The risk factors for hepatitis due to hepatitis B virus (HBV) reactivation in patients positive for hepatitis B surface antigen (HBsAg) treated with autologous hematopoietic cell transplantation (HCT) are unknown. We evaluated 137 consecutive patients (23 positive for HBsAg, 37 positive for hepatitis B surface antibody, and 77 negative for HBV) who underwent HCT. Serial serum ALT were measured before transplant and after transplant at 1 to 4 weekly intervals for the first year and then at 2 to 12 weekly intervals thereafter. Before HCT, basic core promoter (T1762/A1764) and precore (A1896) HBV variants were determined in HBsAg-positive and HBV DNA–positive (by polymerase chain reaction assay) patients by direct sequencing and serum HBV DNA quantitation using the Digene Hybrid Capture II assay. Cox proportional hazards analysis was used to assess the association between pretransplantation HBV virologic and host factors and occurrence of hepatitis due to HBV reactivation. After HCT, hepatitis due to HBV reactivation was more common in HBsAg-positive patients than in HBsAg-negative patients (hazard ratio, 33.3; 95% confidence interval [CI], 7.35-142.86;P < .0001). HBsAg-positive patients with detectable serum HBV DNA before HCT (on Digene assay) had a significantly higher risk of hepatitis due to HBV reactivation than HBsAg-positive patients with no detectable serum HBV DNA (adjusted hazard ratio, 9.35; 95% CI, 1.65-52.6; P = .012). Thus, we found that hepatitis due to HBV reactivation is common in HBsAg-positive patients undergoing autologous HCT. A high HBV DNA level (>105 copies/mL) was the most important risk factor for HBV reactivation, and its lowering by administration of nucleoside analogues before transplantation should be considered.
Introduction
Hepatitis due to reactivation of hepatitis B virus (HBV) is a well-recognized complication in patients with chronic HBV infection undergoing cytotoxic or immunosuppressive therapy. It has been observed in patients positive for hepatitis B surface antigen (HBsAg)1-6 and in HBsAg-negative patients who had HBV infection in the past (hepatitis B surface antibody [HBsAb], HBsAb positive and hepatitis B core antibody [HBcAb], and anti-HBc positive).7-11
In the past few decades, hematopoietic cell transplantation (HCT) has become the standard treatment for various hematologic and oncologic problems.12 In areas such as Southeast Asia, where HBV infection is prevalent, HCT might be complicated by morbidity and mortality related to HBV.13 The median prevalence of HBsAg positivity in Asian Chinese patients undergoing HCT has been reported to be more than 10%.14 Hepatic complications related to HBV infection after HCT are primarily associated with HBV reactivation.2,5,10,11,13,15-17 The underlying pathogenesis of HBV reactivation is likely to be related to the intensive chemotherapy with or without total-body irradiation (TBI) required to ablate the recipients' marrow.18 Prospective serologic testing of patients with chronic HBV infection who have HBV reactivation after chemotherapy showed that immunosuppression enhances viral replication. This is reflected by increases in serum levels of hepatitis B e antigen (HBeAg) and HBV DNA polymerase and naive hepatocyte infection with HBV.6,18,19 Discontinuation of cytotoxic or immunosuppressive drugs restores immune function, resulting in rapid destruction of infected hepatocytes. Clinically, this may manifest as hepatitis and hepatic failure and even cause death.7 20-22
Despite its clinical importance, few data are available on the incidence and risk factors for hepatitis due to HBV reactivation after chemotherapy or immunosuppression. In particular, relation between the various HBV virologic variables and HBV reactivation is unclear. Because these cases of hepatitis are preceded or accompanied by enhanced HBV viral replication, reflected by an increasing serum level of HBV DNA on Digene Hybrid Capture II assays (Murex, Digene, Gaithersburg, MD), it is not known whether HBV-infected patients with a higher HBV viral load have a higher risk of hepatitis due to HBV reactivation after HCT. Other HBV viral factors that might be involved in HBV reactivation are the presence of variants, notably, the G→A mutation at nucleotide 1896 in the precore region (A1896), which converts codon 28 for tryptophan to a stop codon23; and the double mutation in the basic core promoter (BCP) that consists of the A→T mutation at nucleotide 1762 and the G→A mutation at nucleotide 1764 (T1762/A1764).24 The precore A1896 stop mutation was reported to be an Andromeda strain that markedly increased HBV virulence and contributed to the development of fulminant hepatitis.25,26 On the other hand, the BCP regulates transcription of pregenomic RNA, encodes core protein and HBV polymerase, and serves as a replicative intermediate for the synthesis of viral DNA.27 Variants of the BCP (T1762/A1764) have been observed in patients with severe liver disease.28-34
Unlike the situation with hepatitis C virus (HCV) infections, the clinical importance of HBV genotype has received little attention. Seven genotypes of HBV are recognized, distinguished by differences of more than 8% in the entire nucleotide sequence of approximately 3200 nucleotides. They are designated by letters ranging from A to G.35-37 HBV genotypes have distinct geographical distributions.31,38-40 Overall, genotypes B and C are common in Asia, whereas genotypes A and D prevail in Western countries. Genotype E is restricted to Africa, and genotype F is dominant in Central America. Genotype G was described more recently,37and its distribution is still unknown. There is now an increasing awareness of the clinical importance of HBV genotype.29 33
In this study, we investigated the prevalence and risk factors for occurrence of hepatitis due to HBV reactivation after autologous HCT in an area endemic for HBV infection.
Patients, materials, and methods
Patients
We studied 137 consecutively treated patients who underwent autologous HCT at Queen Mary Hospital, Hong Kong, from November 1990 to March 2000 (Table 1). None had received any preemptive antiviral therapy with substantial activity against HBV (such as famciclovir or lamivudine), intravenous immunoglobulin, or HBV vaccination within 12 weeks of transplantation. In accordance with our standard protocols, all recipients who underwent autologous HCT were screened for HBsAg, HBsAb, human immunodeficiency virus (HIV) antibody, and HBV DNA by polymerase chain reaction (PCR) assay. In addition, after July 1993, all recipients were screened for HCV antibody (anti-HCV). Samples of serum obtained before transplantation from recipients who underwent HCT before July 1993 were tested retrospectively for anti-HCV. For all HBsAg-positive recipients, additional PCR serologic testing for HBeAg, hepatitis B e antibody (HBeAb), and serum HBV DNA was done within 4 to 8 weeks of marrow harvest or HCT. In addition, all HBsAg-positive pretransplantation samples of recipients' serum collected after December 1998 were tested for HBV DNA by using the Digene II assay within 4 to 8 weeks of marrow harvest or HCT. All HBsAg-positive or HBV DNA–positive (on PCR assay) pretransplantation samples of recipients' serum collected before December 1998 (and stored at −70°C) were tested retrospectively for HBV DNA by using the Digene assay. The precore and core promoter HBV DNA sequences for all marrow recipients positive for HBsAg or HBV DNA (on PCR assay) were determined by direct DNA sequencing.
Recipients of HCT underwent tests of liver function (including determinations of serum levels of alanine aminotransferase [ALT], albumin, and bilirubin) at least once a week during the first 12 weeks, 1 to 4 times weekly until the end of week 52 after HCT, and at 2- to 12-week intervals until their last follow-up or death. Hepatitis serologic variables (HBsAg, HBeAg, HBeAb, HBV DNA on Digene assay and PCR, and HCV RNA on PCR) were assessed in serum samples collected before and during hepatic events when there was any clinical suspicion of liver damage due to HBV infection. The occurrence of hepatic events (acute hepatitis, chronic hepatitis, anicteric or icteric hepatitis, hepatic failure, and veno-occlusive disease [VOD]) and death were recorded. At least one pretransplantation serum sample (obtained 1-6 weeks before HCT) was available for all HCT recipients in this study. Serial serum samples were collected before HCT and after HCT at 1 to 4 weekly intervals for the first year and then at 2 to 12 weekly intervals until their last follow-up or death. At least 6 posttransplantation serum samples were available for each patient in the study. All serum samples were stored at −70°C. All patients who had hepatitis due to HBV reactivation after HCT were treated with lamivudine (100 mg given once daily). All patients who underwent autologous HCT had high-dose cytoreductive therapy. This consisted of either both busulfan and cyclophosphamide (Cy) or Cy and TBI with a total radiation dose of more than 12 Gy or Cy and bischloroethylnitrosourea (BCNU) with etoposide, high-dose melphalan, melphalan with TBI, etoposide, BCNU, cytarabine, and melphalan.
Hepatitis serologic analysis and HBV DNA assay
HBV serologic markers, including HBsAg, HBsAb, HBeAg, HBeAb, anti-HCV Ab (Electroimmunoassay II), anti–hepatitis-D antibody, and anti-HIV antibody, were tested by using commercially available enzyme immunoassays (Abbott Laboratories, Chicago, IL). HBcAb was tested by radioimmunoassay (Corab; Abbott). Serial serum samples from the same patient were tested in a single run to minimize interassay variation. A nested PCR assay for detection of serum HBV DNA was done by using primer sets from the HBV surface antigen and the core antigen coding region.41 For the surface region, the primers were CCTGCTGGTGGCTCCAGTTC (map position, 58-77) and CAAACGGGCAACATACCTTG (476-457) for the first round of PCR testing and ACATCAGGATTCCTAGGACC (169-188) and CGCAGACACATCCAGCGATA (398-370) for the second round. The corresponding sets of primers for the core region were GGAGTGTGGATTCGCATCCTCC (2269-2288), ATACTAACATTGAGATTCCC (2457-2438), AGACCACCAAATGCCCCTAT (2299-2318), and GATCTTCTGCGACGCGGCGA (2429-2410). Using serial dilution of EuroHep-2 HBV DNA plasma standards, we estimated the detection limit of the PCR assay to be 1 × 102 genomes/mL. Results of the Digene Hybrid Capture II assay were expressed in copies per milliliter, and the manufacturer's stated cut-off limit for detecting HBV viremia in clinical specimens was 0.142 × 106 copies/mL. Reverse transcription–nested PCR for HCV was done in a single tube assay as described previously. The outer primers 57 (5′-AGCGTCTAGCCATGGCGT-3′) and 321 (5′-GCACGGTCTACGAGACCT-3′) and the inner primers 126 (5′-GTGGTCTGCGGAACCGG-3′) and 299 (5′-GGGCACTCGCAAGCACCC-3′) were designed from the highly conserved 5′ noncoding region.
HBV PCR sequencing
The HBV DNA extracted was used to amplify the precore and BCP fragments with PCR using the following primer sets: P7 (5′-TCCTCTGCCGATCCATACTG) and BC1 (5′GGAAAGAAGTCAGAAGGCAA) or P8 (5′-TCTGCCGATCCATACTGCGG) and BC1 (5′GGAAAGAAGTCAGAAGGCAA) at a concentration of 50 pmol. Direct sequencing of the PCR products was carried out with the “fmol” sequencing kit (Promega, Southampton, England). Sequencing primers P9 and BC1 were end-labeled with 25 μCi 32P–adenosine triphosphase (Amersham International, Amersham, England) in a volume of 10 μL using 10 U polynucleotide kinase (Boehringer Mannheim, Penzberg, Germany) for 20 minutes at 37°C, and 1.5 μL of the reaction mix was used directly in sequencing reactions. PCR product (90 μL) was precipitated with an equal volume of 4 M sodium acetate and 2 vol isopropanol for 10 minutes at room temperature, formed into pellets by centrifugation for 10 minutes, and washed twice with 70% ethanol. After resuspension in 20 μL water, 9.5 μL DNA was added to the reaction buffer with the end-labeled primer and 5 U Taq enzyme. Dideoxynucleotide termination sequencing was done according to the manufacturer's instructions. Cycle sequencing was performed for 30 rounds.
The denaturation, annealing, and extension steps were 30 seconds, 30 seconds, and 1 minute in length, respectively. Reproducibility of results was ascertained with selected samples by repeat sequencing with different primers as well as sequencing from both the 5′ and 3′ ends. The PCR band with variants was electroeluted, phosphorylated, and cloned into the SmaI site of pUC18 by using conventional procedures. Recombinant clones were identified by restriction-enzyme digestion, and purified plasmid DNA from individual clones was sequenced in both directions with universal primers by using a commercial sequencing kit (Sequenase 2.0; United States Biochemicals, Cleveland, OH).42 HBV genotyping was done with restriction fragment-length polymorphism of an S region or a pre-S region amplicon, as described by Lindh et al.31
Definition of hepatic events
Hepatitis was defined as a greater than 3-fold elevation in serum aminotransferase above the upper limit of normal on 2 consecutive determinations done at least 5 days apart and in the absence of clinical features suggestive of VOD or infection by cytomegalovirus or herpes simplex virus. Icteric hepatitis was defined as hepatitis associated with clinical jaundice and a serum bilirubin level that exceeded 30 μM (normal, <19 μM). Hepatitis was considered to be transient if the duration was less than 6 months and persistent if the duration was more than 6 months. Hepatitis was assumed to be due to HBV reactivation when it was preceded or accompanied by an elevation in serum HBV DNA to more than 10 times that of the pre-exacerbation baseline value, when serum HBV DNA test results turned from negative to positive, or when HBsAg test results became positive and remained so for 2 consecutive readings 5 days apart. Hepatic failure was defined as the presence of hepatic encephalopathy and deficits in blood coagulation (prothrombin time prolonged for more than 10 seconds). The clinical criteria used for diagnosis of VOD disease were a bilirubin level of at least 34.2 μM, hepatomegaly or right upper quadrant pain of liver origin, and a greater than 2% weight gain due to fluid accumulation; the diagnosis required that 2 of these 3 criteria occurred within 20 days of HCT. Moreover, the diagnosis was made only after hyperacute graft-versus-host disease, sepsis, cardiac failure, and tumor infiltration were ruled out.43
Statistical analysis
Clinical characteristics of patients were quantified and percentages calculated. Differences among groups were analyzed by using the median test for continuous data and the likelihood χ2or Fisher exact test for categorical data. The outcome of interest was the survival time with hepatitis due to HBV reactivation, which was defined as the time from marrow infusion to the time of diagnosis of hepatitis due to HBV reactivation. Patients who died of other causes and patients who were alive without hepatitis due to HBV reactivation were censored. Cumulative survival with hepatitis due to HBV reactivation was analyzed by using Kaplan-Meier curves.44 The Cox proportional hazards model45 was used to compare differences in HBV hepatitis survival between groups by obtaining hazard ratios and 95% confidence intervals (CIs). Virologic factors possibly associated with the rate of occurrence of hepatitis due to HBV reactivation were studied by using a multivariate Cox proportional hazards model, and hazard ratios adjusted for other model factors were obtained together with their 95% CIs. The proportional hazards assumption was checked by examining the martingale and deviance residuals. The SAS statistical program (version 8.0; SAS Institute, Cary, NC) was used. A P value less than .05 was considered to represent a significant difference.
Results
Clinical characteristics of patients undergoing autologous HCT
Before HCT, 23 patients (16.8%) were positive for HBsAg, 37 (27.0%) were positive for anti-HBs (28 were also anti-HBc positive), and 77 (56.2%) were negative for HBV. There were no significant differences in sex, age, underlying disease, conditioning regimen, or percentage of patients with elevated serum ALT levels before HCT (Table2).
Liver-related complications after autologous HCT
One hundred thirty-seven patients were followed for a median of 25 months (range, 0.3-122 months). After autologous HCT, hepatitis developed in 32 patients (23%), with a mean onset at 136 ± 272 days after transplantation. Eleven cases were persistent and 21 were transient. Ten cases of persistent hepatitis (91%) and 3 cases of transient hepatitis (14%) were due to HBV reactivation (P < .0001). Most cases of persistent hepatitis (10 of 11) occurred in patients who were HBsAg positive before HCT, and only one patient negative for HBsAg had persistent hepatitis after HCT (P < .012). All cases of persistent hepatitis were due to HBV reactivation. Of the 32 cases of hepatitis after HCT, 13 were due to HBV reactivation and 13 were related to systemic sepsis; the cause of the remaining 6 cases was undetermined. Hepatitis due to HBV reactivation was more common in patients who were positive for HBsAg before HCT (11 of 23) than in patients who were HBsAg negative (2 of 114; P = .023). Seven of the 11 cases of severe hepatitis (icteric hepatitis or hepatic failure) were due to HBV reactivation, whereas only 6 of the 21 cases of anicteric hepatitis were due to HBV reactivation (P = .072).
The peak HBV DNA level preceded the peak serum ALT level by a median of 6 weeks (range, 0-12 weeks). HBV-related hepatitis resulted in 7 cases of severe hepatitis (54%), whereas there were only 4 cases of severe hepatitis not related to HBV (21%; P = .072 on 2-sided Fisher exact test). The median peak serum ALT levels were higher in patients with HBV-related hepatitis (632 IU/L; range, 120-2410 IU/L) than in those with non-HBV–related hepatitis (208 IU; range, 93-3200 IU/L; P = .029 on median test). However, the median peak bilirubin levels were similar in the HBV-related cases of hepatitis (15 μM/L; range, 5-680 μM/L) and the non-HBV–related cases (24 μM/L; range, 6-380 μM/L; P = 1.000 on median test). The median time to peak ALT levels was 110 days (range, 29-374 days) for HBV hepatitis cases and 27 days (range, 3-1500 days) for non-HBV hepatitis cases (P = .0002 on median test).
Three patients in this series underwent lamivudine treatment for hepatitis reactivation (lamivudine was not available before late 1998). Two patients recovered completely after this treatment. The third patient had complete resolution of hepatitis but died later of leukemia relapse.
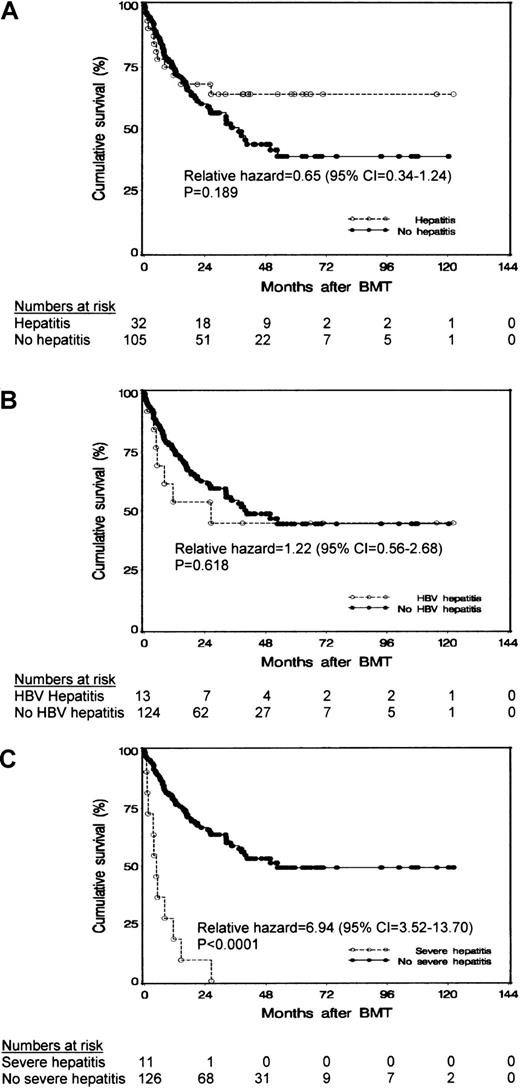
The patients' survival was not affected by the occurrence of hepatitis (P = .189 on log rank test; Figure1A) or HBV-related hepatitis (P = .618 on log rank test; Figure 1B). However, patients who had severe hepatitis after HCT had a poorer survival than patients in whom severe hepatitis did not develop after HCT (P < .0001 on log-rank test; Figure 1C). Eighteen patients (13%) had VOD after transplantation; 4 of these patients (17%) were positive for HBsAg, 7 (19%) were positive for anti-HBs, and 7 (9%) were negative for HBV (P value not significant).
Results of survival analysis.
Cumulative survival of patients after autologous HCT in relation to occurrence of (A) posttransplantation hepatitis, (B) hepatitis due to HBV reactivation, and (C) severe hepatitis.
Results of survival analysis.
Cumulative survival of patients after autologous HCT in relation to occurrence of (A) posttransplantation hepatitis, (B) hepatitis due to HBV reactivation, and (C) severe hepatitis.
Pretransplantation characteristics associated with HBV-related hepatitis after autologous HCT
Pretransplantation characteristics associated with hepatitis due to HBV reactivation after HCT were an elevated serum ALT level, HBsAg positivity, detectable serum HBV DNA (on Digene assay), and BCP (T1762/A1764). Three patients (50%) with elevated serum ALT before HCT and 10 patients (8%) with normal serum ALT before HCT had hepatitis due to HBV reactivation (P < .0001). Eleven patients (48%) who were positive for HBsAg before HCT had hepatitis due to HBV reactivation, where only 2 HBsAg-negative patients (2%) had hepatitis due to HBV reactivation (P < .0001). Nine patients (90%) with detectable serum HBV DNA and only 4 (4%) without detectable serum HBV DNA had hepatitis due to HBV reactivation (P < .0001). Pretransplantation serum HBV DNA levels were also significantly higher in patients with hepatitis due to HBV reactivation (median, 800 pg/mL; range, 0-3800 pg/mL) than in those without hepatitis due to HBV reactivation (median, 0 pg/mL; range, 0-800 pg/mL; P < .0001 on median test). In addition, 7 patients (88%) with BCP (T1762/A1764) and 5 (31%) without BCP (T1762/A1764) had hepatitis due to HBV reactivation (P = .025; Table3).
Patients at risk of posttransplantation hepatitis due to HBV reactivation
The overall cumulative incidence rates of hepatitis due to HBV reactivation were 4.2% (95% CI, 0.6%-7.9%), 10.5% (95% CI, 4.9%-16.2%), and 11.7% (95% CI, 5.7%-17.8%) at 3, 6, and 24 months, respectively, after HCT. Univariate analysis was done to determine whether pretransplantation variables such as sex, age at diagnosis, HBsAg and HBeAg status, HBV DNA positivity on PCR and Digene assays, HBV genotype, BCP (T1762/A1764), and elevated serum ALT level before HCT were associated with an increased cumulative incidence of hepatitis due to HBV reactivation. We found that patients who were positive for HBsAg before HCT had a higher cumulative incidence of hepatitis due to HBV reactivation (51.9%; 95% CI, 30.6%-73.3%) than patients who were negative for HBsAg (2.5%; 95% CI, 0%-6.1%; P < .0001). Other HBV virologic factors found to be associated with posttransplantation hepatitis due to HBV reactivation were detectable serum HBV DNA before HCT (on Digene assay), HBV genotype C, and the presence of BCP (T1762/A1764). Patients with elevated serum ALT levels before HCT also had a higher cumulative incidence of hepatitis due to HBV reactivation. When multivariate Cox regression analysis was used to assess patients positive for HBsAg, detectable HBV DNA on Digene assay before HCT was the only significant and independent risk factor associated with hepatitis due to HBV reactivation after HCT (adjusted hazard ratio, 9.35; 95% CI, 1.65-52.6; Table4).
Discussion
Hepatitis due to HBV reactivation is a serious cause of morbidity and mortality in HBsAg-positive patients undergoing intensive immunosuppression or chemotherapy.1-11 This is a particularly important clinical issue in areas such as Hong Kong, where HBV infection is endemic.14,46 However, the incidence and risk factors for this clinical disorder have not been defined clearly. Hence, in this study, we investigated a cohort of Chinese patients (17% HBsAg positive and 20% anti-HBs and anti-HBc positive) who received intensive chemotherapy in association with autologous HCT. We found that nearly half of the HBsAg-positive patients in our series had hepatitis due to HBV reactivation after autologous HCT. The incidence was comparable to that reported in HBsAg-positive Chinese patients with lymphoma treated with chemotherapy.46
Although most of the posttransplantation cases of hepatitis due to HBV reactivation occurred in patients positive for HBsAg, this disorder also developed in 2 patients who were negative for HBsAg. One of these patients had natural immunity against HBV (anti-HBs and anti-HBc positive). This was probably related to reactivation from latent HBV infection, an assumption consistent with findings of previous studies.7-11 In patients with natural immunity against HBV, HBV DNA could still be detected in serum and peripheral blood mononuclear cells (PBMCs) by using sensitive PCR methods. Moreover, CD69+ (a marker of recent activation) cytotoxic T lymphocytes (CTLs) could still be detected in PBMCs from these patients, indicating the presence of an active CTL control on actively replicating HBV (at a very low level).47 The other HBsAg-negative patient in our study with hepatitis due to HBV reactivation was negative for HBV on PCR assay before HCT, and HBV was probably acquired from the multiple blood products given during HCT.48
The median time to onset of hepatitis due to HBV reactivation was 110 days after marrow infusion, significantly longer than for cases of hepatitis not due to HBV reactivation. The timing of the HBV-related cases of hepatitis coincides with immune reconstitution. Furthermore, peak HBV DNA levels preceded peak ALT levels in patients with hepatitis due to HBV reactivation by a median time of 6 weeks (or they accompanied peak ALT levels), suggesting the presence of an immune mechanism of liver damage.
Because the cases of hepatitis due to HBV reactivation were preceded or accompanied by an increasing level of viral replication, we hypothesized that HBV virologic and host factors implicated to influence HBV viral replication might affect the occurrence of posttransplantation hepatitis due to HBV reactivation. Among those HBV virologic factors, HBV genotype, BCP, and HBV viral load are of particular interest. An association between BCP (T1762/A1764) and severe liver disease was previously reported.29-34 This might be related to the accompanying changes in the secondary structure of the pregenome, which might increase viral replication.34 Furthermore, the BCP (T1762/A1764) double mutation enhanced synthesis of core protein by 15 fold in an expression system.28 In vitro, the mutation was shown to increase transcription of pregenomic RNA by creation of a binding site for hepatocyte nuclear factor 1.49 On the other hand, studies in Taiwan and Japan suggested that, compared with HBV genotype B, HBV genotype C was associated with more severe disease and a lower rate of response to interferon-α therapy.29,33 50 The underlying pathogenetic explanation for this finding remains speculative, and further studies are required to reveal the effect of HBV genotype on HBV virus-host immune interaction.
Using univariate analysis, we found that the risk factors associated with posttransplantation hepatitis due to HBV reactivation were HBsAg, detectable HBV DNA before HCT (on Digene assay), HBV genotype C, and the presence of BCP (T1762/A1764). We also observed that HBV genotype C was associated with the occurrence of BCP (T1762/A1764). These findings are similar to those of studies in Japan,29 Chinese populations,33,51 and Sweden.32 The underlying molecular explanation is unclear but might be related to the stabilization of the stem-loop structure of the encapsidation for various HBV genotypes with these HBV variants.51
Among the few pretransplantation viral and host factors related to the occurrence of hepatitis due to HBV reactivation, a detectable HBV DNA level (on Digene Hybrid Capture II assay) in HBsAg-positive patients was found to be the principal predicting factor on multivariate Cox regression analysis. Moreover, the pretransplantation serum HBV DNA level was also significantly higher in patients with hepatitis due to HBV reactivation than in those without this disorder. Therefore, it is logical to reduce the HBV viral load and to keep it below the detection limit of the Digene assay (105copies/mL) before HCT and immediately afterward. Determining whether additional reductions in the HBV viral load will be beneficial will require studies using more sensitive HBV DNA quantitation methods.52 Treatment with interferon-α53 or nucleoside analogues such as famciclovir54 and lamivudine55 has been shown to reduce the amount of circulating HBV DNA in most patients with chronic HBV infection. Nucleoside analogues are preferable because they do not cause substantial myelosuppression, a possible complication of interferon-α therapy.56 However, prolonged monotherapy with lamivudine or famciclovir was associated with an increase risk of resistant variants,57,58 and emergence of resistant mutants might cause serious liver disease, particularly in immunosuppressed patients.59 It is relevant to this issue that in treatment of HIV infections, resistance has developed as a result of ineffective suppression of viral replication, ie, resistance cannot occur without replication.60 Hence, the use of more potent nucleoside analogues is desirable. Several new nucleoside analogues, including adefovir dipivoxil,61 entecavir,62and emtricitabine,63 have been found to be effective in suppressing HBV replication. Additional clinical trials of these nucleoside analogues are needed to determine their antiviral efficacy in patients. Alternatively, combination nucleoside-analogue therapy with phenotypic reversion and synergism might be useful.60 64
In this series, VOD (Seattle criteria) was observed in 15% of patients after HCT. This finding is consistent with those in some other series,65-67 but the rate is higher than that in a recent European study (3.1%).68 The discrepancy in the incidence of VOD is probably related to our common use of high-dose cytoreductive therapy, which is a known risk factor for VOD43 68; our use of less stringent criteria for the diagnosis of VOD; and the presence of HBV infection in a substantial proportion of our patients.
In conclusion, we found that hepatitis due to HBV reactivation was common in patients positive for HBsAg who underwent autologous HCT. Among such patients, a high HBV DNA level (>105 copies/mL) was the most important risk factor for HBV reactivation. Additional clinical trials of the preemptive use of nucleoside analogues to lower the HBV DNA level before transplantation are needed.
We thank Yuen Wing Sze and Amy Kwok for assistance in data management.
Supported by a grant from the Cheng Si-yuan (China International) Hepatitis Research Foundation to the University of Hong Kong; China National 973 research grant (G 1999 054105 (Y.M.W. and G.K.K.L); an ear-marked grant from the Hong Kong Research Grant Council; and a grant from the University Research Committee (R.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Raymond Liang, Division of Hematology and Oncology, University Department of Medicine, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong SAR, China; e-mail: rliang@hkucc.hku.hk.