Abstract
Early clearance of leukemic cells is a favorable prognostic indicator in childhood acute lymphoblastic leukemia (ALL). However, identification of residual leukemic cells by their morphologic features is subjective and lacks sensitivity. To improve estimates of leukemia clearance, we applied flow cytometric techniques capable of detecting 1 leukemic cell in 10 000 or more normal cells and prospectively measured residual leukemia in bone marrow samples collected on day 19 of remission-induction chemotherapy from 248 children with newly diagnosed ALL. In 134 samples (54.0%), we identified at least 0.01% leukemic cells (0.01%-< 0.1% in 51 samples [20.6%], 0.1%-< 1% in 36 [14.5%], and ≥ 1% in 47 [19.0%]). Among 110 children treated within a single chemotherapy program, the 5-year mean ± SE cumulative incidence of relapse or failure to achieve remission was 32.2% ± 6.5% for the 59 patients with 0.01% residual leukemic cells or greater on day 19 and 6.0% ± 3.4% for the 51 patients with less than 0.01% leukemic cells (P < .001). The prognostic value of day-19 bone marrow status defined by flow cytometry was superior to that defined by morphologic studies and remained significant after adjustment for other clinical and biologic variables. Lack of detectable leukemic cells on day 19 was more closely associated with relapse-free survival than was lack of detectable residual disease at the end of remission induction (day 46). Thus, approximately half of the children with ALL achieve profound clearance of leukemic cells after 2 to 3 weeks of remission-induction chemotherapy, and these patients have an excellent treatment outcome.
Introduction
Treatment outcome in children with acute lymphoblastic leukemia (ALL) is determined by the collective effect of cellular drug resistance, degree of leukemia cell infiltration into pharmacologic sanctuaries, pharmacodynamic profile, and inherited pharmacogenetic features of each patient.1-4 Measurements of any of these variables has prognostic value in childhood ALL, but none predict the course of the disease with absolute precision.5-7
The rate of clearance of leukemic cells from peripheral blood and bone marrow is a reflection of the cumulative effects of leukemia and host factors and should be a valuable indicator of treatment outcome. Indeed, the presence of circulating lymphoblasts after 1 week of single-agent or multiagent remission-induction therapy8-12and the detection of blast cells in the bone marrow by using morphologic criteria during remission-induction therapy13-15 predict a higher incidence of relapse. However, the morphologic features of leukemic lymphoblasts resemble those of normal lymphoid cells, and measurements of residual leukemia by morphologic analysis are inherently subjective and imprecise.16-18 Thus, a considerable proportion of patients with an apparently good early response according to morphologic criteria subsequently relapse.19
In a effort to improve the assessment of early response, we applied flow cytometric techniques based on the identification of immunophenotypes expressed by leukemic cells but not normal cells.20 These techniques are capable of detecting 1 leukemic cell in 104 or more normal cells20,21(a degree of sensitivity currently achievable in at least 90% of children with ALL) and have proved to be useful in monitoring minimal residual disease (MRD) during clinical remission.22 23
Patients, materials, and methods
Patients
From October 1994 to October 2001, 372 children with newly diagnosed ALL were enrolled in Total Therapy studies at our institution. At diagnosis, an immunophenotype that would allow detection by flow cytometry of 1 leukemic cell among 10 000 normal bone marrow cells or more was identified in 269 of the 352 patients (76.4%) with adequate immunophenotypic studies. Of these 269 patients, 248 (92.2%) had residual-disease studies performed by flow cytometry on day 19 of treatment; of the remaining 21 patients, 1 died before day 19 and 20 had insufficient bone marrow sampling for flow cytometric studies. These studies were approved by the St Jude Institutional Review Board, with informed consent obtained from the parents or guardians of each child. Diagnostic immunophenotyping and chromosomal and genetic analyses were performed by using standard techniques.24-26
Treatment protocol
Initial treatment of Total Therapy XIIIB (1994-1998) consisted of methotrexate followed 4 days later by 6-week remission-induction therapy with prednisone, vincristine, daunorubicin, asparaginase, and etoposide plus cytarabine.27 On attaining a complete clinical remission, all patients received 2 weeks of consolidation therapy with methotrexate and mercaptopurine, followed by risk-directed continuation therapy. For patients with higher-risk leukemia, this consisted of administration of multiple drug pairs in a weekly rotation. Those with lower-risk leukemia received daily mercaptopurine administration and weekly administration of methotrexate with prednisone plus vincristine pulse every 4 weeks. High-dose methotrexate was given every 8 weeks to all patients during the first year. Reinduction therapy (similar to that used initially) was administered from weeks 16 to 21. All patients received intrathecal therapy with methotrexate, hydrocortisone, and cytarabine for 1 year. Cranial irradiation was given after 1 year of continuation therapy only to those at very high risk of relapse (18 Gy) or those with a central nervous system (CNS) status 3 condition at diagnosis (24 Gy).28 Five of the 110 patients included in the correlative studies of residual disease on day 19 and treatment outcome were deemed to have very-high-risk leukemia and underwent allogeneic hematopoietic stem cell transplantation while in clinical remission.
Morphologic and flow cytometric assessments of residual leukemia
For morphologic analyses of residual leukemia, bone marrow aspirates collected on day 19 of remission-induction therapy were smeared and slides stained with Wright-Giemsa stain. Slides were observed by at least 2 expert morphologists and the percentage of lymphoblasts was recorded. For flow cytometric studies of residual disease, an aliquot of the same bone marrow aspirate was placed in preservative-free heparin and mononuclear cells were separated on a density step (AccuPrep; Nycomed, Oslo, Norway). Leukemia-associated immunophenotypes (found on leukemic cells but not on normal bone marrow cells) were determined by multivariable flow cytometry, with various combinations of monoclonal antibodies or heterologous antisera conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein, and allophycocyanin.20 The marker combinations currently used for monitoring residual disease in our laboratory are shown in Table 1. With the use of 4-color flow cytometry20 and the introduction of new markers (eg, CD58),29 we successfully studied more than 90% of patients (103 of 112) in the most recent cohort. Matched nonreactive fluorochrome-conjugated antibodies served as controls. The staining procedure was described previously.20 For each case, marker combinations allowing identification of 1 leukemic cell/104 normal nucleated bone marrow cells or more were selected at diagnosis and then applied during clinical remission.20 In the early part of the study, we used a FACScan flow cytometer with Lysis II or Cell Quest software but switched later to a dual laser-FACScalibur flow cytometer with Cell Quest software (all from Becton Dickinson, San Jose, CA).
Antibodies and fluorochromes currently used to study MRD in children with ALL
| Antibodies and fluorochromes* . | Frequency (%)† . |
|---|---|
| T-lineage | |
| Anti-TdT-F/CD5-P/CD3-C‡ | 90-95 |
| CD34-F/CD5-P/CD3-C‡ | 30-50 |
| B-lineage | |
| CD19-A/CD34-C/CD10-P/CD58-F | 40-60 |
| CD19-A/CD34-C/CD10-P/anti-TdT-F | 30-50 |
| CD19-A/CD34-C/CD10-P/CD38-F | 30-50 |
| CD19-A/CD34-C/CD10-P/CD45-F | 30-50 |
| CD19-A/CD34-C/CD10-P/CD22-F | 20-30 |
| CD19-A/CD34-C/CD10-P/CD13-F | 10-20 |
| CD19-A/CD34-C/CD10-P/CD66c-F | 10-20 |
| CD19-A/CD34-C/anti-TdT-F/anti-IgM-P | 10-20 |
| CD19-A/CD34-C/CD10-P/CD15-F | 5-10 |
| CD19-A/CD34-C/CD10-P/CD33-F | 5-10 |
| CD19-A/CD34-C/CD10-P/CD65-F | 5-10 |
| CD19-A/CD34-C/CD10-P/CD21-F | 5-10 |
| CD19-A/CD34-C/CD10-F/7.1-P | 3-5 |
| Antibodies and fluorochromes* . | Frequency (%)† . |
|---|---|
| T-lineage | |
| Anti-TdT-F/CD5-P/CD3-C‡ | 90-95 |
| CD34-F/CD5-P/CD3-C‡ | 30-50 |
| B-lineage | |
| CD19-A/CD34-C/CD10-P/CD58-F | 40-60 |
| CD19-A/CD34-C/CD10-P/anti-TdT-F | 30-50 |
| CD19-A/CD34-C/CD10-P/CD38-F | 30-50 |
| CD19-A/CD34-C/CD10-P/CD45-F | 30-50 |
| CD19-A/CD34-C/CD10-P/CD22-F | 20-30 |
| CD19-A/CD34-C/CD10-P/CD13-F | 10-20 |
| CD19-A/CD34-C/CD10-P/CD66c-F | 10-20 |
| CD19-A/CD34-C/anti-TdT-F/anti-IgM-P | 10-20 |
| CD19-A/CD34-C/CD10-P/CD15-F | 5-10 |
| CD19-A/CD34-C/CD10-P/CD33-F | 5-10 |
| CD19-A/CD34-C/CD10-P/CD65-F | 5-10 |
| CD19-A/CD34-C/CD10-P/CD21-F | 5-10 |
| CD19-A/CD34-C/CD10-F/7.1-P | 3-5 |
MRD indicates minimal residual disease; ALL, acute lymphoblastic leukemia; A, allophycocyanin; C, peridinin chlorophyll protein; F, fluorescein isothiocyanate; and P, phycoerythrin.
All antibodies were against cell-surface markers, except anti-TdT, CD3, and anti-IgM, which require cell-membrane permeabilization.
Proportion of childhood ALL cases of each lineage in which the listed immunophenotype can be used to monitor MRD. In most patients, leukemic cells express more than one abnormal phenotype.
In T-lineage ALL cases, a mixture of CD19, CD33, and anti-HLA-DR antibodies conjugated to allophycocyanin is added to identify B cells and myeloid cells and exclude them from the analysis.
The flow cytometry protocol used for detection of MRD was described in detail previously.20 In all samples, we acquired data from all mononuclear cells in each test tube (> 1 × 105). Flow cytometric data were recorded within 24 hours after sample collection and processing, with the observer having no knowledge of the patient's clinical status or diagnostic features, except for immunophenotype. To compare percentages of leukemic lymphoblasts derived from flow cytometry (obtained from preparations of mononuclear cells) and percentages estimated by morphologic assessment (obtained from smears of whole marrow), we recalculated the latter after excluding segmented leukocytes from the counts.
Statistical analysis
Differences in the distribution of clinical and biologic presenting features according to level of residual disease on day 19 were compared by using exact χ2 and Fisher exact tests. Because of the multiple presenting features (13) with which residual disease status was compared, only associations with a Pvalue lower than .004 (a P value of .05 divided by 13) were deemed significant. The cumulative incidence of ALL relapse was estimated after adjustment for other competing risks (ie, second malignant disease and death while in remission), as described by Kalbfleisch and Prentice,30 and compared by using Grays test.31 The cut-off date for follow-up observations was November 30, 2001. Among the 110 patients included in the correlative studies with treatment outcome, 91 are alive; 84 patients (92%) had complete follow-up information within 1 year of the analysis, and 90 (99%) had complete follow-up data within 1.5 years. To assess the prognostic value of different levels of MRD after adjustment for competing prognostic factors, we stratified the data by treatment and then separately for clinical and biologic presenting features. Patients who underwent hematopoietic stem cell transplantation were followed until they had a relapse or a competing event or until their last follow-up date.
Results
Determination of leukemia cell clearance by flow cytometry
We prospectively measured the percentage of residual leukemic cells among bone marrow mononuclear cells collected on day 19 of remission-induction chemotherapy from 248 children with newly diagnosed ALL. In 134 patients (54.0%), at least 0.01% leukemic cells were identified by flow cytometry (Figure 1). Among these patients, levels of leukemia were 0.01% to less than 0.1% in 51 patients (20.6%), 0.1% to less than 1% in 36 (14.5%) and 1% or more in 47 (19.0%). In the remaining 114 patients, leukemic cells were below the limit of detection of our technique (0.01%).
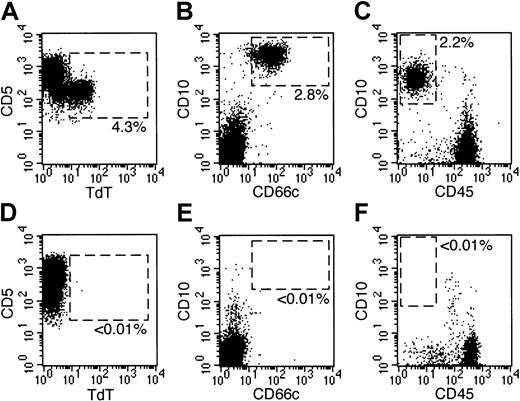
Detection of residual disease by flow cytometry on day 19 of remission-induction therapy.
Bone marrow samples were obtained on day 19 from 6 patients with newly diagnosed ALL. All 6 samples lacked leukemic cells identifiable by morphologic analysis. Dot plots at left show flow cytometric results illustrating expression of TdT (x-axes) and CD5 (y-axes) on gated CD5+, HLA-Dr−, CD19−, CD33− lymphoid cells in 2 patients with T-lineage ALL. Dot plots at middle and right illustrate expression of CD10 (y-axes) and CD66c (x-axes; middle panels) or CD45 (x-axes; right panels) on gated CD19+ lymphoid cells in 4 patients with B-lineage ALL. Dashed areas in all plots correspond to areas that appear empty when normal bone marrow samples are analyzed. In 3 patients (top panels), residual leukemia was present at the percentages indicated; in the other 3 (bottom panels), leukemic cells were undetectable. More than 105 cells were analyzed in each sample.
Detection of residual disease by flow cytometry on day 19 of remission-induction therapy.
Bone marrow samples were obtained on day 19 from 6 patients with newly diagnosed ALL. All 6 samples lacked leukemic cells identifiable by morphologic analysis. Dot plots at left show flow cytometric results illustrating expression of TdT (x-axes) and CD5 (y-axes) on gated CD5+, HLA-Dr−, CD19−, CD33− lymphoid cells in 2 patients with T-lineage ALL. Dot plots at middle and right illustrate expression of CD10 (y-axes) and CD66c (x-axes; middle panels) or CD45 (x-axes; right panels) on gated CD19+ lymphoid cells in 4 patients with B-lineage ALL. Dashed areas in all plots correspond to areas that appear empty when normal bone marrow samples are analyzed. In 3 patients (top panels), residual leukemia was present at the percentages indicated; in the other 3 (bottom panels), leukemic cells were undetectable. More than 105 cells were analyzed in each sample.
Residual leukemia by flow cytometry on day 19 was not significantly related to age, sex, race, leukocyte count, CNS status, or National Cancer Institute risk status (Table 2). Among cellular features, rates of detection did not differ significantly in comparisons based on cell lineage, ploidy, or the presence of t(4;11) or MLL gene rearrangements, t(1;19) orE2A/PBX1 transcripts, or TEL/AML1 transcripts. There was, however, a remarkable association between detection of residual leukemia and the Philadelphia (Ph) chromosome: all 7 patients with this prognostically unfavorable abnormality had positive findings (median percentage of leukemic cells, 3.47%; range, 0.71% to 10.78%;P < .0001).
Patients' presenting features, according to level of residual disease on day 19
| Presenting feature . | Level of residual disease . | P* . | |||
|---|---|---|---|---|---|
| < 0.01% . | 0.01%-< 0.1% . | 0.1%-< 1.0% . | ≥ 1.0% . | ||
| Age (y) | |||||
| < 1 | 4 (3.5) | 0 (0) | 3 (8.3) | 2 (4.3) | .2952 |
| 1 to 9 | 76 (66.7) | 34 (66.7) | 19 (52.8) | 26 (55.3) | |
| ≥ 10 | 34 (29.8) | 17 (33.3) | 14 (38.9) | 19 (40.4) | |
| Sex | |||||
| Male | 58 (50.9) | 30 (58.8) | 19 (52.8) | 36 (76.6) | .0211 |
| Female | 56 (49.1) | 21 (41.2) | 17 (47.2) | 11 (23.4) | |
| Race | |||||
| White | 90 (78.9) | 37 (72.6) | 25 (69.4) | 29 (61.7) | .3242 |
| Black | 13 (11.4) | 10 (19.6) | 6 (16.7) | 11 (23.4) | |
| Other | 11 (9.7) | 4 (7.8) | 5 (13.9) | 7 (14.9) | |
| WBC count | |||||
| < 50 × 109/L | 87 (76.3) | 35 (68.6) | 24 (66.7) | 28 (60.0) | .1769 |
| ≥ 50 × 109/L | 27 (23.7) | 16 (31.4) | 12 (33.3) | 19 (40.4) | |
| CNS status | |||||
| 2 or 3 | 37 (32.5) | 18 (35.3) | 12 (33.3) | 18 (38.3) | .9058 |
| 1 | 77 (67.5) | 33 (64.7) | 24 (66.7) | 29 (61.7) | |
| Lineage | |||||
| T | 20 (17.5) | 13 (25.5) | 9 (25.0) | 14 (29.8) | .3054 |
| B | 94 (82.5) | 38 (74.5) | 27 (75.0) | 33 (70.2) | |
| DNA index | |||||
| ≥ 1.16 | 31 (27.2) | 11 (21.6) | 6 (16.7) | 6 (12.8) | .2050 |
| Other | 83 (72.8) | 40 (78.4) | 30 (83.3) | 41 (87.2) | |
| Ploidy† | |||||
| 51-65 chromosomes | 33 (17.2) | 15 (7.8) | 10 (5.2) | 8 (4.2) | .5627 |
| Others | 61 (31.8) | 23 (12.0) | 17 (8.9) | 25 (13.0) | |
| Ph chromosome‡ | |||||
| Absent | 114 (100) | 49 (96.1) | 35 (97.2) | 40 (85.1) | < .0001 |
| Present | 0 (0) | 0 (0) | 1 (2.8) | 6 (12.8) | |
| t(1;19)/E2A-PBX1‡ | |||||
| Absent | 112 (98.2) | 49 (96.1) | 34 (94.4) | 43 (91.5) | .2031 |
| Present | 2 (1.8) | 0 (0) | 2 (5.6) | 2 (4.3) | |
| t(4;11)/MLLrearrangement‡ | |||||
| Absent | 110 (96.5) | 49 (96.1) | 34 (94.4) | 43 (91.5) | .3861 |
| Present | 4 (3.5) | 0 (0) | 2 (5.6) | 2 (4.3) | |
| TEL/AML1‡ | |||||
| Absent | 81 (71.1) | 37 (72.5) | 28 (77.8) | 37 (78.7) | .0496 |
| Present | 20 (17.5) | 7 (13.7) | 3 (8.3) | 1 (2.1) | |
| NCI risk† | |||||
| Standard | 52 (57.8) | 23 (60.5) | 13 (52.0) | 16 (51.6) | .4700 |
| High | 38 (42.2) | 15 (39.5) | 12 (48.0) | 15 (48.4) | |
| Presenting feature . | Level of residual disease . | P* . | |||
|---|---|---|---|---|---|
| < 0.01% . | 0.01%-< 0.1% . | 0.1%-< 1.0% . | ≥ 1.0% . | ||
| Age (y) | |||||
| < 1 | 4 (3.5) | 0 (0) | 3 (8.3) | 2 (4.3) | .2952 |
| 1 to 9 | 76 (66.7) | 34 (66.7) | 19 (52.8) | 26 (55.3) | |
| ≥ 10 | 34 (29.8) | 17 (33.3) | 14 (38.9) | 19 (40.4) | |
| Sex | |||||
| Male | 58 (50.9) | 30 (58.8) | 19 (52.8) | 36 (76.6) | .0211 |
| Female | 56 (49.1) | 21 (41.2) | 17 (47.2) | 11 (23.4) | |
| Race | |||||
| White | 90 (78.9) | 37 (72.6) | 25 (69.4) | 29 (61.7) | .3242 |
| Black | 13 (11.4) | 10 (19.6) | 6 (16.7) | 11 (23.4) | |
| Other | 11 (9.7) | 4 (7.8) | 5 (13.9) | 7 (14.9) | |
| WBC count | |||||
| < 50 × 109/L | 87 (76.3) | 35 (68.6) | 24 (66.7) | 28 (60.0) | .1769 |
| ≥ 50 × 109/L | 27 (23.7) | 16 (31.4) | 12 (33.3) | 19 (40.4) | |
| CNS status | |||||
| 2 or 3 | 37 (32.5) | 18 (35.3) | 12 (33.3) | 18 (38.3) | .9058 |
| 1 | 77 (67.5) | 33 (64.7) | 24 (66.7) | 29 (61.7) | |
| Lineage | |||||
| T | 20 (17.5) | 13 (25.5) | 9 (25.0) | 14 (29.8) | .3054 |
| B | 94 (82.5) | 38 (74.5) | 27 (75.0) | 33 (70.2) | |
| DNA index | |||||
| ≥ 1.16 | 31 (27.2) | 11 (21.6) | 6 (16.7) | 6 (12.8) | .2050 |
| Other | 83 (72.8) | 40 (78.4) | 30 (83.3) | 41 (87.2) | |
| Ploidy† | |||||
| 51-65 chromosomes | 33 (17.2) | 15 (7.8) | 10 (5.2) | 8 (4.2) | .5627 |
| Others | 61 (31.8) | 23 (12.0) | 17 (8.9) | 25 (13.0) | |
| Ph chromosome‡ | |||||
| Absent | 114 (100) | 49 (96.1) | 35 (97.2) | 40 (85.1) | < .0001 |
| Present | 0 (0) | 0 (0) | 1 (2.8) | 6 (12.8) | |
| t(1;19)/E2A-PBX1‡ | |||||
| Absent | 112 (98.2) | 49 (96.1) | 34 (94.4) | 43 (91.5) | .2031 |
| Present | 2 (1.8) | 0 (0) | 2 (5.6) | 2 (4.3) | |
| t(4;11)/MLLrearrangement‡ | |||||
| Absent | 110 (96.5) | 49 (96.1) | 34 (94.4) | 43 (91.5) | .3861 |
| Present | 4 (3.5) | 0 (0) | 2 (5.6) | 2 (4.3) | |
| TEL/AML1‡ | |||||
| Absent | 81 (71.1) | 37 (72.5) | 28 (77.8) | 37 (78.7) | .0496 |
| Present | 20 (17.5) | 7 (13.7) | 3 (8.3) | 1 (2.1) | |
| NCI risk† | |||||
| Standard | 52 (57.8) | 23 (60.5) | 13 (52.0) | 16 (51.6) | .4700 |
| High | 38 (42.2) | 15 (39.5) | 12 (48.0) | 15 (48.4) | |
Values are numbers (%) of patients unless otherwise indicated.
WBC indicates white blood cell; CNS, central nervous system; Ph, Philadelphia; and NCI, National Cancer Institute.
With χ2 test and Fisher exact test, if necessary.
Only for patients with B-lineage ALL and age at diagnosis of over 1 year.
Data were missing in some cases.
Correlation between flow cytometric and morphologic measurements of residual disease
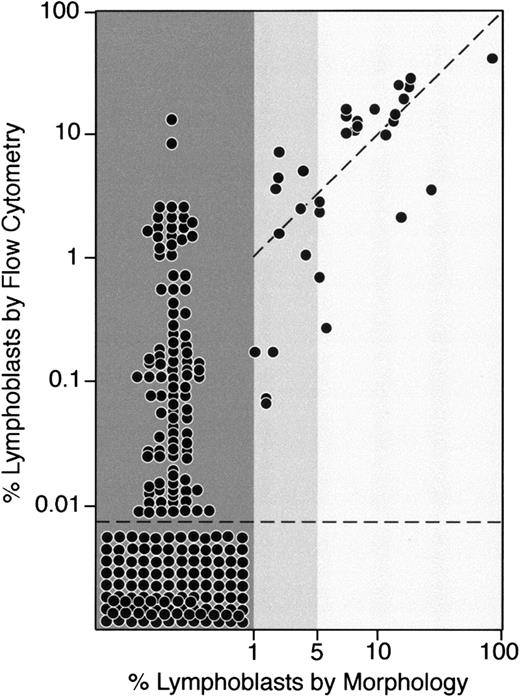
Of the 248 bone marrow samples studied by flow cytometry on day 19 of remission-induction therapy, 32 (12.9%) had leukemic lymphoblasts identifiable by morphologic analysis. In 21 of these samples (8.5%), lymphoblasts represented at least 5% of bone marrow mononuclear cells; in 11, the proportion of lymphoblasts ranged from 1% to 4% (Figure2). In all the 32 morphologically positive samples, at least 0.01% cells expressing leukemia-specific immunophenotypes were detected by flow cytometry. Correlation between morphologic and flow cytometric findings was generally good in samples with at least 5% cells with lymphoblast morphologic features; in 19 of 21 samples, more than 1% leukemic cells were counted by flow cytometry. Discrepancies between morphologic and flow cytometric evaluations were wider among the 11 samples with 1% to 4% lymphoblasts on morphologic analysis (Figure 2).
Comparison between morphologic and flow cytometric assessment of residual disease on day 19 of remission-induction therapy.
Each dot corresponds to the percentage of leukemic cells identified by morphologic analysis (x-axis) and by flow cytometry (y-axis) in bone marrow samples collected on day 19 from 248 children with newly diagnosed ALL
Comparison between morphologic and flow cytometric assessment of residual disease on day 19 of remission-induction therapy.
Each dot corresponds to the percentage of leukemic cells identified by morphologic analysis (x-axis) and by flow cytometry (y-axis) in bone marrow samples collected on day 19 from 248 children with newly diagnosed ALL
Among the 216 samples without leukemic lymphoblasts recognizable by their morphologic features, 114 (52.8%) did not have detectable cells expressing leukemia-associated immunophenotypes. In the remaining 102 samples, however, leukemic lymphoblasts were detected by flow cytometry. Residual levels of disease in these samples ranged from 0.01% to 16% (median, 0.1%; Figure 2). Of note, in the 2 samples with 9% and 16% leukemic cells on flow cytometry, the morphologic analysis revealed only apparently mature normal lymphocytes (9% and 45%, respectively).
Leukemic cell clearance on day 19 and treatment outcome
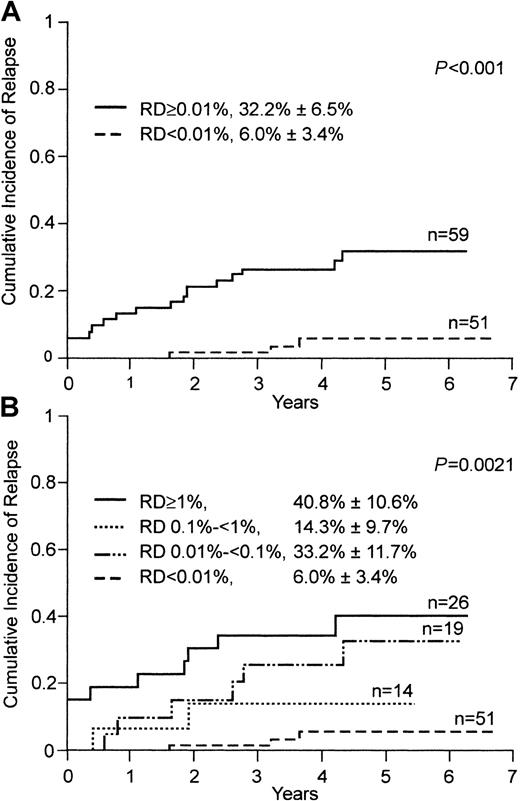
To examine the prognostic effect of lymphoblast clearance assessed by flow cytometry, we analyzed treatment outcome in 110 children treated within a single program of chemotherapy (Total XIIIB) in which MRD status was not used for risk assignment. The 5-year cumulative incidence of relapse or failure to achieve clinical remission in patients with no detectable leukemic cells on day 19 (n = 51) was 6.0% ± 3.4%, whereas it was 32.8% ± 6.5% in the 59 patients with positive findings (P < .001; Figure3A). Notably, 2 of the 3 relapses that occurred in the group with negative findings were extramedullary. The third (hematologic) relapse in this group occurred 11 months after cessation of therapy. In contrast, 10 of the 14 relapses in the group with positive results on day 19 were hematologic, and 7 of these occurred during treatment. This group also included all 4 patients who did not achieve clinical remission within the scheduled 6-week remission-induction therapy.
Cumulative incidence of relapse in children with ALL, according to levels of residual disease (RD) on flow cytometric assessment on day 19 of remission-induction therapy.
Levels of RD were defined by the percentage of mononuclear cells expressing leukemia-specific immunophenotypes. (A) Patients with an RD level of 0.01% or more (solid line) compared with patients with an RD level below 0.01% (broken line). (B) Cumulative incidence of relapse in patients with different levels of detectable RD.
Cumulative incidence of relapse in children with ALL, according to levels of residual disease (RD) on flow cytometric assessment on day 19 of remission-induction therapy.
Levels of RD were defined by the percentage of mononuclear cells expressing leukemia-specific immunophenotypes. (A) Patients with an RD level of 0.01% or more (solid line) compared with patients with an RD level below 0.01% (broken line). (B) Cumulative incidence of relapse in patients with different levels of detectable RD.
Next, we determined whether higher levels of residual disease were associated with a higher risk of treatment failure. Patients with at least 1% leukemic cells (n = 26) fared worse: the 5-year cumulative incidence of relapse or failure to achieve remission was 40.8% ± 10.6%, and all 4 patients with remission failure were in this group (Figure 3B). However, lower levels of residual disease were not proportionally related to risk of failure in this analysis: the incidence of relapse or failure to achieve remission in the 14 patients with 0.1% to less than 1% residual disease was 14.3% ± 9.7%, whereas that in the 19 patients with 0.01% to less than 0.1% residual disease was 33.2% ± 11.7% (Figure 3B).
Prognostic importance of leukemic cell clearance and other prognostic factors of childhood ALL
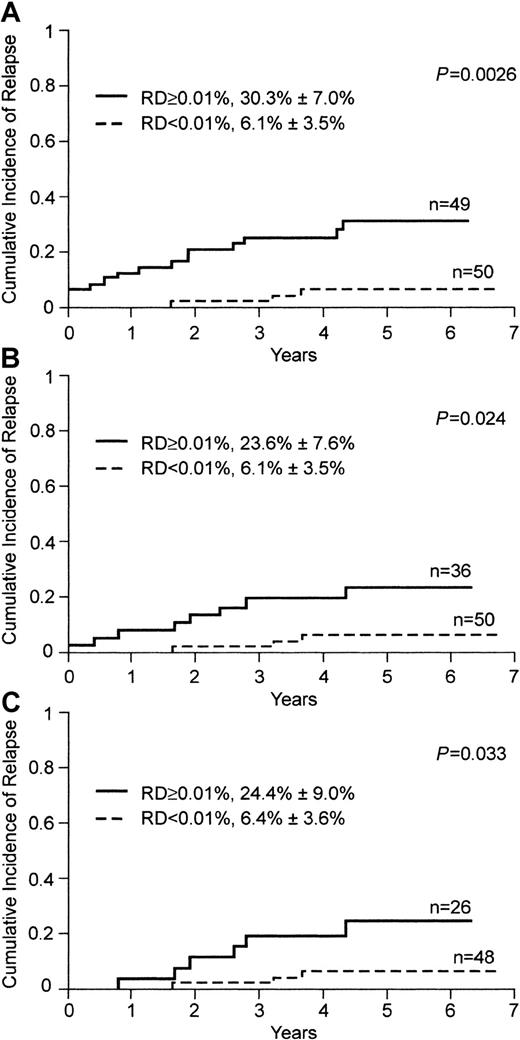
Bone marrow status on day 19 as assessed by flow cytometry remained a significant prognostic factor after adjustment for known prognostic factors of childhood ALL, such as age, leukocyte counts, immunophenotype, ploidy, t(4;11) or MLL gene rearrangements, Ph chromosome, and TEL/AML1. Moreover, bone marrow status indicated by flow cytometry on day 19 was a significant predictor of relapse in a subgroup of patients that excluded those with high-risk leukemia indicated by presenting criteria (age < 1 year, n = 6; Ph chromosome, n = 5; P = .0026; Figure4A).
Cumulative incidence of relapse in children with ALL, according to levels of residual disease (RD) on flow cytometric assessment on day 19
. Levels of RD were defined by the percentage of mononuclear cells expressing leukemia-specific immunophenotypes; the solid line indicates 0.01% or more, and the broken line, less than 0.01%. (A) Patients 1 year of age or older in whom leukemic cells lacked the Ph chromosome. (B) Patients with less than 1% lymphoblasts on morphologic analysis on day 19. (C) Patients who had clinical remission and less than 0.01% MRD at the end of remission-induction therapy (day 46).
Cumulative incidence of relapse in children with ALL, according to levels of residual disease (RD) on flow cytometric assessment on day 19
. Levels of RD were defined by the percentage of mononuclear cells expressing leukemia-specific immunophenotypes; the solid line indicates 0.01% or more, and the broken line, less than 0.01%. (A) Patients 1 year of age or older in whom leukemic cells lacked the Ph chromosome. (B) Patients with less than 1% lymphoblasts on morphologic analysis on day 19. (C) Patients who had clinical remission and less than 0.01% MRD at the end of remission-induction therapy (day 46).
Residual leukemic cells detected by morphologic assessment after 2 weeks of remission-induction therapy have been reported to be associated with treatment outcome.19 In our series, 16 of 110 patients had at least 5% leukemic cells identified by morphologic analysis, the standard cut-off value for identifying high-risk patients by this criterion. The 5-year cumulative incidence of relapse or failure to achieve remission in these patients was 47.9% ± 14.8%, whereas the incidence of relapse in the 94 patients with less than 5% blasts was 15.5% ± 3.9% (P = .0037). It was previously observed that patients with less than 1% blasts on morphologic analysis may have a superior outcome.15 In our series, 86 patients met this criterion, but their incidence of relapse remained relatively high (13.4% ± 3.8%) and the group included one patient who did not have remission.
To determine whether flow cytometric measurements of residual disease on day 19 provided more information than that provided by standard morphologic analysis, we focused our analysis on the 86 patients with completely negative morphologic findings (< 1% blasts) on day 19. Fifty of the 86 patients had residual leukemia levels below 0.01% on flow cytometry. These patients had a significantly lower cumulative incidence of relapse at 5 years than the 36 patients with 0.01% leukemic cells or greater by flow cytometry (P = .024; Figure 4B), a group that included a patient who had not achieved morphologic remission by day 46 after the scheduled remission-induction treatment.
Leukemic cell clearance on day 19 and MRD during clinical remission
We previously found that measurements of MRD using flow cytometry at the end of remission induction (day 46 after diagnosis) provide a strong and independent prediction of treatment outcome.23We observed, however, that relapse still occurred in approximately 10% of patients with negative MRD findings at this time. To test whether earlier measurements of residual disease (ie, on day 19) could better identify patients with excellent treatment outcome, we analyzed the group of patients who were negative for MRD (< 0.01% leukemic cells by flow cytometry) at the end of remission-induction therapy. Of the 110 patients included in our series, 106 achieved complete morphologic remission and 74 were also MRD negative at this time. Measurements of leukemic cell clearance on day 19 were helpful in identifying a subset of patients with an excellent treatment outcome. Forty-eight of the 74 patients negative for MRD at the end of induction had less than 0.01% leukemic cells on day 19, and these patients had a cumulative incidence of relapse that was significantly lower than that in the 26 patients with detectable leukemic cells on day 19 (P = .03; Figure 4C).
Table 3 shows the relation between residual disease measured on day 19 and subsequent findings of MRD during clinical remission in the same cohort of patients. Patients with less than 0.01% leukemic cells on day 19 who were studied during continuation therapy were generally MRD negative (< 0.01%), with 2 exceptions: one patient who had detectable MRD (0.02%) at the end of remission induction but subsequently became MRD negative and remains in continuous complete remission 27 months off therapy and one patient who had conversion to MRD positivity (0.01%) in week 56, 7 months before clinical relapse. Among patients with detectable residual leukemia on day 19, 50.9% were MRD positive at the end of remission induction; MRD positivity was found in 28.6%, 2.4%, and 7.0% of these patients at weeks 14, 32, and 56, respectively, of continuation therapy.
Relation between residual disease on day 19 and minimal residual disease (MRD) during clinical remission
| Residual disease on day 19 . | MRD at end of induction . | MRD during continuation therapy . | ||||||
|---|---|---|---|---|---|---|---|---|
| Week 14 . | Week 32 . | Week 56 . | ||||||
| ≥ 0.01% . | < 0.01% . | ≥ 0.01% . | < 0.01% . | ≥ 0.01% . | < 0.01% . | ≥ 0.01% . | < 0.01% . | |
| ≥ 0.01% | 27 | 26 | 14 | 35 | 1 | 40 | 3 | 40 |
| < 0.01% | 1 | 49 | 0 | 49 | 0 | 47 | 1 | 48 |
| Residual disease on day 19 . | MRD at end of induction . | MRD during continuation therapy . | ||||||
|---|---|---|---|---|---|---|---|---|
| Week 14 . | Week 32 . | Week 56 . | ||||||
| ≥ 0.01% . | < 0.01% . | ≥ 0.01% . | < 0.01% . | ≥ 0.01% . | < 0.01% . | ≥ 0.01% . | < 0.01% . | |
| ≥ 0.01% | 27 | 26 | 14 | 35 | 1 | 40 | 3 | 40 |
| < 0.01% | 1 | 49 | 0 | 49 | 0 | 47 | 1 | 48 |
MRD data were not available for all patients at all assessment times.
Discussion
We found that approximately half of children with ALL achieve a profound clearance of leukemic cells (< 0.01% leukemic cells among bone marrow mononuclear cells) after only 2 to 3 weeks of remission-induction chemotherapy. These patients have an excellent treatment outcome and their likelihood of remaining in continuous complete remission approaches 95%. The probability of relapse in patients with detectable residual disease at this time point is significantly higher, particularly for those with 1% or greater leukemic lymphoblasts among bone marrow mononuclear cells. Notably, 6 of the 7 patients with the Ph chromosome were among this group, a finding that highlights the resistance of leukemic cells with this karyotype to conventional antileukemic drugs. Elucidation of the clinical importance of the intermediate levels of residual leukemia (0.01% to less than 1%) on day 19 will probably require additional study of a larger number of patients. Although relapse rates were higher among patients with detectable residual leukemia below the 1% levels than among those with no detectable disease, we did not observe a linear relation between levels of residual disease and risk of relapse in this group.
An important question raised by these results is whether flow cytometric measurements of residual disease provide information beyond that provided by conventional morphologic assessment of treatment response. The results of our analysis restricted to patients with completely negative morphologic findings (ie, those with no identifiable lymphoblasts) indicate that flow cytometry does indeed provide additional information. Among this group of patients, those who had less than 0.01% leukemic cells according to the flow cytometric criteria had a significantly better treatment outcome.
In general, our results are in concordance with those reported by Panzer-Grumayer et al32 in a series of 68 children with ALL in whom treatment response was studied on day 15 by polymerase chain reaction (PCR) amplification of antigen receptor genes. However, there was a difference in the proportions of children who achieved the lowest levels of MRD: 14 of 68 (21%) in the series of Panzer-Grumayer et al and 114 of 248 (46%) in ours. Because the level of residual leukemic cells used to define maximum leukemic cell clearance was similar in the 2 studies (1 in 104), this difference cannot be ascribed to a different sensitivity of the 2 assessment techniques. Rather, it may be attributable to differences in chemotherapy or to the inability of PCR methods to distinguish between viable and apoptotic leukemic cells. The flow cytometric methods used in our study can make this distinction readily.20 They are also rapid and easily applicable in most cancer centers, a feature that should facilitate their incorporation in treatment protocols. In our study, sensitive flow cytometric analyses (ie, those capable of detecting 1 leukemic cell among 10 000 or more normal bone marrow cells) were applicable to approximately 75% of all patients with newly diagnosed ALL during the time span of the study, which began in 1994. However, more recent improvements in flow cytometric methods, including the use of 4-color analysis and the introduction of new markers of leukemia,29 currently allow us to study at least 90% of patients with the desired levels of sensitivity, even in regenerating bone marrow with a high proportion of normal lymphoid progenitors or “hematogones.”33 34 Because of the lower background level of hematogones, residual disease in bone marrow samples collected on day 19 is typically more evident than in bone marrow samples collected after a pause in chemotherapy or when the patient is off treatment.
Assuming that the total leukemic burden at diagnosis is on average 1012 leukemic cells,16 a leukemic cell level below 0.01% should correspond to a leukemia burden of less than 108 total leukemic cells. Thus, in patients with no residual leukemic cells detectable by flow cytometry, a cytoreduction in excess of 4 logs is likely to have occurred. We found that such massive response occurs in nearly half of the children with newly diagnosed ALL after 2 to 3 weeks of remission-induction chemotherapy and that it is associated with an excellent treatment outcome. Patients with early and profound cytoreductions may therefore become candidates for future studies designed to test less intense and hence less toxic regimens of chemotherapy.
We thank Peixin Liu and Mo Mehrpooya for technical assistance and Yinmei Zhou for assistance with the statistical analysis.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0006.
Supported by grants CA60419, CA21765, and CA20180 from the National Cancer Institute, by the Rizzo Memorial Grant from the Leukemia Research Foundation, and by the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dario Campana, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 North Lauderdale, Memphis, TN 38105; e-mail: dario.campana@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal