1 × 1012 particles per milliliter and expressed as optical particle units (opu) as previously described.41 Adenovirus was administered to the lungs by transoral intubation and tracheal instillation as previously described.29 In vitro infection of cultured AMs was done as previously described.40
FcγR-mediated phagocytosis assay
Albumin-coated fluorescent latex beads (referred to as unopsonized beads) were prepared using 2-μm–diameter fluorescein isothiocyanate (FITC)–labeled latex microspheres (Spherotech, Libertyville, IL) by incubation with bovine serum albumin (BSA) (faction V; Sigma, St Louis, MO; 10 mg/mL, 37°C, 60 minutes) followed by washing (3 ×) and resuspension in phosphate-buffered saline (PBS) at a concentration of 1.5 × 109beads per milliliter. IgG-opsonized beads were prepared by incubating albumin-coated beads with antialbumin antibody (Pharmingen; 1500 μg/mL, 37°C, 30 minutes) followed by washing and resuspension as above. FcγR-mediated phagocytosis was quantified in primary AMs ex vivo immediately after recovery by BAL20 and adherence to plastic42 and in cultured AM cell lines plated the day prior to analysis. Cells (105 per well, plated in 35-mm dishes) were exposed to unopsonized beads or IgG-opsonized beads at a concentration of 0.5 × 107/mL for 1 hour. In some experiments, cells were preincubated with rat antimouse CD16/32 (Pharmingen) for 30 minutes prior to and during incubation with IgG-opsonized beads to block FcγRII/FcγRIII-mediated opsonophagocytosis. Cells were then washed extensively in FACS buffer to remove noninternalized particles, detached by brief trypsinization, and evaluated by flow cytometry on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Results were analyzed using CellQuest software (Becton Dickinson) on a Macintosh microcomputer. The phagocytic index was calculated from the following formula: phagocytic index = percent of cells containing beads × mean fluorescence of cells containing beads. All determinations represent the mean of at least 3 separate measurements, and each experiment was performed 2 or more times with similar results.
FcγR expression on AMs
Primary AMs collected by BAL or cultured AMs collected by scraping in Versene (Life Technologies, Grand Island, NY) were resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS, 0.2% BSA, 0.01% sodium azide), washed in FACS buffer, counted, and divided into aliquots (105 cells) in 100 μL FACS buffer. Cells were incubated with phycoerythrin-conjugated antibody directed at mouse FcγR (rat antimouse CD16/32; Pharmingen; 30 minutes, 4°C). As controls, cells were also evaluated with primary isotype- and species-matched antimouse immunoglobulins. After incubation, immunostained cells were washed twice in FACS buffer and kept on ice and then analyzed by single-color flow cytometry using a FACScan flow cytometer as above. Fluorescence data were collected using logarithmic amplification on 10 000 viable cells as determined by forward light scattering. To determine if AMs in GM−/−mice were capable of responding to IFN-γ by increasing FcγR expression, primary AMs were obtained by BAL, collected by centrifugation, and freed of nonadherent cells by adherence in plastic dishes for 45 minutes as previously described.42 AMs were then exposed to IFN-γ (200 U/mL, 24 hours) and detached with Versene, and cell-surface FcγR levels were assessed by FACS as above. In other experiments, AM FcγR levels were also similarly assessed by FACS 48 hours after intrapulmonary administration of IL-18 (100 ng per mouse in 60 μL 0.9% NaCl) to stimulate increased levels of IFN-γ levels in the lung. All determinations were done on primary AMs from 4 mice per group or 4 plates of cultured AMs analyzed separately. Results were similar in corresponding samples, and representative examples are shown. Each experiment was done twice.
Reverse transcriptase–polymerase chain reaction amplification
Messenger RNA transcript levels were quantified in primary or cultured AMs using reverse transcriptase–polymerase chain reaction (RT-PCR) as previously described.20 29 The following primer sets were used: glyceraldehyde-3-phosphate dehydrogenase [GAPDH]: 5′-ATTCTACCCACGGCAAGTTCAATGG-′3 and 5′-AGGGGCGGAGATGATGACCC-3′; IL-18: 5′-AGACCTGGAATCAGACAACTTTGG-′3 and 5′-AAACTCCATCTTGTTGTGTCCTGG-3′; FcγRIA: 5′-GAGCAGGGAAAGAAAGCAAATTCC-3′ and 5′-TTAAGAGTTGCATGCCATGGTCC-3′; FcγRIIB: 5′-CCCAAGTCCAGCAGGTCTTTACC-3′ and 5′-TTCTGGCTTGCTTTTCCCAATGCC-3′; and FcγRIIIA: 5′-GATCCAGCAACTACATCCTCCATC-3′ and 5′-GCCTTGAACTGGTGATCCTAAGTC-3′. All determinations were done in triplicate using either primary AMs from 3 mice analyzed separately or plates of cultured AMs. Each experiment was done twice.
Cytokine levels
IFN-γ, IL-18, and IL-12 concentrations in the lungs were measured by enzyme-linked immunosorbent assay (ELISA) as previously described.29 Briefly, BAL fluid was obtained from groups of mice (4-6 per group) and cleared of cells and debris by low-speed centrifugation (450g, 10 minutes, 4°C). Cleared BAL fluid from each mouse was then assayed individually for various cytokines by using the appropriate murine Quantikine kit (R&D Systems, Minneapolis, MN) as directed by the manufacturer. Assessments of IL-18 and IL-12 levels were done twice. IFN-γ levels in the BAL fluid were also similarly assessed in mice 48 hours after receiving IL-18 via pulmonary administration as described above. In vitro determination of IL-18 release by cultured AMs (MH-S, mAM, and mAM) was done essentially as described.40Briefly, cells (2.5 × 105 per well in 24-well plates) were incubated in the absence or presence of adenovirus (1010 optical particle units [opu] per well) for 24 hours. Culture supernate was then aspirated, cleared by low-speed centrifugation, and evaluated for the presence of IL-18 by ELISA as above. All determinations represent the mean of 4 determinations done on separate plates of cells per group. Experiments with mAM and mAM cells were done twice.
Statistics
Numeric data are presented as mean ± SEM. Statistical comparisons were made using the Student t test. Statistical calculations were performed with Sigma Plot (version 7.0) software on an IBM-compatible microcomputer.
Results
GM-CSF is required for FcγR-mediated phagocytosis by primary AMs
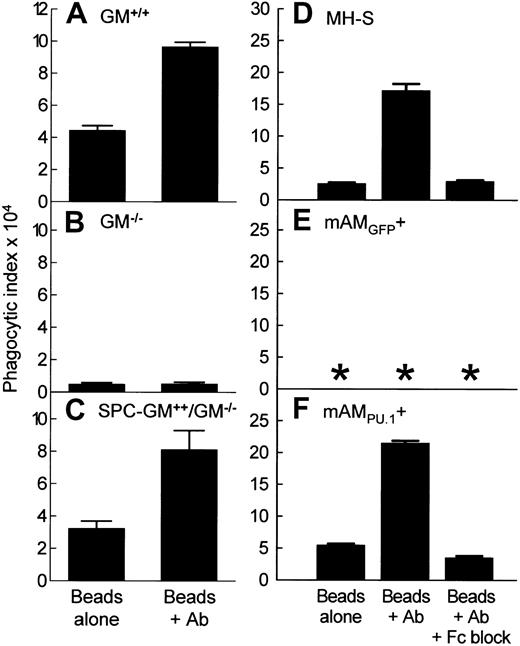
The role of GM-CSF in FcγR-mediated phagocytosis by AMs was assessed in primary AMs from GM+/+, GM−/−, and SPC-GM+/+/GM−/− mice by ex vivo challenge with fluorescent latex beads coated either with albumin alone (unopsonized beads) or with albumin and then antialbumin antibody (IgG-opsonized beads) followed by flow cytometry. In GM+/+AMs, FcγR-mediated phagocytosis was demonstrated by a phagocytic index for IgG-opsonized beads 117% ± 4% greater than that for unopsonized beads (Figure 1A). In contrast, FcγR-mediated phagocytosis was absent in GM−/− AMs (Figure 1B). Phagocytosis of unopsonized beads was also reduced in GM−/− AMs. Pulmonary expression of GM-CSF restored FcγR-mediated phagocytosis as demonstrated in SPC-GM+/+/GM−/− AMs, which had a phagocytic index for IgG-opsonized beads 150% ± 23% greater than that for unopsonized beads (Figure 1C). Thus, exposure to GM-CSF in the lung is required for FcγR-mediated phagocytosis by primary AMs.
FcγR-mediated phagocytosis by alveolar macrophages (AMs) is regulated by GM-CSF in the lungs and expression of PU.1 in AMs.
(A-C) Primary AMs were recovered by BAL from mice in which GM-CSF expression was normal (GM+/+), absent (GM−/−), or present only in the lungs (SPC-GM+/+/GM−/−) and challenged with unopsonized beads (Beads alone) or IgG-opsonized beads (Beads + Ab) as described in “Materials and methods.” Phagocytic indices are shown. Data represent means ± SEM; n = 4 (GM−/−and SPC-GM+/+/GM−/−) or n = 3 (GM−/−) mice per group; AMs from each mouse were analyzed individually. Differences in phagocytic indices for FcγR-mediated and non-FcγR–mediated phagocytosis (Beads + Ab, Beads alone, respectively) by AMs from GM+/+ and SPC-GM+/+/GM−/− were significant (P < .01). Corresponding phagocytic indices for AMs from GM−/− mice were not significantly different (P = .86). (D-F) Cultured AM cell lines were challenged as above except that plates of cells were also challenged in the presence of FcγR-blocking antibody (Beads + Ab +Fc block). Phagocytic indices are shown. Data represent means ± SEM; n = 4 determinations per group. Differences in phagocytic indices for FcγR-mediated and non-FcγR–mediated phagocytosis (Beads + Ab, Beads alone, respectively) in MH-S and mAM cells were significant (P < .0001). FcγR-mediated phagocytosis was completely blocked by addition of anti-FcγR antibody. Phagocytosis was not detected in mAM cells (*).
FcγR-mediated phagocytosis by alveolar macrophages (AMs) is regulated by GM-CSF in the lungs and expression of PU.1 in AMs.
(A-C) Primary AMs were recovered by BAL from mice in which GM-CSF expression was normal (GM+/+), absent (GM−/−), or present only in the lungs (SPC-GM+/+/GM−/−) and challenged with unopsonized beads (Beads alone) or IgG-opsonized beads (Beads + Ab) as described in “Materials and methods.” Phagocytic indices are shown. Data represent means ± SEM; n = 4 (GM−/−and SPC-GM+/+/GM−/−) or n = 3 (GM−/−) mice per group; AMs from each mouse were analyzed individually. Differences in phagocytic indices for FcγR-mediated and non-FcγR–mediated phagocytosis (Beads + Ab, Beads alone, respectively) by AMs from GM+/+ and SPC-GM+/+/GM−/− were significant (P < .01). Corresponding phagocytic indices for AMs from GM−/− mice were not significantly different (P = .86). (D-F) Cultured AM cell lines were challenged as above except that plates of cells were also challenged in the presence of FcγR-blocking antibody (Beads + Ab +Fc block). Phagocytic indices are shown. Data represent means ± SEM; n = 4 determinations per group. Differences in phagocytic indices for FcγR-mediated and non-FcγR–mediated phagocytosis (Beads + Ab, Beads alone, respectively) in MH-S and mAM cells were significant (P < .0001). FcγR-mediated phagocytosis was completely blocked by addition of anti-FcγR antibody. Phagocytosis was not detected in mAM cells (*).
PU.1 expression rescues FcγR-mediated phagocytosis by cultured GM−/− AMs
The role of PU.1 in the regulation of FcγR-mediated phagocytosis by AMs, downstream of GM-CSF, was assessed as above in cultured GM−/− AM cell lines wherein PU.1 expression was absent (mAM) or restored by retroviral gene transfer (mAM) and a cultured GM+/+ AM cell line expressing PU.1 normally (MH-S). In MH-S cells, FcγR-mediated phagocytosis was demonstrated by a phagocytic index for IgG-opsonized beads 574% ± 40% greater than that for unopsonized beads (Figure1D). Rat antimurine FcγRII/III antibody completely blocked FcγR-dependent uptake. In GM−/− mAM cells, FcγR-mediated phagocytosis did not occur in the absence of PU.1 (mAM; Figure 1E) but was restored by PU.1 expression (mAM; Figure 1F). FcγR-mediated phagocytosis in mAMPU.1+ cells was completely blocked by antimurine FcγRII/III antibody. Because pulmonary GM-CSF is required for expression of PU.1 in AMs,20 these data indicate that GM-CSF regulates FcγR-mediated phagocytosis by AMs via PU.1.
GM-CSF, via PU.1, regulates constitutive FcγR levels on AMs
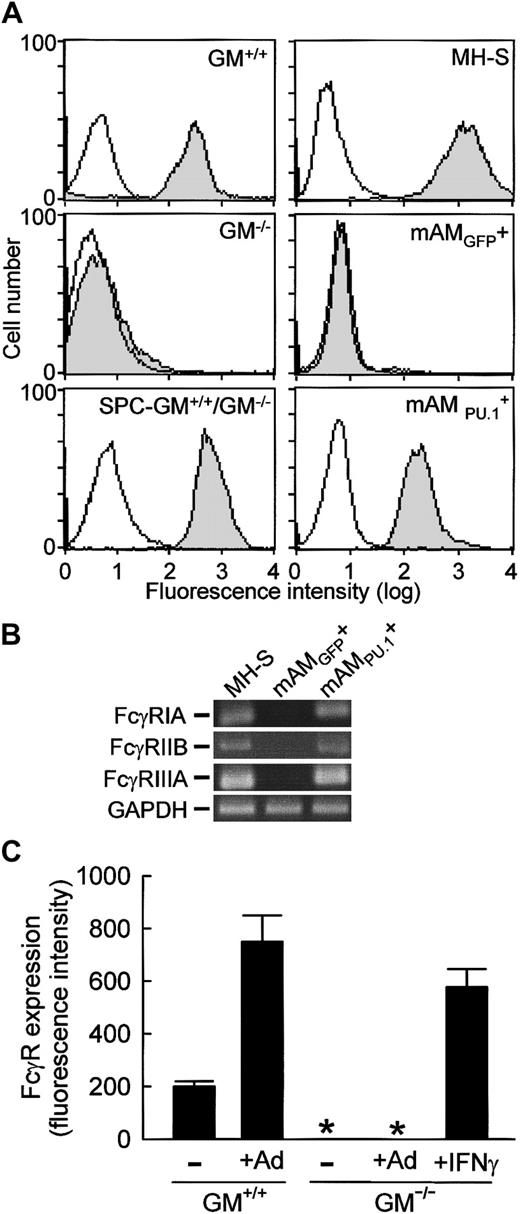
To determine if impaired FcγR-mediated phagocytosis by GM−/− AMs could be explained by failure to stimulate expression of FcγRs, levels of cell-surface FcγRs on primary and cultured AMs were assessed by flow cytometry. FcγRs were present on all primary GM+/+ AMs as demonstrated using a rat antimouse FcγRII/III antibody (Figure 2A). In contrast, FcγRs were undetectable on GM−/− AMs but were restored by pulmonary GM-CSF expression as demonstrated with AMs from SPC-GM+/+/GM−/− mice. Because PU.1 stimulates transcription of the human FcγRIB43 and murine FcγRIIIA44 genes in myeloid cell lines, the role of PU.1 in regulation of AM FcγR levels was assessed using MH-S, mAM, and mAM cells. FcγRs were readily and uniformly detected on cultured MH-S cells as demonstrated using the anti-FcγRII/III antibody (Figure 2A). In sharp contrast, FcγRs were absent on mAM cells but were restored by PU.1 expression in mAM cells (Figure 2A). Importantly, mAM cells did not contain detectable mRNA encoding either FcγRIIIA or FcγRIIB (Figure 2B), both of which are detected by the antimurine FcR antibody45 46 used for flow cytometry above. Nor did these cells contain mRNA for FcγRIA. In contrast, both wild-type (MH-S) and PU.1-expressing GM−/−(mAM) cultured AMs expressed mRNA for all 3 FcγRs (Figure 2B). Together with the results from primary AMs, these data indicate that GM-CSF, via PU.1, is required for expression of both activating FcRs (FcγRIA, FcγRIIIA) and inhibiting FcRs (FcγRIIB) in AMs.
FcγR expression on AMs is regulated by levels of GM-CSF in the lungs and expression of PU.1 in AMs, and levels are increased during pulmonary infection by adenovirus.
(A) FcγR expression on primary AMs (GM+/+, GM−/−, SPC-GM+/+/GM−/−) or cultured AMs (MH-S, mAM, mAM) was quantified by FACS as described in “Materials and methods.” Shown are data for FcγRII/III-specific antibodies (shaded histogram) and isotype controls (open histogram). Differences in autofluorescence among cultured AM cell lines, due in part to the presence of the GFP marker in mAM and mAM, have been compensated. Results are representative of 4 separate determinations in AMs from mice analyzed individually. (B) The cultured AM cell line from GM−/− mice (mAM) failed to express mRNA for either activating (FcγRIA, FcγRIIIA) or inhibiting (FcγRIIB) FcRs, but expression was stimulated by PU.1 as shown in mAM cells. Total RNA was prepared from cultured AM cell lines (MH-S, mAM, mAM) and subjected to RT-PCR analysis using gene-specific primers as described in “Materials and methods.” Shown are photographs of ethidium bromide–stained RT-PCR reaction products separated on 2% agarose gels. H20 PCR controls were negative (not shown). This experiment was repeated twice with identical results. (C) Primary AMs were recovered 36 hours after pulmonary adenoviral infection of GM+/+ or GM−/− mice, and levels of FcγR expression were assessed as above. Levels of FcγR on AMs are represented as the mean fluorescence intensity as determined using the FcγRII/III-specific antibody. Data represent means ± SEM; n = 4 mice per group; AMs from each mouse were analyzed individually. Differences in FcγR expression on AMs from infected and uninfected GM+/+ mice were significant (P < .001). FcγR expression was not detected (*) on GM−/− AMs in the absence or presence of adenovirus infection. AMs recovered from GM−/− mice and exposed ex vivo to IFN-γ for 24 hours showed a marked up-regulation of cell-surface FcγRII/III expression at levels significantly different from untreated mice (P < .001).
FcγR expression on AMs is regulated by levels of GM-CSF in the lungs and expression of PU.1 in AMs, and levels are increased during pulmonary infection by adenovirus.
(A) FcγR expression on primary AMs (GM+/+, GM−/−, SPC-GM+/+/GM−/−) or cultured AMs (MH-S, mAM, mAM) was quantified by FACS as described in “Materials and methods.” Shown are data for FcγRII/III-specific antibodies (shaded histogram) and isotype controls (open histogram). Differences in autofluorescence among cultured AM cell lines, due in part to the presence of the GFP marker in mAM and mAM, have been compensated. Results are representative of 4 separate determinations in AMs from mice analyzed individually. (B) The cultured AM cell line from GM−/− mice (mAM) failed to express mRNA for either activating (FcγRIA, FcγRIIIA) or inhibiting (FcγRIIB) FcRs, but expression was stimulated by PU.1 as shown in mAM cells. Total RNA was prepared from cultured AM cell lines (MH-S, mAM, mAM) and subjected to RT-PCR analysis using gene-specific primers as described in “Materials and methods.” Shown are photographs of ethidium bromide–stained RT-PCR reaction products separated on 2% agarose gels. H20 PCR controls were negative (not shown). This experiment was repeated twice with identical results. (C) Primary AMs were recovered 36 hours after pulmonary adenoviral infection of GM+/+ or GM−/− mice, and levels of FcγR expression were assessed as above. Levels of FcγR on AMs are represented as the mean fluorescence intensity as determined using the FcγRII/III-specific antibody. Data represent means ± SEM; n = 4 mice per group; AMs from each mouse were analyzed individually. Differences in FcγR expression on AMs from infected and uninfected GM+/+ mice were significant (P < .001). FcγR expression was not detected (*) on GM−/− AMs in the absence or presence of adenovirus infection. AMs recovered from GM−/− mice and exposed ex vivo to IFN-γ for 24 hours showed a marked up-regulation of cell-surface FcγRII/III expression at levels significantly different from untreated mice (P < .001).
FcγR expression is deficient on primary AMs in GM−/− mice following adenoviral infection
In GM+/+ mice, pulmonary adenovirus infection augmented FcγR levels on AMs by 253% ± 95% as determined by flow cytometry using the anti-FcγRII/III antibody (Figure 2C). In contrast, FcγRs were absent on GM−/− AMs following pulmonary adenoviral infection. Importantly, ex vivo exposure of GM−/− AMs to IFN-γ restored FcγR expression as indicated by the marked increase in fluorescence of anti-FcγRII/III antibody–stained cells (Figure 2C). Thus, enhancement of FcγR II/III expression on AMs following adenoviral infection does not occur in GM−/− mice and is not due to an inability of GM−/− AMs to respond to IFN-γ.
Impairment of IFN-γ, IL-18, and IL-12 expression in GM−/− mice during pulmonary adenoviral infection
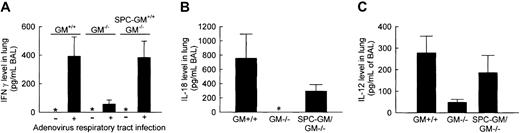
To determine whether GM-CSF is required for regulation of IFN-γ expression by IL-18/IL-12, levels of these cytokines were quantified in the lungs after pulmonary adenoviral infection. IFN-γ was not detected in the lungs of uninfected GM+/+, GM−/−, or SPC-GM+/+/GM−/− mice (Figure 3A). In GM+/+ or SPC-GM+/+/GM−/− mice, but not GM−/− mice, high levels of IFN-γ were detected after infection (392 ± 136, 384 ± 114, and 57 ± 29 pg/mL, respectively; Figure 3A). Thus, pulmonary GM-CSF expression is required for the high-level expression of IFN-γ accompanying pulmonary adenoviral infection.
The increase in IFN-γ, IL-18, and IL-12 levels in the lungs stimulated by pulmonary adenovirus infection is severely impaired in GM−/− mice.
(A) BAL fluid was collected from GM+/+, GM−/−, and SPC-GM+/+/GM−/− mice 36 hours after pulmonary adenovirus infection and evaluated for the presence of IFN-γ by ELISA. Asterisks indicate that no IFN-γ was detected in the lungs of any mice in the absence of adenovirus lung infection. Data represent means ± SEM; n = 4 (GM−/− or SPC-GM+/+/GM−/−) or n = 3 (GM+/+) mice per group; BAL fluid from each mouse was analyzed individually. Differences in IFN-γ levels in infected (+) and uninfected (−) GM+/+ and SPC-GM+/+/GM−/− mice were significant (P < .02) but were not significant in GM−/−mice (P = .10). (B) Mice exposed to adenovirus as above were also evaluated for the presence of IL-18 in BAL. IL-18 levels in BAL fluid of infected animals are shown. Data represent the means ± SEM; n = 6 (GM−/− or SPC-GM+/+/GM−/−) or n = 3 (GM+/+) mice per group; BAL fluid from each mouse was analyzed individually. IL-18 was not detected in BAL fluid from adenovirus-exposed GM−/− mice (*). Differences in IL-18 levels in GM+/+ and GM−/− mice were significant (P < .05). The adenovirus-simulated increase in IL-18 levels in the lungs was restored in SPC-GM+/+/GM−/− mice. (C) The same animals evaluated in panel A were also evaluated for the presence of IL-12 in BAL. IL-12 levels in BAL fluid of infected animals are shown. Data represent the means ± SEM; n = 7 mice per group. Differences in IL-12 levels in GM+/+ and GM−/− mice were significant (P < .05). The adenovirus-simulated increase in IL-18 levels in the lungs was restored in SPC-GM+/+/GM−/− mice.
The increase in IFN-γ, IL-18, and IL-12 levels in the lungs stimulated by pulmonary adenovirus infection is severely impaired in GM−/− mice.
(A) BAL fluid was collected from GM+/+, GM−/−, and SPC-GM+/+/GM−/− mice 36 hours after pulmonary adenovirus infection and evaluated for the presence of IFN-γ by ELISA. Asterisks indicate that no IFN-γ was detected in the lungs of any mice in the absence of adenovirus lung infection. Data represent means ± SEM; n = 4 (GM−/− or SPC-GM+/+/GM−/−) or n = 3 (GM+/+) mice per group; BAL fluid from each mouse was analyzed individually. Differences in IFN-γ levels in infected (+) and uninfected (−) GM+/+ and SPC-GM+/+/GM−/− mice were significant (P < .02) but were not significant in GM−/−mice (P = .10). (B) Mice exposed to adenovirus as above were also evaluated for the presence of IL-18 in BAL. IL-18 levels in BAL fluid of infected animals are shown. Data represent the means ± SEM; n = 6 (GM−/− or SPC-GM+/+/GM−/−) or n = 3 (GM+/+) mice per group; BAL fluid from each mouse was analyzed individually. IL-18 was not detected in BAL fluid from adenovirus-exposed GM−/− mice (*). Differences in IL-18 levels in GM+/+ and GM−/− mice were significant (P < .05). The adenovirus-simulated increase in IL-18 levels in the lungs was restored in SPC-GM+/+/GM−/− mice. (C) The same animals evaluated in panel A were also evaluated for the presence of IL-12 in BAL. IL-12 levels in BAL fluid of infected animals are shown. Data represent the means ± SEM; n = 7 mice per group. Differences in IL-12 levels in GM+/+ and GM−/− mice were significant (P < .05). The adenovirus-simulated increase in IL-18 levels in the lungs was restored in SPC-GM+/+/GM−/− mice.
Because IL-18 and IL-12 stimulate IFN-γ production in the lung during pulmonary adenovirus infection,30 these cytokines were also quantified after pulmonary adenoviral infection. Neither IL-18 nor IL-12 was detected in the lungs of uninfected mice (data not shown). In GM+/+ and SPC-GM+/+/GM−/− mice, but not GM−/− mice, pulmonary adenovirus infection increased IL-18 levels (756 ± 340, 294 ± 93, 0 ± 0 pg/mL BAL, respectively; Figure 3B). Thus, GM-CSF is required for IL-18 production during pulmonary adenovirus infection. Further, the absence of IL-18–stimulated IFN-γ production in GM−/− mice following adenoviral infection explains the lack of augmentation of FcγR expression on primary AMs. To confirm this hypothesis, IL-18 was administered to the lungs of GM−/− mice and IFN-γ levels were measured 48 hours later. IL-18 significantly stimulated IFN-γ levels (84 ± 29 pg/mL BAL fluid; n = 7;P < .04) and restored FcγR levels on primary AMs (mean fluorescence intensity of anti-FcγRII/III antibody–stained AMs 92 ± 11; n = 7; P < .001) compared with controls (n = 5; data not shown). In GM+/+ and SPC-GM+/+/GM−/− mice, pulmonary adenovirus infection also increased IL-12 levels, but in GM−/− mice levels were elevated to a much lower degree (213 ± 129, 134 ± 60, 26 ± 7.3 pg/mL BAL, respectively; Figure 3C). These data indicate that GM-CSF is required for stimulation of IL-18 and IL-12 production in the lung during pulmonary adenovirus infection and provide a molecular explanation for the decreased IFN-γ levels seen during adenovirus infection in GM−/− mice.
PU.1 rescues IL-18 production in cultured GM−/−AMs
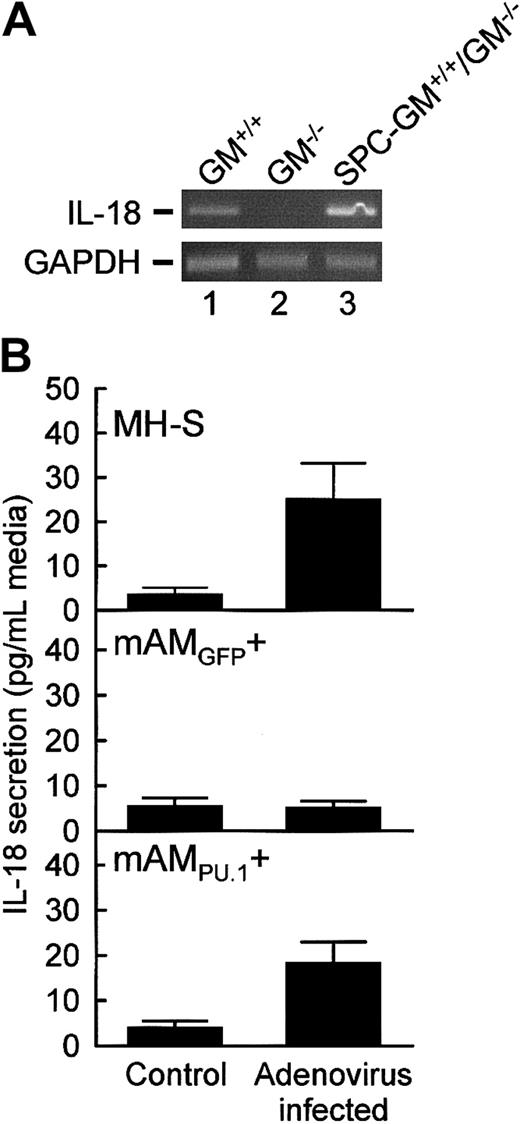
The role of GM-CSF/PU.1 in regulation of IL-18 production by AMs was assessed in primary and cultured AMs. Consistent with the ELISA data above, IL-18 mRNA was detected in primary AMs from GM+/+ and SPC-GM+/+/GM−/− mice but not GM−/− mice (Figure4A). IL-18 mRNA was more abundant in primary AMs from SPC-GM+/+/GM−/− than from GM+/+ mice, likely reflecting the markedly increased pulmonary GM-CSF levels in SPC-GM+/+/GM−/−mice.34 To further assess the role of GM-CSF/PU.1 in regulation of IL-18 expression in AMs, IL-18 release from adenovirus-infected and uninfected MH-S, mAM, and mAM cells was quantified by ELISA. Adenoviral infection of MH-S cells, but not mAM cells, markedly increased IL-18 release (25.2 ± 7.9 vs 5.3 ± 1.2 pg/mL medium, respectively; Figure 4B). PU.1 restored IL-18 release by mAM cells following adenoviral infection (18.5 ± 4.5 pg/mL medium). Because pulmonary GM-CSF is required for expression of PU.1 in primary AMs,20 these data indicate that GM-CSF regulates IL-18 production by AMs via PU.1.
IL-18 expression in AMs from GM−/− mice is severely impaired but was restored by retrovirus-mediated expression of PU.1.
(A) Primary AMs from GM+/+, GM−/−, and SPC-GM+/+/GM−/− mice were assessed for the presence of mRNA transcripts encoding IL-18 or GAPDH, as a control to demonstrate evaluation of equal amounts of total RNA, using RT-PCR as described in “Materials and methods.” Photographs of ethidium bromide–stained agarose electrophoresis gels of the PCR products are shown. Each lane represents AMs from one mouse. The experiment was repeated twice with the same results. (B) Cultured AMs were exposed to adenovirus (Adenovirus infected) or not exposed (Control) for 24 hours, and then IL-18 release into the medium was quantified by ELISA as described in “Materials and methods.” The sensitivity of detection of IL-18 was 5 pg/mL. Data represent means ± SEM; n = 3 to 6 (uninfected) or 4 to 10 (infected). Differences in IL-18 release by adenovirus-infected and uninfected MH-S and mAM cells were significant (P < .03). IL-18 release by adenovirus-infected and uninfected mAM cells was not significantly different (P = .88).
IL-18 expression in AMs from GM−/− mice is severely impaired but was restored by retrovirus-mediated expression of PU.1.
(A) Primary AMs from GM+/+, GM−/−, and SPC-GM+/+/GM−/− mice were assessed for the presence of mRNA transcripts encoding IL-18 or GAPDH, as a control to demonstrate evaluation of equal amounts of total RNA, using RT-PCR as described in “Materials and methods.” Photographs of ethidium bromide–stained agarose electrophoresis gels of the PCR products are shown. Each lane represents AMs from one mouse. The experiment was repeated twice with the same results. (B) Cultured AMs were exposed to adenovirus (Adenovirus infected) or not exposed (Control) for 24 hours, and then IL-18 release into the medium was quantified by ELISA as described in “Materials and methods.” The sensitivity of detection of IL-18 was 5 pg/mL. Data represent means ± SEM; n = 3 to 6 (uninfected) or 4 to 10 (infected). Differences in IL-18 release by adenovirus-infected and uninfected MH-S and mAM cells were significant (P < .03). IL-18 release by adenovirus-infected and uninfected mAM cells was not significantly different (P = .88).
Discussion
The present study was designed to determine the role of GM-CSF in FcγR-mediated opsonophagocytosis by AMs and the mechanism by which GM-CSF influences FcγR expression during pulmonary infection. FcγR-mediated phagocytosis by AMs from GM−/−mice was severely impaired in vivo and in vitro and was restored by expression of GM-CSF in the lungs or by constitutive expression of PU.1 in primary and cultured AMs, respectively. Impaired opsonophagocytosis was associated with the absence of FcγRs on AMs in vivo and in vitro, and AM FcγR expression was dependent on PU.1. Enhancement of AM FcγR expression following pulmonary adenoviral infection did not occur in GM−/− mice due to impaired IFN-γ production, which was associated with impaired IL-18/IL-12 production. Pulmonary expression of GM-CSF restored adenovirus-stimulated production of all 3 cytokines and augmented FcγR levels on AMs. GM−/− AMs did not express IL-18 mRNA or protein, but expression was restored by PU.1 expression in cultured AMs in vitro, and pulmonary administration of IL-18 in GM−/− mice stimulated both IFN-γ production in vivo and augmented FcγR levels on primary AMs. These data show that GM-CSF, via PU.1, regulates AM FcγR expression and opsonophagocytosis and also the positive feedback mechanism by which pathogen-exposed AMs enhance FcγR expression during pulmonary infection.
The correlation between FcγR expression and opsonophagocytosis by primary and cultured AMs observed here suggests that the presence or absence of the FcγR may itself be a principal point of control in the molecular mechanism regulating the constitutive opsonophagocytic capacity of AMs. Thus, GM-CSF, via PU.1, may program differentiating myeloid cells for opsonophagocytosis primarily by stimulating expression of FcγRs. Several lines of evidence support this concept. First, PU.1 expression was required for expression of FcγR mRNA and protein in cultured AMs. Further, FcγRs were present on primary AMs in vivo only when GM-CSF was present in the lung, consistent with the prior observation that the pulmonary GM-CSF regulates PU.1 expression in primary AMs in vivo.20 Thus, opsonophagocytosis and expression of GM-CSF, PU.1, and FcγR were precisely correlated in the present study. These results are consistent with reports showing that GM-CSF stimulates FcγR expression and opsonophagocytosis in murine macrophages21 and increased FcγR mRNA expression in monocytes during in vitro differentiation47 and also with data showing that PU.1 stimulates transcription of FcγRIA in a human monocytic cell line43 and FcγRIIIA in a murine peritoneal macrophage cell line.44 Second, transfection-mediated FcγRI expression in COS cells confers opsonophagocytic capacity, demonstrating that cells that are not professional phagocytes and do not normally express FcγRs have the molecular machinery for phagocytosis.5 Finally, PU.1 is a principal regulator of AM terminal differentiation and stimulates expression of other receptors on AMs, including the mannose receptor and several Toll-like receptors.20 Although PU.1 was previously shown to stimulate FcγRIA and FcγRIIIA expression in myeloid cells, our data demonstrate that PU.1 is required for expression of these activating receptors, and also the inhibiting receptor FcγRIIB. Further, their parallel regulation by GM-CSF/PU.1 suggests that coordinate expression of the balanced activation and inhibitory Fc signaling pathways during myeloid differentiation may be important to macrophage opsonophagocytosis and/or FcγR signaling. Our findings do not rule out the possibility that other critical molecular events may also be simultaneously regulated by GM-CSF and/or PU.1 in AMs and thus may also be rate limiting in GM−/− AMs. For example, several distinct protein tyrosine kinase families are important in the regulation of opsonophagocytosis, including Syk,48 Src,49and phosphatidylinositol 3-kinase.50,51 Consistent with this latter possibility is the finding that, although FcγR expression in COS cells did promote opsonophagocytosis, it did not restore activity to a level typical of professional phagocytes.5Thus, while GM-CSF/PU.1–stimulated FcγR expression in AMs is necessary, it may not be sufficient to confer opsonophagocytosis in AMs, and other factors could potentially be rate limiting. Our data also do not exclude the theoretical possibility that FcγR-mediated phagocytosis may be regulated by GM-CSF through additional pathways not involving PU.1. Because GM−/− AMs have abnormally low adhesion, collection of primary AMs by adherence to plastic dishes could have influenced our findings if subsets with differential adherence were present. However, we have no evidence for such heterogeneity and, importantly, similar findings in primary and cultured GM−/− AMs argue against this theoretical possibility.
The present studies support a model in which adenoviral enhancement of FcγR expression on AMs is mediated by GM-CSF–dependent IL-18/IL-12 production, which stimulates pulmonary IFN-γ production, in turn enhancing AM FcγR levels (Figure 5). The observation that adenoviral infection increases IFN-γ levels in the lungs and FcγR levels on AMs in GM+/+ mice is consistent with reports showing that adenovirus stimulates production of IFN-γ, IL-18, and IL-12 during lung infection29,30,52and reports showing that IFN-γ enhances both AM FcγR expression and opsonophagocytosis.53 Further, IFN-γ production by local inflammatory cells (eg, NK, TH1 lymphocytes) was stimulated by IL-12 or IL-18,54,55 and both of these cytokines are released by macrophages during infection.55 The effects of GM-CSF on AM expression of FcγRs and pathogen-stimulated production of IL-18/IL-12 are both mediated by PU.1, the expression of which is maintained in AMs by GM-CSF. In GM−/− mice, AMs do not express PU.1 or secrete IL-18 during adenovirus infection. Because pulmonary adenoviral infection stimulated higher IL-18 mRNA levels in primary AMs in SPC-GM+/+/GM−/− than GM+/+ mice and GM-CSF levels are much higher in the lungs of the former,34 these data suggest that pulmonary GM-CSF levels may regulate the capacity of the AM IL-18 response to infection. However, pulmonary IL-18 levels in SPC-GM+/+/GM−/− and GM+/+ mice following pulmonary adenoviral infection were not statistical different, possibly due to minor differences in pulmonary adenoviral infection in these separate experiments. Thus, further studies will be required to define the precise regulation of this pathway. IL-12 production is also severely attenuated, but not absent, in adenoviral-infected GM−/− mice, and the low levels of this cytokine likely explain the low levels of IFN-γ in the lungs of these mice. Thus, neither IFN-γ production nor FcγR enhancement on AMs occurs in GM−/− mice. Together, these data show that GM-CSF/PU.1 is required for cytokine-mediated augmentation of mechanisms regulating opsonophagocytosis by AMs during lung infection.
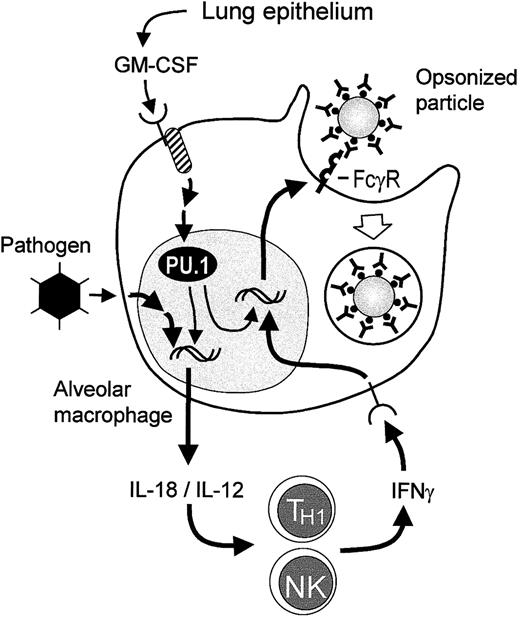
Proposed model for regulation of FcγR-mediated phagocytosis in resting and activated AMs.
In GM+/+ mice, GM-CSF is released constitutively by respiratory epithelium and other cells in the lung and interacts with receptors on AM precursors, maintaining the presence of the transcription factor PU.1 in AMs. High levels of PU.1 in AMs promote their terminal differentiation,20 including stimulation of expression of FcγRs, thus enabling constitutive FcγR-mediated phagocytosis. Exposure to pathogens such as adenovirus stimulates AMs to release IL-18 and IL-12, both of which are potent stimulators of IFN-γ release by TH1 and NK cells during adenovirus infection of the respiratory tract.30 IFN-γ then interacts with receptors on AMs and stimulates increased FcγR expression, resulting in increased FcγR-mediated phagocytosis. In GM−/− mice, PU.1 levels in AMs are deficient due to the absence of GM-CSF in the lungs.20 Because IL-18 expression in AMs requires PU.1, pathogen exposure of AMs in the lungs of GM−/− mice does not result in release of IL-18. Although NK cells from GM−/− mice are capable of cytokine-stimulated IFN-γ release,32 in the absence of such a stimulus IFN-γ release does not occur in pathogen-exposed GM−/− mice and thus is not present to stimulate FcγR or FcγR-mediated phagocytosis by AMs.
Proposed model for regulation of FcγR-mediated phagocytosis in resting and activated AMs.
In GM+/+ mice, GM-CSF is released constitutively by respiratory epithelium and other cells in the lung and interacts with receptors on AM precursors, maintaining the presence of the transcription factor PU.1 in AMs. High levels of PU.1 in AMs promote their terminal differentiation,20 including stimulation of expression of FcγRs, thus enabling constitutive FcγR-mediated phagocytosis. Exposure to pathogens such as adenovirus stimulates AMs to release IL-18 and IL-12, both of which are potent stimulators of IFN-γ release by TH1 and NK cells during adenovirus infection of the respiratory tract.30 IFN-γ then interacts with receptors on AMs and stimulates increased FcγR expression, resulting in increased FcγR-mediated phagocytosis. In GM−/− mice, PU.1 levels in AMs are deficient due to the absence of GM-CSF in the lungs.20 Because IL-18 expression in AMs requires PU.1, pathogen exposure of AMs in the lungs of GM−/− mice does not result in release of IL-18. Although NK cells from GM−/− mice are capable of cytokine-stimulated IFN-γ release,32 in the absence of such a stimulus IFN-γ release does not occur in pathogen-exposed GM−/− mice and thus is not present to stimulate FcγR or FcγR-mediated phagocytosis by AMs.
Results of the present study support the concept that GM-CSF is required for terminal differentiation of AMs in the lungs in vivo.20 This is consistent with previous findings demonstrating that rescue of a wild-type AM phenotype was associated with restoration of GM-CSF only in the lungs in SPC-GM+/+/GM−/− mice.33 FcγRs provide useful markers of macrophage differentiation because their presence is correlated phenotypically with terminal differentiation and also functionally with a primary AM function, ie, enhancing clearance of microbial organisms. The role of FcγRs in stimulation of inflammatory cytokine signaling pathways in macrophages underscores the importance of a second principal function, recruitment and activation of other innate immune cells as well as cells involved in the adaptive immune responses. The finding that GM-CSF and PU.1 are critical to regulation of this inflammatory cytokine mechanism augmenting opsonophagocytosis in a positive feedback loop involving AM release of IL-18/IL-12 further supports the broad nature of the effects of GM-CSF/PU.1 on AM functional capabilities. Thus, GM-CSF, via PU.1, is a critical regulator of constitutive AM FcγR-mediated clearance and an important molecular mechanism that connects innate and adaptive immunity in the lung and, among other functions, amplifies AM opsonophagocytosis.
We thank Dr Harinder Singh for the gift of the PU.1/GFP- and GFP-expressing retroviral vectors and thank Z. Chroneos, who provided the GM−/− AM cell line mAM.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1102.
Supported by the National Institutes of Health (NIH) R01 HL69549 (B.C.T.), NIH SCOR HL56387 (J.A.W.), Fondation Suisse de Bourse en Medecine et Biologie (FSBMB) (P.-Y.B.), TCHRF Procter Scholarship (P.-Y.B.), M. Carvajal Steuer (P.-Y.B.), and the Cystic Fibrosis Foundation, Research and Development Program (J.A.W. and B.C.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce C. Trapnell, Children's Hospital Medical Center, Division of Pulmonary Biology, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail:bruce.trapnell@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal