Abstract
We determined the prognostic importance of morphologically identifiable persistent disease at day 15 and days 22 to 25 of remission induction in childhood acute lymphoblastic leukemia (ALL). Among 546 patients entered on 2 consecutive protocols, 397 patients had evaluable bone marrow (BM) examinations on day 15 (± 1 day) and 218 on days 22 to 25 (± 1 day). Fifty-seven patients (14%) had persistent lymphoblasts (≥ 1%) in the BM on day 15 and 27 patients (5.5%) had persistent lymphoblasts on days 22 to 25. The 5-year event-free survival (EFS) was significantly worse for patients with lymphoblasts on day 15 (40% ± 6%) or on days 22 to 25 (4% ± 3%) as compared to those without lymphoblasts on these dates (78% ± 2% and 76% ± 2%, respectively, P < .001 for both comparisons). A worse prognosis was observed even for patients with a low percentage of lymphoblasts (ie, 1%-4%) at either day 15 (5-year EFS = 56% ± 8%) or days 22 to 25 (5-year EFS = 0%) compared to those without morphologically identifiable persistent lymphoblasts at these times (P < .001 for both comparisons). The prognostic impact of persistent lymphoblasts on both dates remained significant after adjusting for other known risk factors, including treatment protocol, age, white blood cell count, DNA index, cell lineage, and central nervous system status, and National Cancer Institute/Rome criteria simultaneously. Hence, persistence of lymphoblasts (even 1%-4%) on day 15 of remission induction was associated with a poor prognosis and on days 22 to 25 signified a particularly dismal outcome; these very high-risk patients require novel or more intensive therapy to improve outcome.
Introduction
Significant improvement in the ability to successfully treat children with acute lymphoblastic leukemia (ALL) is in part based on the sequential development of risk-adapted therapy.1,2 Over the past 30 years, various factors have been identified that have helped identify children who are at increased risk for treatment failure and, thus, are eligible for novel or more intensive therapy. For many years, clinical and laboratory features such as age and white blood cell (WBC) count have been used to assign therapy. Subsequently, biologic features such as the DNA index (DI) and the presence or absence of certain genetic abnormalities (t(9;22), t(4;11), t(1;19), and MLL or TEL gene rearrangements) were shown to have prognostic significance and improve risk assignment.3-20 However, even risk assignment based on genetic abnormalities lacks precision, because some children with high-risk features do well, and vice versa.
Early response to therapy, another important indicator of treatment outcome, has also been used widely to assign treatment.21-29 For example, in a Berlin-Frankfurt-Münster (BFM) trial, a blast cell count of 1000/μL or more in the peripheral blood after a 7-day exposure of prednisone and one intrathecal dose of methotrexate identified a group of patients with a significantly worse prognosis. In a subsequent trial, this group of patients was targeted for more aggressive therapy.26 Persistence of circulating blasts 7 days after multiagent remission induction was also associated with a poor prognosis in a St Jude Children's Research Hospital study.27 Others have assessed the bone marrow (BM) lymphoblast percentage to assess early response to therapy.22-26,28,29 The Children's Cancer Group (CCG) has examined the prognostic importance of persistent lymphoblasts on day 7 and day 14 of a 4- or 5-drug induction therapy for patients with high-risk ALL (presence of lymphomatous features). They found that among high-risk cases, the day 7 result had greater prognostic impact than the day 14 result; although the day 14 result provided additional information regarding the prognosis of the day 7 slow responders.28
There remain unanswered questions regarding early response evaluation. What are the optimal time points for measuring early response? What degree of persistent disease is clinically significant? Conventionally, persistent BM disease is arbitrarily defined as greater than or equal to 5% lymphoblasts. Does a smaller degree of residual disease (ie, 1%-4% lymphoblasts) have prognostic importance? To address these issues, we retrospectively reviewed the results of day 15 and days 22 to 25 BM examinations for patients with ALL of all risk groups entered on 2 consecutive institutional trials for ALL.
Patients and methods
Patients
From 1984 to 1991, 546 evaluable patients were entered on 2 consecutive protocols for newly diagnosed ALL (St Jude Total Therapy Studies XI and XII).30 31 These studies were approved by the St Jude Children's Research Hospital institutional review board and informed consent was provided according to institutional and federal guidelines. These included 288 boys and 258 girls with a median age of 6.8 years (range, 0.1-18.8 years).
There were 358 evaluable patients treated on study XI and 188 on study XII. Study XI30 included an induction phase consisting of weekly vincristine times 4, l-asparaginase given 3 times per week for 2 weeks, daunorubicin weekly for 2 weeks, prednisone daily for 28 days, and teniposide plus cytarabine on days 22, 25, and 29. Intrathecal methotrexate, hydrocortisone, and cytarabine were given to all patients on days 2, 22, and 43; additional doses were given on days 8 and 15 to those with central nervous system (CNS) leukemia. High-dose methotrexate (2 g/m2 per week for 2 weeks) with leucovorin rescue was administered as consolidation therapy. Patients were then stratified by risk classification and randomized to receive different schedules of continuation therapy for 120 weeks (including intrathecal therapy during the first year), as previously described.30 Cranial irradiation and 5 doses of triple intrathecal therapy were added after 1 year of continuous complete response (CR) for higher-risk patients (18 Gy) and for those with CNS leukemia (24 Gy) as defined by blasts in the cerebrospinal fluid (CSF) with WBC counts 5 cells/μL or higher. Study XII included an induction phase similar to study XI and continuation phase consisting of daily oral mercaptopurine (75 mg/m2) and weekly parenteral methotrexate (40 mg/m2) interrupted every 6 weeks for alternating pulses of high-dose methotrexate and teniposide plus cytarabine (5 pulses each).31 Treatment of subclinical CNS leukemia included intrathecal methotrexate, hydrocortisone, and cytarabine given during the first year of therapy, with added cranial irradiation at 1 year for those with high-risk leukemia (18 Gy) or CNS leukemia (24 Gy). In both studies, patients with any identifiable lymphoblasts in the day 15 bone marrow received 3 additional doses of l-asparaginase plus one dose of daunorubicin during the third week of remission induction. Day 22 marrows were done if lymphoblasts were present in the day 15 BM or if the absolute neutrophil count (ANC) was less than 300/μL on day 22. If lymphoblasts were identified, teniposide and cytarabine were given as scheduled; if there were no identifiable lymphoblasts, teniposide, and cytarabine were held until the ANC was 300/μL or higher.
Only those BM results obtained within 1 day of the scheduled BM examination were included in the analysis. Each BM examination was reviewed by both an experienced laboratory technician and a hematopathologist. The designation of lymphoblast was made on the basis of morphology alone. Flow cytometry was not available during the study period to confirm the result reported by morphologic examination. The blast morphology in day 15/22 to 25 BMs was compared to that of leukemic blasts in diagnostic samples. BMs were considered not evaluable if they lacked marrow particles or cellular elements or both. A total of 397 patients had evaluable BM examinations on day 15 (± 1 day) and 218 on days 22 to 25 (± 1 day). For patients with both day 15 and days 22 to 25 BM examinations performed (n = 107), there were none who were negative for lymphoblasts on the day 15 examination who became positive by days 22 to 25; therefore, patients who did not have a day 22 BM examination were assumed to have no lymphoblasts in that marrow, provided that their day 15 BM was negative for lymphoblasts. There were 143 such patients from study XI and 134 such patients from study XII. A total of 126 patients did not have a day 15 BM examination. Study XI originally required all patients to receive a BM examination on day 25 of induction therapy; however, an amendment to this study replaced the required day 25 exam with a day 15 exam for all patients and a subsequent day 22 exam for patients with residual disease on day 15.
Statistical methods
Event-free survival (EFS) was measured from the date the patient went on study to the first failure of any kind (relapse, second malignancy, or death) or the date of last follow-up. Patients who did not achieve a CR by day 43 were assigned an EFS value of zero. Distributions of EFS were estimated by the method of Kaplan and Meier32 and were compared by the Mantel-Haenszel statistic.33 The Mantel-Haenszel test was used to evaluate associations between EFS and the presence/absence of blasts on day 15 and days 22 to 25, separately, and a number of presenting prognostic features that were determined a priori. The Wald statistic from a Cox proportional hazards regression model34 was used to evaluate the significance of having blasts on day 15 and days 22 to 25 with respect to EFS while controlling for other prognostic factors. To examine the reproducibility of the morphologic examinations, we randomly selected 50 cases to review blindly by a hematopathologist and a technologist. The weighted κ coefficient was used to assess the level of agreement between the original results and the results of the blinded rereview by comparing the data, which were categorized on the same ordinal scale. All analyses were conducted using SAS Release 6.12 (SAS Institute Incorporated, Cary, NC).
Results
Fifty-seven patients (14%) had persistent lymphoblasts (≥ 1%) in the BM on day 15 and 27 patients (5.5%) had persistent lymphoblasts on days 22 to 25 (Table 1). Of the 117 patients who actually received day 25 BM examinations, 14 were outside the acceptable range of days and one was considered to be not evaluable. Of the remaining 102 patients with eligible and evaluable day 25 marrows, 10 (9.8%) were positive for lymphoblasts. Persistence of BM lymphoblasts on day 15 or days 22 to 25 of induction was correlated with a number of adverse presenting features (Table2): high WBC count, unfavorable age, and T-cell immunophenotype on day 15, and WBC count, unfavorable age, T-cell immunophenotype, unfavorable DI (ie, < 1.16 or > 1.60) and the presence of the Philadelphia chromosome on days 22 to 25.MLL gene rearrangement and CNS status at diagnosis were not associated with persistent disease on either day 15 or 22 to 25.
Frequency and degree of BM involvement by time of examination during induction therapy
| % lymphoblasts . | Day 15, n = 397 (%) . | Day 22-25, n = 495 (%) . |
|---|---|---|
| 1 to 4% | 34 (8.6) | 11 (2.2) |
| At least 5% | 23 (5.8) | 16 (3.2) |
| Any amount (≥ 1%) | 57 (14.4) | 27 (5.4) |
| % lymphoblasts . | Day 15, n = 397 (%) . | Day 22-25, n = 495 (%) . |
|---|---|---|
| 1 to 4% | 34 (8.6) | 11 (2.2) |
| At least 5% | 23 (5.8) | 16 (3.2) |
| Any amount (≥ 1%) | 57 (14.4) | 27 (5.4) |
Association of persistent BM disease with presenting features
| Presenting feature . | Day 15 . | P . | Day 22-25 . | P . | ||
|---|---|---|---|---|---|---|
| % patients with listed feature . | % patients with listed feature . | |||||
| BM + . | BM − . | BM + . | BM − . | |||
| WBC (greater than 50 × 109/L) | 33 | 22 | .06 | 48 | 24 | < .01 |
| Age younger than 1 y, 10 y or older | 44 | 27 | .01 | 56 | 28 | < .01 |
| T-cell | 31 | 15 | < .01 | 33 | 17 | .02 |
| DI less than 1.16, greater than 1.60 | 84 | 78 | .30 | 96 | 77 | .02 |
| t(9;22) | 5 | 4 | .75 | 11 | 4 | .03 |
| Presenting feature . | Day 15 . | P . | Day 22-25 . | P . | ||
|---|---|---|---|---|---|---|
| % patients with listed feature . | % patients with listed feature . | |||||
| BM + . | BM − . | BM + . | BM − . | |||
| WBC (greater than 50 × 109/L) | 33 | 22 | .06 | 48 | 24 | < .01 |
| Age younger than 1 y, 10 y or older | 44 | 27 | .01 | 56 | 28 | < .01 |
| T-cell | 31 | 15 | < .01 | 33 | 17 | .02 |
| DI less than 1.16, greater than 1.60 | 84 | 78 | .30 | 96 | 77 | .02 |
| t(9;22) | 5 | 4 | .75 | 11 | 4 | .03 |
The day 43 CR rate for patients with any lymphoblasts (≥ 1%) in the day 15 BM was significantly lower than that for other patients (89% versus 99%, P = .001). The day 43 CR rate for children with 1% lymphoblasts or more on days 22 to 25 was even lower (59%). The CR rates varied according to degree of BM involvement (ie, 1% to 4% versus ≥ 5%): 97% versus 78% for day 15 results and 64% versus 56% for days 22 to 25 results.
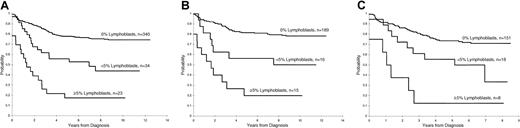
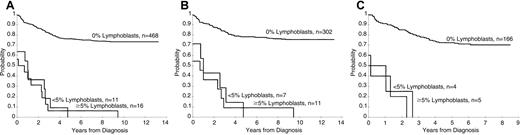
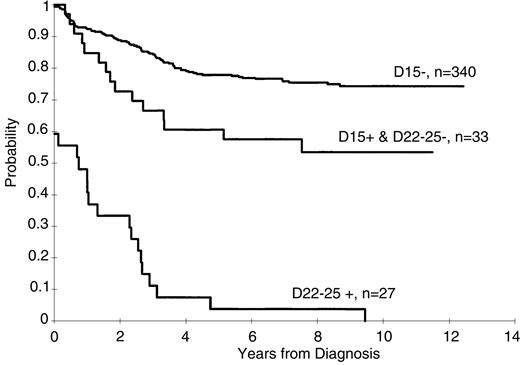
Persistence of lymphoblasts (≥ 1%) on the day 15 and days 22 to 25 BM was associated with a poorer treatment result (P < .001 for both comparisons, Figures1 and 2). The 5-year EFS rates ± 1 SE for patients with and without lymphoblasts on day 15 were 40% ± 6% and 78% ± 2%, respectively, and on days 22 to 25 they were 4% ± 3% and 76% ± 2%, respectively. A worse prognosis was observed even for those patients with a low percentage of persistent lymphoblasts (ie, 1%-4%) at either day 15 (5-year EFS = 56% ± 8%) or days 22 to 25 (5-year EFS = 0%) compared to patients who did not have morphologically identifiable persistent lymphoblasts at these times (P < .001 for both comparisons). The EFS for patients with lymphoblasts (≥ 1%) on day 15 that cleared by days 22 to 25 was significantly (P = .006) worse than that for patients with no lymphoblasts on day 15 (5-year rate = 61% ± 8% versus 78% ± 2%, respectively (Figure 3).
Day 15 estimates of EFS in patients with ALL.
(A) Kaplan-Meier estimate of EFS according to the percentage of lymphoblasts on the day 15 BM in studies XI and XII combined. (B) Kaplan-Meier estimates of EFS by day 15 BM blast percentage in study XI. (C) Kaplan-Meier estimates of EFS by day 15 BM blast percentage in study XII.
Day 15 estimates of EFS in patients with ALL.
(A) Kaplan-Meier estimate of EFS according to the percentage of lymphoblasts on the day 15 BM in studies XI and XII combined. (B) Kaplan-Meier estimates of EFS by day 15 BM blast percentage in study XI. (C) Kaplan-Meier estimates of EFS by day 15 BM blast percentage in study XII.
Day 22 estimates of EFS.
(A) Kaplan-Meier estimates of EFS according to the percentage of lymphoblasts in studies XI and XII. (B) Kaplan-Meier estimates of EFS by day 22 BM blast percentage in study XI. (C) Kaplan-Meier estimates of EFS by day 22 BM blast percentage in study XII.
Day 22 estimates of EFS.
(A) Kaplan-Meier estimates of EFS according to the percentage of lymphoblasts in studies XI and XII. (B) Kaplan-Meier estimates of EFS by day 22 BM blast percentage in study XI. (C) Kaplan-Meier estimates of EFS by day 22 BM blast percentage in study XII.
Kaplan-Meier estimates of EFS for patients with no lymphoblasts on day 15, for those with lymphoblasts on day 15 but with no lymphoblasts on day 22, and for those with lymphoblasts on day 22.
Kaplan-Meier estimates of EFS for patients with no lymphoblasts on day 15, for those with lymphoblasts on day 15 but with no lymphoblasts on day 22, and for those with lymphoblasts on day 22.
A Cox proportional hazards model revealed the independent prognostic importance of persistent lymphoblasts (≥ 1%) in the day 15 and days 22 to 25 marrow; those with persistent disease on day 15 and day 22 had a 3-fold and 10-fold higher risk of failure, respectively (Table3). These results were significant even after adjusting for age, WBC count, National Cancer Institute (NCI)/Rome risk criteria, DI, lineage, CNS status, and treatment protocol, simultaneously. The sites of relapse are summarized in Table4.
Adverse prognostic factors in Cox proportional hazards model
| Feature . | Day 15 . | Days 22-25 . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio3-150 . | 95% CI . | P . | Hazard ratio3-150 . | 95% CI . | P . | |
| At least 1% lymphoblasts | 3.14 | (2.06, 4.79) | < .001 | 9.83 | (6.12, 15.79) | < .001 |
| CNS status at diagnosis | 1.88 | (1.26, 2.81) | .002 | 2.34 | (1.54, 3.24) | < .001 |
| DI | 1.85 | (1.03, 3.32) | .040 | 1.56 | (0.91, 2.67) | .11 |
| Age at diagnosis, y | 1.13 | (0.63, 2.04) | .68 | 1.05 | (0.66, 1.66) | .84 |
| WBC at diagnosis | 0.95 | (0.55, 1.64) | .85 | 1.21 | (0.76, 1.93) | .42 |
| Lineage | 0.99 | (0.60, 1.62) | .96 | 1.15 | (0.75, 1.75) | .53 |
| NCI/Rome risk criteria | 1.42 | (0.71, 2.85) | .32 | 1.52 | (0.83, 2.79) | .17 |
| Feature . | Day 15 . | Days 22-25 . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio3-150 . | 95% CI . | P . | Hazard ratio3-150 . | 95% CI . | P . | |
| At least 1% lymphoblasts | 3.14 | (2.06, 4.79) | < .001 | 9.83 | (6.12, 15.79) | < .001 |
| CNS status at diagnosis | 1.88 | (1.26, 2.81) | .002 | 2.34 | (1.54, 3.24) | < .001 |
| DI | 1.85 | (1.03, 3.32) | .040 | 1.56 | (0.91, 2.67) | .11 |
| Age at diagnosis, y | 1.13 | (0.63, 2.04) | .68 | 1.05 | (0.66, 1.66) | .84 |
| WBC at diagnosis | 0.95 | (0.55, 1.64) | .85 | 1.21 | (0.76, 1.93) | .42 |
| Lineage | 0.99 | (0.60, 1.62) | .96 | 1.15 | (0.75, 1.75) | .53 |
| NCI/Rome risk criteria | 1.42 | (0.71, 2.85) | .32 | 1.52 | (0.83, 2.79) | .17 |
Stratifying for treatment protocol and simultaneously adjusting for age, WBC, NCI/Rome risk criteria, lineage, DI, and CNS status at diagnosis.
Types of failures for those with persistent disease on days 15 and 22-25 during remission induction
| . | Day 15 (n = 57)4-150 . | Day 22-25 (n = 27)4-150 . |
|---|---|---|
| Hematologic relapse | 16 | 12 |
| CNS relapse | 7 | 2 |
| Testicular relapse | 1 | 0 |
| Hematologic and CNS relapse | 2 | 1 |
| Second malignancy | 4 | 1 |
| Mediastinal relapse | 1 | 0 |
| . | Day 15 (n = 57)4-150 . | Day 22-25 (n = 27)4-150 . |
|---|---|---|
| Hematologic relapse | 16 | 12 |
| CNS relapse | 7 | 2 |
| Testicular relapse | 1 | 0 |
| Hematologic and CNS relapse | 2 | 1 |
| Second malignancy | 4 | 1 |
| Mediastinal relapse | 1 | 0 |
Total number of patients with persistent disease at these time points.
In the blinded rereview, blast cells were found in all 26 cases originally categorized to have 1% or more blasts, whereas of the other 22 cases initially categorized to have less than 1% blasts, only 4 had more than 1% blasts on rereview (P < .001).
Discussion
Among patients entered in 2 consecutive institutional ALL protocols for patients of all risk groups, persistent morphologically identifiable disease was present in 57 (14.4%) patients on day 15 and in 27 (5.5%) patients on days 22 to 25. In approximately 50% of these cases (both at day 15 and day 22-25), the percentage of lymphoblasts was less than 5% (ie, 1%-4%). The 6% frequency of 5% lymphoblasts or more on day 15 in our study is similar to the 8% frequency on day 14, reported by CCG (study 105) for average-risk patients receiving similar 4-drug induction therapy.35 In a subsequent CCG study (CCG 123) for patients with high-risk ALL as defined by the presence of lymphomatous features, 13% to 16% of patients had at least 5% blasts at day 14.28 The frequency of residual disease on day 22 has not been previously described; it is therefore unclear how the 5.5% frequency compares to other treatment protocols.
Conventionally, persistent disease in a day 15 BM examination is generally defined by 5% or more lymphoblasts. In our study, the outcome for patients with 5% or more lymphoblasts at day 15 was significantly worse than for those with no lymphoblasts, a finding consistent with that reported by others.29 Of interest was our finding that the adverse prognostic effect of persistent lymphoblasts in the day 15 BM exists even for cases with only 1% to 4% lymphoblasts. Thus, any lymphoblasts (≥ 1%) detected by an experienced hematopathologist on a day 15 BM examination was associated with a significantly poorer treatment outcome even if they were undetectable by day 22. In most treatment regimens, the next time point for evaluating response is at the end of induction. However, we have done BM examinations on days 22 to 25 for patients who have blasts on day 15 and for those with an ANC less than 300/μL to determine scheduling of etoposide and cytarabine administration. The persistence of lymphoblasts on days 22 to 25 was associated with a particularly dismal treatment outcome, even for patients with 1% to 4% lymphoblasts. In fact, virtually all patients with any amount of detectable lymphoblasts (ie, ≥ 1%) on day 22 experienced a treatment failure even though the majority achieved a CR by day 43 induction.
The prognostic importance of persistent lymphoblasts on days 15 and 22 to 25 remained significant after adjusting for other factors including age, WBC count, DI, cell lineage, CNS status, and treatment protocol. This supports the conclusions of Gaynon et al29 that early response to therapy is a consistent independent prognostic factor in childhood ALL. In our study, children with persistent disease on day 15 had a 3-fold higher risk of failure than those with no identifiable lymphoblasts. A new finding in the current study is that the prognosis was even worse for those with persistent disease at days 22 to 25, because these patients had a 10-fold greater risk of failure compared to those with no persistent disease. Therefore, persistent disease on day 15 or days 22 to 25 remains the strongest predictor of treatment failure among the factors studied (Table 3). The adverse prognosis associated with a low percentage of lymphoblasts (1%-4%) in the current study indicates that the BM smears should be reviewed by an experienced hematopathologist or technologist to discern this low level of residual disease. Indeed, we have demonstrated a remarkable reproducibility of the detection of low percentages of blast cells even with the archival samples. We attributed the small discrepancy to the generally poor quality of the archival material. This finding notwithstanding, it is preferable to use immunophenotypic studies by flow cytometry to confirm persistent lymphoblasts when the percentage is less than 5%, particularly if significant changes in treatment (ie, beyond an additional dose of daunomycin or 3 doses ofl-asparaginase during induction) rest on these decisions. Coustan-Smith et al36 have demonstrated that immunologically detection minimal residual disease at day 43 of induction and week 14 of continuation therapy are associated with a poorer treatment result. Thus, it will be important to establish the relationship between the early response data with minimal residual disease studies performed at later time points during therapy.
We have shown that persistence of lymphoblasts (even 1%-4%) on day 15 of remission induction was associated with a poor prognosis and that residual disease on days 22 to 25 signified a particularly dismal outcome, suggesting that these very high-risk patients require novel or more intensive therapy to improve outcome.
Supported in part by National Cancer Institute grants CA 20180 and CA 21765 and the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John T. Sandlund, Department of Hematology/Oncology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail: john.sandlund@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal