Selectin ligands are glycan structures that participate in leukocyte trafficking and inflammation. At least 6 ST3Gal sialyltransferases (I-VI) have been identified that may contribute to selectin ligand formation. However, it is not known which of these sialyltransferases are involved in vivo and whether they may differentially regulate selectin function. We have produced and characterized mice genetically deficient in ST3Gal-I, ST3Gal-II, ST3Gal-III, and ST3Gal-IV. Unlike mice bearing severe defects in selectin ligand formation, there was no finding of leukocytosis with these single ST3Gal deficiencies. Among neutrophils, only ST3Gal-IV was found to play a role in the synthesis of selectin ligands. In vitro rolling of marrow-derived neutrophils on E- or P-selectins presented by Chinese hamster ovary cells was reduced in the absence of ST3Gal-IV. However, in a tumor necrosis factor α (TNF-α)–induced inflammation model in vivo, no defect among P-selectin ligands was observed. Nevertheless, the number of leukocytes rolling on postcapillary venules in an E-selectin–dependent manner was decreased while E-selectin–dependent rolling velocity was increased. We propose that multiple ST3Gal sialyltransferases contribute to selectin ligand formation, as none of these ST3Gal deficiencies recapitulated the degree of E- and P-selectin ligand deficit observed on neuraminidase treatment of intact neutrophils. Our findings indicate a high degree of functional specificity among sialyltransferases and a substantial role for ST3Gal-IV in selectin ligand formation.
Introduction
Leukocytes in the bloodstream use a carbohydrate adhesion system involving the selectins for cell tethering and rolling on the vascular endothelium (reviewed in Lowe1 and Springer2). This lectin-ligand system is essential for the subsequent transmigration of adherent cells through the endothelium, thereby contributing to the development and function of leukocytes at secondary lymphoid organs and sites of inflammation. The expression pattern of the 3 selectin molecules E, L, and P reflects the physiologic role that each plays in vivo. E- and P-selectin are induced on the vascular endothelium during inflammation, whereas P-selectin is also found on platelets, and L-selectin is expressed on the surface of leukocytes. Absence of these molecules, singly or in combination, yields defects to varying degrees in leukocyte homeostasis, trafficking, and innate immune responses during inflammation.3-7
The carbohydrate (glycan) ligands of the selectins are less well defined. The prototypical selectin ligand structure is a terminal tetrasaccharide (Siaα2-3Galβ1-4[Fucα1-3]GlcNAc-) termed sialyl Lewis X (sLex; Figure 1A; reviewed in Varki8). Evidence from gene ablation studies indicates that multiple glycosyltransferases control selectin ligand biosynthesis. For example, fucosyltransferase-VII (FucT-VII) is required for functional E-, P-, and L-selectin ligand formation, and its absence in mice provokes deficits involving both neutrophil trafficking in inflammation and lymphocyte homing and colonization of the lymph nodes.9 In contrast, FucT-IV contributes to E-selectin ligand function, mostly in concert with FucT-VII as evident in the FucT-VII–deficient background.10 Moreover, the core 2 N-acetylglucosaminyltransferase-I (C2GlcNAcT-I) is required for the formation of a subset of selectin ligands involved in neutrophil recruitment during inflammation but not in lymphocyte trafficking to peripheral lymph nodes.11
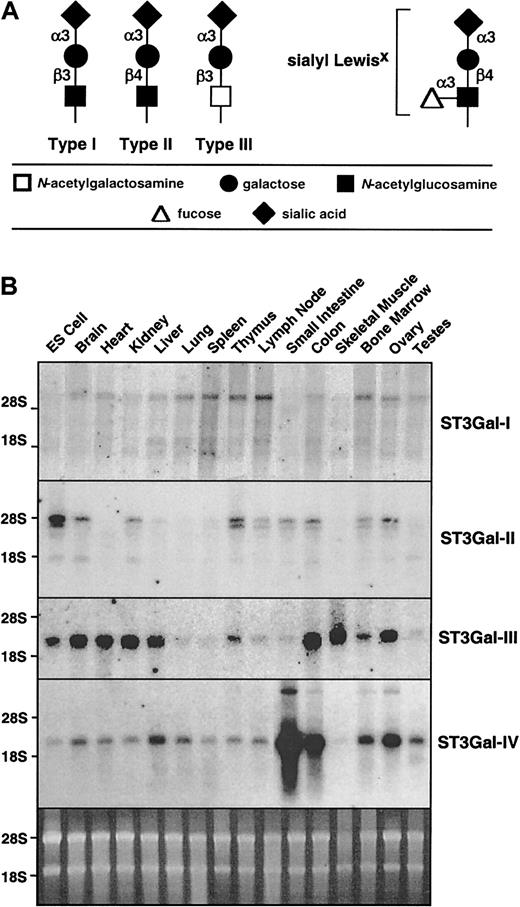
Sialylated glycan chain termini and expression of ST3Gal-I, -II, -III, and -IV sialyltransferase RNA.
(A) ST3Gal sialyltransferases add sialic acid to the terminus of type I, II, or III glycan chains, as occurs in formation of selectin ligands such as sialyl Lewis X. (B) Total RNA from various mouse tissues was hybridized with cDNA probes specific for each of the sialyltransferases. The sybergreen-stained gel (bottom) indicates similar loading of the RNA.
Sialylated glycan chain termini and expression of ST3Gal-I, -II, -III, and -IV sialyltransferase RNA.
(A) ST3Gal sialyltransferases add sialic acid to the terminus of type I, II, or III glycan chains, as occurs in formation of selectin ligands such as sialyl Lewis X. (B) Total RNA from various mouse tissues was hybridized with cDNA probes specific for each of the sialyltransferases. The sybergreen-stained gel (bottom) indicates similar loading of the RNA.
Six genes have been identified in the mammalian genome that encode Golgi-resident sialyltransferases that form α2-3 sialic acid linkages (ST3Gal-I-VI) potentially involved in selectin ligand formation.12-14 Three of those sialytransferases (ST3Gal-III, ST3Gal-IV, and ST3Gal-VI) are known to sialylate type II (Galβ1-4GlcNAc) oligosaccharides in vitro, consistent with involvement in the formation of sLex.14,15 In fact, ST3Gal-III expression has been correlated with the formation of sLex in some cancer tissues.16However, ST3Gal-III exhibits strongest substrate preference for type I (Galβ1-3GlcNAc) oligosaccharides, which could result in the formation of sLea on leukocytes.17 ST3Gal-I and ST3Gal-II activity in vitro is predominantly toward type III oligosaccharides, whereas ST3Gal-II prefers glycolipid substrates, and neither has detectable in vitro activity toward type II chains that form the foundation for sLex structures on leukocytes.12,18 ST3Gal-V is also known as GM3synthase and has a substrate preference for glycolipids.13Any of these ST3Gal sialyltransferases may be involved to some degree in selectin ligand formation among various cell types in vivo.
Sialyltransferase function may also be influenced by competition in the Golgi with other glycosyltransferases that operate in concurrent biosynthetic and branching steps, potentially affecting the formation of downstream terminal branch structures. For example, it is known that ST3Gal-I effectively competes with C2GlcNAcT-I for the same substrate, and thereby reduces core 2 O-glycan branch formation in vivo.19 Such characterization of genetically altered mice has revealed the essential and modulatory role of specific glycosyltransferases and their glycan products in leukocyte-endothelial recognition by the selectins.20 We have furthered this line of investigation by producing mice deficient inST3Gal-II and -III genes using Cre-loxP mutagenesis and comparing selectin ligand formation among these mice as well as those already available lacking ST3Gal-I and ST3Gal-IV.19 21 Our findings show that only ST3Gal-IV of the 4 analyzed plays a substantial role in selectin ligand formation in vivo.
Materials and methods
Tissue Northern
RNA expression levels in various tissues of normal mice were analyzed as previously described,22 using ST3Gal cDNAs I to IV, each containing the entire protein coding sequence.
Gene targeting and mutant mouse production
Genomic clones of the ST3Gal-II andST3Gal-III were isolated from a 129/SvJ phage library (Stratagene, La Jolla, CA), and Cre-loxP gene targeting constructs prepared by described approaches and procedures (Figure 1B).23 Mice bearing mutant genotypes were produced and bred by previously described procedures.22 Genotyping was performed by polymerase chain reaction (PCR) by using oligonucleotide primers: LE-120 (5′-CCCTGTCTGACCTGGAACACAC) and LE-121 (5′-CACTGAGAGCTCTCAGGAGGCTGAG) to detect the 220–base pair (bp) ST3Gal-II wild-type allele, LE-120 and rlox (5′-CTCGAATTGATCCCCGGGTAC) to detect the 170 bp ST3Gal-II Δ allele, LE-110 (5′-CCAGCCAGCAGAGGATCTGATAC) and LE-115 (5′-CGCAGGGGGCGTTTCTAGAC) to detect the 450-bp ST3Gal-III wild type allele, and LE-110 and rlox to detect the 300-bp ST3Gal-III Δ allele.
Hematology
Blood was collected from the tail vein of anesthetized mice into EDTA (ethylenediaminetetraacetic acid) microtubes (Becton Dickinson, Mountain View, CA) and analyzed with a CELL-DYN 3500 calibrated with normal mouse blood. Differential blood counts were also performed on Wright-Giemsa–stained blood smears.
Isolation of peripheral blood leukocytes and flow cytometry
Blood from wild-type or mutant mice was collected as above into lithium heparin microtubes (Becton Dickinson) and diluted 1:1 in phosphate-buffered saline (PBS). An equal volume of 2% dextran T500 (Pharmacia, Uppsala, Sweden) in PBS was added, and the cells were incubated at 37°C for 10 minutes. The upper layer containing peripheral blood leukocytes (PBLs) was removed and washed in PBS. Red blood cells were lysed with PharM lyse (Pharmingen, San Diego, CA), and PBLs were resuspended in PBS containing 0.1% bovine serum albumin (PBS/BSA). Cells were incubated with 0.5 μg/mL Fc block (Pharmingen) prior to antibody, immunoglobulin M (IgM)–chimera, and lectin staining. Ricinus communis agglutinin-I (RCA-I),Erythrina crystagalli lectin (ECA), or Peanut agglutinin (PNA; all from Vector Laboratories, Burlingame, CA) were incubated with PBLs in combination with antibody Gr1 (Pharmingen) for 10 minutes prior to analysis by flow cytometry on a FACScalibur (Becton Dickinson). Mouse P- and E-selectin cDNAs were linked to the CH2, CH3, and CH4 domains of human IgM to construct P- and E-selectin IgM chimeras.9 Supernatants from transfected COS cells were diluted 1:20 for P-selectin IgM or 1:30 for E-selectin IgM chimeras in PBS/BSA. An antihuman IgM fluorescein isothiocyanate (FITC) antibody (Sigma Chemical, St Louis, MO) was added at 1:1000 for 15 minutes, and labeled selectin chimeras were added to PBLs in the presence of Gr1 for 10 minutes prior to flow cytometry. In other experiments, PBLs from wild-type littermates were treated with Arthrobacter ureafaciens neuraminidase (Sigma) in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 140 mM NaCl, pH 7.0, for 1 hour at 37°C prior to incubation with selectin chimeras.
In vitro rolling assay
Monolayers of Chinese hamster ovary (CHO) cells stably transfected with either human P- or E-selectin24 served as the rolling substrate in a parallel plate flow chamber (Glycotech, Rockville, MD). Bone marrow neutrophils, prepared as previously described,24 were introduced into the flow chamber at a concentration of 1 × 106 cells/mL. Wall shear stress was maintained at 1.5 dynes/cm2, and images were obtained with a Nikon Eclipse TE300 inverted microscope (Nikon, Melville, NY). Rolling events, defined as a rolling cell that can be tracked between sequential images separated by a time delay of 2 seconds, were measured and analyzed as described.25
Antibodies and cytokines
The P-selectin monoclonal antibody (mAb) RB40.34 (rat IgG1, 30 μg/mouse)26 was used to block P-selectin–dependent leukocyte adhesion and rolling in vivo. The rat antimouse E-selectin mAb 9A9 (rat IgG1, 30 μg/mouse)27was used to block E-selectin function in vitro and E-selectin–dependent rolling in vivo. For the in vivo model, recombinant murine tumor necrosis factor α (TNF-α) (500 ng/mouse; R&D, Minneapolis, MN) was diluted in 0.3 mL normal saline and injected intrascrotally 2 hour prior to the experiment.
Intravital microscopy and cremaster muscle preparation.
Mice were anesthetized with an intraperitoneal injection of ketamine (125 mg/g body weight, Ketalar; Parke-Davis, Morris Plains, NJ), xylazine (12.5 mg/g body weight; Phoenix Scientific, St Joseph, MO), and atropine sulfate (0.025 mg/g body weight; Elkins-Sinn, Cherry Hill, NJ). Mice were then placed on a heating pad to maintain body temperature. Intravital microscopy experiments were conducted with a microscope (Axioskop; Zeiss, Thornwood, NY) equipped with a saline immersion objective (SW 40/0.75 numerical aperture) and connected to a charged-coupled device (CCD) camera (model VE-1000CD; Dage-MTI, Michigan City, IN) and a video recorder (Panasonic, Secausus, NJ). After tracheal intubation, the left carotid artery was cannulated for systemic administration of anesthetics and mAbs and for taking blood samples during the experiment.
The cremaster muscle was prepared as described earlier28 and superfused with thermocontrolled (35°C) bicarbonate-buffered saline. Systemic blood samples (10 μL) were taken after each mAb injection and stained with Kimura to assess systemic white blood cell counts. Leukocyte rolling was observed in venules with diameters ranging from 20 μm to 45 μm. Microvessel diameters, lengths, and rolling leukocyte velocities were measured by using a digital image processing system.29,30 The number of rolling cells was counted in each 100-μm segment of postcapillary venules. Centerline blood flow velocity was measured by using a dual photodiode and a digital online cross-correlation program (Circusoft Instrumentation, Hockessin, DE) and converted to mean blood flow velocity by multiplying with an empirical factor of 0.625.31 Wall shear rates (γw) were estimated as 2.12 (8 vb/d), where vb is the mean blood flow velocity, d is the diameter of the vessel, and 2.12 is a median empirical correction factor obtained from velocity profiles measured in microvessels in vivo.31 Leukocyte rolling velocities (> 5 leukocytes per venule) were measured as averages over a 2-second time window.
Statistics
Statistical analysis was performed by using Sigma-Stat 2.0 software package (SPSS Science, Chicago, IL). Average vessel diameter, leukocyte rolling, leukocyte rolling velocities, and wall shear rates between groups and treatments were compared with the one-way analysis of variance (ANOVA) on ranks (Kruskal-Wallis) with a multiple pairwise comparison test (Dunn test). Leukocyte rolling between untreated and antibody-treated groups was compared with Studentt test or by the Wilcoxon rank-sum test as appropriate. Statistical significance was set at P < .05, indicated by asterisk (*).
Results
Sialyltransferase tissue distribution and targeted gene disruption
The ST3Gal family of sialyltransferases appears to consist of a total of 6 genes in mammals. All encode type II transmembrane proteins residing in the Golgi apparatus and bearing a common sialylmotif that is essential for donor substrate binding and catalytic activity.32 ST3Gal-I to -IV RNA expression is broadly distributed with variations in levels observed among distinct tissues (Figure 1B). Multiple RNA transcripts are noted in some cases, as has been described.12 Although the patterns of RNA expression were different for each ST3Gal gene studied, all tissues surveyed expressed multiple ST3Gal sialyltransferases.
The production and initial characterization of ST3Gal-I– and ST3Gal-IV–mutant mice has been described.19,21 Both are fertile and without overt developmental and morphologic abnormalities. Herein, we have similarly generated mice lacking functional ST3Gal-II and -III sialyltransferases by Cre-loxP gene targeting to produce deletions of either the large sialylmotif or transmembrane domain, respectively (Figure 2A,B,E,F). The loxP-flanked and deleted alleles of each gene were confirmed by Southern blot analysis of embryonic stem cell DNA (Figure 2C,G). Correctly targeted embryonic stem (ES) cells were used to generate mutant mice bearing the deleted allelic structures (Figure2D,H), as previously described.22 Heterozygous ST3Gal-IIwt/Δ and -IIIwt/Δ mice were bred to the C57BL/6 strain for more than 5 generations prior to crossing to produce homozygotes and littermate controls for studies.
ST3Gal-II and ST3Gal-III mutagenesis.
(A,E) Genomic clones bearing wild type ST3Gal-II and -III allelic structure, respectively, were used to construct targeting constructs using the pflox vector. In the ST3Gal-II, exons containing the large sialylmotif were flanked by loxP sites (ST3Gal-IIF[tkneo]), whereas loxP sites flanked the transmembrane domain in the ST3Gal-III gene (ST3Gal-IIIF[tkneo]). Restriction enzyme sites indicated are BamHI (B), Avr II (A), Cla I (C), EcoRI (E),HindIII (H), Kpn I (K), Nhe I (N), Sal I (Sa), Stu I (St), and XbaI (X). (B,F) Transient Cre expression in ES cells that have undergone homologous recombination with ST3Gal-II or -III targeting vector yielded subclones with a ST3Gal-II or -IIIΔ/Δ (systemic-null) or ST3Gal-II or -IIIF (conditional-null) mutation. (C,G) Southern blot analysis of ES cell DNA probed with a loxP probe confirmed the expected recombined genomic structures. Wild-type RI ES cell DNA did not hybridize with the loxP probe. Three loxP sites were present parental clones of ST3Gal-II and -III (2-3 and 2-6, respectively). One loxP site is present in each of 2 ST3Gal-IIΔ/Δ subclones (2-3B4 and 2-3B5) and ST3Gal-IIIΔ/Δ subclones (2-6A5 and 2-6D5). Two loxP sites are present in the ST3Gal-IIFsubclones (2-3B3 and 2-3B6) and ST3Gal-IIIF subclones (2-6A1 and 2-6A3). (D,H) PCR analyses of tail DNA from offspring of parental mice heterozygous for the ST3Gal-II Δ allele reveals the 230-bp wild-type (wt) fragment and the 190-bp deleted (Δ) fragment. PCR analyses of tail DNA from offspring of parental mice heterozygous for the ST3Gal-III Δ allele indicates the 370-bp wt allele and the 260-bp Δ allele.
ST3Gal-II and ST3Gal-III mutagenesis.
(A,E) Genomic clones bearing wild type ST3Gal-II and -III allelic structure, respectively, were used to construct targeting constructs using the pflox vector. In the ST3Gal-II, exons containing the large sialylmotif were flanked by loxP sites (ST3Gal-IIF[tkneo]), whereas loxP sites flanked the transmembrane domain in the ST3Gal-III gene (ST3Gal-IIIF[tkneo]). Restriction enzyme sites indicated are BamHI (B), Avr II (A), Cla I (C), EcoRI (E),HindIII (H), Kpn I (K), Nhe I (N), Sal I (Sa), Stu I (St), and XbaI (X). (B,F) Transient Cre expression in ES cells that have undergone homologous recombination with ST3Gal-II or -III targeting vector yielded subclones with a ST3Gal-II or -IIIΔ/Δ (systemic-null) or ST3Gal-II or -IIIF (conditional-null) mutation. (C,G) Southern blot analysis of ES cell DNA probed with a loxP probe confirmed the expected recombined genomic structures. Wild-type RI ES cell DNA did not hybridize with the loxP probe. Three loxP sites were present parental clones of ST3Gal-II and -III (2-3 and 2-6, respectively). One loxP site is present in each of 2 ST3Gal-IIΔ/Δ subclones (2-3B4 and 2-3B5) and ST3Gal-IIIΔ/Δ subclones (2-6A5 and 2-6D5). Two loxP sites are present in the ST3Gal-IIFsubclones (2-3B3 and 2-3B6) and ST3Gal-IIIF subclones (2-6A1 and 2-6A3). (D,H) PCR analyses of tail DNA from offspring of parental mice heterozygous for the ST3Gal-II Δ allele reveals the 230-bp wild-type (wt) fragment and the 190-bp deleted (Δ) fragment. PCR analyses of tail DNA from offspring of parental mice heterozygous for the ST3Gal-III Δ allele indicates the 370-bp wt allele and the 260-bp Δ allele.
Sialyltransferase mutations result in increased β-linked galactose exposure on peripheral blood leukocytes
Sialic acids are terminal modifications to β-linked galactose residues present among various glycan classes. Lectins that bind β-linked galactose were used to assess the loss of ST3Gal function among myeloid and lymphoid cell types. These studies revealed differential increases in the exposure of β-linked galactose on specific peripheral blood leukocytes among all 4 homozygous mutant genotypes (Figure 3A). Binding of the RCA-I lectin, which has a preference for unsialylated terminal galactose on type II and type III glycans,33 was increased on the surface of neutrophils among mice homozygous for deletions in the genes encoding ST3Gal-I, -II and, -IV. Increased binding to ECA lectin, which is specific for unsialylated type II chains,34 occurred to a substantial extent only among cells homozygous for the ST3Gal-IV deletion. PNA lectin binding, which primarily discriminates between sialylated and unsialylated type III glycans,35 revealed an increase in unsialylated Galβ1-3GalNAc- among neutrophils from mice homozygous for deletions in ST3Gal-I, ST3Gal-II, and ST3Gal-IV and CD8+ T cells from mice homozygous for deletions in ST3Gal-I and ST3Gal-IV. PNA can also recognize unsialylated type I chains to some extent,36 and the increase in binding to CD8+ T cells from mice homozygous for the ST3Gal-III deletion may reflect this additional binding specificity. Neutrophils from ST3Gal-III–mutant mice did not show any binding changes with the use of RCA-I, ECA, or PNA lectins, suggesting the possibility that ST3Gal-III is not expressed in Gr1+ cells, or perhaps that other sialyltransferases may fully compensate for ST3Gal-III deficiency. In addition to providing data on the cell types in which these ST3Gal sialyltransferases operate in vivo, the results obtained reflect closely the described substrate specificity and preferences from in vitro enzymatic studies that have been described.12
ST3Gal I-IV sialyltransferase deficiencies result in differential degrees of exposed galactose and selectin–immunoglobulin chimera binding to blood neutrophils.
(A) RCA-I, ECA, and PNA lectin binding to circulating Gr1+cells (mostly neutrophils) or CD8+ T cells was assessed by flow cytometry. Reduced sialylation resulting in exposed β-linked galactose was observed differentially among leukocytes and specific sialyltransferase mutations. (B) P- and E-selectin–immunoglobulin chimera binding to circulating Gr1+ leukocytes of mice deficient in ST3Gal-I, -II, -III, and -IV was analyzed by flow cytometry and compared with C2GlcNAcT-I deficiency. Panels A and B are representative of 3 separate experiments. Filled histograms represent selectin binding to neutrophils of mutant mice and are compared with wild-type littermates in the same panel.
ST3Gal I-IV sialyltransferase deficiencies result in differential degrees of exposed galactose and selectin–immunoglobulin chimera binding to blood neutrophils.
(A) RCA-I, ECA, and PNA lectin binding to circulating Gr1+cells (mostly neutrophils) or CD8+ T cells was assessed by flow cytometry. Reduced sialylation resulting in exposed β-linked galactose was observed differentially among leukocytes and specific sialyltransferase mutations. (B) P- and E-selectin–immunoglobulin chimera binding to circulating Gr1+ leukocytes of mice deficient in ST3Gal-I, -II, -III, and -IV was analyzed by flow cytometry and compared with C2GlcNAcT-I deficiency. Panels A and B are representative of 3 separate experiments. Filled histograms represent selectin binding to neutrophils of mutant mice and are compared with wild-type littermates in the same panel.
Hematologic findings in ST3Gal deficiencies
Hematologic analyses of all 4 mutations bred to homozygosity revealed erythroid profiles within normal limits, as compared with wild type littermates (Table 1). Although the numbers of lymphocytes in circulation were also normal, we noted a consistent increase in circulating monocytes and a likely decrease in eosinophils in ST3Gal-I–deficient mice. However, none of the ST3Gal deficiencies resulted in leukocytosis. Interestingly, both ST3Gal-I and ST3Gal-IV deficiencies resulted in thrombocytopenia. In the absence of ST3Gal-IV, exposure of galactose occurs in a manner among some plasma constituents that triggers asialoglycoprotein receptor (ASGPR) clearance mechanisms and thereby reduces levels of von Willebrand factor and platelets in circulation.21
Peripheral blood hematology
| . | Wild type . | ST3Gal-I′ . | ST3Gal-II′ . | ST3Gal-III′ . | ST3Gal-IV′ . |
|---|---|---|---|---|---|
| WBC (cells/μL) | 6263 ± 2646 | 6510 ± 2217 | 5027 ± 1600 | 6993 ± 1644 | 5454 ± 2732 |
| Neutrophils (%) | 15 ± 10 | 24 ± 14 | 12 ± 6 | 9 ± 4 | 18 ± 16 |
| Lymphocytes (%) | 79 ± 12 | 60 ± 20 | 81 ± 8 | 85 ± 5 | 74 ± 17 |
| Monocytes (%) | 3.9 ± 3.4 | 13.9 ± 8.3* | 3.2 ± 2.1 | 3.1 ± 2.6 | 5.8 ± 3.9 |
| Eosinophils (%) | 1.3 ± 1.9 | 0.3 ± 0.3 | 1.1 ± 1.7 | 1.6 ± 1.6 | 1.3 ± 1.4 |
| Basophils (%) | 1.2 ± 1.6 | 2.3 ± 2.1 | 2.8 ± 1.9 | 1.5 ± 1.5 | 1.0 ± 2.2 |
| RBC (× 106/mL) | 8.9 ± 0.5 | 8.4 ± 1.7 | 8.9 ± 0.6 | 8.5 ± 0.6 | 8.5 ± 0.6 |
| PLT (× 103/mL) | 1021 ± 281 | 406 ± 191* | 893 ± 300 | 1000 ± 192 | 363 ± 182* |
| MPV (fL) | 5.7 ± 1.4 | 11 ± 2.3* | 6.9 ± 0.4 | 6.9 ± 0.4 | 8.4 ± 2.5* |
| Sample size, n | 64 | 14 | 10 | 19 | 45 |
| . | Wild type . | ST3Gal-I′ . | ST3Gal-II′ . | ST3Gal-III′ . | ST3Gal-IV′ . |
|---|---|---|---|---|---|
| WBC (cells/μL) | 6263 ± 2646 | 6510 ± 2217 | 5027 ± 1600 | 6993 ± 1644 | 5454 ± 2732 |
| Neutrophils (%) | 15 ± 10 | 24 ± 14 | 12 ± 6 | 9 ± 4 | 18 ± 16 |
| Lymphocytes (%) | 79 ± 12 | 60 ± 20 | 81 ± 8 | 85 ± 5 | 74 ± 17 |
| Monocytes (%) | 3.9 ± 3.4 | 13.9 ± 8.3* | 3.2 ± 2.1 | 3.1 ± 2.6 | 5.8 ± 3.9 |
| Eosinophils (%) | 1.3 ± 1.9 | 0.3 ± 0.3 | 1.1 ± 1.7 | 1.6 ± 1.6 | 1.3 ± 1.4 |
| Basophils (%) | 1.2 ± 1.6 | 2.3 ± 2.1 | 2.8 ± 1.9 | 1.5 ± 1.5 | 1.0 ± 2.2 |
| RBC (× 106/mL) | 8.9 ± 0.5 | 8.4 ± 1.7 | 8.9 ± 0.6 | 8.5 ± 0.6 | 8.5 ± 0.6 |
| PLT (× 103/mL) | 1021 ± 281 | 406 ± 191* | 893 ± 300 | 1000 ± 192 | 363 ± 182* |
| MPV (fL) | 5.7 ± 1.4 | 11 ± 2.3* | 6.9 ± 0.4 | 6.9 ± 0.4 | 8.4 ± 2.5* |
| Sample size, n | 64 | 14 | 10 | 19 | 45 |
Hematology values are presented as means ± SD. WBC, white blood cells; RBC, red blood cells; PLT, platelet; MPV, mean platelet volume. Significant differences between appropriate wild-type or heterozygous controls and/genotypes are indicated. (*)P < .01. Wild-type values are pooled as littermates of all mutant genotypes.
P- and E-selectin ligand deficiency on Gr1+ leukocytes from ST3Gal-IV–mutant mice
The binding of selectin chimera immunoglobulin (IgM) Fc fusion proteins was used in flow cytometric analyses as a measure of selectin ligand levels on the cell surface.9 When compared with Gr1+ cells, which are primarily mature neutrophils, from wild-type littermates, P-selectin—immunoglobulin chimera binding was reduced by approximately 50% among ST3Gal-IV–deficient samples with mean peak fluorescence intensity measurements (MFIs) of 207 and 97, respectively (Figure 3B). A slightly greater reduction in E-selectin binding to approximately 40% of control levels was found (MFI, 96 and 37, respectively). However, neither of these reductions in E- and P-selectin ligand levels were as great as those observed in the absence of the glycosyltransferase C2GlcNAcT-I11 (Figure 3B). Interestingly, a slight but reproducible increase of approximately 20% in P-selectin chimera binding was observed in ST3Gal-I–deficient mice. In contrast to these changes, no alterations in selectin ligand levels were observed among Gr1+ cells from ST3Gal-II– and ST3Gal-III–deficient mice.
Multiple sialyltransferases in E- and P-selectin ligand formation
The possibility that only ST3Gal-IV contributes to E- and P-selectin ligand formation was investigated in vitro by using neuraminidase (sialidase) treatment of intact cells, followed by flow cytometry as described above. Sialidase treatment of ST3Gal-IV–deficient Gr1+ cells further reduced P- and E-selectin–immunoglobulin chimera binding, suggesting that other sialyltransferases, in addition to ST3Gal-IV, are also involved in E- and P-selectin ligand formation (Figure4).
Sialidase treatment and compound ST3Gal deficiencies implicate multiple sialyltransferases in the formation of selectin ligands in vivo.
E- and P-selectin–immunoglobulin chimera binding was assessed following treatment of peripheral blood Gr1+ cells from ST3Gal-IV–deficient mice with Arthrobacter ureafaciensneuraminidase.
Sialidase treatment and compound ST3Gal deficiencies implicate multiple sialyltransferases in the formation of selectin ligands in vivo.
E- and P-selectin–immunoglobulin chimera binding was assessed following treatment of peripheral blood Gr1+ cells from ST3Gal-IV–deficient mice with Arthrobacter ureafaciensneuraminidase.
Reduced rolling of ST3Gal-IVΔ/Δ neutrophils on P- and E-selectin
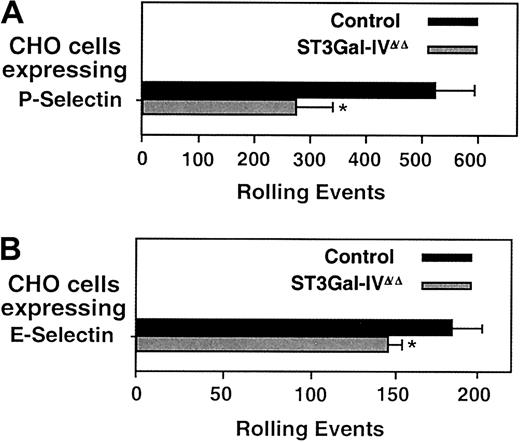
To examine the biologic effect of reduced selectin ligands on ST3Gal-IVΔ/Δ neutrophils, we used a parallel plate attachment and rolling assay, as previously described in studies of mice with C2GlcNAcT-I deficiency.24 Transfected CHO cells used as a monolayer in this assay express E-selectin at levels approximating those found on human umbilical vein endothelial cells activated by TNF, whereas P-selectin–expressing CHO cells express levels 2 to 3 times above that.24 The assay was carried out in a flow chamber with a wall shear stress maintained at 1.5 dynes/cm2 as previously described.25Consistent with a partial effect on P-selectin ligands measured by flow cytometry, bone marrow–derived ST3Gal-IV–deficient neutrophils showed a 50% ± 16% reduction in rolling on P-selectin expressed by CHO cells (Figure 5A). ST3Gal-IVΔ/Δ neutrophils showed a 22% ± 9% reduction in rolling on E-selectin in this assay (Figure 5B), also consistent with the flow cytometry assay.
Leukocyte rolling in vitro.
(A,B) Rolling of purified bone marrow neutrophils on CHO cells expressing either P-selectin or E-selectin at 1.5 dynes/cm2. Data are presented as the means ± SEM of total rolling events and are derived from 8 independent experiments. * indicates significant difference; P < .05.
Leukocyte rolling in vitro.
(A,B) Rolling of purified bone marrow neutrophils on CHO cells expressing either P-selectin or E-selectin at 1.5 dynes/cm2. Data are presented as the means ± SEM of total rolling events and are derived from 8 independent experiments. * indicates significant difference; P < .05.
Leukocyte rolling in vivo is impaired in ST3Gal-IV–deficient mice
Intrascrotal injection of TNF-α leads to the expression of E-selectin and enhances the expression of P-selectin on venular endothelial cells of the cremaster muscle.37 To compare in vivo rolling with the above results from the flow chamber and cytometry experiments, we analyzed leukocyte rolling in the TNF-α–pretreated cremaster muscle. We studied leukocyte rolling in 23 venules of 8 TNF-α–treated mice deficient in ST3Gal-IV and compared the results with rolling in 19 venules of 5 littermate control mice. Hemodynamic and microvascular parameters for both groups indicate similar vessel diameters, centerline velocities, and wall shear rates (Table2). The results indicated a reduction in the number of rolling cells per 100-μm vessel length in ST3Gal-IV–deficient mice compared with wild-type littermates when treated with the blocking P-selectin mAb RB40.34 (Figure6A). The reduction involving E-selectin–mediated rolling in ST3Gal-IVΔ/Δ mice was consistent with the reduction in E-selectin–mediated rolling of ST3Gal-IVΔ/Δ leukocytes in the flow chamber (Figure 5). In contrast, blocking E-selectin with mAb 9A9 in vivo, which leads to P-selectin–mediated rolling, revealed a similar number of rolling cells per vessel length in both groups (Figure 6A).
Hemodynamic and microvascular parameters in TNF-α–induced inflammation
| Mouse genotype . | Mice (n) . | Venules (n) . | Diameter (μm) . | Centerline velocity (μm/s) . | Wall shear rate (s−1) . |
|---|---|---|---|---|---|
| ST3Gal-IV′ | 8 | 23 | 33 ± 1 | 2800 ± 300 | 880 ± 80 |
| Control | 5 | 19 | 37 ± 2 | 2900 ± 300 | 840 ± 100 |
| Mouse genotype . | Mice (n) . | Venules (n) . | Diameter (μm) . | Centerline velocity (μm/s) . | Wall shear rate (s−1) . |
|---|---|---|---|---|---|
| ST3Gal-IV′ | 8 | 23 | 33 ± 1 | 2800 ± 300 | 880 ± 80 |
| Control | 5 | 19 | 37 ± 2 | 2900 ± 300 | 840 ± 100 |
Venule diameters, centerline velocity, and wall shear rate presented as mean ± SEM.
Altered in vivo leukocyte rolling in ST3Gal-IVΔ/Δ mice during TNF-α–induced vascular inflammation.
(A) Leukocyte rolling per 100 μm vessel segment length was assessed in ST3Gal-IVΔ/Δ mice (▪) and control mice (░) treated with either P-selectin blocking mAb RB40.34 or E-selectin blocking mAb 9A9. Data are presented as the mean ± SEM. * indicates significant difference; P < .05. (B-D) Cumulative velocity distribution for ST3Gal-IVΔ/Δ mice (solid line) and wild-type littermates (dotted line) with (B) no treatment, (C) P-selectin blocking mAb RB40.34, and (D) E-selectin blocking mAb 9A9. Significant differences in leukocyte velocity (*P < .05) between ST3Gal-IVΔ/Δ mice and wild-type mice were observed for anti–P-selectin–treated mice and mice without antibody treatment, indicating an E-selectin ligand defect.
Altered in vivo leukocyte rolling in ST3Gal-IVΔ/Δ mice during TNF-α–induced vascular inflammation.
(A) Leukocyte rolling per 100 μm vessel segment length was assessed in ST3Gal-IVΔ/Δ mice (▪) and control mice (░) treated with either P-selectin blocking mAb RB40.34 or E-selectin blocking mAb 9A9. Data are presented as the mean ± SEM. * indicates significant difference; P < .05. (B-D) Cumulative velocity distribution for ST3Gal-IVΔ/Δ mice (solid line) and wild-type littermates (dotted line) with (B) no treatment, (C) P-selectin blocking mAb RB40.34, and (D) E-selectin blocking mAb 9A9. Significant differences in leukocyte velocity (*P < .05) between ST3Gal-IVΔ/Δ mice and wild-type mice were observed for anti–P-selectin–treated mice and mice without antibody treatment, indicating an E-selectin ligand defect.
Leukocyte rolling velocities in TNF-α–treated cremaster muscle venules were next investigated. TNF-α–treated ST3Gal-IVΔ/Δ mice showed substantially higher rolling velocities (Vavg 13 ± 1 μm/s) than control mice (Vavg 10 ± 1 μm/s; Figure 6B). E-selectin–mediated rolling was measured after treatment with the P-selectin–blocking mAb RB40.34 and found to be faster in ST3Gal-IV–deficient mice (Vavg 9 ± 1 μm/s) than in control mice (Vavg 6 ± 1 μm/s; Figure 6C). In contrast, there was no difference in rolling velocity in P-selectin–dependent rolling after injection of the E-selectin–blocking antibody 9A9 (Figure 6D), suggesting that sialylation by ST3Gal-IV contributes to characteristic slow rolling mediated by E-selectin.
Discussion
Recognition of selectin ligands by leukocytes and endothelial cells of the vasculature forms the basis for a substantial component of the inflammatory response and contributes to leukocyte homeostasis. Although single genes exist to produce each of the selectins, the formation of selectin ligands requires the orchestrated action of many distinct glycosyltransferase genes that encode enzymes operating in the secretory pathway, primarily within the Golgi apparatus. Changes in the normal expression profile of glycosyltransferases in various cell types can alter glycan branching and influence terminal modifications in the Golgi, thereby providing multiple regulatory points in selectin ligand formation that can act to partition the physiologic activities of selectins.11,38 Among the 19 sialyltransferase genes found in the mammalian genome to date, 6 are ST3Gal sialyltransferases that may operate either singly or in combination in producing selectin ligands.12,39-41 We have rendered mice deficient in 4 of these 6 candidates: ST3Gal-I, ST3Gal-II, ST3Gal-III, and ST3Gal-IV, and have analyzed the relative contribution of each to selectin ligand formation among neutrophils. All ST3Gal deficiencies studied result in exposure of galactose termini; however, each ST3Gal operated to a different degree among leukocyte cell types studied. None of the ST3Gal deficiencies caused leukocytosis, which is a phenotype found in glycosyltransferase–mutant mice with severe deficiencies of selectin ligands.9,11 42
We have noted a concurrence of our in vivo findings with data acquired from in vitro enzymatic analyses that proposes ST3Gal-I predominantly sialylates type III glycan chain termini (Galβ1-3GalNAc-), provoking a dramatic increase in PNA binding to ST3Gal-I–deficient leukocytes.12,19 Because sLex is constructed with type II glycan chains (Galβ1-4GlcNAc-), it was considered unlikely that ST3Gal-I would be directly involved in selectin ligand formation. Nevertheless, ST3Gal-I expression has been found to effectively compete with the action of C2GlcNAcT-I for the same substrate in vivo. Thus, ST3Gal-I deficiency might lead to increased selectin ligand formation by increasing the availability of core 2O-glycans that bear type II glycan termini.19 43 Our findings indeed indicate that ST3Gal-I deficiency results in an increase in P-selectin ligand formation by approximately 20% on neutrophils. Moreover, levels of core 2O-glycans recognized by antibody 1B11 are also increased (data not shown).
ST3Gal-II deficiency results in increased PNA binding to peripheral blood neutrophils, as well as increased binding of RCA and to a lessor extent ECA. Unlike ST3Gal-I, no change in PNA binding to CD8+ T cells was observed. ST3Gal-II prefers glycolipid substrates, and, although studies have indicated that selectins can recognize glycolipids,44 no evidence of a deficiency in selectin ligands on neutrophils was found in the absence of ST3Gal-II. These findings do not preclude the possibility that glycolipids bear selectin ligands, and perhaps in some cases among tumor cells.45
ST3Gal-III levels have been associated with the formation of sLex in lung carcinoma.16 However, ST3Gal-III prefers to sialylate type I (Galβ1-3GlcNAc) glycan chains. Moreover, we did not observe any decrease in selectin ligands among ST3Gal-III–deficient neutrophils. We did observe increased binding of PNA on ST3Gal-III–deficient CD8+ T cells, consistent with a defect in sialylation due to ST3Gal-III mutagenesis. The unsialylated type 1 chain can be recognized to some extent by PNA.36Therefore, the increase in PNA reactivity observed among ST3Gal-III–deficient CD8+ T cells is distinct from the undersialylation that occurs with ST3Gal-I deficiency that involves type III glycan chains and that leads to a defect in CD8+T-cell homeostasis.19 No defect in CD8+ T-cell homeostasis was observed in ST3Gal-III–deficient mice. Although we find that ST3Gal-III is also not essential for synthesis of P- and E-selectin ligands on peripheral blood neutrophils in normal circumstances, this sialyltransferase may perhaps participate in selectin ligand formation in other cell types and in tumorigenic contexts.
ST3Gal-IV deficiency was unique among the 4 ST3Gal sialyltransferase mutations studied by substantially reducing the formation of selectin ligands on circulating neutrophils. However, this reduction was only partial when compared with C2GlcNAcT-I–deficient neutrophils. In addition, a further reduction in selectin binding to ST3Gal-IV–deficient neutrophils was noted following neuraminidase treatment in vitro. This finding indicates the likelihood that other sialyltransferases are involved in selectin ligand formation in vivo. It is also possible, although perhaps unlikely, that this finding reflects conformational alterations in glycoproteins occurring on de-sialylation that alter E- and P-selectin–IgM chimera binding independent of the role of α2-3 sialic acid in selectin ligand formation.
We have investigated the role of ST3Gal sialyltransferases in selectin ligand formation by first applying flow cytometry as a screen to detect changes in selectin ligand expression levels. This screen has been found to be a valuable initial approach as flow cytometric findings of decreased selectin ligands are found associated with defects in neutrophil rolling in vitro on synthetic and cell-based selectin substrates.9 11 However, neutrophil rolling and recruitment in vitro and in vivo can provide functional data regarding alterations observed in selectin ligand expression. We observed a substantial decrement in rolling on cell monolayers bearing P-selectin, as well as E-selectin, using bone marrow–derived neutrophils. These findings are similar in scope to the flow cytometric results and indicate a functional role for ST3Gal-IV in selectin ligand formation.
The number of neutrophils rolling per length of inflamed vascular endothelium following TNF-α treatment in vivo revealed data that corroborated the in vitro evidence for an effect on E-selectin ligands but contradicted findings suggesting P-selectin ligand deficiencies. The effect of ST3Gal-IV on E-selectin–mediated leukocyte interactions was also remarkably similar to that reported for FucT-IV deficiency.46 The leukocyte rolling flux fraction, a measure of the net balance between leukocyte attachment to the endothelium (increasing leukocyte rolling flux fraction) and firm leukocyte adhesion resulting in transmigration (removing leukocytes from the rolling pool), was normal in mice deficient in both FucT-IV46 and ST3Gal-IV (data not shown). However, an increase in E-selectin–dependent rolling velocity is observed in both strains, suggesting that these 2 glycosyltransferases may collaborate to form E-selectin ligands important in slow rolling of leukocytes. A role for ST3Gal-IV in P-selectin ligand function in vivo was not evident. The different findings regarding P-selectin interactions between the in vitro and in vivo rolling assays are not fully resolved at this time; however, several possible explanations exist. Because subtle differences exist in P-selectin glycoprotein ligand 1 (PSGL-1) glycosylation between mouse and humans, the human P-selectin used in the in vitro rolling assay may recognize slightly different glycosylation patterns on mouse PSGL-1, resulting in reduced binding efficiency in the absence of ST3Gal-IV. Nevertheless, the selectin-immunoglobulin chimeras used in cytometric analysis were of mouse origin, and these reagents also revealed a decrement in P-selectin ligand formation. Another possible explanation involves the fact that leukocytes in the in vitro flow chamber are subjected to lower shear stresses than leukocytes in vivo, which may be important in altering the availability and conformation of molecules at the cell surface. In addition, the differences observed may be due to the more complex and dynamic molecular interactions in vivo that include contributions to neutrophil rolling by leukocyte factor antigen-1 (LFA-1) and αMβ2 integrin (Mac-1).47
The initial step of leukocyte tethering to the endothelium during inflammation is largely dependent on P-selectin interactions. No effect of ST3Gal-IV deficiency was found on E- or P-selectin ligands in this process. Because PSGL-1 is the major ligand for P-selectin and in vivo rolling is markedly reduced in PSGL-1–deficient mice,48these data suggest that ST3Gal-IV does not contribute to functional selectin ligands on PSGL-1 in vivo. In contrast, we found that E-selectin–dependent leukocyte rolling velocity was increased in ST3Gal-IVΔ/Δ mice. Previous studies have reported a requirement for E-selectin in slow leukocyte rolling on the vessel wall in the TNF-α–treated cremaster model of inflammation.28Our findings reveal an important role for ST3Gal-IV in forming E-selectin ligands necessary for reducing the rolling velocity of circulating leukocytes as they enter sites of inflammation. Such ligands have not been found on PSGL-1.48
Other sialyltransferase mutations not yet produced or examined may also be informative in resolving the degree of contribution to selectin function by sialic acid linkages. Of the 6 ST3Gal sialyltransferases identified thus far, and the 2 remaining to be analyzed in this manner for selectin ligand formation, ST3Gal-V bears a strong glycolipid substrate preference, like ST3Gal-II, but specifically generates the ganglioside GM3.13 In contrast, ST3Gal-VI is similar to ST3Gal-IV with specificity for type II glycan chains.14 We would therefore hypothesize that ST3Gal-VI will be found to play a substantial role in selectin ligand formation in vivo.
Selectin expression and selectin ligand formation provide multiple points of regulation pertaining to cell type communication during leukocyte homeostasis and innate immune responses. Distinct physiologic outcomes emerge from the characterization of mice inheriting genetic deficiencies of various selectins and glycosyltransferases operating in selectin ligand formation, including FucT-VII, C2GlcNAcT-I, and FucT-IV.5,9-11,25 48-50 We have herein provided evidence of a functional segregation involving ST3Gal sialyltransferase activity in the formation of selectin ligands in vivo. Among ST3Gal-I, -II, -III, and -IV sialyltransferases, only ST3Gal-IV provides a substantial degree of selectin ligand formation in vivo. Our data suggest that ST3Gal-IV contributes to the characteristic slow rolling velocity observed for E-selectin–mediated rolling during inflammation without substantially affecting E-selectin–mediated capturing of leukocytes. These findings reveal a substantial degree of specificity among ST3Gal sialyltransferases in vivo in the formation of selectin ligands on neutrophils.
We thank John Lowe for providing the selectin chimera supernatants, Dietmar Vestweber for providing monoclonal antibody RB40.34, and Barry Wolitzky for providing monoclonal antibody 9A9.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-04-1007.
Supported by the National Institutes of Health program project grant PO1-HL57345 (J.D.M.), HL58710 (G.S.K.), HL54136 (K.L.), and F32CA79130 (L.G.E.). M. S. is supported by a stipend from the German Research Foundation (DFG) SP 621/1-1. J.D.M. acknowledges support as an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jamey D. Marth, Howard Hughes Medical Institute, 9500 Gilman Dr 0625, CMM-W Building, Room 333, University of California San Diego, La Jolla, CA 92093; e-mail:jmarth@ucsd.edu.

![Fig. 2. ST3Gal-II and ST3Gal-III mutagenesis. / (A,E) Genomic clones bearing wild type ST3Gal-II and -III allelic structure, respectively, were used to construct targeting constructs using the pflox vector. In the ST3Gal-II, exons containing the large sialylmotif were flanked by loxP sites (ST3Gal-IIF[tkneo]), whereas loxP sites flanked the transmembrane domain in the ST3Gal-III gene (ST3Gal-IIIF[tkneo]). Restriction enzyme sites indicated are BamHI (B), Avr II (A), Cla I (C), EcoRI (E),HindIII (H), Kpn I (K), Nhe I (N), Sal I (Sa), Stu I (St), and XbaI (X). (B,F) Transient Cre expression in ES cells that have undergone homologous recombination with ST3Gal-II or -III targeting vector yielded subclones with a ST3Gal-II or -IIIΔ/Δ (systemic-null) or ST3Gal-II or -IIIF (conditional-null) mutation. (C,G) Southern blot analysis of ES cell DNA probed with a loxP probe confirmed the expected recombined genomic structures. Wild-type RI ES cell DNA did not hybridize with the loxP probe. Three loxP sites were present parental clones of ST3Gal-II and -III (2-3 and 2-6, respectively). One loxP site is present in each of 2 ST3Gal-IIΔ/Δ subclones (2-3B4 and 2-3B5) and ST3Gal-IIIΔ/Δ subclones (2-6A5 and 2-6D5). Two loxP sites are present in the ST3Gal-IIFsubclones (2-3B3 and 2-3B6) and ST3Gal-IIIF subclones (2-6A1 and 2-6A3). (D,H) PCR analyses of tail DNA from offspring of parental mice heterozygous for the ST3Gal-II Δ allele reveals the 230-bp wild-type (wt) fragment and the 190-bp deleted (Δ) fragment. PCR analyses of tail DNA from offspring of parental mice heterozygous for the ST3Gal-III Δ allele indicates the 370-bp wt allele and the 260-bp Δ allele.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood-2002-04-1007/4/m_h82223420002.jpeg?Expires=1764020087&Signature=HCtql49r4Ws2oGERSqNjkH7GUhYS32h2dgDrCNUAu853kcxN5ZmSkzR156WKmYK7S83DXyAE-bQcbcwyZIbdWLvV5g3L-Mxq07D9~9EkVXbWNojgeDhQ20r5G1z7tsmylbg4gGFYNnMF~d-ZbrRVEFeoppfQI1JGsI2oMS1zU0v-PZspJpNCFnpKyFDhcuot7CUOPKN1ZG2SPhxrW7NY~U8C95H2raWqFkrHLrZdaqUR7VbGTh2vQpEqOLaUcvHehjHqX4su2O2mT1IpDJqJQRKPJn-1a1D6bEOgIYbL0n9XMn4-jL4JgPd~L8dUK3~oBigZFiMQjxQC7Mna5IFlAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal