GATA-2 is considered to be essential for the development, maintenance, and function of hematopoietic stem cells (HSCs). However, it was also reported that GATA-2 inhibits the growth of HSCs. To examine the role of GATA-2 in the growth of hematopoietic cells, we introduced an estradiol-inducible form of GATA-2 (GATA-2/estrogen receptor [ER]) into interleukin 3 (IL-3)–dependent cell lines, Ba/F3, 32D, and FDC-P1. Estradiol-induced GATA-2 suppressed c-myc mRNA expression and inhibited IL-3–dependent growth in these clones. As for this mechanism, GATA-2 was found to inhibit ubiquitin/proteasome-dependent degradation of p21WAF1 and p27Kip1 and to induce their accumulation by repressing the expression of Skp2 and Cul1, both of which are components of the ubiquitin ligase for p21WAF1 and p27Kip1. Overexpression of c-myc restored the expression of Skp2 and Cul1 mRNA, reduced the amounts of p21WAF1 and p27Kip1 proteins, and canceled GATA-2–induced growth suppression, suggesting that down-regulation of c-mycexpression may be primarily responsible for GATA-2–induced growth suppression. Next, we transduced retrovirus containing GATA-2/ER into murine bone marrow mononuclear cells (MNCs) and stem/progenitor (Sca-1+Lin−) cells. GATA-2/ER suppressed cytokine-dependent growth of MNCs and Sca-1+Lin− cells by about 70%, which was also accompanied by the reduced expression of c-myc, Skp2, and Cul1 mRNA and the accumulation of p21WAF1 and p27Kip1 proteins. In addition, the amount of GATA-2 protein was found to decline in hematopoietic stem/progenitor cells that were promoted to enter cell cycle by the stimulation with cytokines. These results suggest that GATA-2 may regulate expression levels of p21WAF1 and p27Kip1, thereby contributing to the quiescence of hematopoietic stem/progenitor cells.
Introduction
GATA family transcription factors are composed of 6 members (GATA-1 through GATA-6) and play essential roles in the development of diverse cell types according to their unique tissue distribution (for a review, see Weiss and Orkin1). These factors bind to a GATA motif (A/T)GATA(A/G), in the regulatory region of their target genes through 2 highly conserved zinc finger domains. The carboxyl (C) finger is absolutely required for the DNA binding, whereas the amino (N) finger stabilizes the DNA binding and confers full specificity.2,3 Among GATA family members, at least 3 GATA factors (GATA-1, GATA-2, and GATA-3) are critically involved in different aspects of hematopoiesis. GATA-1 is highly expressed in erythroid cells, mast cells, and megakaryocytes.4-7 GATA-1 is essential for primitive and definitive erythropoiesis, because targeted disruption of GATA-1 gene in mice causes fatal embryonic anemia due to a block in erythroid maturation and apoptosis.8-10 Furthermore, Shivdasani et al11 showed that GATA-1 is also required for megakaryopoiesis by generating lineage-selective GATA-1 knockout mice. GATA-3 is exclusively expressed in T lymphocytes and indispensable for the development of T lymphocytes.12,13 These results suggest that both GATA-1 and GATA-3 are essential for the lineage commitment or the subsequent maturation of committed hematopoietic progenitor cells. In contrast, GATA-2 is highly expressed in pluripotent hematopoietic stem cells (HSCs),14 whereas it is also expressed in early stages of erythroid cells, mast cells, and megakaryocytes.15-18 GATA-2 knockout mice die around embryonic day 9.5 to 10.5 due to a pan–hematopoietic deficit, suggesting that GATA-2 would be a key regulator of development, maintenance, or function of HSCs.19 Furthermore, Tsai and Orkin20 showed that GATA-2 was necessary for proliferation and survival of early hematopoietic cells by analyzing GATA-2−/− embryonic stem cells in vitro. In contrast, it was also reported that retrovirus-transferred GATA-2 inhibited the growth of normal hematopoietic progenitor cells.21Moreover, an estrogen-inducible form of GATA-2, GATA-2/estrogen receptor (ER) suppressed cytokine-dependent growth of normal hematopoietic progenitor cells in response to estradiol.22Therefore, the function of GATA-2 in HSCs, especially in terms of growth control, remains largely unknown.
Cell growth is tightly regulated by cell cycle regulatory molecules, including cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors (CKIs).23 Cell cycle progression from G1 to S phase is regulated by CDK4, CDK6, and CDK2. The catalytic activities of CDK4 and CDK6 are up-regulated in the mid G1 to late G1 phases dependent on the formation of the complexes with D-type cyclins, whereas they are also negatively regulated by INK4 family of CKIs included in these complexes (p16INK4A, p15INK4B, p18INK4C, and p19INK4D). CDK2 activities are induced at late G1 phase through its interaction with cyclin D or cyclin E and are suppressed by KIP family of CKIs (p21WAF1, p27Kip1, and p57Kip2). At early G1 phase, a dephosphorylated form of retinoblastoma protein (Rb) binds to E2F-1, thereby inhibiting transcriptional activities of E2F-1. From the mid G1 to late G1 phase, Rb is sequentially phosphorylated by CDK4, CDK6, or CDK2, and phosphorylated Rb, in turn, releases E2F-1, which initiates transcription of its target genes such as cyclin D, cyclin E, cyclin A, c-myc, c-myb, and DNA polymerase that are required for G1/S transition or DNA synthesis. Expression levels of these cell cycle regulatory molecules are regulated at both transcriptional and posttranscriptional levels, and the ubiquitin (Ub)/proteasome system plays central roles in the later step.24 25 Ub/proteasome-mediated proteolysis is executed through a series of enzymatic reactions. First, Ub is charged with adenosine triphosphate (ATP) by a Ub-activating enzyme (E1), and then transferred to the substrate by a Ub-conjugating enzyme (E2). An element within the target protein termed a degron is recognized by E2 either alone or in combination with a Ub ligase (E3). After recognition of the degron, the Ub target protein conjugates are formed via an isopeptide bond between the carboxyl-terminal glysine of Ub and a lysine residue on the target protein. Repeated rounds of ubiquitination results in the highly ubiquitinated target protein, which is recognized and degraded by 26S proteasome. At present, a number of cell cycle regulatory molecules such as E2Fs, cyclin D1, cyclin E, cyclin A, cyclin B, CDC25B, p21WAF1, p27Kip1, and p53 are known to be the targets of the Ub/proteasome system.
Pluripotent HSCs are characterized by their abilities for self-renewal and multipotentiality.26,27 By using these abilities, HSCs maintain their own population and keep supplying mature blood cells in all lineages throughout the life period. Most of HSCs are kept dormant under physiologically normal conditions, whereas these cells show dramatic proliferative activities in response to hematopoietic injury such as the treatment with myelotoxic reagents and irradiation. In a previous study, Cheng et al28 reported that p21WAF1 was required to keep HSCs in quiescence and to prevent their exhaustion, indicating that cell cycle control would affect biologic properties and fate of HSCs profoundly. However, the precise mechanism governing the cell cycle in HSCs still remains to be determined.
In an attempt to clarify the roles of GATA-2 in the growth regulation of HSCs, in this study we examined the effects of GATA-2 on the expression and the function of cell cycle regulatory molecules. We found here that enforced expression of GATA-2 inhibited cytokine-dependent growth of normal hematopoietic stem/progenitor cells, which was linked with the suppression of c-myc mRNA expression and the accumulation of p21WAF1 and p27Kip1 proteins. As for this mechanism, GATA-2 was found to inhibit c-myc–dependent expressions of Skp2 and Cul1, which act as components of E3 toward p21WAF1 and p27Kip1 and participate in their degradation. In addition, we found that the expression of GATA-2 protein was reduced in hematopoietic stem/progenitor cells that were enforced to enter cell cycle by the cytokine stimulation. These results suggest that GATA-2 may induce growth suppression of HSCs through up-regulated expressions of p21WAF1 and p27Kip1 proteins, and raise the possibility that GATA-2 may contribute to the quiescence of HSCs.
Materials and methods
Reagents and antibodies
Highly purified human interleukin 6 (hIL-6), human erythropoietin (hEPO), murine (m) IL-3, and murine stem cell factor (mSCF) were provided by Kirin Brewery Company (Tokyo, Japan). The antibodies (Abs) against GATA-2, CDK2 and p21WAF1, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-p27Kip1 Ab was purchased from New England Biolabs (Beverly, MA).
Plasmid constructs and cDNAs
The expression vectors of GATA-2/estrogen receptor (ER), pEF-BOS-GATA-2/ER-puro, and pMX-GATA-2/ER-neo were described previously.22 Expression vectors of CDK2, CDK4, and CDK6 were gifts from Dr E. Harlow (Massachusetts General Hospital, Boston). Other cDNAs were kindly provided from investigators as follows: cyclin D1, cyclin D2, and cyclin D3 from Dr H. Matsushime (Nippon Roche Research Institute, Kanagawa, Japan); cyclin A, cyclin B, CDC2, and p27Kip1 from Dr H. Kiyokawa (University of Illinois, Chicago); p21WAF1 and p53 from Dr B. Vogelstein (Johns Hopkins Oncology Center, Baltimore, MD); p57Kip2 from Dr J. Massague (Memorial Sloan-Kettering Cancer Center, New York, NY); p16INK4A from Dr T. Hama (Cancer Research Institute, Tokyo, Japan); p15INK4B from Dr K. Kataoka (Institute of Medical Science Tokyo University, Tokyo, Japan); p18INK4C and p19INK4D from Dr C. Sherr (Howard Hughes Medical Institute, Memphis, TN); Skp1 and Skp2 from Dr H. Zhang (Yale University, New Haven, CT); and Cul1 from Dr R. A. DePinho (Dana Farber Cancer Institute, Boston, MA). The cDNAs of murine Skp1 and Skp2 for Northern blot analysis were obtained by the polymerase chain reaction (PCR) method.
Cell lines and cultures
Murine IL-3–dependent cells lines Ba/F3, 32D, and FDC-P1 were cultured in RPMI (Nakarai Tesq, Kyoto, Japan) supplemented with 10% fetal calf serum (FCS; Flow, North Ryde, Australia) and 1 ng/mL mIL-3. NIH3T3 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS.
Flow cytometry
DNA content of cultured cells was quantitated by staining with propidium iodide (PI) and analyzed on FACSort (Beckon Dickinson, Oxnard, CA). Cell cycle analysis was performed with a program Modfit LT2.0 (Beckon Dickinson).
Northern blot analysis
The methods for isolation of total cellular RNA and Northern blotting were described previously.29
Cell proliferation assays
To quantitate proliferation of cultured cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, St Louis, MO) rapid colorimetric assays were performed as previously reported.30 In brief, triplicate aliquots of 2.0 × 104 cells were suspended in 96-well flat-bottom microtiter plates and cultured for 72 hours in the presence or absence of 2 μM estradiol. MTT (10 μL of a 5-mg/mL solution in phosphate-buffered saline [PBS]) was added for the final 4 hours of the culture, and then 100 μL acid isopropanol (0.04 N HCL in isopropanol) was added and mixed. The optical density (OD) was measured on the Microelisa plate reader (Corona Electric, Ibaragi, Japan) with a test wavelength of 540 nm and a reference wavelength of 620 nm.
Immunoprecipitation and immunoblotting
Isolation of total cellular lysates, immunoprecipitation, gel electrophoresis, and immunoblotting were performed according to the methods described previously.29 Immunoreactive proteins were visualized with the enhanced chemiluminescence detection system (Dupont NEN, Boston, MA).
Metabolic labeling and measurement of protein turnover
To examine the half-lives of p21WAF1 and p27Kip1 proteins, a pulse chase experiment was performed according to the procedures described previously.31 In short, 1 × 107 cells for each sample were radiolabeled with 200 μCi (7.4 MBq) [35S]-methionine in 1 mL methionine-free DMEM supplemented with 10% dialyzed FCS for 60 minutes. Then, the cells were washed and resuspended in DMEM containing 2 mM unlabeled methionine. During the culture with or without estradiol, total cellular lysates were prepared at the time indicated. p21WAF1 and p27Kip1 were immunoprecipitated and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were dried and subjected to the autoradiography.
Preparation of stable transfectants from Ba/F3, 32D, and FDC-P1
To stably introduce an expression vector for GATA-2/ER into Ba/F3, 32D, and FDC-P1, we transfected 30 μg pEF-BOS-GATA-2/ER-puro by electroporation (250 V, 960 μFD; Bio-Rad Laboratory, Richmond, CA). After the culture with puromycin at a concentration of 1.2 μg/mL, several clones, in which GATA-2/ER was effectively expressed, were selected and subjected to analyses. We further introduced an expression vector for c-myc, CDK2, CDK4, or CDK6 into Ba/F3/GATA-2/ER, and these clones were selected by the culture with G418 (1.0 mg/mL).
Luciferase assays
Luciferase assays were performed with a Dual-Luciferase Reporter System (Promega, Madison, WI) as previously described.29In short, NIH3T3 cells (2 × 105 cells seeded in a 60-mm dish) were transfected with 2 μg pEF-BOS-GATA-2/ER-puro or an empty mock vector together with 2 μg of a reporter gene for GATA (named 3 × MαP-Luc) and 10 ng pRL-CMV-Rluc, an expression vector of renilla luciferase, by the calcium phosphate coprecipitation method. After 12 hours, the cells were washed, serum-deprived for 12 hours, and then cultured with 2 μM estradiol for 8 hours. Then, the cells were lysed in lysis buffer supplied by the manufacturer, followed by the measurement of the firefly andrenilla luciferase activities on a luminometer LB96P (Berthold Japan, Tokyo, Japan). The relative firefly luciferase activities were calculated by normalizing transfection efficiency according to the renilla luciferase activities. The experiments were performed in triplicate and the similar results were obtained from at least 3 independent experiments.
Immune complex kinase assays
Immune complex kinase assays were performed according to the procedure described previously.32 Briefly, CDK2 was immunoprecipitated from 100 μg total cellular lysates. Kinase reactions were initiated by the addition of 20 μCi (0.74 MBq) γ-[32P]ATP (6000 Ci/mmol [222 GBq/mmol]) into 20 μL kinase buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] at pH 7.5, 10 mM MgCl2, 1 mM DTT [dichlorodiphenyltrichloroethane], 1 mM EGTA [ethyleneglycoltetraacetic acid], 0.4 mM NaVO4, 0.4 mM NaF, and 40 μM of nonradioactive ATP) and performed at 30°C for 20 minutes (within a linear incorporation kinetics) by using 3 μg Histone H1 (Boehringer Mannheim, Mannheim, Germany) as a substrate. After addition of the protein-loading buffer, samples were boiled, and subjected to SDS-PAGE. The gels were stained with Coomassie blue to confirm the amounts of the immunoprecipitates, destained, dried, and subjected to autoradiography.
Semiquantitative reverse transcription–PCR analyses
Total cellular RNA was extracted from cultured cells (about 104 cells) and reverse transcribed (RT) into cDNA with oligo(dT) primers (Pharmacia, Piscataway, NJ) by using SuperScript reverse transcriptase according to the manufacturer's instruction (Gibco BRL, Gaithersburg MD). The cDNA product (1 μL) was resuspended in 20 μL of the PCR reaction buffer containing 0.5 UTaqGold DNA polymerase (Perkin Elmer, Foster City, CA), 2 mM MgCl2, 200 μM deoxynucleoside triphosphate (dNTP) mix, 30 μCi (1.11 MBq) α-[32P] deoxycytidine triphosphate (dCTP), and 15 pmol forward and reverse primers. The primer sets to amplify c-myc, Cul1, and β-actin are as follows: c-myc, a sense primer 5′-TCACCAGCACAACTACGCCG-3′ and a reverse primer 5′-CAGGATGTAGGCGGTGGCTT-3′; Cul1, a sense primer 5′-GGACTGAAGCAGATCGGTCTT-3′ and a reverse primer 5′-AATGTCTATTGAGGTAGGCA-3′; β-actin, a sense primer 5′-CATCACTATTGGCAACGAGC-3′ and a reverse primer 5′-ACGCAGCTCAGTAACAGTCC-3′. The samples were denatured at 94°C for 10 minutes, followed by 20 to 32 cycles of amplification (94°C, 30 seconds for denaturation; 55°C, 30 seconds for annealing; 72°C, 30 seconds for extension). At first, we adjusted the amounts of cDNA products in several samples. Briefly, the aliquots (5 μL) of β-actin products obtained after 26, 28, 30, and 32 PCR cycles from each sample were size fractionated on 12% polyacrylamide gels, dried, and autoradiographed. Because β-actin was amplified exponentially during 28 to 34 cycles of PCR in all of the samples, we quantified the amounts of cDNA by measuring the intensities of β-actin products obtained after 30 cycles of PCR with a densitometer. After adjustment of the amounts of cDNA products, the equal amount of cDNA products were subjected to the PCR. The amounts of c-myc and Cul1 mRNA were evaluated after 28 to 34 cycles of PCR, during which PCR products from all of the samples were exponentially amplified.
Preparation of the conditioned media containing high-titer virus particles
The conditioned media containing high-titer virus particles were prepared as described previously.33 Briefly, the plasmid pMX-GATA-2/ER-neo, an empty pMX-neo or pMX-GFP (green fluorescent protein) was transfected into an ecotropic packaging cell line Plat-E by the calcium phosphate coprecipitation method. After 12 hours, the cells were washed, and then cultured for 48 hours in DMEM supplemented with 10% FCS. The supernatant containing virus particles was collected, centrifuged at 6900 rpm for 16 hours, and concentrated by 50-fold in volume.
Retrovirus transfection into murine bone marrow cells
Bone marrow cells were harvested from 9- to 12-week-old Balb/c mice pretreated with 150 mg/kg 5-fluoruracil (5-FU) for 2 days. Mononuclear cells were isolated by density gradient centrifugation and cultured in DMEM supplemented with 10% FCS in the presence of mIL-3 (10 ng/mL), mSCF (100 ng/mL), and hIL-6 (50 ng/mL) for 48 hours. Then, the cells were supplemented with 5% volume of conditioned media containing high-titer retrovirus and polybrene (3 μg/mL). After 24 hours, the cells were washed and cultured in DMEM supplemented with 10% FCS containing cytokines (as described above) and 1 mg/mL G418 for 72 hours. After the selection with G418, retrovirus-infected cells were further cultured with or without 2 μM estradiol for 72 hours, and subjected to MTT assays, RT-PCR analyses, and Western blot analyses. To assess the transfection efficiency, we infected retrovirus containing pMX-GFP and examined the proportion of GFP+ cells by flow cytometric analysis.
Isolation of murine bone marrow stem/progenitor cells
We isolated Lin− cells by negative selection of CD3+, CD20+, Ter-119+, Mac-1+, and Gr-1+ cells with BioMAG (Polysciences, Warrington, PA). Lin−Sca-1+cells were isolated from murine bone marrow mononuclear cells (MNCs) by using MACS (Miltenyl Biotec, Bergisch Gladbach, Germany).
Results
Effects of GATA-2 on the growth of Ba/F3, 32D, and FDC-P1
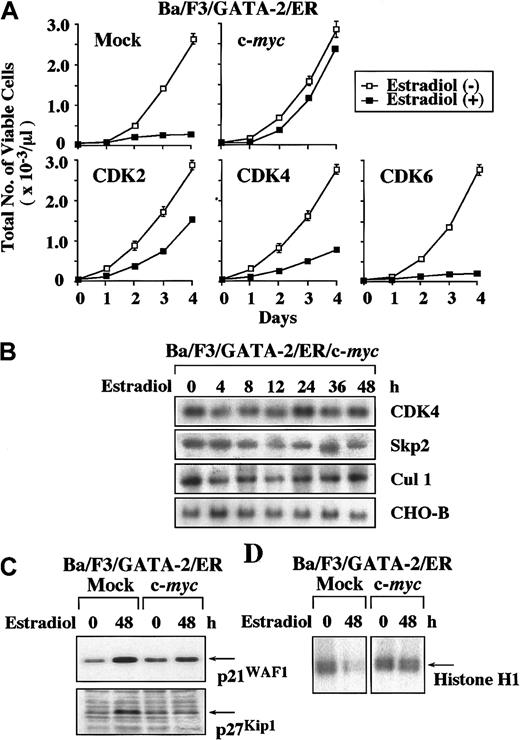
To examine the effects of GATA-2 on the growth of hematopoietic cells in a ligand-inducible system, we used GATA-2/ER, a chimeric molecule of GATA-2 consisting of full-length GATA-2 and the ligand-binding domain of ER. At first, we performed luciferase assays with a reporter gene for GATA, 3 × MαP-Luc, in NIH3T3 cells. As shown in Figure 1A, the estradiol treatment stimulated 3 × MαP-Luc by 4.7-fold in GATA-2/ER–transfected cells, whereas it did not show any effect in mock-transfected cells. These results indicate that GATA-2/ER reveals its activities only when the cells are treated with estradiol. Next, we stably introduced GATA-2/ER into mIL-3–dependent cell lines (a pro–B cell line Ba/F3, a myeloid cell line 32D, and a hematopoietic progenitor cell line FDC-P1); each clone was designated Ba/F3/GATA-2/ER, 32D/GATA-2/ER, and FDC-P1/GATA-2/ER, respectively. At first, we compared the expression levels of GATA-2/ER with those of endogenous GATA-2 in these clones by immunoblot analysis. The expression level of GATA-2/ER was almost equivalent to that of endogenous GATA-2 in FDC-P1/GATA-2/ER and about 2-fold in 32D/GATA-2/ER, whereas Ba/F3/GATA-2/ER scarcely expressed endogenous GATA-2 protein (Figure 1B). Under the culture without estradiol, these clones proliferated in response to IL-3 with the growth curves similar to those of their parental clones (data not shown). In contrast, the estradiol treatment inhibited IL-3–dependent growth of Ba/F3/GATA-2/ER, 32D/GATA-2/ER, and FDC-P1/GATA-2/ER almost completely (Figure 1C), whereas it did not affect the growth of mock (an empty vector)–transfected Ba/F3, 32D, or FDC-P1 (data not shown). In DNA content analysis, the estradiol treatment reduced the cells in S phase in all of the clones (changes in the proportion of the cells in S phase before and after the estradiol treatment: Ba/F3/GATA-2/ER, 59% to 5%; 32D/GATA-2/ER, 49% to 6%; FDC-P1/GATA-2/ER, 42% to 4%; Figure 1D). Similar results were obtained from at least 3 GATA-2/ER–transfected clones of Ba/F3, 32D, and FDC-P1 (data not shown).
Effects of GATA-2 on the growth of mIL-3–dependent cell lines, Ba/F3, 32D, and FDC-P1.
(A) NIH3T3 cells were transfected with 2 μg of a reporter gene for GATA, 3 × MαP-Luc, 10 ng pRL-CMV-Rluc, and 2 μg of an expression vector of GATA-2/ER or an empty expression vector. After 12 hours, the cells were serum deprived and cultured for 24 hours. Then, the cells were stimulated with 1 μM estradiol for 8 hours and lysed in the lysis buffer, followed by the measurement of the firefly and therenilla luciferase activities. The relative firefly luciferase activities were calculated by normalizing transfection efficiency according to the renilla luciferase activities. The results are shown as the means ± SD of triplicate experiments. (B) The expression levels of ectopic (Ect) GATA-2/ER and endogenous (End) GATA-2 were examined in the transfectants by immunoblot analysis. (C) The cells of Ba/F3, 32D, and FDC-P1 each transfected with an expression vector of GATA-2/ER were seeded at a cell density of 100/μL and cultured with (▪) or without (■) 1 μM estradiol. Total number of viable cells was counted by trypan blue dye exclusion method at the times indicated. The results are shown as means ± SD of triplicate cultures. (D) Cells cultured as described above were subjected to PI staining, and DNA content was analyzed on FACSort. Cell cycle analysis was performed with a program Modfit LT2.0. The results shown are representative of 3 independent experiments.
Effects of GATA-2 on the growth of mIL-3–dependent cell lines, Ba/F3, 32D, and FDC-P1.
(A) NIH3T3 cells were transfected with 2 μg of a reporter gene for GATA, 3 × MαP-Luc, 10 ng pRL-CMV-Rluc, and 2 μg of an expression vector of GATA-2/ER or an empty expression vector. After 12 hours, the cells were serum deprived and cultured for 24 hours. Then, the cells were stimulated with 1 μM estradiol for 8 hours and lysed in the lysis buffer, followed by the measurement of the firefly and therenilla luciferase activities. The relative firefly luciferase activities were calculated by normalizing transfection efficiency according to the renilla luciferase activities. The results are shown as the means ± SD of triplicate experiments. (B) The expression levels of ectopic (Ect) GATA-2/ER and endogenous (End) GATA-2 were examined in the transfectants by immunoblot analysis. (C) The cells of Ba/F3, 32D, and FDC-P1 each transfected with an expression vector of GATA-2/ER were seeded at a cell density of 100/μL and cultured with (▪) or without (■) 1 μM estradiol. Total number of viable cells was counted by trypan blue dye exclusion method at the times indicated. The results are shown as means ± SD of triplicate cultures. (D) Cells cultured as described above were subjected to PI staining, and DNA content was analyzed on FACSort. Cell cycle analysis was performed with a program Modfit LT2.0. The results shown are representative of 3 independent experiments.
Effects of GATA-2 on the expression of growth regulatory molecules
To clarify the mechanism of GATA-2–induced growth suppression, we examined the expression levels of GATA-2/ER and growth-promoting molecules in Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER during the estradiol treatment by Northern blot analysis. As shown in Figure 2A, the expression of GATA-2/ER mRNA was kept constant in both clones during the test period. After the addition of estradiol into the culture medium, the expression of c-myc decreased as early as 4 hours in both clones (Figure2A). Then, the expression of CDK4, cyclin A, and cyclin B declined gradually. In contrast, we did not detect an apparent change in the expression levels of CDK2 or CDK6 (Figure 2A) in addition to cyclin D2 or cyclin D3 (data not shown). Furthermore, neither cyclin D1 nor cyclin E was expressed in these clones during the test period (data not shown). We also examined the expression of growth inhibitory molecules during GATA-2–induced growth suppression. As shown in Figure 2A, the expression levels of p21WAF1 and p27Kip1 mRNA did not change for up to 120 hours. Moreover, we did not detect a significant change in the expression of other CDK inhibitors (p16INK4A, p15INK4B, p18INK4C, p19INK4D, and p57Kip2), p19ARF, or p53 (data not shown). Next, we examined the effects of GATA-2 on the expression levels of CKIs by Western blot analysis. Although the expression levels of p21WAF1 and p27Kip1 mRNA were kept constant during the estradiol treatment, the amount of p21WAF1 and p27Kip1 proteins increased in both Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER (Figure 2B). To investigate the significance of up-regulated expression of p21WAF1 and p27Kip1 proteins, we evaluated changes of CDK2 activities with immune complex kinase assays by using Histone H1 as a substrate. As shown in Figure 2C, CDK2 activities were reduced by the estradiol treatment in both Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER, probably due to the increased expression of p21WAF1 and p27Kip1proteins. In contrast, the estradiol treatment did not affect the expression of these growth regulatory molecules at mRNA or protein levels in mock-transfected Ba/F3 or FDC-P1 (data not shown).
Effect of GATA-2 on the expression and function of cell cycle regulatory molecules.
(A) The cells of Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER were cultured with mIL-3 in the presence of 1 μM estradiol and subjected to Northern blot analysis at the time indicated. Similar results were observed in 2 other clones of GATA-2/ER–transfected Ba/F3 and FDC-P1. (B,C) The expression levels of p21WAF1 and p27Kip1 proteins were examined by Western blot analysis on total cellular lysates. CDK2 was immunoprecipitated from equal amounts of the cell lysates. Immune complex kinase assays were performed in kinase buffer containing 5 μg Histone H1 and 20 μCi (0.74 MBq) [γ-32P]ATP for 30 minutes at 30°C. Then, samples were subjected to SDS-PAGE. The gels were stained with Coomassie blue, destained, dried, and subjected to autoradiography. The results shown in panels B and C are representative of 3 independent experiments.
Effect of GATA-2 on the expression and function of cell cycle regulatory molecules.
(A) The cells of Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER were cultured with mIL-3 in the presence of 1 μM estradiol and subjected to Northern blot analysis at the time indicated. Similar results were observed in 2 other clones of GATA-2/ER–transfected Ba/F3 and FDC-P1. (B,C) The expression levels of p21WAF1 and p27Kip1 proteins were examined by Western blot analysis on total cellular lysates. CDK2 was immunoprecipitated from equal amounts of the cell lysates. Immune complex kinase assays were performed in kinase buffer containing 5 μg Histone H1 and 20 μCi (0.74 MBq) [γ-32P]ATP for 30 minutes at 30°C. Then, samples were subjected to SDS-PAGE. The gels were stained with Coomassie blue, destained, dried, and subjected to autoradiography. The results shown in panels B and C are representative of 3 independent experiments.
GATA-2 inhibits Ub/proteasome-dependent degradation of p21WAF1 and p27Kip1 through the suppression of Skp2 and Cul1 mRNA expression
Next, we investigated the effects of GATA-2 on the half-lives of p21WAF1 and p27Kip1 proteins by a pulse chase experiment. [35S]-methionine–labeled FDC-P1/GATA-2/ER and Ba/F3/GATA-2/ER cells were cultured with or without estradiol for the time indicated, and changes in the expression levels of p21WAF1 and p27Kip1 proteins were examined in each clone. As shown in Figure3A, both 35S-labeled p21WAF1 and p27Kip1 were degraded rapidly under the culture without estradiol (percent degradation assessed by the densitometric analysis: p21WAF1, 62% at 40 minutes and 93% at 80 minutes; p27Kip1, 32% at 40 minutes and 91% at 80 minutes). In contrast, the estradiol treatment maintained the expression levels of 35S-labeled p21WAF1 and p27Kip1 for up to 80 minutes (Figure 3A), whereas it did not retain their expression levels in mock-transfected FDC-P1 or Ba/F3 (data not shown). These results indicate that GATA-2 would enhance the protein stability of p21WAF1 and p27Kip1. Because degradation of p21WAF1 and p27Kip1 is executed by the Ub/proteasome system,34-36 we examined the effects of a proteasome inhibitor, lactacystin, on the amounts of p21WAF1 and p27Kip1 in FDC-P1/GATA-2/ER and Ba/F3/GATA-2/ER. Under the culture without estradiol, the treatment with lactacystin augmented the amounts of p21WAF1 and p27Kip1 in each clone (Figure 3B, lane 1 versus lane 2), implying that both p21WAF1 and p27Kip1 are constantly degraded by Ub/proteasome pathways under normal culture conditions. Meanwhile, the estradiol treatment augmented the amounts of p21WAF1 and p27Kip1 proteins as efficiently as lactacystin (Figure 3B, lane 2 versus lane 3), and lactacystin could not further augment their amounts in the estradiol-treated cells (Figure 3B, lane 3 versus lane 4). These results suggest that GATA-2 may inhibit Ub/proteasome-dependent degradation of p21WAF1and p27Kip1. Next, we analyzed the mechanism through which GATA-2 inhibits Ub/proteasome-dependent degradation of p21WAF1 and p27Kip1. Because Skp1, Skp2, and Cul1 have been reported to form a complex and to act as a Ub ligase (E3) toward p21WAF1 and p27Kip1, we examined their expression in Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER during the estradiol treatment by Northern blot analysis.34-36 As shown in Figure 3C, the expression of Skp1 was retained in both Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER during the estradiol treatment. In contrast, the expression of Skp2 and Cul1 was gradually suppressed by the estradiol treatment in Ba/F3/GATA-2/ER. Also, in FDC-P1/GATA-2/ER, the expression of Skp2 and Cul1 was reduced by the estradiol treatment after 12 hours, whereas that of Skp2 was transiently up-regulated from 4 to 8 hours. Although the results shown in Figure 3 were obtained from a single cell clone, similar results were observed in 2 other clones of GATA-2/ER–transfected Ba/F3 and FDC-P1, respectively (data not shown). Together, these results suggest that GATA-2 might inhibit Ub/proteasome-dependent degradation of p21WAF1 and p27Kip1 through the suppression of Skp2 and Cul1 expression.
Effects of GATA-2 on the Ub/proteasome-dependent degradation of p21WAF1 and p27Kip1 in FDC-P1/GATA-2/ER and Ba/F3/GATA-2/ER.
(A) To examine the half-lives of p21WAF1 and p27Kip1 proteins, cells (1 × 107 cells for each sample) were radiolabeled with [35S]methionine for 30 minutes. Then, the labeled cells were washed, resuspended in DMEM containing 2 mM unlabeled methionine, and cultured with or without 2 μM estradiol for the times indicated. p21WAF1 and p27Kip1 were immunoprecipitated from total cellular lysates and subjected to SDS-PAGE. The gels were dried and subjected to the autoradiography. (B) The cells were cultured in the presence or absence of 1 μM estradiol and/or 0.1 μM lactacystin for 24 hours. The protein expression levels of p21WAF1 and p27Kip1 were examined by Western blot analysis on total cellular lysates. (C) The cells were cultured with 1 μM estradiol for the times indicated and subjected to Northern blot analysis on the expressions of Skp1, Skp2, and Cul1. The results show representative data from 3 independent experiments.
Effects of GATA-2 on the Ub/proteasome-dependent degradation of p21WAF1 and p27Kip1 in FDC-P1/GATA-2/ER and Ba/F3/GATA-2/ER.
(A) To examine the half-lives of p21WAF1 and p27Kip1 proteins, cells (1 × 107 cells for each sample) were radiolabeled with [35S]methionine for 30 minutes. Then, the labeled cells were washed, resuspended in DMEM containing 2 mM unlabeled methionine, and cultured with or without 2 μM estradiol for the times indicated. p21WAF1 and p27Kip1 were immunoprecipitated from total cellular lysates and subjected to SDS-PAGE. The gels were dried and subjected to the autoradiography. (B) The cells were cultured in the presence or absence of 1 μM estradiol and/or 0.1 μM lactacystin for 24 hours. The protein expression levels of p21WAF1 and p27Kip1 were examined by Western blot analysis on total cellular lysates. (C) The cells were cultured with 1 μM estradiol for the times indicated and subjected to Northern blot analysis on the expressions of Skp1, Skp2, and Cul1. The results show representative data from 3 independent experiments.
Reduced expression of c-myc mRNA is primarily responsible for GATA-2–induced growth suppression
In this study, GATA-2 suppressed the expression of c-myc and CDK4 mRNA (Figure 2A). GATA-2 also inhibited the expression of Skp2 and Cul1 mRNA (Figure 3C), thereby inducing the accumulation of p21WAF1 and p27Kip1 proteins. In previous studies, c-myc was reported to induce the expression of CDK4 and Cul1 mRNA.37 38 Therefore, we speculated that down-regulation of c-myc expression might be primarily responsible for the reduced expression of CDK4 and Cul1 and for the subsequent accumulation of p21WAF1 and p27Kip1. To verify this hypothesis, we overexpressed c-myc, CDK2, CDK4, and CDK6 in GATA-2/ER–transfected Ba/F3 cells. As compared with the growth inhibition observed in the mock clone transfected with an empty vector, overexpression of c-myc, CDK2, and CDK4 canceled GATA-2–induced growth suppression by 90%, 40%, and 20%, respectively (Figure4A). In contrast, overexpression of CDK6 showed little effect. Next, we examined the effects of overexpressed c-myc on the expression of CDK4, Skp2, and Cul1 mRNA in c-myc–introduced Ba/F3/GATA-2/ER. In contrast to the data obtained from Ba/F3/GATA-2/ER (Figures 2A and 3C), the expression of CDK4, Skp2, and Cul1 mRNA was maintained in Ba/F3/GATA-2/ER/c-myc even during the estradiol treatment (Figure 4B). In addition, the accumulation of p21WAF1 and p27Kip1 proteins and the suppression of CDK2 activities that are induced by the estradiol treatment in the mock clone were canceled in Ba/F3/GATA-2/ER/c-myc (Figure 4C-D). Although the results shown in Figure 4 were obtained from a single cell clone, similar results were observed in 2 other clones of each transfectant (data not shown). Together, these results suggest that the suppression of c-myc expression by GATA-2 may be primarily responsible for the accumulation of p21WAF1 and p27Kip1 and consequent growth inhibition.
Effects of overexpression of c-
myc, CDK2, CDK4, and CDK6 on GATA-2–induced growth suppression of Ba/F3 cells. (A) Ba/F3/GATA-2/ER clones each transfected with an expression vector of c-myc, CDK2, CDK4, CDK6, or an empty vector were cultured with or without 1 μM estradiol. Total number of viable cells was counted by trypan blue dye exclusion method at the time indicated. Similar results were obtained from at least 3 independent clones in each transfectant. The results are shown as means ± SD of triplicate cultures. (B) The cells of Ba/F3/GATA-2/ER overexpressing c-myc were cultured with 1 μM estradiol and subjected to Northern blot analysis on the expression of CDK4, Skp2, and Cul1 at the times indicated. (C) The expression levels of p21WAF1 and p27Kip1proteins were examined by Western blot analysis before and after the 48-hour culture with 1 μM estradiol in mock- and c-myc–transfected Ba/F3/GATA-2/ER. (D) CDK2 activities were evaluated before and after the 48-hour culture with estradiol in mock- and c-myc–transfected Ba/F3/GATA-2/ER with immune complex kinase assays by using Histone H1 as a substrate. The results shown in panels B, C, and D are representative of 3 independent experiments.
Effects of overexpression of c-
myc, CDK2, CDK4, and CDK6 on GATA-2–induced growth suppression of Ba/F3 cells. (A) Ba/F3/GATA-2/ER clones each transfected with an expression vector of c-myc, CDK2, CDK4, CDK6, or an empty vector were cultured with or without 1 μM estradiol. Total number of viable cells was counted by trypan blue dye exclusion method at the time indicated. Similar results were obtained from at least 3 independent clones in each transfectant. The results are shown as means ± SD of triplicate cultures. (B) The cells of Ba/F3/GATA-2/ER overexpressing c-myc were cultured with 1 μM estradiol and subjected to Northern blot analysis on the expression of CDK4, Skp2, and Cul1 at the times indicated. (C) The expression levels of p21WAF1 and p27Kip1proteins were examined by Western blot analysis before and after the 48-hour culture with 1 μM estradiol in mock- and c-myc–transfected Ba/F3/GATA-2/ER. (D) CDK2 activities were evaluated before and after the 48-hour culture with estradiol in mock- and c-myc–transfected Ba/F3/GATA-2/ER with immune complex kinase assays by using Histone H1 as a substrate. The results shown in panels B, C, and D are representative of 3 independent experiments.
GATA-2 inhibits cytokine-dependent growth of normal hematopoietic cells
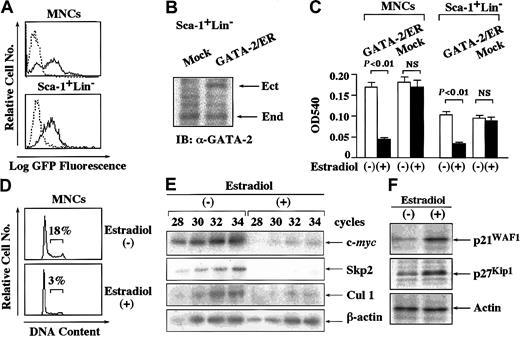
To examine the effects of GATA-2 on cytokine-dependent growth of normal hematopoietic cells, we isolated MNCs from bone marrow of mice treated with 5-FU. We also purified Sca-1+Lin−cells from MNCs. After culture with various cytokines, we infected retrovirus containing pMX-GFP into these cells and assessed the transfection efficiency after 72 hours. As shown in Figure5A, about 55% to 65% of MNCs and Sca-1+Lin− cells were found to be GFP+ by flow cytometric analysis. Next, we infected retrovirus containing pMX-GATA-2/ER-neo or pMX-neo (a mock vector) into these cells. After the selection with G418 for 3 days, we examined the expression level of GATA-2/ER relative to endogenous GATA-2 in Sca-1+Lin− cells by immunoblot analysis. The expression level of GATA-2/ER was almost equivalent to that of endogenous GATA-2 in Sca-1+Lin− cells (Figure5B). We further cultured G418-resistant cells with or without estradiol for 72 hours and subjected them to a proliferation assay, cell cycle analysis, Western blot analysis, and RT-PCR analysis. As shown in Figure 5C, GATA-2/ER inhibited the growth of MNCs by 75% (P < .01) and that of Sca-1+Lin−cells by 65% (P < .01) in an MTT assay although the estradiol treatment did not affect the growth of mock-infected MNCs or Sca-1+Lin− cells. In agreement with this result, the estradiol treatment reduced the proportion of the cells in S phase from 18% to 3% in GATA-2/ER–transfected MNCs (Figure 5D).
Effects of GATA-2 on the growth of normal hematopoietic cells.
(A) Normal MNCs and Sca-1+Lin− cells were isolated from bone marrow of 5-FU–treated mice and infected with retrovirus containing pMX-GATA-2/ER-neo, pMX-neo, or pMX-GFP. The expression of GFP was examined in pMX-GFP–infected MNCs and Sca-1+Lin− cells. Dashed-line histograms indicate mock-infected cells; solid-line histograms, pMX-GFP–infected cells. (B) The cells infected with retrovirus containing GATA-2/ER or an empty vector (Mock) were cultured with G418 for 72 hours and subjected to immunoblot analysis with an anti–GATA-2 Ab. IB indicates immunoblot; Ect, ectopic GATA-2/ER; End, endogenous GATA-2. (C) After 72 hours of culture with G418, retrovirus-transduced cells were also cultured with or without estradiol for 72 hours and subjected to an MTT colorimetric assay. The results are shown as the means ± SD of triplicate cultures. NS indicates statistically not significant. (D) Cell cycle analysis of the cultured cells was performed by PI staining. (E) The cultured cells were subjected to semiquantitative RT-PCR analysis on the expression of c-mycand Cul1 mRNA. Total cellular RNA was isolated at the time indicated and cDNA was synthesized. The amounts of cDNA products were normalized according to the amounts of PCR products of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The adjusted amounts of cDNA products from each sample were subjected to PCR for c-myc, Cul1, and GAPDH. After the PCR cycles indicated, the PCR products were size-fractionated on 3.5% polyacrylamide gels, dried, and autoradiographed. (F) The expression levels of p21WAF1 and p27Kip1proteins in the cultured cells were examined by Western blot analysis. Results representative of 3 independent experiments are shown.
Effects of GATA-2 on the growth of normal hematopoietic cells.
(A) Normal MNCs and Sca-1+Lin− cells were isolated from bone marrow of 5-FU–treated mice and infected with retrovirus containing pMX-GATA-2/ER-neo, pMX-neo, or pMX-GFP. The expression of GFP was examined in pMX-GFP–infected MNCs and Sca-1+Lin− cells. Dashed-line histograms indicate mock-infected cells; solid-line histograms, pMX-GFP–infected cells. (B) The cells infected with retrovirus containing GATA-2/ER or an empty vector (Mock) were cultured with G418 for 72 hours and subjected to immunoblot analysis with an anti–GATA-2 Ab. IB indicates immunoblot; Ect, ectopic GATA-2/ER; End, endogenous GATA-2. (C) After 72 hours of culture with G418, retrovirus-transduced cells were also cultured with or without estradiol for 72 hours and subjected to an MTT colorimetric assay. The results are shown as the means ± SD of triplicate cultures. NS indicates statistically not significant. (D) Cell cycle analysis of the cultured cells was performed by PI staining. (E) The cultured cells were subjected to semiquantitative RT-PCR analysis on the expression of c-mycand Cul1 mRNA. Total cellular RNA was isolated at the time indicated and cDNA was synthesized. The amounts of cDNA products were normalized according to the amounts of PCR products of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The adjusted amounts of cDNA products from each sample were subjected to PCR for c-myc, Cul1, and GAPDH. After the PCR cycles indicated, the PCR products were size-fractionated on 3.5% polyacrylamide gels, dried, and autoradiographed. (F) The expression levels of p21WAF1 and p27Kip1proteins in the cultured cells were examined by Western blot analysis. Results representative of 3 independent experiments are shown.
GATA-2 suppresses the expression of c-myc and Cul1 mRNA and induces the accumulation of p21WAF1and p27Kip1proteins in normal hematopoietic cells
Next, we examined the effects of GATA-2 on the expression of c-myc, Skp2, and Cul1 in normal MNCs by semiquantitative RT-PCR analysis. After normalization of the amounts of cDNA according to the amounts of the β-actin products, we evaluated their expression after 28 to 34 cycles of PCR, during which these PCR products were amplified exponentially (Figure 5D). In agreement with the results obtained from Ba/F3 and FDC-P1, the estradiol treatment severely reduced the expression of c-myc, Skp2, and Cul1 mRNA in normal hematopoietic cells (Figure 5E). Also, the estradiol treatment induced the accumulation of p21WAF1 and p27Kip1proteins in normal MNCs (Figure 5F).
The expression of GATA-2 protein decreases in cycling hematopoietic stem/progenitor cells
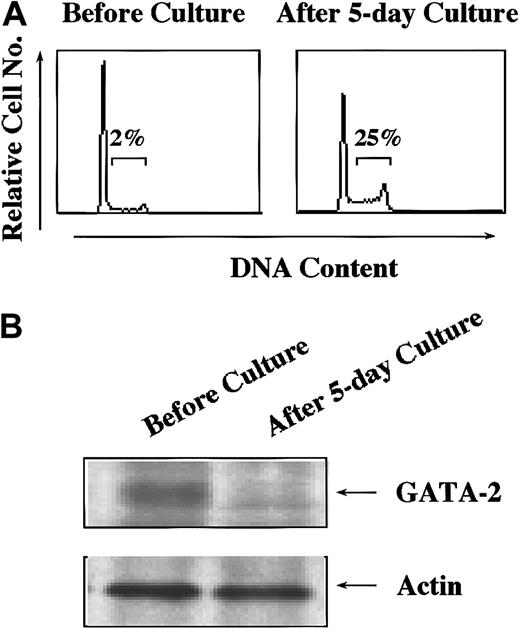
To assess the roles of endogenous GATA-2 expressed in hematopoietic stem/progenitor cells, we compared expression levels of GATA-2 protein in quiescent and cycling stem cells. We isolated Lin− cells from murine bone marrow cells and cultured with mSCF, mIL-3, hIL-6, and mFLT3 ligand for 5 days because this combination was reported to expand HSCs without spoiling their mutipotentiality.39 Surface phenotypes of the cells were essentially the same before and after the culture (data not shown). However, the proportion of the cells in S phase increased from 2% to 25% after the culture (Figure 6A). The expression of GATA-2 protein was found to decrease in the cultured cells (Figure 6B), suggesting a possibility that down-regulated expression of GATA-2 in hematopoietic stem/progenitor cells might be prerequisite for their cell cycle entry.
The expression levels of GATA-2 in dormant and cycling hematopoietic stem/progenitor cells.
Lin− cells were purified from bone marrow of 5-FU–treated mice and cultured with mIL-3 (10 ng/mL), mSCF (100 ng/mL), hIL-6 (50 ng/mL), and FLT3 ligand (50 ng/mL) for 5 days. (A) Lin−cells were subjected to cell cycle analysis by PI staining before and after the culture. (B) The expression levels of GATA-2 were examined in Lin− cells before and after the culture. Results shown are representative of 3 independent experiments.
The expression levels of GATA-2 in dormant and cycling hematopoietic stem/progenitor cells.
Lin− cells were purified from bone marrow of 5-FU–treated mice and cultured with mIL-3 (10 ng/mL), mSCF (100 ng/mL), hIL-6 (50 ng/mL), and FLT3 ligand (50 ng/mL) for 5 days. (A) Lin−cells were subjected to cell cycle analysis by PI staining before and after the culture. (B) The expression levels of GATA-2 were examined in Lin− cells before and after the culture. Results shown are representative of 3 independent experiments.
Discussion
In a previous study, Heyworth et al22 reported that an estradiol-inducible form of GATA-2 (GATA-2/ER) inhibited cytokine-dependent growth of normal hematopoietic progenitor cells in vitro. Similarly, in this study, we found that GATA-2/ER inhibited the growth of normal hematopoietic cells and various cell lines. However, considering the fact that GATA-2/ER is an artificial form, we have to pay special attention to the possibility that the ER portion of the conditional protein might reveal some artificial effects that are different from those of wild-type GATA-2. Yet, supporting the data obtained by GATA-2/ER, Persons et al21 showed that enforced expression of wild-type GATA-2 inhibited the expansion of HSCs in vitro and in vivo. They introduced wild-type GATA-2 into murine primitive Sca-1+Lin− cells by retrovirus, in which the expression level of GATA-2 was up-regulated by 2-fold as compared with that in mock-transfected cells. When these cells were planted into lethally irradiated mice, GATA-2–transduced Sca-1+Lin− cells neither expanded nor underwent differentiation, whereas mock-transfected cell expanded over 40-fold and reconstituted hematopoietic cells in all lineages. Together, these results suggest that enforced expression of GATA-2 would induce growth suppression of hematopoietic cells. However, it is certainly true that GATA-2 is essential for development, maintenance, or physiologic function of HSCs because GATA-2−/− mice are embryonic lethal phenotype due to the lack of definitive hematopoiesis.19 Regarding this contradiction, we speculate that the function of GATA-2 in formation of the hematopoietic system and that in already committed progenitors may be different. In previous studies, GATA factors have been reported to not only act as transcription factors but also interact with various nuclear regulatory proteins such as promyelocytic leukemia protein (PML), CREB-binding protein (CBP), PU.1, c-Myb, erythroid Kruppel-like factor (EKLF), Lmo2, and Sp1 through their highly conserved zinc finger domains.40-46 In addition, these interacting molecules have been reported to positively or negatively regulate activities of GATA factors and vise versa. Because the expression pattern of these GATA-2–interacting molecules is supposed to be different between developing HSCs and committed HSCs, GATA-2 might play a distinct role in the growth control of HSCs dependently on its binding partners. Another possibility is that GATA-2 might control the growth of HSCs both positively and negatively according to its amount. Supporting this hypothesis, it was reported that small Maf transcription factors, which participate in platelet production, could act as both positive and negative regulators of transcription according to their amounts within the range from 0- to 2-fold of normal amount.47 In agreement with the report by Persons et al, we found here that about 2-fold increase of GATA-2 led to the growth inhibition of normal Sca-1+Lin− cells. However, we have to be deliberate to evaluate this result properly, because this result does not necessarily exclude a possibility that a physiologic amount of GATA-2 may promote the growth of HSCs. Yet, because we found that the amount of GATA-2 was reduced in cycling hematopoietic stem/progenitor cells as compared with that in dormant corresponding cells, it was assumed that the physiologic amount of GATA-2 expressed in HSCs might contribute to their quiescence and that down-regulation of GATA-2 expression might be prerequisite for their cell cycle entry.
With regard to the mechanisms of GATA-2–induced growth suppression, Persons et al21 could not detect an apparent effect of GATA-2 on cell cycle profiles in normal hematopoietic progenitor cells, whereas they found that GATA-2 inhibited their growth severely. On the other hand, in the present study, we found that GATA-2 reduced the proportion of the cells in S phase in normal hematopoietic cells as well as in various hematopoietic cell lines. As for this mechanism, we found that GATA-2 suppressed the expression of c-myc, CDK4, and Cul1 mRNA and thereby induced the accumulation of p21WAF1 and p27Kip1 proteins. Because overexpression of c-myc restored these changes and consequently canceled GATA-2–induced growth suppression, the suppression of c-myc expression was considered to be primarily responsible for GATA-2–mediated growth inhibition. However, it still remains unresolved how GATA-2 suppressed the expression of c-myc mRNA. Up to the present, GATA-2 has been reported to act as a transcriptional repressor on promoters of erythropoietin and peroxisome proliferator-activated receptor γ 2 (PPARγ2) genes.48,49 However, in our preliminary experiments, GATA-2 did not suppress basal activities of c-myc promoter in luciferase assays (data not shown), denying a possibility that GATA-2 would act as a direct transcriptional repressor on c-myc promoter. Alternatively, because GATA factors influence the functions of various nuclear factors through the protein-protein interactions as described above, it was plausible that GATA-2 might inhibit the function of the nuclear factor that activates c-myc promoter. Because c-myc promoter is activated by Ras/Raf signaling and gp130-activated STAT3,50 51 it may be interesting to examine the effects of GATA-2 on the cytokine signaling and to identify the binding partner of GATA-2.
We found here that GATA-2 augments the amounts of p21WAF1and p27Kip1 proteins. Because p21WAF1 and p27Kip1 were reported to play essential roles to keep HSCs and progenitor cells in quiescence, respectively,28 52 it was speculated that GATA-2 may play some role in their cell cycle regulation through modulating the expression levels of p21WAF1 and p27Kip1 proteins.
In summary, we demonstrate here that enforced expression of GATA-2 inhibits cytokine-dependent growth of hematopoietic cells through the down-regulation of c-myc mRNA expression and the up-regulation of p21WAF1 and p27Kip1 protein expression.
We thank Drs E. Harlow, H. Matsushime, H. Kiyokawa, B. Vogelstein, J. Massague, T. Hama, K. Kataoka, C. Sherr, H. Zhang, and R. A. DePinho for providing the plasmids.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-04-1177.
Supported by grants from ministry of Education, Science and Culture of Japan, Mochida Foundation, Ichiro Kanehara Foundation, Uehara Memorial Foundation, Naito Foundation, and the Japan Medical Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
After we had made a revision of this manuscript, Kitajima et al53 reported that the functions of GATA-2/ER were somewhat different from those of wild-type GATA-2.
Author notes
Itaru Matsumura, Department of Hematology and Oncology (C9), Osaka University Graduate School of Medicine, 2-2, Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail:matumura@bldon.med.osaka-u.ac.jp


![Fig. 2. Effect of GATA-2 on the expression and function of cell cycle regulatory molecules. / (A) The cells of Ba/F3/GATA-2/ER and FDC-P1/GATA-2/ER were cultured with mIL-3 in the presence of 1 μM estradiol and subjected to Northern blot analysis at the time indicated. Similar results were observed in 2 other clones of GATA-2/ER–transfected Ba/F3 and FDC-P1. (B,C) The expression levels of p21WAF1 and p27Kip1 proteins were examined by Western blot analysis on total cellular lysates. CDK2 was immunoprecipitated from equal amounts of the cell lysates. Immune complex kinase assays were performed in kinase buffer containing 5 μg Histone H1 and 20 μCi (0.74 MBq) [γ-32P]ATP for 30 minutes at 30°C. Then, samples were subjected to SDS-PAGE. The gels were stained with Coomassie blue, destained, dried, and subjected to autoradiography. The results shown in panels B and C are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood-2002-04-1177/4/m_h82223385002.jpeg?Expires=1769158874&Signature=HGz1X~E5TLt58NYSCPetBCjE16HAeVcNTaWGVUiflVCaARS2V5ubBB09vG2jP9RuyIqss6ikykfQzaPOeKQUZ6slyGmZh~gkfux9kvTR5OL1yc2X2jfnCWlHIwRsjeGID1xASbM2aI7b-SONh8rjGeunUj8pBAZGAq-6fs53Beek8kTOrAD8SMlISu7Krs1Oo~8omyuL-mgHlAdQOEor9h1yIWFb2Es9lE9Gk1smNECbuFeNCNEk7XVElKRpueG5GB2FUjSmiA8WfHHMbUjyXlAAMhwZmeUj50iQoiC80ZSVDc0w2kOamsqchk8hbQhSv~Dqdm245M1IxHpjBkx-2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of GATA-2 on the Ub/proteasome-dependent degradation of p21WAF1 and p27Kip1 in FDC-P1/GATA-2/ER and Ba/F3/GATA-2/ER. / (A) To examine the half-lives of p21WAF1 and p27Kip1 proteins, cells (1 × 107 cells for each sample) were radiolabeled with [35S]methionine for 30 minutes. Then, the labeled cells were washed, resuspended in DMEM containing 2 mM unlabeled methionine, and cultured with or without 2 μM estradiol for the times indicated. p21WAF1 and p27Kip1 were immunoprecipitated from total cellular lysates and subjected to SDS-PAGE. The gels were dried and subjected to the autoradiography. (B) The cells were cultured in the presence or absence of 1 μM estradiol and/or 0.1 μM lactacystin for 24 hours. The protein expression levels of p21WAF1 and p27Kip1 were examined by Western blot analysis on total cellular lysates. (C) The cells were cultured with 1 μM estradiol for the times indicated and subjected to Northern blot analysis on the expressions of Skp1, Skp2, and Cul1. The results show representative data from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood-2002-04-1177/4/m_h82223385003.jpeg?Expires=1769158874&Signature=mVCJxOUMI74AYZjVrIMIcPFQO2LUulIfbBp5urkfL3KKRIGD586thge9iobxpHeuvbksgQVtJExZkcaipEH50KsYDAm9KWIRlqO16IR5CDNr1hK40hqozLD3KxT79uNFzt3ZiHdM-y1CsTLq-Q-pcHa7Y7kK0FxAGnhnXYoxXr7HzXQtkd1z3s3lpoENqbMrvR4tibVDap4uhELwQxQCmvkCl751mPYFmXQ2e-so~ZpoXcgr1SEVQA40CoiquRnGUnkFXqUdbUN2vjxZcLXDl8UMo~2uzqNYASOyyWYSOgywIKDtFpNGO1O~wkgejJnl9baBL5ShISmq8jkt~OFtQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal