Abstract
Results of previous studies have suggested that transplantation-related mortality among patients with chronic graft-versus-host disease (GVHD) may be reduced by combined treatment with cyclosporine (CSP) and prednisone rather than by prednisone alone. In a randomized trial, we assessed the efficacy of cyclosporine plus prednisone versus prednisone alone as initial therapy for chronic GHVD among patients whose platelet counts were higher than 100 000/μL. Prednisone was administered initially at a dose of 1.0 mg/kg per day orally, followed by a prolonged taper, and cyclosporine was administered at 6 mg/kg orally twice daily every other day. The cumulative incidence of transplantation-related mortality at 5 years from enrollment was 17% (95% CI, 0.11-0.23) in the CSP plus prednisone arm and 13% (95% CI, 0.08-0.19) in the prednisone arm. The hazards of transplantation-related mortality, overall mortality, recurrent malignancy, secondary therapy, and discontinuation of all immunosuppressive therapy were not significantly different between the 2 arms, but survival without recurrent malignancy was lower in the 2-drug arm (P = .03). Avascular necrosis developed in 18 (13%) of the 142 patients in the CSP plus prednisone arm and in 32 (22%) of the 145 patients in the prednisone arm (P = .04). Treatment with CSP plus prednisone may reduce the risk for steroid-related toxicity, but results of the current study do not substantiate the hypothesis that the administration of CSP reduces transplantation-related mortality among patients with chronic GVHD.
Introduction
Chronic GHVD is the major late complication following allogeneic transplantation.1,2 Systemic treatment is not necessary when clinical manifestations are not severe and are limited to a single organ, but prolonged immunosuppressive treatment is indicated when clinical manifestations extend to more than one organ. Among patients with clinical extensive chronic GVHD, thrombocytopenia was the first factor identified as an indicator of poor prognosis.3,4 In an early trial, the survival rate at 3 years after transplantation was only 26% among 38 patients with chronic GVHD and thrombocytopenia when prednisone was used for treatment.1 3
Long-term treatment with high-dose prednisone is associated with a high risk for morbidity. Complications prominently include avascular necrosis, glucose intolerance requiring administration of insulin, infections, hypertension, weight gain, changes in body habitus, cutaneous atrophy and striae, cataracts, osteoporosis, emotional lability, interference with sleep, and growth retardation in children. Some of these complications can be ameliorated by alternate-day administration of prednisone as opposed to daily administration,5 but alternate-day administration might be less effective in controlling chronic GVHD. These considerations led to the hypothesis that a regimen of cyclosporine (CSP) and prednisone administered on alternating days might have better efficacy and less toxicity than prednisone alone for the treatment of chronic GVHD.
In support of this hypothesis, survival at 3 years after transplantation was 52% among 40 patients with chronic GVHD and thrombocytopenia when an alternate-day regimen of CSP and prednisone was used for treatment.1,6 Encouraged by these results among patients with thrombocytopenia, we designed a clinical trial to compare CSP alternating with prednisone every other day versus alternate-day prednisone alone as treatment for newly diagnosed clinical extensive chronic GVHD in patients with platelet counts higher than 100 000/μL. Interim results have been published.1
Patients and methods
The diagnosis and staging of chronic GVHD were established by using previously published clinical, histologic, and laboratory criteria.7 8 All patients gave institutional review board–approved written, informed consent for randomization of treatment to prednisone alone versus CSP plus prednisone. All patients received prednisone as a single morning dose of 1 mg/kg orally for 2 weeks. The dosage was gradually tapered on alternate days to reach an every other day regimen of administration after 6 weeks. The dosage of prednisone was then maintained at 1.0 mg/kg every other day until week 20 and was tapered to 0.5 mg/kg by week 22. This dosage was maintained until week 40 and then was tapered to discontinuation if there was no clinical evidence of chronic GVHD. CSP was given at a dosage of 6 mg/kg orally in the morning and evening every other day until week 40 and then was gradually tapered. Patients treated with CSP at the time of enrollment had the administration of CSP tapered to discontinuation within 6 to 9 weeks if they were assigned to receive prednisone alone. Criteria for treatment failure included exacerbation of chronic GVHD manifestations in sites previously affected by the disease or development of manifestations in previously unaffected sites. The occurrence of avascular necrosis was monitored by reviewing correspondence from referring physicians and by including this item in questionnaires sent to the referring physician for each patient at annual intervals.
Patients were enrolled consecutively between September 1985 and February 1992. The primary endpoint of this study was transplantation-related mortality, defined as death from any cause other than recurrent malignancy. With 150 patients enrolled per arm, there was at least 90% power to detect a hazard ratio of 0.65 for the primary endpoint in the CSP plus prednisone arm compared with the prednisone arm, assuming a 2-sided type 1 error rate of .05 and a 20% incidence of transplantation-related mortality in the prednisone arm.3
Time-to-event outcomes were dated from enrollment in the study. Cumulative incidence rates9 were used to estimate the probabilities of transplantation-related mortality, recurrent malignancy, secondary therapy (defined as the administration of any systemic immunosuppressive treatment not prescribed as part of the original randomized assignment), and discontinuation of all immunosuppressive treatment before the onset of recurrent malignancy. Because direct evaluation at regular intervals was not possible for the high proportion of patients who lived long distances from Seattle and because there are no validated scales for assessment of response in patients with chronic GVHD, permanent discontinuation of all immunosuppressive treatment was used as a surrogate for resolution of the disease. Similarly, the administration of secondary therapy was used as a surrogate for failure of the protocol-assigned treatment. The Kaplan-Meier method was used to estimate overall survival and survival without recurrent malignancy.10 Because there was some imbalance between study arms with respect to sex of patient, GVHD prophylaxis, and onset of chronic GVHD, multivariable Cox proportional hazards models were used to evaluate adjusted hazard ratios and Pvalues for comparisons between arms. Two-sidedP < .05 was considered statistically significant.
Results
A total of 307 patients were randomized, 154 to the CSP plus prednisone arm and 153 to the prednisone arm. Twenty patients were subsequently excluded from analysis—14 because of ineligibility, 5 because informed consent could not be documented, and 1 because of inadequate records. With 3 exceptions, demographic characteristics of the remaining 287 patients were similar in the 2 groups (Table1). The CSP plus prednisone arm contained a higher proportion of female patients (P = .05, χ2 test), a lower proportion of patients who received both methotrexate and CSP for GVHD prophylaxis (P = .05, Fisher exact test), and a lower proportion of patients with progressive onset of chronic GVHD (P = .04, χ2test).
Characteristics of patients, according to treatment arm
| Characteristic . | Study arm . | |
|---|---|---|
| Prednisone (N = 145) . | CSP plus prednisone (N = 142) . | |
| Patient age at transplantation, median y (range) | 30.0 (2.3-56.5) | 30.8 (0.9-57.1) |
| Disease risk, N (%)* | ||
| Low | 61 (42) | 54 (38) |
| Intermediate | 46 (32) | 50 (35) |
| High | 38 (26) | 38 (27) |
| Donor type, N (%) | ||
| HLA-identical sibling | 99 (68) | 95 (67) |
| HLA-mismatched relative | 32 (22) | 28 (20) |
| Unrelated | 14 (10) | 19 (13) |
| Patient/donor sex, N (%) | ||
| Female/female | 30 (21) | 43 (30) |
| Female/male | 22 (15) | 24 (17) |
| Male/female | 53 (37) | 35 (25) |
| Male/male | 40 (28) | 40 (28) |
| Prophylaxis for acute GVHD, N (%) | ||
| Methotrexate | 9 (6) | 15 (11) |
| Cyclosporine ± glucocorticoids | 10 (7) | 20 (14) |
| Methotrexate plus cyclosporine | 122 (84) | 106 (75) |
| Other† | 4 (3) | 1 (1) |
| Onset type, N (%)‡ | ||
| De novo | 51 (35) | 44 (31) |
| Quiescent | 65 (45) | 82 (58) |
| Progressive | 29 (20) | 16 (11) |
| Immunosuppressive medications at enrollment, N (%) | ||
| Cyclosporine alone | 56 (39) | 38 (27) |
| Cyclosporine plus prednisone | 23 (16) | 31 (22) |
| Prednisone | 12 (8) | 9 (6) |
| None | 54 (37) | 64 (45) |
| Interval from transplantation to onset of chronic GVHD, N (%) | ||
| < 100 days | 41 (28) | 33 (23) |
| ≥ 100 days | 104 (72) | 109 (77) |
| Characteristic . | Study arm . | |
|---|---|---|
| Prednisone (N = 145) . | CSP plus prednisone (N = 142) . | |
| Patient age at transplantation, median y (range) | 30.0 (2.3-56.5) | 30.8 (0.9-57.1) |
| Disease risk, N (%)* | ||
| Low | 61 (42) | 54 (38) |
| Intermediate | 46 (32) | 50 (35) |
| High | 38 (26) | 38 (27) |
| Donor type, N (%) | ||
| HLA-identical sibling | 99 (68) | 95 (67) |
| HLA-mismatched relative | 32 (22) | 28 (20) |
| Unrelated | 14 (10) | 19 (13) |
| Patient/donor sex, N (%) | ||
| Female/female | 30 (21) | 43 (30) |
| Female/male | 22 (15) | 24 (17) |
| Male/female | 53 (37) | 35 (25) |
| Male/male | 40 (28) | 40 (28) |
| Prophylaxis for acute GVHD, N (%) | ||
| Methotrexate | 9 (6) | 15 (11) |
| Cyclosporine ± glucocorticoids | 10 (7) | 20 (14) |
| Methotrexate plus cyclosporine | 122 (84) | 106 (75) |
| Other† | 4 (3) | 1 (1) |
| Onset type, N (%)‡ | ||
| De novo | 51 (35) | 44 (31) |
| Quiescent | 65 (45) | 82 (58) |
| Progressive | 29 (20) | 16 (11) |
| Immunosuppressive medications at enrollment, N (%) | ||
| Cyclosporine alone | 56 (39) | 38 (27) |
| Cyclosporine plus prednisone | 23 (16) | 31 (22) |
| Prednisone | 12 (8) | 9 (6) |
| None | 54 (37) | 64 (45) |
| Interval from transplantation to onset of chronic GVHD, N (%) | ||
| < 100 days | 41 (28) | 33 (23) |
| ≥ 100 days | 104 (72) | 109 (77) |
Low-risk diseases included chronic myeloid leukemia in chronic phase, refractory anemia, aplastic anemia, and Blackfan-Diamond syndrome. Intermediate-risk diseases included chronic myeloid leukemia in accelerated phase or in chronic phase after blast phase, acute leukemia or lymphoma in remission, refractory anemia with excess blasts, chronic lymphocytic leukemia, and paroxysmal nocturnal hemoglobinuria. High-risk diseases included chronic myeloid leukemia in blast phase, juvenile chronic myeloid leukemia, acute leukemia or lymphoma in relapse, refractory anemia with excess blasts in transformation, and myeloma.
Two patients in the prednisone arm underwent T cell–depleted second marrow transplantation with no posttransplantation immunosuppression, and 2 underwent unmodified second marrow transplantation with glucocorticoids or no posttransplantation immunosuppression for the prevention of acute GVHD. One patient in the CSP plus prednisone arm underwent a second marrow transplantation, with glucocorticoids and ATG administered for the prevention of acute GVHD.
De novo onset indicates the absence of prior acute GVHD. Quiescent onset indicates a history of acute GVHD that resolved before the onset of chronic GVHD. Progressive onset indicates a history of acute GVHD that did not resolve before the onset of chronic GVHD.
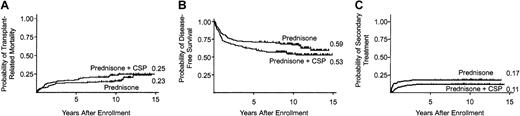
The cumulative incidence of death before recurrent malignancy at 5 years was 17% (95% CI, 0.11-0.23) in the CSP plus prednisone arm and 13% (95% CI, 0.8-0.19) in the prednisone arm (Figure1A). The risks for transplantation-related mortality were not significantly different in the 2 arms (Table 2).
Administration of CSP at a dose of 12 mg/kg every other day did not add benefit when given with prednisone for treatment of newly diagnosed chronic GVHD.
(A) Mortality from causes other than recurrent malignancy, where recurrent malignancy is considered a competing risk (P = .11). (B) Survival without recurrent malignancy (P = .03). (C) Secondary therapy for chronic GVHD, where recurrent malignancy is considered a competing risk (P = .35). Tic marks indicate censors at date of last contact, and numbers at the end of each curve indicate point estimates at the end of follow-up.
Administration of CSP at a dose of 12 mg/kg every other day did not add benefit when given with prednisone for treatment of newly diagnosed chronic GVHD.
(A) Mortality from causes other than recurrent malignancy, where recurrent malignancy is considered a competing risk (P = .11). (B) Survival without recurrent malignancy (P = .03). (C) Secondary therapy for chronic GVHD, where recurrent malignancy is considered a competing risk (P = .35). Tic marks indicate censors at date of last contact, and numbers at the end of each curve indicate point estimates at the end of follow-up.
Outcomes according to treatment arm
| Outcome . | HR* . | 95% CI . | P . |
|---|---|---|---|
| Death before recurrent malignancy | 1.55 | 0.90-2.65 | .11 |
| Secondary immunosuppressive therapy | 0.73 | 0.38-1.37 | .35 |
| Discontinuation of immunosuppression | 1.17 | 0.86-1.58 | .32 |
| Recurrent malignancy | 1.17 | 0.68-2.02 | .57 |
| Mortality | 1.35 | 0.91-2.01 | .13 |
| Transplantation-related mortality or relapse | 1.51 | 1.03-2.21 | .03 |
| Outcome . | HR* . | 95% CI . | P . |
|---|---|---|---|
| Death before recurrent malignancy | 1.55 | 0.90-2.65 | .11 |
| Secondary immunosuppressive therapy | 0.73 | 0.38-1.37 | .35 |
| Discontinuation of immunosuppression | 1.17 | 0.86-1.58 | .32 |
| Recurrent malignancy | 1.17 | 0.68-2.02 | .57 |
| Mortality | 1.35 | 0.91-2.01 | .13 |
| Transplantation-related mortality or relapse | 1.51 | 1.03-2.21 | .03 |
Hazard ratios (HR) compare results for the CSP plus prednisone arm with those for the prednisone arm, with adjustments for sex of patient, GVHD prophylaxis, and type of onset. Results were similar to those reported in the table when disease risk and sex of patient/donor were added to the model (data not shown).
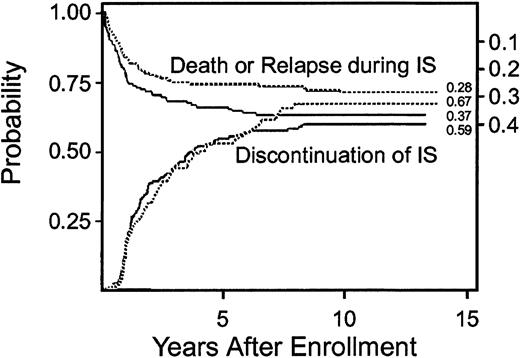
Cumulative incidence of secondary therapy at 5 years was 11% (95% CI, 0.6-0.16) in the CSP plus prednisone arm compared to 17% (95% CI, 0.11-0.23) in the prednisone arm. The risks for secondary therapy were not significantly different in the 2 arms (Table 2). Eighty-four patients in the prednisone plus CSP arm and 97 patients in the prednisone arm discontinued all immunosuppressive therapy before death or the onset of recurrent malignancy. The median interval time from enrollment in the study to discontinuation of immunosuppressive treatment among these patients was 1.6 years (range, 0.3-8.2 years) in the CSP plus prednisone arm and 2.2 years (range, 0.2-7.9 years) in the prednisone arm (P = .26). The cumulative incidence of discontinued immunosuppressive treatment at 5 years was 54% (95% CI, 0.46-0.62) in the CSP plus prednisone arm and 53% (95% CI, 0.45-0.61) in the prednisone arm (Figure 2). Eighteen (13%) patients in the CSP plus prednisone arm and 32 (22%) patients in the prednisone arm acquired avascular necrosis (P = .04). Other complications of glucocorticoid treatment were not compared in the 2 arms.
Differences in the probability of discontinued immunosuppressive treatment in patients treated with prednisone alone (- - -) or with CSP plus prednisone (—) were not statistically significant.
Lower curves and left-side scale in each panel show the cumulative incidence of discontinued immunosuppressive treatment (IS) before death or recurrent malignancy (P = .32). Upper curves and right-side scale show the cumulative incidence of death or recurrent malignancy during continued immunosuppressive treatment as competing risks. The space between the 2 curves indicates the proportion of patients surviving without recurrent malignancy and continuing with immunosuppressive treatment. Numbers at the end of each curve indicate point estimates at the end of follow-up.
Differences in the probability of discontinued immunosuppressive treatment in patients treated with prednisone alone (- - -) or with CSP plus prednisone (—) were not statistically significant.
Lower curves and left-side scale in each panel show the cumulative incidence of discontinued immunosuppressive treatment (IS) before death or recurrent malignancy (P = .32). Upper curves and right-side scale show the cumulative incidence of death or recurrent malignancy during continued immunosuppressive treatment as competing risks. The space between the 2 curves indicates the proportion of patients surviving without recurrent malignancy and continuing with immunosuppressive treatment. Numbers at the end of each curve indicate point estimates at the end of follow-up.
Cumulative incidence of recurrent malignancy at 5 years was 39% (95% CI, 0.29-0.51) in the CSP plus prednisone arm compared with 37% (95% CI, 0.23-0.51) in the prednisone arm. The risks for recurrent malignancy across the entire length of follow-up were not significantly different in the 2 arms (Table 2). Survival at 5 years was 67% (95% CI, 0.58-0.74) in the CSP plus prednisone arm compared with 72% (95% CI, 0.64-0.79) in the prednisone arm. The hazards of mortality across the entire length of follow-up were not statistically different in the 2 arms (Table 2). Survival without recurrent malignancy at 5 years was 61% (95% CI, 0.53-0.69) in the CSP plus prednisone arm, compared with 71% (95% CI, 0.63-0.78) in the prednisone arm (Figure 1C). Analysis across the entire length of follow-up indicated a statistically significant higher composite risk for transplantation-related mortality or recurrent malignancy in the CSP plus prednisone arm (Table 2), resulting in a lower probability of survival without recurrent malignancy.
Patients with a progressive onset of chronic GVHD are now recognized as having increased risk for transplantation-related mortality than those with de novo or quiescent onset (defined in Table1).1,4 11 Because the CSP plus prednisone arm contained a lower proportion of patients with progressive onset of chronic GVHD, we analyzed results of this study separately among patients with progressive onset and among those with de novo or quiescent onset. Fifteen (12%) of 126 patients with de novo or quiescent onset chronic GVHD in the CSP plus prednisone arm acquired avascular necrosis, compared with 27 (23%) of 116 patients in the prednisone arm. Among patients with de novo or quiescent onset chronic GVHD, there was no statistically significant difference in any other outcome measure between the 2 arms (data not shown).
Demographic characteristics of the progressive onset subsets were similar in the 2 arms (data not shown). Avascular necrosis developed in 3 of 16 (19%) patients with progressive onset chronic GVHD in the CSP plus prednisone arm compared with 5 of 29 (17%) patients in the prednisone arm. The cumulative incidence of secondary therapy and the cumulative incidence of discontinued immunosuppressive treatment were not significantly different between the 2 arms in the progressive onset subset (data not shown). Within this subset, the cumulative incidence of death from causes other than recurrent malignancy at 5 years was 38% (95% CI, 0.14-0.61) in the CSP plus prednisone arm compared with 14% (95% CI, 0.1-0.26) in the prednisone arm. Survival at 5 years was 43% (95% CI, 0.19-0.65) in the CSP plus prednisone arm compared with 76% (95% CI, 0.56-0.88) in the prednisone arm. Among the 16 progressive onset patients in the CSP plus prednisone arm, there were 11 deaths, 4 caused by recurrent malignancy, 1 by accident, and 6 by infections (2 bacterial, 1 cytomegalovirus, 1 hepatitis C, 1 influenza, and 1 Aspergillus). Five of the 6 patients with fatal infection were taking cyclosporine at the onset of infection. Among the 29 progressive onset patients in the prednisone arm, there were 9 deaths, 4 caused by recurrent malignancy, 1 by secondary malignancy, and 4 by infection (1 bacterial, 1 cytomegalovirus, 1 herpes simplex virus, 1 Pneumocystis). Three of the 4 patients with fatal infection were taking CSP at the onset of infection.
Discussion
The data from this study support the hypothesis that at least one major toxicity caused by glucocorticoid treatment can be prevented in patients with chronic GVHD by alternate-day treatment with CSP plus prednisone as opposed to treatment with prednisone alone. Although detailed information about the dose of prednisone was not collected, we suspect that the amount of glucocorticoid treatment was lower in the 2-drug arm. In patients with de novo or quiescent onset chronic GVHD, continued administration of CSP had no statistically significant effect on the duration of immunosuppressive treatment, the need for secondary treatment, the risks for transplantation-related mortality or recurrent malignancy, or survival.
The similarity in duration of immunosuppressive treatment and the need for secondary therapy between the 2 arms suggests no improvement in efficacy of the 2-drug regimen for controlling chronic GVHD compared with prednisone alone. Results of previous randomized clinical trials suggested that the incidence of chronic GVHD was not affected by early discontinuation of CSP administration in patients who did not have acute GVHD on day 60 after transplantation12 or by prolongation of CSP administration in patients who did not have chronic GVHD on day 80 after transplantation.13 Thus, the regimens of CSP tested in the 2 previous studies and in the present study appear to have little effect on the development or resolution of chronic GVHD in the populations tested.
Among the subgroup of patients with progressive onset chronic GVHD, results in the CSP plus prednisone arm were similar to those of a previous single-arm study,6 in which approximately 40% of patients with thrombocytopenia at the time of diagnosis died of causes other than recurrent malignancy, and long-term survival was approximately 50%. Results for the progressive onset subset in the prednisone arm of the present study were much better than those of a previous single-arm study in which patients were treated with prednisone alone.3 In that study, 47% of patients with thrombocytopenia at the time of diagnosis died of causes other than recurrent malignancy compared with 14% in the present study, and long-term survival was only 26% compared with 76% in the present study. Thirteen of 38 (34%) patients in the previous study died of infection, and in 6 patients the infections were caused by bacteria. In both studies, patients were given trimethoprim-sulfa with or without penicillin to prevent infection.
Indicators of poor prognosis and the immunosuppressive regimens given to prevent acute GVHD were different in the 2 studies. In the previous study,3 12 of the 38 patients with thrombocytopenia as an indicator of poor prognosis also had a progressive onset of chronic GVHD. In the present study, none of the patients with progressive onset chronic GVHD had thrombocytopenia. The difference in number of risk factors between the 2 studies might explain the better survival in the present study.4 In the previous study,3 only 2 of 38 patients treated with prednisone alone received a CSP-containing regimen for the prevention of acute GVHD, compared with 26 of 29 patients with progressive onset chronic GVHD in the prednisone arm of the present study. The better outcome in the present study might suggest that the use of CSP in the GVHD prophylaxis regimen could have made subsequent chronic GVHD more amenable to treatment with glucocorticoids.
The number of patients with progressive onset chronic GVHD was not large enough to determine whether the alternate-day regimen of CSP and prednisone had improved efficacy in controlling chronic GVHD compared to prednisone alone, as determined by the proportions of patients who discontinued immunosuppression or required secondary immunosuppressive therapy. By these criteria, we found no evidence to suggest strikingly improved efficacy for the 2-drug regimen among patients with de novo or quiescent onset chronic GVHD. This finding argues against the possibility that the 2-drug regimen might have such an effect among patients with progressive onset chronic GVHD after receiving CSP for the prevention of acute GVHD. Differences between the 2 arms of the present study suggest that continued treatment with CSP might impair immune function, thereby increasing the risk for fatal infections in patients with progressive onset chronic GVHD.
Although we cannot conclude definitively from the subset analysis that cyclosporine causes harm in patients with progressive onset chronic GVHD, our data certainly do not support the hypothesis that treatment with CSP provides the survival benefit that was anticipated from the results of earlier studies.1,3,6 For patients with de novo or quiescent onset chronic GVHD, the alternate-day regimen of CSP plus prednisone might decrease the risk for glucocorticoid-related morbidity without increasing the risk for complications caused by too much immunosuppression. For patients with progressive onset chronic GVHD, alternative approaches are needed to improve the efficacy of treatment and to reduce the risks for glucocorticoid-related morbidity.14
We thank the physicians and nurses who cared for patients, Aurora Brandvold, RN, Judy Campbell, RN, and Muriel Siadak, PAC, for assistance with data collection, Chris Davis for assistance with data management, and Deborah Anderson for assistance in preparing the figures.
Supported by National Institutes of Health grants CA15704, HL36444, CA18221, and CA18029.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul J. Martin, Fred Hutchinson Cancer Research Center D2-100, PO Box 19204, Seattle, WA 98109-1024; e-mail:pmartin@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal