Abstract
Clinical results after T-cell–depleted allografts might be improved by modifying the graft content of progenitor and accessory cells. Although the association of the number of donor T cells with the clinical outcome has been studied extensively, the optimum number of progenitor cells that should be administered to patients is unknown. The characteristics of 84 consecutive human leukocyte antigen (HLA)–identical sibling transplants of granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood progenitor cells depleted of T cells by CD34+ positive selection (allo-PBT/CD34+) were analyzed for their effect on clinical outcome. After a median follow-up of 24 months (range, 1-70 months), 50 patients remain alive (59.5%) and 34 have died (21 [25%] as a result of the transplant and 13 [15.5%] due to disease relapse). The median number of CD34+ cells administered to the patients was 3.9 × 106/kg (range, 1.2-14.3 × 106/kg). A number of CD34+ cells in the inoculum of 1 × 106/kg to 3 × 106/kg was associated with increased survival: 21 of 28 (75%) patients are alive, as compared with 29 of 56 (52%) patients receiving more than 3 × 106/kg (actuarial probability 75% vs. 42%, respectively; P = .01). In the multivariate analysis, the independent prognostic variables for survival were CD34+cell dose 1 × 106/kg to 3 × 106/kg (RR = 4.8; P = .0008), sex-pairing match (RR = 3.2;P = .002), and early stage of disease (RR = 2.8;P = .007). From these results it appears that, in allo-PBT/CD34+ from HLA-identical siblings, a number of CD34+ cells in the inoculum between 1 × 106/kg to 3 × 106/kg is an important factor for better survival, and that higher CD34+ cell doses might be associated with a poorer outcome.
Introduction
Allogeneic transplants can cure patients with hematologic malignancies, but the mortality associated with the procedure is of concern. Several prognostic factors have been identified, but most of them, such as age of the patient, stage of the disease, or human leukocyte antigen (HLA)–matching compatibility, are inherent to the characteristics of the donor and/or the recipient and cannot be modified.1 For this reason, the finding in some studies that a higher nucleated cell dose in the marrow graft is associated with reduced transplant-related mortality and better disease-free survival has been encouraging.1-6 This association has been attributed to shorter neutropenia, faster immunorecovery, and enhanced graft-versus-leukemia effect.7 It is not clear, however, whether the beneficial effect of a higher nucleated cell dose is a result of the higher content of either progenitor or accessory cells.
The influence of the number of progenitor cells on outcome is of particular relevance in T-cell–depleted allogeneic marrow transplants, in which, as a result of manipulation of the graft, the inoculum may contain a suboptimal number of stem cells. Thus, Mavroudis et al, in a series of T-cell–depleted marrow transplants, found that a very low CD34+ cell dose (< 1 × 106/kg) was associated with increased mortality and lower disease-free survival.8 The use of granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood progenitor cells (PBPCs) and CD34+ positive selection as T-cell–depletion method (allo-PBT/CD34+) circumvents this problem, since a number of CD34+ cells higher than 3 × 106/kg is easily obtained.9 10
The possibility of infusing very high numbers of progenitor cells raises the question of the quantity of CD34+ cells that, beyond a minimum of 1 × 106/kg, should be left in the graft for allogeneic transplants with T-cell depletion from HLA-identical siblings. Although a beneficial effect of increasing numbers of CD34+ cells on engraftment and survival has been demonstrated for autografting,3,11,12 that association might be different in the context of allogeneic transplantation, where CD34+ cells not only participate in engraftment, but also have an immunogenic role.13-16 With this background, we have analyzed the effect on the clinical outcome of the number of CD34+ cells in 84 consecutive adult patients submitted to allo-PBT/CD34+ from HLA-identical siblings. This study, for the first time, reports that a high CD34+ cell dose not only does not improve the clinical result, but actually might have a deleterious effect.
Patients, materials, and methods
Patients and donors
This study, performed from March 1995 to December 2000 in a single institution (Hospital Clı́nic of Barcelona), included a total of 84 consecutive adult patients with hematologic malignancies submitted to allo-PBT/CD34+ from an HLA-identical sibling donor (Table 1). No second transplants were included. The conditioning regimen consisted of cyclophosphamide (120 mg/kg of body weight) and total body irradiation (TBI) (13 Gy in 4 fractions of 325 cGy each) in 75 (90%) patients. TBI (12 Gy) and melphalan (110 mg/m2) were administered to 3 patients diagnosed with multiple myeloma, and 6 patients received busulphan (16 mg/kg) and cyclophosphamide (120 mg/kg) because they had received locoregional irradiation beforehand. No growth factors were administered after the transplant. Donors received G-CSF (Filgrastim; Amgen, Thousand Oaks, CA) at a dose of 10 μg/kg every 12 hours per day subcutaneously for 4 to 7 days. On day 5 to 8, donors underwent a median of 10 L leukapheresis with the Fenwall CS-3000 plus separator (Baxter, Deerfield, IL). Leukaphereses were initiated 12 to 18 hours following the fourth dose of G-CSF and were performed in all cases through 2 peripheral vein accesses.
Graft-versus-host disease prophylaxis
CD34+ cells were positively selected using an immunoadsorption biotin-avidin column (Ceprate SC System; CellPro, Bothell, WA) in 46 cases (55%). From July 1998, when this device was no longer available, CD34+ positive selection was performed by indirect immunomagnetic beads (CliniMACS, Miltenyi Biotec, Bergisch Gladbach, Germany), for a total of 38 (45%) cases. Immunophenotyping was performed with a FACScan flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA). The antibodies used were: 8G12-PE (HPCA-2/CD34), HLe-1-FTIC (CD45), Leu-M3-PE (CD14), Leu-4-FTIC, (CD3), and phycoerythrin (PE)– and fluorescein isothiocyanate FTIC–conjugated irrelevant isotype-specific antibodies. CD34+ cells and CD3+ cells were quantified as previously published.17 The CD34+ positive fraction was infused to the patients, with previous cryopreservation in 34 cases (40.5%), as the sole source of progenitor cells. The median number of CD34+cells and CD3+ cells infused to the patients was 3.9 × 106/kg (range, 1.2-14.3 × 106/kg) and 0.3 × 106/kg (range, 0.1-2), respectively. In cases in which CD34+ selection was performed with indirect immunomagnetic beads, CD3+ cells were added to the graft to achieve a similar quantity of T cells to that infused using the immunoadsorption biotin-avidin column. Thus, the great majority of patients (77 of 84; 92%) received a quantity of CD3+ cells ranging between 0.1 × 106/kg and 0.5 × 106/kg. Graft-versus-host disease (GVHD) posttransplant prophylaxis consisted of cyclosporine A (CsA), which was started at 1.5 mg/kg intravenously every 12 hours from day −1 and adjusted to maintain therapeutic blood levels of 250 ng/mL to 400 ng/mL, and methylprednisolone 0.5 mg/kg days +7 to +14, 1 mg/kg days +15 to +28, followed by tapered doses. The cyclosporine dose was reduced if renal function decreased, regardless of cyclosporine blood levels. Cyclosporine was given orally as soon as oral mucositis disappeared, and was stopped by day 180 after transplantation. From July 1998, CsA methylprednisolone was substituted with CsA alone.
Evaluation and definitions
Patients with chronic myeloid leukemia in chronic phase, acute leukemia in first remission, and other hematologic malignancies in remission were designated as being in early stage. Patients with acute leukemia in second or subsequent remission, or other hematologic malignancies in relapse, were designated as being in advanced stage. The day of CD34+ selected transfusion was defined as day 0. Engraftment was documented by increasing neutrophil and platelet counts unsupported by transfusions. Time to neutrophil engraftment was assessed by determining the number of days after day 0 for patients to achieve more than 500/μL. Time to platelet engraftment was assessed by determining the number of days after day 0 to maintain an untransfused platelet count of more than 20 000/μL or greater. The diagnosis and grading of acute and chronic GVHD was established according to the Seattle criteria.18 Chronic GVHD was defined as the presence of GVHD after day 90. Skin biopsies were taken from all patients with skin rashes. Transplant-related mortality (TRM) was defined as death due to causes different from disease relapse. Survival was determined by measuring the time from transplantation until death from any cause. This study was approved by the local ethic committee and by the Spanish Health Department. Detailed written informed consent was obtained from all donors and patients before beginning the procedure.
Statistical methods
Actuarial curves were obtained by the Kaplan-Meier method and statistically compared using the log-rank test. All reportedP values are 2-sided, and a significance level of α = 0.05 was used. The characteristics considered in this study were donor sex, patient sex, sex-pairing, donor age, patient age, pretransplant cytomegalovirus (CMV) serology of donor and patient, diagnosis, disease stage at time of transplant (early vs. advanced), number of CD34+ cells and of CD3+cells infused, CD34+ selection method, cryopreservation of the CD34+ positive fraction, and type of GVHD posttransplant prophylaxis. In considering the association of the number of CD34+ cells in the graft with clinical outcome, 2 groups were established (1 × 106/kg to 3 × 106/kg vs. > 3 × 106/kg) based on previous clinical results, showing that, by administering a CD34+ cell dose of 1 × 106/kg to 3 × 106/kg, outcome was improved8 (Table2). All prognostic variables in the univariate analysis (Kaplan-Meier method) with a P value less than or equal to .2 (Table 2) were included for the multivariate analysis to eliminate the redundancy among highly correlated characteristics, each of which may be individually significant. This was performed using the stepwise proportional hazard Cox regression model. The proportional hazard assumption of the Cox model was checked separately for each covariate before performing the regression analysis. Such checking was done by a graphical and analytical method. The graphs of loge [-loge S (t)] vs. loge (t) for each dichotomous covariate were obtained in order to check that the curves were roughly parallel. Additionally, for each covariate, a time-dependent covariate (covariate × t) was obtained and checked, in order to verify whether the coefficient of the latter differed significantly from 0. The proportional hazard assumption was not rejected for any one of the covariates included in the Cox model. Statistical studies were performed by means of SPSS 9.0.1 (1999; SPSS, Chicago, IL) statistical software.
Results
Engraftment
All patients reached an absolute neutrophil count (ANC) of 500/μL and an untransfused platelet count of more than 20 000/μL. The median day to achieve an ANC of more than 500/μL and an untransfused platelet count of more than 20 000/μL was 15 (range, 9-32 days) and 14 (range, 5-65 days), respectively. The absolute counts of neutrophils, monocytes, platelets, and lymphocytes in the first 9 months after transplantation, with monthly time points, were checked and compared according to the CD34+ cell dose (1 × 106/kg-3 × 106/kg vs. > 3 × 106/kg). Platelet counts were significantly higher at all time points in the more than 3 × 106/kg CD34+ cell group. In contrast, absolute counts of neutrophils, monocytes, and lymphocytes did not significantly differ at any time point between both groups (data not shown). Six patients (7%) developed graft failure, between 30 to 178 days after transplantation, for an actuarial probability of this complication of 8% (95% CI: 3%-13%). The range of CD34+ cells and CD3+cells (× 106/kg) infused to the patients with graft failure was 1.3 to 6.3 and 0.1 to 0.2, respectively.
Graft-versus-host disease
Twenty-four patients (28%) developed acute GVHD (aGVHD) clinical grade I, and 6 (7%) aGVHD clinical grade II. In no single patient was aGVHD III-IV observed. The actuarial probability for aGVHD I-IV and II-IV was 35% (95% CI: 25%-45%) and 7% (95% CI: 2%-12%), respectively. Eight patients (9.5%) developed limited and 5 (6%) extensive chronic GVHD (cGVHD); the actuarial probability for extensive cGVHD was 7% (95% CI: 2%-12%). The 1 × 106/kg to 3 × 106/kg CD34+ cell group had, as compared with the more than 3 × 106/kg group, a trend for a lower actuarial probability of aGVHD I-IV (28% vs. 38%,P = .2), a later appearance of this complication (median of 46 days after transplantation vs. 29 days, P = .04; Mann-Whitney U test), and a lower actuarial probability of extensive cGVHD (0% vs. 12%, P = .05).
CMV infection
Forty-four patients (52.4%) received preemptive treatment with ganciclovir (5 mg/kg IV twice a day for 14 days) or foscarnet (60 mg/kg IV twice a day for 14 days) for CMV antigenemia, which was observed at a median of 55 days (range, 27-270 days) after transplantation, with an actuarial probability for CMV antigenemia of 55% (95% CI: 45%-65%). The actuarial probability for CMV antigenemia was identical in both CD34+ groups. Six patients developed features of CMV disease, causing the death of the patient in 4 cases.
Recipient and donor characteristics associated with clinical outcome
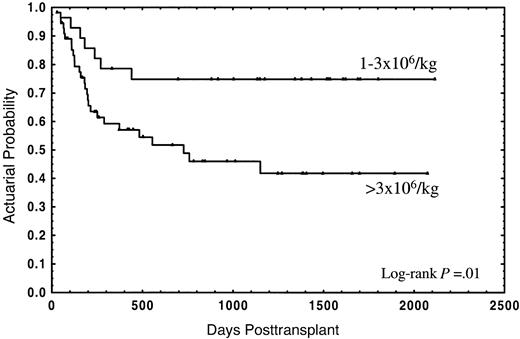
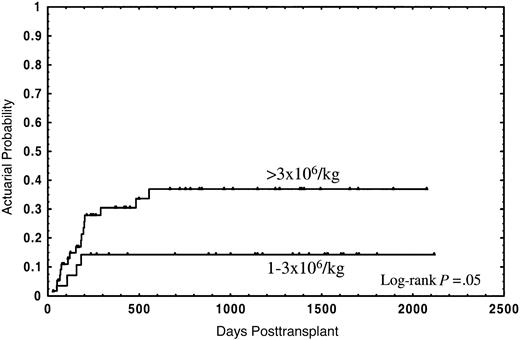
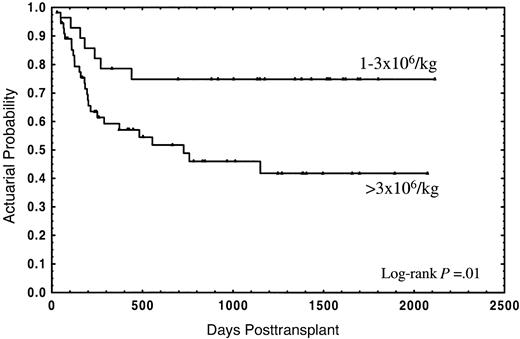
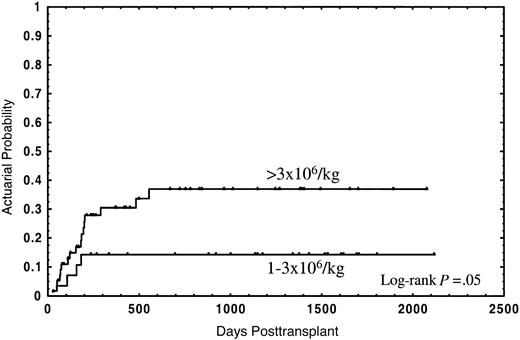
The median follow-up for the whole group was 2 years (range in months, 1-70), and the median follow-up for patients still alive was 3 years (range in months, 1-70). Thirty-four patients (40.5%) died; 21 (25%) due to the transplant and 13 (15.5%) from disease relapse. The actuarial probability for survival and TRM was 55% (95% CI: 44%-66%) and 28% (95% CI: 18%-38%), respectively. In the univariate analysis, overall survival was not significantly affected by either median age of donor or recipient, diagnosis, sex relation male to female or female to male, stage of disease, CD34+selection method, number of CD3+ cells, cryopreservation, or type of GVHD posttransplant prophylaxis (Table 2). In this analysis an association of decreasing rates of survival with increasing numbers of CD34+ cells was found, with the strongest correlation of CD34+ cell dose and impaired survival in the group of patients receiving a number of CD34+ cells of more than 3 × 106/kg (Table 2). Twenty-one out of 28 patients (75%) receiving 1 × 106/kg to 3 × 106/kg CD34+ cells survived, as compared with 29 out of 56 patients (51.8%) receiving more than 3 × 106/kg, the actuarial probability being 75% and 42%, respectively (P = .01) (Figure1). Four patients (14.3%) in the first group and 17 (30.4%) in the second died due to the transplant, at a median of 126 days (range, 58-182 days) and 177 days (range, 29-555 days), respectively; the actuarial probability for TRM being 14% vs. 37%, respectively (P = .05) (Figure2). In contrast, the relapse rate was very similar in both groups (actuarial probability 34% vs. 36%, respectively, P = NS). Causes of death in both groups are shown in Table 3. Two other factors were found to be associated with increased survival in the univariate analysis: sex-pairing match, and CMV-seronegative patients having received the graft from a CMV-seronegative donor (Table 2).
Actuarial probability of overall survival for patients receiving a CD34+ cell dose of 1 × 106/kg to 3 × 106/kg or greater than 3 × 106/kg.
Actuarial probability of overall survival for patients receiving a CD34+ cell dose of 1 × 106/kg to 3 × 106/kg or greater than 3 × 106/kg.
Actuarial probability of transplant-related mortality for patients receiving a CD34+ cell dose of 1 × 106/kg to 3 × 106/kg or greater than 3 × 106/kg.
Actuarial probability of transplant-related mortality for patients receiving a CD34+ cell dose of 1 × 106/kg to 3 × 106/kg or greater than 3 × 106/kg.
Predictive variables
We introduced into regression all variables with a Pvalue of less than or equal to .2 in the univariate analysis (Table 2): sex-pairing match, median age of donors and patients, negative CMV serology for both donor and patient, stage of disease, CD34+ cells 1 × 106/kg to 3 × 106/kg, and type of GVHD posttransplant prophylaxis. As shown in Table 4, 3 variables entered into regression at a significant level: CD34+ cells 1 × 106/kg to 3 × 106/kg (RR = 4.8;P = .0008), sex-pairing match (RR = 3.2;P = .002), and early stage of disease (RR = 2.8;P = .007).
Confounding variables
To determine whether the CD34+ cell dose was independent of other variables that could affect transplant outcome, in addition to the multivariate analysis we compared characteristics of the 2 groups (1 × 106/kg to 3 × 106/kg vs. > 3 × 106/kg) (Table5). Patients in the 1 × 106/kg to 3 × 106/kg CD34+ cell group had a significantly longer follow-up, and there was a trend of fewer cases with sex-pairing match and in early stage. As mentioned in “Patients, materials, and methods,” as of mid-1998, the CD34+ selection method and the type of GVHD posttransplant prophylaxis were modified. Although neither of the 2 factors was associated with the outcome in the univariate and the multivariate analysis, we performed a second univariate and multivariate study restricted to the group of patients transplanted before that date (n = 54); very similar results were obtained, with the same covariates entering into the regression model. In particular, the covariate CD34+ cell dose 1 × 106/kg to 3 × 106/kg retained the strongest association with improved survival (RR = 4.9; 95% CI: 1.7%-14.6%;P = .004).
Discussion
GVHD is one of the major impediments to the success of allogeneic transplants. T-cell depletion of the graft effectively reduces this complication, but is related to a higher incidence of graft failure and leukemic relapse, so that the final outcome is very similar to that of unmanipulated allogeneic transplantation.19 It has been suggested that these results might be improved by modifying the graft content of progenitor and accessory cells. In such a context, we have recently shown the association of increasing numbers of CD3+ cells in the inoculum with decreasing graft failure rates.20 However, the optimum number of CD34+cells that should be left in the graft to improve the outcome is unknown.
In this study we have analyzed the association of characteristics from the donor, recipient, and the cell content of the graft with the outcome in 84 consecutive adult patients with hematologic malignancies submitted to allo-PBT/CD34+ from HLA-identical siblings in our institution. Besides well-known factors influencing the clinical outcome, such as the stage of the disease or CMV serology of donor and recipient,21 a strong association of the number of donor CD34+ cells with survival was observed. Thus, the actuarial probability of survival for patients receiving a CD34+ cell dose 1 × 106/kg to 3 × 106/kg was 75%, as compared with 42% for patients receiving more than 3 × 106/kg (P = .01) (Figure 1), this association was also evident in the multivariate analyses (Table 4). Mavroudis et al, in a series of T-cell–depleted allogeneic bone marrow transplants, also found an actuarial probability of survival of 74% for patients receiving 1 × 106/kg to 3 × 106/kg CD34+ cells.8Because that study did not include patients receiving more than 3 × 106/kg CD34+ cells, the question of whether the clinical outcome could be further improved by increasing the CD34+ cell dose remained unanswered. Here we report that a higher CD34+ cell dose might have a deleterious effect on the clinical outcome.
In addition to the multivariate analysis, which excluded a competing effect of other variables associated with the outcome, we further sought confounding factors that might explain the differences in survival between both CD34+ cell dose groups. Follow-up in the 1 × 106/kg to 3 × 106/kg CD34+ group was significantly longer, with other characteristics being well distributed (Table 5). As of mid-1998, the technique for CD34+ selection and the type of GVHD posttransplant prophylaxis were modified. The possibility that one of these 2 factors could influence the results was discarded, because they were not significantly associated with outcome in either the univariate or the multivariate analysis. Moreover, we performed a multivariate study restricted to the group of patients who received their transplants before that date (n = 54): again, CD34+ cell dose 1 × 106/kg to 3 × 106/kg was the covariate most strongly associated with better survival.
The detrimental effect of infusing high numbers of CD34+cells is intriguing. A possible explanation is that, in allogeneic transplants, CD34+ cells do not simply participate in engraftment.13-16 Thus, experimental data show that CD34+ cells are enriched for dendritic cell progenitors and induce T-cell proliferation, in the presence of tumor necrosis factor alpha (TNFα) or allogeneic mononuclear cells.13,14 Of note, an association of the number of donor dendritic cell progenitors with impaired outcome after allogeneic transplantation has been reported.22 Moreover, it has been shown that the immunogenic role of highly enriched CD34+ cells disappears in the presence of autologous T cells.13,14 Thus, the results presented here might not be extrapolative to unmanipulated allogeneic transplants, in which accessory cell effects could obscure the effect of CD34+ cells on transplant outcome. There is also the possibility that the clinical relevance of the number of nucleated cells from bone marrow2,3,5,6 is different from that of the number of nucleated cells from G-CSF–mobilized PBPCs, because CD34+ cell and accessory cell function and cytokine patterns from both sources are different.23-27 Although these possibilities are conjectural, the clinical observation herein presented is of interest in the general context of allogeneic transplants, and suggests the convenience of analyzing not only CD34+ cells and CD3+ cells in the allograft, but also other important accessory cells, for example, dendritic cells. The potential effect of these cell subsets on clinical outcome after allogeneic stem cell transplantation needs to be studied.
The high CD34+ cell dose impaired survival as a result of higher TRM. (Figure 2). This might be due to the fact that this group of patients had a higher incidence, and earlier appearance, of GVHD, which is associated with increased TRM due to severe infections, in particular in patients receiving a T-cell–depleted graft.28 Although an association of CD34+ cell dose with GVHD has been observed,29-31 and experimental data show the capacity of CD34+ cells to present certain antigens to autologous T cells,16 a formal demonstration of the participation of CD34+ cells in GVHD is still pending.
To summarize, our results support the concept that, in HLA-identical sibling transplants of G-CSF–mobilized PBPCs depleted of T cells, a CD34+ cell dose of 1 × 106/kg to 3 × 106/kg is associated with improved outcome. The principal finding in this report, however, is that increasing the numbers of CD34+ cells beyond these values not only does not improve the clinical result, but might make it worse. These observations should be taken into consideration when designing and evaluating trials in which highly enriched CD34+ cells from G-CSF–mobilized PBPCs from HLA-identical siblings are administered.
We are indebted to Dr Llorens Quintó from the Statistical Department of the Hospital Clinic of Barcelona for his supervision of the statistical study.
Supported in part by grants FIJC-00/P-EM and FIJC-00/P-CR from the José Carreras International Leukaemia Foundation (Barcelona, Spain), grant 2000SGR 00121 from the Generalitat de Catalunya (Barcelona, Spain), and grants FIJC-98/0995 and FIS-98/0380 from the Fondo de Investigaciones Sanitarias de la Seguridad Social, Spanish Ministry of Health (Madrid, Spain).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alvaro Urbano-Ispizua, Institute of Hematology and Oncology, Department of Hematology, Hospital Clı́nic, University of Barcelona, Villarroel 170, 08036 Barcelona, Spain; e-mail:aurbano@clinic.ub.es.