Abstract
In previous analyses of transferrin saturation data in African Americans and Caucasians from the second National Health and Nutrition Examination Survey (NHANES II), subpopulations were found consistent with population genetics for common loci that influence iron metabolism. The goal of this new study was to determine if these transferrin saturation subpopulations have different levels of iron stores. Statistical mixture modeling was applied to transferrin saturation data for African Americans and Caucasians from the third National Health and Nutrition Examination Survey (NHANES III), and then the mean serum ferritin concentrations were determined for the transferrin saturation subpopulations that were identified. After adjustment for diurnal variation, 3 subpopulations of transferrin saturation were identified in each racial group. Satisfying Hardy-Weinberg conditions for major locus effects, in both racial groups the sum of the square roots of the proportion with the lowest mean transferrin saturation and the proportion with the highest mean transferrin saturation was approximately 1. When weighted to reflect the US adult population as a whole, these subpopulations of increasing transferrin saturations had progressively increasing mean age-adjusted serum ferritin concentration values in each ethnic grouping as stratified by sex (trend test, P < .002 for all). These results are consistent with the concept that population transferrin saturation subpopulations reflect different levels of storage iron.
Introduction
We previously used mixture modeling of transferrin saturations measured in the second National Health and Nutrition Examination Survey (NHANES II) to study possible genetic influences on iron metabolism in the African American population1 and the Caucasian population2 of the United States. In the study of NHANES II Caucasian data, we analyzed the distribution of transferrin saturation after removing values for individuals possibly homozygous for the gene responsible for hemochromatosis and found evidence for 2 subpopulations, one postulated to be predominantly unaffected by the gene for hemochromatosis and the other predominantly heterozygous for hemochromatosis.2 More recently, we examined NHANES II data from African Americans from which markedly elevated transferrin saturation values were not removed, and mixture modeling provided evidence for 3 subpopulations of transferrin saturation. Although the type of hemochromatosis found in Caucasians may be rare in African Americans, our findings for African Americans were consistent with population genetics for a common locus that influences iron metabolism.1
Transferrin saturation can be reduced with inflammation as well as iron deficiency3 and the same measurement can be elevated by increased iron stores and a variety of other conditions.4Therefore, it is not completely clear that, on a population basis, elevated transferrin saturation reflects increased iron stores. In fact, in a recent analysis of data from the third National Health and Nutrition Examination Survey (NHANES III), only 11% to 22% of adults with elevated serum transferrin saturation as defined by various cutoff values had a concurrently elevated serum ferritin level.5In the present study, we analyzed data from the NHANES III to determine if subpopulations based on transferrin saturation have different levels of storage iron as assessed by measurements of serum ferritin concentration.
Materials and methods
Sources of data
The primary source of data for this study was NHANES III, which surveyed a representative sample of the noninstitutionalized US population in 1988–1994. There were 31 311 persons examined from 89 primary sampling units (county or small group of contiguous counties). The lower age limit was set at 2 months with no upper limit in age. Based on an interviewer's observation, each person was classified by race as non-Hispanic black, non-Hispanic white, Mexican American, or other.6 For the purposes of this study, we did not analyze data from Mexican Americans because of the lack of estimates of the admixture of Caucasian genes in this population. Although NHANES III provides the categories of non-Hispanic black and non-Hispanic white, in this paper we refer to these categories as African American and Caucasian, respectively.
Serum iron concentration and total iron-binding capacity were measured colorimetrically by using an Alpkem RFA analyzer (Alpkem, Clackamas, OR); a 1% thiourea solution was added to complex Cu++ to prevent copper interference. Serum transferrin saturation was calculated by dividing the serum iron level by total iron-binding capacity and multiplying by 100. Information regarding methodologic variation over the assays was provided by Gunter and colleagues.7 The coefficients of variation for analyses of control pools averaged 3.1% and 3.3% for serum iron and total iron-binding capacity, respectively. Serum ferritin concentration was measured by using the Quantimmune IRMA kit (Biorad Laboratories, Hercules, CA). The coefficient of variation for serum ferritin control pools covering a range of medium to high concentrations (approximately 25-440 ng/mL) was 5.4% on average, and for low-concentration pools (5-10 ng/mL) it was 10.5%.7
Selection criteria
Data were extracted from the NHANES CD-ROM.7 For the analysis, we selected transferrin saturation and serum ferritin values from 19 263 men and nonpregnant women who were at least 20 years of age. These included 10 507 Caucasians and 8756 African Americans. We excluded subjects with abnormally low hemoglobin or hematocrit values because anemias of various causes are associated with abnormally high8-10 or low3,11 transferrin saturations and serum ferritin concentrations. We excluded subjects with abnormally low mean corpuscular volume (MCV) levels because a low MCV can be associated with iron deficiency and inflammation,3 either of which can lead to altered transferrin saturation and serum ferritin values.10-13 It has been shown that hemochromatosis probands have mean MCV values significantly higher than wild-type control subjects,14and therefore we did not exclude subjects with MCV values above the reference range. We excluded subjects with elevated erythrocyte protoporphyrin because this finding is associated with iron deficiency and inflammation.15,16 We excluded subjects whose liver function tests were twice the upper limit of the normal reference range (serum alanine aminotransferase, serum aspartate aminotransferase, serum γ-glutamyl transferase, serum total bilirubin, and serum lactate dehydrogenase) or who had a positive or borderline serum hepatitis B surface antigen or positive serum hepatitis C antibody because transferrin saturation and serum ferritin concentration elevations can be associated with hepatocellular damage attributable to concurrent viral disease and alcohol or drug usage.17 We excluded subjects with an increased serum C-reactive protein level because inflammation can affect measures of iron status.18 19 In this study we were unable to account for intraindividual variation in transferrin saturation due, in part, to diet at the time of the last meal before the blood collection.
Adjustment of transferrin saturations for diurnal variation
Because transferrin saturation has a diurnal variation,12,20 including samples obtained at different times of the day, without appropriate adjustment, might alter the distribution of transferrin saturation. To address this possibility, regression methods were used to adjust transferrin saturation values for blood samples drawn in the afternoon or evening to the predicted values for blood samples drawn in the morning in the same individuals.1 For each data set, transferrin saturation values were divided into deciles and a regression equation was determined to predict the adjusted average value for morning transferrin saturation for each decile. For blood samples drawn in the afternoon or evening, the predicted average transferrin saturation value was computed. The final race- and gender-specific data sets consisted of the actual transferrin saturation values from samples drawn in the morning and the predicted values for samples drawn in the afternoon or evening.
Adjustment of the data from African Americans to account for a possible admixture of Caucasian hereditary hemochromatosis genes
The data were modified to allow for the possibility that the distribution of transferrin saturations among African Americans is affected by individuals who are heterozygotes or homozygotes for hereditary hemochromatosis. The gene frequency for the hereditary hemochromatosis locus in the Caucasian population is estimated to be 0.067.4 Assuming a 25% admixture of Caucasian genes in the African American population,21 the gene frequency for the hereditary hemochromatosis locus in African Americans would be 25% of 0.067 or 0.017. The actual frequency for the Cys282Tyr mutation among African Americans was somewhat lower in 2 geographic areas of the United States, a reported 0.011 in San Diego, California22 and 0.007 in Jefferson County, Alabama.23 Assuming a nationwide gene frequency of 0.017, population genetics would then project a proportion of homozygotes for the hereditary hemochromatosis gene of 3/10 000 (0.0172) and a proportion of heterozygotes of 330/10 000. We assumed that for African Americans, the transferrin saturation values from heterozygotes for the hereditary hemochromatosis locus would be normally distributed with the same means and SDs as found in our previous study of Caucasian Americans in NHANES II.2 Under this assumption, to adjust for hemochromatosis heterozygosity, we removed 3.3% of the 1301 values from the data set for men (n = 43) that were closest to 43 random values generated from a normal distribution with a mean of 46.5% and an SD of 7%. Similarly, we removed 3.3% of the 1089 values from the data set for women (n = 36) that were closest to 36 random values generated from a normal distribution with a mean of 43.4% and an SD of 7%. In the case when several transferrin saturation values were equidistant from a randomly generated value, one of these values was selected randomly. Because the number of transferrin saturations arising from homozygotes for hereditary hemochromatosis was projected to be less than one for men and women, no further adjustments for possible admixture were made to the data sets.
Statistical modeling of transferrin saturations
Data sets for transferrin saturation modeling consisted of 1258 African American men, 1053 African American women, 2328 Caucasian men, and 2483 Caucasian women (Table 1). As described previously, mixture distribution modeling was applied to determine the best fit of each grouped frequency distribution1,2,24 using the DISFIT computer program.25 In brief, transferrin saturation values were sorted into intervals of 2% and the frequency of values within each interval was computed. The physiologic models we considered were a single normal distribution, a mixture of 2 normal distributions, and a mixture of 3 normal distributions. We have previously established that transferrin saturations in a homogeneous population follow a normal distribution.2 The expectation-maximization algorithm was applied to the distributions of transferrin saturation values for parameter estimation.26,27 The statistical test used to determine the best fitting model was based on the likelihood ratio statistic. Using a hierarchical structure to analyze each observed distribution, the maximized log-likelihood function for a mixture of 3 normal distributions was evaluated (Log L3) and compared with the maximized log-likelihood function for 2 normal distributions (Log L2) and for single normal distribution (Log L1). Significance of the likelihood ratio statistics [−2Log (L3/L2)] and [−2Log (L3/L1)] at the 0.01 level was assessed using a resampling technique.28 For the 3-population model, the methods of Crump and Howe were used to compute confidence intervals for proportions.29
Exclusions from the total sample of Caucasian and African Americans at least 20 y of age
| Exclusion . | African American . | Caucasian . | ||
|---|---|---|---|---|
| Men . | Women . | Men . | Women . | |
| Total sample | 4121 | 4635 | 4921 | 5586 |
| Excluded for | ||||
| Age younger than 20 y | 2002 | 2045 | 1634 | 1752 |
| Pregnant | 0 | 90 | 0 | 70 |
| Transferrin saturation not measured | 183 | 208 | 119 | 180 |
| Abnormal hemoglobin level* | 230 | 610 | 347 | 272 |
| Abnormal hematocrit† | 0 | 52 | 48 | 68 |
| Protoporphyrin greater than or equal to 70 μg/dL red blood cells | 41 | 148 | 121 | 358 |
| MCV less than 80 fL | 127 | 96 | 50 | 55 |
| Serum alanine aminotransferase greater than 80 | 15 | 15 | 11 | 20 |
| Serum aspartate aminotransferase greater than 74 U/L | 18 | 9 | 7 | 4 |
| Serum γ-glutamyl transferase greater than 102 | 71 | 82 | 64 | 90 |
| Serum total bilirubin greater than 2.0 mg/dL | 6 | 2 | 23 | 4 |
| Serum lactate dehydrogenase greater than 546 | 0 | 0 | 1 | 1 |
| Serum C-reactive protein greater than 1.0 mg/dL | 69 | 163 | 124 | 207 |
| Serum hepatitis B surface antigen positive or borderline | 17 | 6 | 5 | 4 |
| Serum hepatitis C antibody positive | 41 | 20 | 39 | 18 |
| Subtotal | 1301 | 1089 | 2328 | 2483 |
| Possible heterozygosity for hemochromatosis (non-Hispanic blacks only) | 43 | 36 | 0 | 0 |
| Sample for transferrin saturation modeling | 1258 | 1053 | 2328 | 2483 |
| Serum ferritin not measured | 6 | 6 | 6 | 8 |
| Sample for serum ferritin analysis | 1252 | 1047 | 2322 | 2475 |
| Exclusion . | African American . | Caucasian . | ||
|---|---|---|---|---|
| Men . | Women . | Men . | Women . | |
| Total sample | 4121 | 4635 | 4921 | 5586 |
| Excluded for | ||||
| Age younger than 20 y | 2002 | 2045 | 1634 | 1752 |
| Pregnant | 0 | 90 | 0 | 70 |
| Transferrin saturation not measured | 183 | 208 | 119 | 180 |
| Abnormal hemoglobin level* | 230 | 610 | 347 | 272 |
| Abnormal hematocrit† | 0 | 52 | 48 | 68 |
| Protoporphyrin greater than or equal to 70 μg/dL red blood cells | 41 | 148 | 121 | 358 |
| MCV less than 80 fL | 127 | 96 | 50 | 55 |
| Serum alanine aminotransferase greater than 80 | 15 | 15 | 11 | 20 |
| Serum aspartate aminotransferase greater than 74 U/L | 18 | 9 | 7 | 4 |
| Serum γ-glutamyl transferase greater than 102 | 71 | 82 | 64 | 90 |
| Serum total bilirubin greater than 2.0 mg/dL | 6 | 2 | 23 | 4 |
| Serum lactate dehydrogenase greater than 546 | 0 | 0 | 1 | 1 |
| Serum C-reactive protein greater than 1.0 mg/dL | 69 | 163 | 124 | 207 |
| Serum hepatitis B surface antigen positive or borderline | 17 | 6 | 5 | 4 |
| Serum hepatitis C antibody positive | 41 | 20 | 39 | 18 |
| Subtotal | 1301 | 1089 | 2328 | 2483 |
| Possible heterozygosity for hemochromatosis (non-Hispanic blacks only) | 43 | 36 | 0 | 0 |
| Sample for transferrin saturation modeling | 1258 | 1053 | 2328 | 2483 |
| Serum ferritin not measured | 6 | 6 | 6 | 8 |
| Sample for serum ferritin analysis | 1252 | 1047 | 2322 | 2475 |
Hemoglobin (Hb) level exclusions: African American men, Hb less than 13 g/dL; African American women, Hb less than 11.5 g/dL; Caucasian men, Hb less than 13.5 g/dL; Caucasian women, Hb less than 12 g/dL.
Hematocrit (Hct) exclusions: African American men, Hct less than 34%; African American women, Hct less than 30%; Caucasian men, Hct less than 40%; Caucasian women, Hct less than 36%.
Estimation of gene frequency for possible loci that influence iron status
Accepting the possibility that our findings might represent the presence of loci that influence iron metabolism, we assumed that the 3 normal distributions of transferrin saturation in our analyses would represent predominantly a subpopulation of normal homozygotes, a subpopulation of heterozygotes, and a subpopulation of affected homozygotes. We estimated the proportions of normal homozygotes, heterozygotes, and affected homozygotes as the proportions in the populations with the lowest, intermediate, and highest mean transferrin saturations, respectively. According to the Hardy-Weinberg equilibrium equation, p2 (the proportion of normal homozygotes) + 2pq (the proportion of heterozygotes) + q2 (the proportion of abnormal homozygotes) = 1. We estimated the gene frequency of the abnormal allele (q) as the square root of the proportion from the population with the highest mean transferrin saturation. We estimated the gene frequency of the normal homozygotes (p) as the square root of the proportion from the population with the lowest mean transferrin saturation. We then examined whether the model distributions were consistent with population genetics for a major locus effect in which p + q = 1.
The means for transferrin saturation within corresponding subpopulations were compared between African Americans and Caucasians of the same gender using the independent sample t test.
Comparison of mean serum ferritin concentrations within subpopulations determined on the basis of transferrin saturation
A goal of our study was to determine if subpopulations of individuals, determined on the basis of transferrin saturation, have significantly different mean iron stores. Considering the transferrin saturation data sets used for mixture modeling, 26 subjects did not have measurements of serum ferritin concentration. After removal of values for these individuals, the data sets for analysis of serum ferritin concentration consisted of 1252 African American men, 1047 African American women, 2322 Caucasian men, and 2475 Caucasian women. Because of the marked skewness in the distributions of serum ferritin concentration, the square root transformation was applied to each data set. Because serum ferritin concentration tends to increase with age,30-37 we developed a method for age standardization based on statistical regression analysis. Linear, piece-wise linear, and nonlinear regression models, stratified by race and gender, were examined by regressing the square root of the observed serum ferritin concentration on the age at interview. The best fitting models showed an increase to middle age followed by steady state or decline in serum ferritin. Because of the lack of a biologic explanation for age-related decline in serum ferritin in terms of reduction of iron stores, we adopted a conditional approach to the modeling. For individuals below 60 years of age we used race- and gender-specific linear regression equations to calculate the predicted serum ferritin at age 60. For individuals 60 years of age or older, the observed serum ferritin was used in further analyses.
The probability that an individual serum ferritin concentration value belonged to one of the 3 transferrin saturation subpopulations was computed. Given the total sample size, the number of expected observations within each transferrin saturation interval was calculated. Within a given transferrin saturation interval, each observation was then assigned a probability by random assignment from a uniform (0,1) distribution and these probabilities were used to assign individual serum ferritin concentration values to a given subgroup according to the expected proportions within transferrin saturation subpopulations. Two hundred repetitions of the assignment of serum ferritin concentration values to subpopulations were made and the average mean and variance for serum ferritin concentration within subpopulations were computed. The parametric trend test38was used to compare the mean square root of serum ferritin concentration within each of 3 transferrin saturation subgroups, taking into account the unequal sample sizes for subgroups of subjects.
Weighting the results to reflect the US population as a whole
The assumptions underlying our analysis were that transferrin saturation values are independent and identically distributed; that is, each observation has an equal chance of being selected and that all observations come from the same distribution. However, because individuals in the NHANES III sample did not have an equal probability of selection, sample weights must be used to calculate parameter estimates that reflect the US population. For NHANES III, a multistage estimation procedure was used to calculate sample weights so that point estimates would reflect the US population. The sampling weights were based on the March 1990 and March 1993 Current Population Survey, adjusted for undercounts, for the civilian noninstitutionalized US population.39 The methods described above were used to compute parameter estimates from the weighted transferrin saturation distributions to reflect results for African American and Caucasian men and women in the US population fitting our exclusion criteria. It was not possible to adjust the variance estimates to account for the complex design of NHANES III.
Results
Analysis of unweighted transferrin saturation data
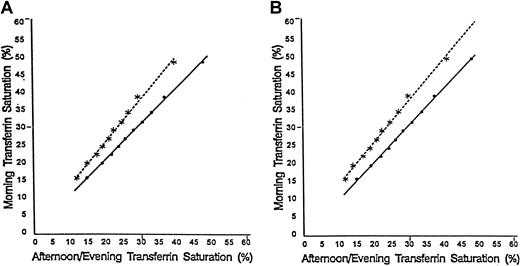
The primary analysis was performed on transferrin saturations for 7122 individuals. The transferrin saturations had been adjusted for diurnal variation and for the possible presence of an hereditary hemochromatosis allele in African Americans. Figure1 shows the effect of diurnal variation on measurement of transferrin saturation for African American men. The graphs indicate the relationship between decile means for transferrin saturations, determined from blood drawn in morning examination sessions, and the corresponding mean transferrin saturations determined from blood drawn in afternoon sessions (Pearson correlation coefficient, r = 0.999) and evening sessions (r = 0.997). The regression equations based on the unweighted data (Figure 1A) are as follows:
Decile means for transferrin saturations.
The relationship is shown between decile means for transferrin saturations determined from blood drawn in morning examination sessions and those of corresponding afternoon (••••) and evening (∗∗∗∗) examination sessions for African American men. Panel A shows unweighted data and panel B data weighted to represent the US population as a whole. The regression lines are shown in each panel.
Decile means for transferrin saturations.
The relationship is shown between decile means for transferrin saturations determined from blood drawn in morning examination sessions and those of corresponding afternoon (••••) and evening (∗∗∗∗) examination sessions for African American men. Panel A shows unweighted data and panel B data weighted to represent the US population as a whole. The regression lines are shown in each panel.
Morning session transferrin saturation = 0.6 + 1.0 × (Afternoon session transferrin saturation).
Morning session transferrin saturation = 1.5 + 1.2 × (Evening session transferrin saturation).
These regression lines differed significantly as judged by the F test for the equality of regression lines (F = 262.2 with numerator and denominator degrees of freedom of 2 and 16, respectively;P < .0001). Similar results were found for data from African American women and Caucasian men and women. For each group, the correlation between decile means for transferrin saturations, determined from blood drawn in morning examination sessions, and the corresponding mean transferrin saturations determined from blood drawn in afternoon sessions and evening sessions was strong (r > 0.99 for all) and the regression lines differed significantly as judged by the F test for the equality of regression lines (F test,P < .0001 for all).
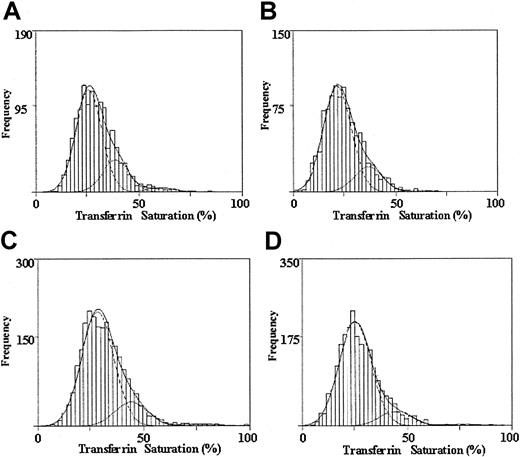
Results of statistical mixture modeling indicated that the fit of the unweighted data to a mixture of 3 normal populations with unequal variances was significantly better than the fit to a mixture of 2 normal populations for African American men (likelihood ratio statistic, 24.1, P < .01) and women (18.8,P < .01) as well as for Caucasian men (35.3,P < .01) and women (39.7, P < .01). Similarly, the fit of the data to a mixture of 3 normal populations was significantly better than the fit to a single normal population for all: African American men, likelihood ratio statistic, 221.1,P < .01, and women, 119.7, P < .01; Caucasian men, 416.9, P < .01, and women, 469.4,P < .01. Figure 2 shows the distribution of transferrin saturation for African American men (A) and women (B) and for Caucasian men (C) and women (D). The fitted subpopulations are superimposed over the histograms of the observed data. Table2 gives mixture model parameter estimates for transferrin saturation subpopulations and shows that for each ethnic subgroup and gender, transferrin saturation subpopulations were identified with increasing means (trend test P < .0001 for all). The findings are consistent with population genetics for single major loci affecting the distribution of transferrin saturations. For example, for African American men the sum of the square roots of the proportion with the lowest transferrin saturation (p = 0.854) and of the proportion with the highest transferrin saturation (q = 0.146) was approximately 1 (p+q = 1.000). Corresponding results were found for African American women (p = 0.905, q = 0.095) and for Caucasian men (p = 0.902, q = 0.098) and women (p = 0.927, q = 0.073) wherep+q = 1.000 for all.
The observed and modeled distributions of transferrin saturation values are shown for each ethnicity and sex.
Distribution of transferrin saturation values for 1301 African American men (A), 1089 African American women (B), 2328 Caucasian men (C), and 2483 Caucasian women (D). A histogram of the observed data is given. The dashed lines represent the fitted normal distributions representing 3 subpopulations. The overall fitted mixture distribution is shown with a solid line.
The observed and modeled distributions of transferrin saturation values are shown for each ethnicity and sex.
Distribution of transferrin saturation values for 1301 African American men (A), 1089 African American women (B), 2328 Caucasian men (C), and 2483 Caucasian women (D). A histogram of the observed data is given. The dashed lines represent the fitted normal distributions representing 3 subpopulations. The overall fitted mixture distribution is shown with a solid line.
Parameter estimates for transferrin saturation and age-standardized serum ferritin concentration from the unweighted samples
| Ethnic subgroup . | Transferrin saturation (%) . | Serum ferritin concentration* (ng/mL) . | ||||
|---|---|---|---|---|---|---|
| Mixture distribution models . | Subpopulation estimates . | |||||
| Percent of population . | Mean . | SD . | N . | Mean . | 95% CI . | |
| African American men | 72.9 | 25.0 | 6.21 | 914 | 231 | (220, 240) |
| 24.9 | 37.4 | 6.38 | 309 | 256 | (240, 272) | |
| 2.1 | 63.8 | 8.54 | 29 | 313 | (234, 404) | |
| Caucasian men | 81.4 | 28.9 | 7.52 | 1898 | 174 | (169, 180) |
| 17.6 | 45.3 | 8.55 | 396 | 193 | (180, 207) | |
| 0.9 | 77.5 | 11.09 | 28 | 289 | (199, 396) | |
| African American women | 81.9 | 21.6 | 6.66 | 857 | 132 | (125, 139) |
| 17.2 | 35.6 | 6.85 | 182 | 156 | (142, 172) | |
| 0.9 | 63.9 | 7.29 | 8 | 213 | (135, 310) | |
| Caucasian women | 86.0 | 25.9 | 7.66 | 2137 | 88 | (85, 92) |
| 13.5 | 45.7 | 8.73 | 318 | 117 | (106, 128) | |
| 0.5 | 79.5 | 9.23 | 20 | 135 | (100, 169) | |
| Ethnic subgroup . | Transferrin saturation (%) . | Serum ferritin concentration* (ng/mL) . | ||||
|---|---|---|---|---|---|---|
| Mixture distribution models . | Subpopulation estimates . | |||||
| Percent of population . | Mean . | SD . | N . | Mean . | 95% CI . | |
| African American men | 72.9 | 25.0 | 6.21 | 914 | 231 | (220, 240) |
| 24.9 | 37.4 | 6.38 | 309 | 256 | (240, 272) | |
| 2.1 | 63.8 | 8.54 | 29 | 313 | (234, 404) | |
| Caucasian men | 81.4 | 28.9 | 7.52 | 1898 | 174 | (169, 180) |
| 17.6 | 45.3 | 8.55 | 396 | 193 | (180, 207) | |
| 0.9 | 77.5 | 11.09 | 28 | 289 | (199, 396) | |
| African American women | 81.9 | 21.6 | 6.66 | 857 | 132 | (125, 139) |
| 17.2 | 35.6 | 6.85 | 182 | 156 | (142, 172) | |
| 0.9 | 63.9 | 7.29 | 8 | 213 | (135, 310) | |
| Caucasian women | 86.0 | 25.9 | 7.66 | 2137 | 88 | (85, 92) |
| 13.5 | 45.7 | 8.73 | 318 | 117 | (106, 128) | |
| 0.5 | 79.5 | 9.23 | 20 | 135 | (100, 169) | |
For individuals younger than 60 years, serum ferritin concentration values were standardized to those expected at age 60.
Analysis of unweighted serum ferritin concentration data
Our analysis of transferrin saturations demonstrated that 3 subpopulations of individuals could be detected within each ethnic subgroup and gender. We then compared the age-standardized mean serum ferritin concentrations for these subpopulations (Table 2). Trend tests demonstrated an increase in mean serum ferritin concentration, standardized to age 60, with increasing mean transferrin saturation for African American men (trend test χ2 statistic with 1 degree of freedom, 1385, P < .0001) and women (858,P < .0001) and for Caucasian men (1746,P < .0001) and women (418, P < .0001). The mean transferrin saturations within the middle and upper subpopulations for African American men and women are significantly lower than those for Caucasians (Table 2). For the second subpopulation, the mean transferrin saturation value for African American men was 8% lower in absolute value than that of Caucasian men (95% confidence interval [CI] 6.8%, 9.1%). The mean transferrin saturation for African American women was 10% lower in absolute value than that of Caucasian women (8.7%, 11.6%). Considering the upper subpopulation, the mean transferrin saturation values were 14% (8.2%, 19.2%) lower in absolute value for African American men than in Caucasian men and 16% (8.4%, 22.9%) lower in African American women compared to Caucasian women.
Weighted results for African American and Caucasian populations
Figure 1B confirms the effect of diurnal variation on measurement of transferrin saturation values using the weighted data. For African American men, the regression lines differed significantly (F = 192.9 with numerator and denominator degrees of freedom of 2 and 16, respectively; P < .0001). Table3 gives the mixture modeling results after the data (adjusted for diurnal variation and age effects in African American and Caucasian individuals and for the potential presence of hemochromatosis in African Americans) were weighted to reflect the US population as a whole. The weighted results for transferrin saturation are similar to our primary results using unweighted data, which suggests that the unequal probability of selection in NHANES III did not have a major input on the transferrin saturation and serum ferritin concentration distributions. For example, for African Americans, the findings for weighted data are consistent with population genetics for a single major locus affecting the distribution of transferrin saturations. For men, the sum of the square roots of the proportion with the lowest transferrin saturation (p = 0.854) and of the proportion with the highest saturation (q = 0.146) is approximately 1 (1.000). Similarly, for women the sum of the square roots of the proportion with the lowest transferrin saturation (p = 0.904) and of the proportion with the highest saturation (q = 0.104) is also approximately 1 (1.0085). For both ethnic subgroups and each gender, trend tests demonstrated an increase in mean serum ferritin concentration, standardized to age 60, with increasing mean transferrin saturation (Table 3; P < .002 for all).
Parameter estimates for transferrin saturation and serum ferritin concentration for weighted population data
| Ethnic subgroup . | Transferrin saturation (%) . | Serum ferritin concentration3-150 (ng/mL) . | |||
|---|---|---|---|---|---|
| Mixture distribution models . | Subpopulation estimates . | ||||
| Percent of population . | Mean . | SD . | No. . | Mean . | |
| African American men | 73.0 | 24.7 | 6.34 | 3 807 940 | 237.2 |
| 24.9 | 37.1 | 6.45 | 1 300 491 | 256.0 | |
| 2.1 | 64.0 | 8.91 | 111 071 | 309.8 | |
| Caucasian men | 86.1 | 29.6 | 7.88 | 43 514 385 | 187.7 |
| 13.4 | 48.3 | 9.21 | 6 752 746 | 207.4 | |
| 0.5 | 82.0 | 10.59 | 262 248 | 282.2 | |
| African American women | 81.8 | 21.7 | 6.34 | 3 567 584 | 134.6 |
| 17.3 | 36.0 | 6.45 | 755 516 | 153.8 | |
| 1.1 | 64.4 | 8.90 | 40 010 | 187.7 | |
| Caucasian women | 86.5 | 25.9 | 7.55 | 41 075 383 | 82.8 |
| 13.0 | 45.5 | 8.60 | 6 175 671 | 106.1 | |
| 0.5 | 79.3 | 9.10 | 232 193 | 118.8 | |
| Ethnic subgroup . | Transferrin saturation (%) . | Serum ferritin concentration3-150 (ng/mL) . | |||
|---|---|---|---|---|---|
| Mixture distribution models . | Subpopulation estimates . | ||||
| Percent of population . | Mean . | SD . | No. . | Mean . | |
| African American men | 73.0 | 24.7 | 6.34 | 3 807 940 | 237.2 |
| 24.9 | 37.1 | 6.45 | 1 300 491 | 256.0 | |
| 2.1 | 64.0 | 8.91 | 111 071 | 309.8 | |
| Caucasian men | 86.1 | 29.6 | 7.88 | 43 514 385 | 187.7 |
| 13.4 | 48.3 | 9.21 | 6 752 746 | 207.4 | |
| 0.5 | 82.0 | 10.59 | 262 248 | 282.2 | |
| African American women | 81.8 | 21.7 | 6.34 | 3 567 584 | 134.6 |
| 17.3 | 36.0 | 6.45 | 755 516 | 153.8 | |
| 1.1 | 64.4 | 8.90 | 40 010 | 187.7 | |
| Caucasian women | 86.5 | 25.9 | 7.55 | 41 075 383 | 82.8 |
| 13.0 | 45.5 | 8.60 | 6 175 671 | 106.1 | |
| 0.5 | 79.3 | 9.10 | 232 193 | 118.8 | |
For individuals younger than 60 years, serum ferritin concentration values were standardized to those expected at age 60.
An estimated proportion of 0.861 of Caucasian men studied were included in a subpopulation with a mean transferrin saturation of 29.6%, whereas 0.134 comprised a subpopulation with an intermediate mean saturation of 48.3% and 0.005 formed a subpopulation with a mean saturation of 82.0%. An estimated proportion of 0.865 of Caucasian women studied were included in a subpopulation with a mean saturation of 25.9%, whereas 0.130 comprised a subpopulation with an intermediate mean saturation of 45.5% and 0.005 formed a subpopulation with a mean saturation of 79.3%. From these results, the estimated gene frequencies for hereditary hemochromatosis were 0.072 (0.070-0.075) for Caucasian men and 0.070 (0.060-0.081) for Caucasian women corresponding to prevalences of 5.2/1000 and 4.9/1000, respectively.
Discussion
In the present study, we identified 3 transferrin saturation subpopulations in African Americans and Caucasians by using specialized modeling of data from NHANES III. The proportions of these subpopulations are consistent with Hardy-Weinberg criteria for a major, common genetic influence on iron metabolism in each of these 2 broad ethnic groups.
We previously analyzed transferrin saturation data for African Americans in the NHANES II study and obtained similar findings regarding a possible major locus affecting iron metabolism.1 In this regard, it is of interest that an iron-loading genetic defect, distinct from the Cys282Tyr mutation in HFE that is responsible for most hemochromatosis in Caucasians,40 may be present in the sub-Saharan African population,41,42 and that mutations in HFE are very rare in the African American population.41 The present results are also consistent with the possibility of an iron-loading genetic defect in the African American population distinct from that in the Caucasian population and producing different phenotypes. For example, the mean transferrin saturations within the middle and upper subpopulations for African American men and women were from 8% (95% CI, 6.8%-9.1%) to 16% (8.4%-22.9%) lower, whereas mean serum ferritin concentrations were substantially higher than those for Caucasians of the same gender.
In the present study, the estimated gene frequencies for hereditary hemochromatosis in Caucasians based on transferrin saturation subpopulations were 7.2% (7.0%-7.5%) for men and 7.0% (6.0%-8.1%) for women corresponding to prevalence estimates of 5.2/1000 for men and 4.9/1000 for women. These results are consistent with the reported prevalence of hereditary hemochromatosis and the frequency of the Csy282Tyr mutation in the HFE gene. For example, a meta-analysis by Lucotte and Mercier, comprising the results of recent studies conducted in 40 European populations, showed an overall frequency of 5.5% for the Cys282Tyr allele, corresponding to a prevalence estimate of 3/1000 (0.303%) for Cys282Tyr homozygotes.43
Since NHANES III was conducted before the discovery of theHFE gene, a limitation of this study is that testing for the Cys282Tyr mutation and other mutations was not performed. About 28% of Caucasians have the His63Asp mutation in the HFEgene.22,44,45 Heterozygotes for His63Asp have only minor changes in the transferrin saturation22 and are probably included in the lower Caucasian subpopulation of the analysis of the present study, whereas homozygotes for His63Asp and compound heterozygotes (Cys282Tyr/His63Asp) have elevations in transferrin saturation similar to Cys282Tyr heterozygotes22,46 and are probably included in the middle Caucasian subpopulation. Only about 10% of African Americans have the His63Asp mutation with most being heterozygotes and less than 0.5% being homozygotes.22 In the present analysis of transferrin saturations from African Americans, it is possible that His63Asp heterozygotes were included in the lower subpopulation, whereas His63Asp homozygotes were included in the middle subpopulation. Among Caucasian women, our finding of an elevation in mean serum ferritin for the middle subpopulation is consistent with the results found for Cys282Tyr heterozygotes in the large study conducted by Beutler and colleagues,22 but in contrast to other investigations in which Caucasian women, heterozygous for the Cys282Tyr mutation, did not have elevations in ferritin as compared to normal homozygotes.44 46
We have considered the possibility that adjustment of transferrin saturations for diurnal variation potentially could introduce an element of artifact in the analyses. To evaluate this possibility, we reanalyzed the data using the unadjusted values. As in the analyses of the adjusted data, these reanalyses identified 3 transferrin saturation subpopulations with increasing means for each ethnic subgroup and gender (trend test P < .0001 for all), although the estimated percentage of cells in corresponding subpopulations differed slightly (results not shown). Similarly, before correction for diurnal variation, the means for transferrin saturation within the middle and upper subpopulations for African American men and women were 5% (4.4%-6.4%) to 16% (7.3%-24.1%) lower, and the means for serum ferritin concentration were substantially higher than those for Caucasians of the same gender. Trend tests also demonstrated an increase in mean serum ferritin concentration, standardized to age 60, with increasing mean transferrin saturation for each group (P < .0001 for all), consistent with the results observed in the analyses of the adjusted data.
Even among Cys282Tyr homozygotes there is considerable variability in disease expression,22,47 and the place of testing for the Cys282Tyr mutation in screening for hemochromatosis has not been determined. Although serum ferritin concentration was once suggested as a screening marker for homozygosity for the hemochromatosis allele,48 transferrin saturation has been regarded for some time as the best single screening test for hemochromatosis.49,50 Recent large studies of Caucasians in which both phenotypic testing with transferrin saturation and genotypic testing for the Cys282Tyr mutation were performed have shown that screening with transferrin saturation does not identify all Cys282Tyr homozygotes,22 47 underscoring the fact that the ideal approach to phenotypic screening for hemochromatosis has not yet been developed. In our study of NHANES III data using statistical modeling methodology, subpopulations identified on the basis of increasing transferrin saturation had progressively increasing mean age-adjusted serum ferritin levels in each ethnic group, stratified by gender. These results suggest that it may be possible to develop a practical approach to the initial screening for iron overload that includes the combination of transferrin saturation and serum ferritin concentration measurements.
We thank Lamman Doan for statistical computing support. We gratefully acknowledge the contributions of Jacqueline Lovingood, CDC analyst who performed specimen analyses for serum iron and total iron-binding capacity in the NHANES Laboratory, and Della Twite, CDC analyst who performed analyses for serum ferritin.
Supported in part by grants and contracts from the National Institutes of Health, HL-508203 (to C.E.M.), N01-HC-05190 (to C.E.M. and G.D.M.), UH1 HL03679-03 (to V.R.G.), and N01-HC-05186 (to V.R.G). Additional support was provided by the Department of Veterans Affairs (to G.D.M. and C.E.M.) and the National Center for Health Statistics (to C.E.M. and V.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christine E. McLaren, University of California, Irvine, Epidemiology Division, Dept of Medicine, 224 Irvine Hall, Irvine, CA 92697-7550; e-mail: cmclaren@uci.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal