Abstract
Bone marrow involvement is reported in approximately 25% of patients with newly diagnosed acquired immunodeficiency syndrome–related lymphoma (ARL). Studied were 291 patients with ARL, diagnosed and treated at one medical center between 1984 and 1998. Clinical, immunologic, and pathologic characteristics of patients with bone marrow involvement were compared with those of patients without marrow involvement. Bone marrow involvement was present in 55 patients (19%). Small noncleaved lymphoma was diagnosed in 38% of the entire group and was the most common pathologic subtype in patients with bone marrow involvement (55% versus 34%;P = .008). Analysis of complete blood counts revealed a median hemoglobin level of 10.6 g/dL in both marrow-positive and marrow-negative groups. In contrast, a platelet count lower than 100 000/μL was more common in patients with bone marrow involvement (27% versus 11%; P = .02). Patients with marrow involvement were more likely to have leptomeningeal (cerebrospinal fluid [CSF]) lymphoma than patients whose marrows were uninvolved (24% versus 7%; P < .001) and were also more likely to have high lactate dehydrogenase (LDH) (P = .002), bone involvement (P < .001), and/or systemic B symptoms including fever, night sweats, and/or weight loss (P = .05). Median survival did not differ between marrow-positive and marrow-negative groups. On multivariate analysis, factors associated with decreased survival of marrow-positive patients included greater than 50% involvement (P = .002), systemic B symptoms (P = .008), and high-grade histologic type (P = .035). Marrow involvement in ARL correlates with small noncleaved pathology, thrombocytopenia lower than 100 000 mm3, high LDH, and lymphomatous involvement of the CSF. Survival is statistically shorter in patients with greater than 50% marrow involvement, high-grade pathology, and/or systemic B symptoms.
Introduction
Systemic lymphoma of high-grade B-cell type was included as a criterion for the diagnosis of acquired immunodeficiency syndrome (AIDS) in 1985.1 Since then, many reports of AIDS-related non-Hodgkin lymphoma (NHL) have documented the widespread nature of the disease at the time of diagnosis, the tendency for extranodal involvement, and the short median survival.2-10 Bone marrow involvement has been reported in approximately 20% to 30% of patients with AIDS-related NHL at initial diagnosis3,10 and in approximately 20% of human immunodeficiency virus (HIV)–negative patients with diffuse large-cell NHL.11-13 Bone marrow involvement has been considered a poor prognostic factor in AIDS-related lymphoma (ARL), associated with shorter survival.9 10 However, the full characteristics of marrow involvement in patients with AIDS-related lymphomas have not yet been described. We therefore examined the clinical, immunologic, and pathologic characteristics of lymphoma in HIV-infected patients with bone marrow involvement and compared them with similar patients without marrow involvement.
Patients, materials, and methods
Patients
This is a retrospective study of 291 patients, diagnosed and treated at Los Angeles County–University of Southern California (USC) Medical Center and the USC/Norris Cancer Hospital and Research Institute between 1984 and 1998. All patients were HIV seropositive by enzyme-linked immunosorbent assay with confirmation by Western blot assay.14 All patients had biopsy or cytologically proven NHL and were staged prior to treatment by means of the Ann Arbor staging system.15 A total of 392 patients were identified as having a diagnosis of NHL. The analyses excluded 48 patients with primary central nervous system (CNS) lymphomas, although those with systemic lymphoma and CNS involvement were included. Of the 344 patients with systemic lymphoma, 53 did not undergo bone marrow biopsy at this institution and were therefore excluded from the analysis.
Staging evaluation
Bone marrow aspirates, clot sections, and/or trephine biopsies and hemograms were performed in all patients included in this report. Chest radiographs; computed axial tomography scans of the chest, abdomen, and pelvis; and lumbar puncture with cerebrospinal fluid (CSF) analysis were performed on all patients. A complete history, complete physical examination, and routine blood work, as well as analysis of T-cell subtypes in the peripheral blood, were performed at the time of diagnosis. Hospital records, bone marrow pathology reports, laboratory data, treatment regimens, and response to therapy were reviewed.
Pathologic assessment of marrow involvement
Careful morphologic assessment was employed on all bone marrow specimens, including both aspirates and biopsy material. If a marrow sample was considered morphologically positive for lymphoma, routine immunostains and/or flow cytometry or molecular studies were performed as required or requested to further characterize the case. If a marrow sample was considered equivocal on morphologic grounds, we obtained immunostains and/or flow or a molecular study to clarify the diagnosis. If a marrow sample was considered clearly negative on morphologic grounds, routine performance of flow cytometry or immunostains was not done, in accordance with community standards.
The bone marrow biopsies from 24 patients (10%) showing no morphologic involvement by NHL in the hematoxylin and eosin sections were randomly selected for immunohistochemical stains to ascertain whether involvement by lymphoma could be detected solely by this technique. All biopsies were fixed in B5 solution and 10% formalin, routinely processed and embedded in paraffin.
All marrow aspirates and biopsies underwent central pathology review by 3 of us (B.N.N., J.A.C., R.K.B.), with the use of both Lukes-Collins16 and World Health Organization criteria.17 The criteria used to differentiate large-cell lymphoma from small noncleaved lymphoma were as follows: Large noncleaved cells are more than 3 times the size of a small lymphocyte or a red blood cell in surface area, not diameter, and contain multiple small nucleoli that are usually located on the nuclear membranes and have scanty cytoplasm. Small noncleaved cells are 2 to 3 times the size of a red blood cell or a small lymphocyte (in surface area). The nuclei exhibit multiple, more prominent nucleoli that may or may not be placed on the nuclear membranes. The cytoplasm of the small noncleaved cells is relatively less abundant and often deeply staining. Once a diagnosis of small noncleaved lymphoma was made, if the tumor was very monomorphic with respect to size, shape, and nuclear chromatin structure, it was termed a Burkitt lymphoma. If there was greater variability in the size of the cells, shape of the nuclei, and number of nucleoli, it was subclassified as a non-Burkitt (Burkitt-like) lymphoma.
Immunohistochemistry
The monoclonal antibodies used were anti-CD20 (L26, 1:100) (Biogenex, San Ramon, CA); anti-CD30 (Ber-H2, 1:25) (Dako, Carpinteria, CA); and anti-CD3 (1:250) (Dako), which was polyclonal. Control sections were immunostained under identical conditions with buffer solution substituted for the primary antibody. Tissue sections were mounted onto charged slides (Surgipath, Richmond, IL), baked at 56°C for 60 minutes, deparaffinized with xylene, and rehydrated with graded ethanols to distilled water. Sections underwent antigen retrieval consisting of submersion of the sections in buffer followed by 1 hour of steaming and 10 minutes of cooling. For CD3 and CD20, citrate buffer at pH 6.0 (Citra Solution, Biogenex) was used; for CD30, the buffer was EDTA at pH 8.0 (Zymed, South San Francisco, CA). Reactivity was demonstrated by a strepavidin-biotin immunoperoxidase detection system (Super Sensitive Immunodetection System, Biogenex) employing 3′,3′diaminobenzidine-tetrahydrochloride dihydrate as the chromogen.
Treatment
Owing to intercurrent infection and/or refusal of systemic therapy, 27 patients never received treatment for their lymphoma. One patient received radiation therapy alone for cord compression and refused any further treatment. Of the remaining 263 patients, 83 (31.6%) received a CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)–based regimen18,19; 149 patients (57%) received an mBACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone)–based regimen20-22; and 31 patients received either a dose-intensive acute lymphoblastic leukemia–based regimen,23 suramin,24 single-agent chlorambucil, BACOP (bleomycin, doxorubicin, cyclophosphamide, vincristine, prednisone), or other regimens. Thus, 232 (88%) of 263 treated patients received either mBACOD- or CHOP-based regimens.
Statistical analysis
Comparisons between HIV characteristics, lymphoma characteristics, and bone marrow findings were performed by means of Fisher exact chi-square test for discrete data and a t test for continuous data.25 Survival curves that use the methods of Kaplan and Meier were constructed.26 Study entry parameters were examined for their impact on survival by means of the log-rank test.27 In addition to bone marrow involvement, those factors with univariate impact on survival were evaluated by the multivariate Cox proportional hazards regression model.28
Results
Patient demographics
A total of 291 patients were included in this study, and their characteristics are shown in Table 1. Fifty-five (19%) patients had lymphomatous involvement of bone marrow; this group consisted of 53 males and 2 females, with a median age of 38 years (range, 25-64 years).
Demographic characteristics of 291 patients with acquired immunodeficiency syndrome–related systemic lymphoma
| . | BM+ . | BM− . | Total . | P . |
|---|---|---|---|---|
| No. | 55 | 236 | 291 | |
| Age, y | ||||
| Median | 38 | 39 | 39 | |
| Range | 25-64 | 22-65 | 22-65 | |
| Sex, no. (%) | 1.00 | |||
| Male | 53 (96) | 225 (95) | 278 (96) | |
| Female | 2 (4) | 11 (5) | 13 (4) | |
| Race, no. (%) | .80 | |||
| Caucasian | 30 (54) | 117 (50) | 147 (51) | |
| Hispanic/Latino | 19 (35) | 97 (41) | 116 (40) | |
| African American | 5 (9) | 18 (8) | 23 (8) | |
| Asian | 1 (2) | 4 (1) | 5 (2) | |
| HIV risk, no. (%) | ||||
| MSM | 37 (67) | 180 (76) | 217 (75) | .17* |
| Injection drug use | 3 (5) | 12 (5) | 15 (5) | |
| Prostitute contact | 3 (5) | 4 (2) | 7 (2) | |
| Heterosexual | 9 (16) | 12 (5) | 21 (7) | |
| Transfusion | 2 (4) | 3 (1) | 5 (2) | |
| Other/unknown | 1 (2) | 25 (11) | 26 (9) | |
| Prior AIDS, no. (%) | 17 (31) | 110 (47) | 137 (47) | .04 |
| OI only | 9 (16) | 88 (37) | 98 (34) | |
| KS only | 0 (0) | 10 (4) | 10 (3) | |
| OI + KS | 8 (15) | 12 (5) | 20 (7) | |
| CD4+, cells/μL | ||||
| Median | 140 | 73 | 86 | .005 |
| Range | 12-1927 | 0-978 | 0-1927 | |
| CD8+, cells/μL | ||||
| Median | 794 | 529 | 547 | .07 |
| Range | 43-7542 | 20-4318 | 20-7542 |
| . | BM+ . | BM− . | Total . | P . |
|---|---|---|---|---|
| No. | 55 | 236 | 291 | |
| Age, y | ||||
| Median | 38 | 39 | 39 | |
| Range | 25-64 | 22-65 | 22-65 | |
| Sex, no. (%) | 1.00 | |||
| Male | 53 (96) | 225 (95) | 278 (96) | |
| Female | 2 (4) | 11 (5) | 13 (4) | |
| Race, no. (%) | .80 | |||
| Caucasian | 30 (54) | 117 (50) | 147 (51) | |
| Hispanic/Latino | 19 (35) | 97 (41) | 116 (40) | |
| African American | 5 (9) | 18 (8) | 23 (8) | |
| Asian | 1 (2) | 4 (1) | 5 (2) | |
| HIV risk, no. (%) | ||||
| MSM | 37 (67) | 180 (76) | 217 (75) | .17* |
| Injection drug use | 3 (5) | 12 (5) | 15 (5) | |
| Prostitute contact | 3 (5) | 4 (2) | 7 (2) | |
| Heterosexual | 9 (16) | 12 (5) | 21 (7) | |
| Transfusion | 2 (4) | 3 (1) | 5 (2) | |
| Other/unknown | 1 (2) | 25 (11) | 26 (9) | |
| Prior AIDS, no. (%) | 17 (31) | 110 (47) | 137 (47) | .04 |
| OI only | 9 (16) | 88 (37) | 98 (34) | |
| KS only | 0 (0) | 10 (4) | 10 (3) | |
| OI + KS | 8 (15) | 12 (5) | 20 (7) | |
| CD4+, cells/μL | ||||
| Median | 140 | 73 | 86 | .005 |
| Range | 12-1927 | 0-978 | 0-1927 | |
| CD8+, cells/μL | ||||
| Median | 794 | 529 | 547 | .07 |
| Range | 43-7542 | 20-4318 | 20-7542 |
BM indicates bone marrow; HIV, human immunodeficiency virus; MSM, men who have sex with men; AIDS, acquired immunodeficiency syndrome; OI, opportunistic infection; KS, Kaposi sarcoma.
In a comparison of MSM versus all other HIV-risk groups.
Characteristics of HIV disease
In patients with marrow involvement, prior AIDS-defining illness was reported in 31%, while 47% without marrow involvement gave a history of AIDS prior to lymphoma (Table 1) (P = .04). The median CD4+ lymphocyte count at diagnosis was 140/μL (range, 12-1927 μL) in patients with bone marrow involvement, compared with 73/μL (range, 0-978 μL) in those without (P = .005).
Characteristics of lymphoma at diagnosis
Characteristics of lymphoma in the marrow-positive versus marrow-negative cases are provided in Table2. High-grade, small noncleaved lymphoma was the most common pathologic subtype, diagnosed in 111 (38%) of all patients. This subtype was significantly more common in patients with bone marrow involvement (55%), when compared with patients without such involvement (34%) (P = .008). As shown in Table 2, B symptoms (fever, night sweats, and/or weight loss) were also more common in bone marrow–positive patients. Patients with marrow involvement had higher serum lactate dehydrogenase (LDH) levels than those without (median, 479 U/dL versus 256 U/dL;P = .002). When all other extranodal sites of disease were considered, involvement of the CSF was significantly more common in the bone marrow–positive group (24% versus 7%;P < .001). Similarly, bone involvement was also correlated with involvement of the marrow (Table 2).
Characteristics of acquired immunodeficiency syndrome–related lymphoma at diagnosis
| . | BM+ . | BM− . | Total . | P . |
|---|---|---|---|---|
| Pathology, no. (%) | .01* | |||
| High grade | ||||
| Small noncleaved | 30 (55) | 81 (34) | 111 (38) | .008† |
| Immunoblastic | 11 (20) | 78 (33) | 89 (31) | |
| High grade NOS | 1 (2) | 24 (10) | 25 (9) | |
| Peripheral T cell | 1 (2) | 0 | 1 (< 1) | |
| Intermediate, no. (%) | ||||
| Diffuse large cell | 6 (11) | 48 (20) | 56 (19) | |
| Diffuse mixed cell | 1 (2) | 1 (0.4) | 2 (< 1) | |
| Follicular large cell | 0 | 2 (1) | 2 (< 1) | |
| Low grade, no. (%) | ||||
| MALT | 0 | 1 (0.4) | 1 (< 1) | |
| Small lymphocytic | 2 (4) | 0 | 2 (< 1) | |
| Small cleaved | 3 (5) | 1 (0.4) | 4 (1) | |
| B symptoms, no. (%) | 43 (78) | 136 (58) | 179 (62) | .05 |
| LDH, U/dL, median | 479 | 256 | 279 | .002 |
| Range | 142-8480 | 110-3650 | 110-8480 | |
| > 300, no. (%) | 36 (65) | 69 (29) | 105 (36) | < .001 |
| Other sites of extranodal disease, no. (%) | ||||
| CSF | 13 (24) | 16 (7) | 39 (13) | < .001 |
| Bone | 13 (24) | 9 (4) | 22 (8) | < .001 |
| Liver | 17 (31) | 44 (19) | 61 (21) | .14 |
| Pleura | 6 (11) | 18 (8) | 24 (8) | .40 |
| Rectum | 4 (7) | 20 (9) | 24 (8) | 1.00 |
| Stomach | 3 (5) | 30 (13) | 33 (11) | .16 |
| Kidney | 5 (9) | 10 (4) | 15 (5) | .18 |
| Oral cavity | 2 (4) | 22 (10) | 24 (8) | .19 |
| Lung | 2 (4) | 15 (6) | 17 (6) | .50 |
| Skin | 2 (4) | 8 (3) | 10 (3) | 1.00 |
| . | BM+ . | BM− . | Total . | P . |
|---|---|---|---|---|
| Pathology, no. (%) | .01* | |||
| High grade | ||||
| Small noncleaved | 30 (55) | 81 (34) | 111 (38) | .008† |
| Immunoblastic | 11 (20) | 78 (33) | 89 (31) | |
| High grade NOS | 1 (2) | 24 (10) | 25 (9) | |
| Peripheral T cell | 1 (2) | 0 | 1 (< 1) | |
| Intermediate, no. (%) | ||||
| Diffuse large cell | 6 (11) | 48 (20) | 56 (19) | |
| Diffuse mixed cell | 1 (2) | 1 (0.4) | 2 (< 1) | |
| Follicular large cell | 0 | 2 (1) | 2 (< 1) | |
| Low grade, no. (%) | ||||
| MALT | 0 | 1 (0.4) | 1 (< 1) | |
| Small lymphocytic | 2 (4) | 0 | 2 (< 1) | |
| Small cleaved | 3 (5) | 1 (0.4) | 4 (1) | |
| B symptoms, no. (%) | 43 (78) | 136 (58) | 179 (62) | .05 |
| LDH, U/dL, median | 479 | 256 | 279 | .002 |
| Range | 142-8480 | 110-3650 | 110-8480 | |
| > 300, no. (%) | 36 (65) | 69 (29) | 105 (36) | < .001 |
| Other sites of extranodal disease, no. (%) | ||||
| CSF | 13 (24) | 16 (7) | 39 (13) | < .001 |
| Bone | 13 (24) | 9 (4) | 22 (8) | < .001 |
| Liver | 17 (31) | 44 (19) | 61 (21) | .14 |
| Pleura | 6 (11) | 18 (8) | 24 (8) | .40 |
| Rectum | 4 (7) | 20 (9) | 24 (8) | 1.00 |
| Stomach | 3 (5) | 30 (13) | 33 (11) | .16 |
| Kidney | 5 (9) | 10 (4) | 15 (5) | .18 |
| Oral cavity | 2 (4) | 22 (10) | 24 (8) | .19 |
| Lung | 2 (4) | 15 (6) | 17 (6) | .50 |
| Skin | 2 (4) | 8 (3) | 10 (3) | 1.00 |
BM indicates bone marrow; NOS, not otherwise specified; MALT, mucosal associated lymphoid tissue; B symptoms, fever, night sweats, and/or weight loss; LDH, lactate dehydrogenase; CSF, cerebrospinal fluid.
In a comparison of high versus intermediate versus low-grade pathologies.
In a comparison of small noncleaved versus all other pathologies.
Laboratory characteristics
The findings on the complete blood count at the time of bone marrow biopsy and aspirate are provided in Table3. The median hemoglobin in both groups was 10.6 g/dL, and ranges were similar as well. There was no difference in any of the red blood cell indices when patients with and without bone marrow involvement were compared. Bone marrow–positive patients had a higher median white blood cell count (WBC) (5000/μL) than the marrow-negative group (4400/μL) (P = .03).
Laboratory characteristics of 291 patients with systemic acquired immunodeficiency syndrome–related lymphoma
| . | BM+ . | BM− . | P . |
|---|---|---|---|
| Hgb, g/dL | |||
| Median | 10.6 | 10.6 | .90 |
| Range | 4.2-17.0 | 4.0-17.3 | |
| < 10.0 | 22 (40%) | 95 (40%) | .88 |
| < 8.0 | 5 (9%) | 27 (11%) | .80 |
| Hct, % | |||
| Median | 30.7 | 31.0 | .55 |
| Range | 12.8-50.0 | 15.0-50.1 | |
| RBC, millions/μL | |||
| Median | 3.3 | 3.3 | .71 |
| Range | 1.9-5.2 | 1.7-6.0 | |
| WBC, 1000/μL | |||
| Median | 5.0 | 4.4 | .03 |
| Range | 1.4-27.5 | 0.6-18.7 | |
| ANC, per μL | |||
| Median | 2879 | 2683 | .60 |
| Range | 420-11 730 | 324-15 521 | |
| Plat, 1000/μL | |||
| Median | 169 | 200 | .09 |
| Range | 2-523 | 5-764 | |
| < 100 000 | 14 (27%) | 27 (11%) | .02 |
| < 50 000 | 9 (16%) | 11 (5%) | .005 |
| MCV, fL | |||
| Median | 85.8 | 89.0 | .12 |
| Range | 40-114 | 63-119 | |
| MCH, pg | |||
| Median | 29.5 | 30.3 | .84 |
| Range | 26.1-39.3 | 23.0-37.0 | |
| MCHC, g/dL | |||
| Median | 34.0 | 34.0 | .44 |
| Range | 26.4-35.4 | 22.4-37.0 | |
| RDW, % | |||
| Median | 13.9 | 15.0 | .24 |
| Range | 12.0-25.3 | 11.0-35.2 |
| . | BM+ . | BM− . | P . |
|---|---|---|---|
| Hgb, g/dL | |||
| Median | 10.6 | 10.6 | .90 |
| Range | 4.2-17.0 | 4.0-17.3 | |
| < 10.0 | 22 (40%) | 95 (40%) | .88 |
| < 8.0 | 5 (9%) | 27 (11%) | .80 |
| Hct, % | |||
| Median | 30.7 | 31.0 | .55 |
| Range | 12.8-50.0 | 15.0-50.1 | |
| RBC, millions/μL | |||
| Median | 3.3 | 3.3 | .71 |
| Range | 1.9-5.2 | 1.7-6.0 | |
| WBC, 1000/μL | |||
| Median | 5.0 | 4.4 | .03 |
| Range | 1.4-27.5 | 0.6-18.7 | |
| ANC, per μL | |||
| Median | 2879 | 2683 | .60 |
| Range | 420-11 730 | 324-15 521 | |
| Plat, 1000/μL | |||
| Median | 169 | 200 | .09 |
| Range | 2-523 | 5-764 | |
| < 100 000 | 14 (27%) | 27 (11%) | .02 |
| < 50 000 | 9 (16%) | 11 (5%) | .005 |
| MCV, fL | |||
| Median | 85.8 | 89.0 | .12 |
| Range | 40-114 | 63-119 | |
| MCH, pg | |||
| Median | 29.5 | 30.3 | .84 |
| Range | 26.1-39.3 | 23.0-37.0 | |
| MCHC, g/dL | |||
| Median | 34.0 | 34.0 | .44 |
| Range | 26.4-35.4 | 22.4-37.0 | |
| RDW, % | |||
| Median | 13.9 | 15.0 | .24 |
| Range | 12.0-25.3 | 11.0-35.2 |
BM indicates bone marrow; Hgb, hemoglobin; Hct, hematocrit; RBC, red blood cells; WBC, white blood cell count; ANC, absolute neutrophil count; Plat, platelet count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width.
There was no significant difference in the median platelet count between the 2 groups. However, patients with marrow involvement were more likely to have severe thrombocytopenia, a platelet count lower than 100 000/μL (P = .02) or lower than 50 000/μL (P = .005).
Pattern and extent of marrow involvement
To ascertain if the pattern of marrow involvement provided further information, we employed 5 categories: diffuse, interstitial, nodular, paratrabecular, and necrotic. The pattern of marrow involvement in 52 available cases revealed a nodular pattern in 28 (54%), a diffuse pattern in 16 (31%), an interstitial pattern in 5 (10%), a necrotic pattern in 2 (4%), and a paratrabecular pattern in only 1 case (2%).
The extent of marrow involvement varied from less than 25% in 31 patients (56%) to greater than 75% in 16 (29%) patients. Three patients had between 25% and 50% involvement, while 5 patients (9%) had between 50% and 75% marrow involvement by lymphoma.
Results of immunostains in morphologically uninvolved marrow
To determine if occult lymphoma was present in bone marrow biopsies that were considered morphologically uninvolved, we randomly selected 10% of the negative cases (no. = 24) for immunohistochemical analysis. All tissue sections and immunostains were evaluated independently by 3 expert hematopathologists (B.N.N., J.A.C., R.K.B.). No immunohistochemical evidence of lymphoma was detected in any of these morphologically negative cases.
Treatment
The majority of our patients (149 of 291 or 51%) received the mBACOD regimen, with 127 receiving low-dose mBACOD20,21and 22 receiving the standard dose.22 The next largest group (No. = 83, or 29%) received CHOP; 58 received low-dose and 25 standard-dose CHOP.18 19 A total of 28 patients received no systemic therapy at all, while 18 received various intensive, high-dose regimens, and 14 received various low-intensity regimens, such as chlorambucil plus prednisone, since the majority of this latter group were diagnosed with low-grade lymphoma. In the 185 patients who received low-dose combination therapy (mBACOD or CHOP), the median survival with marrow involvement was 7.83 months, versus 8.16 months in those without marrow involvement (P = .83). In the 47 patients who received standard-dose CHOP or mBACOD, the median survival with marrow involvement was 8.12 months, versus 6.97 months in those without marrow involvement (P = .74).
Survival
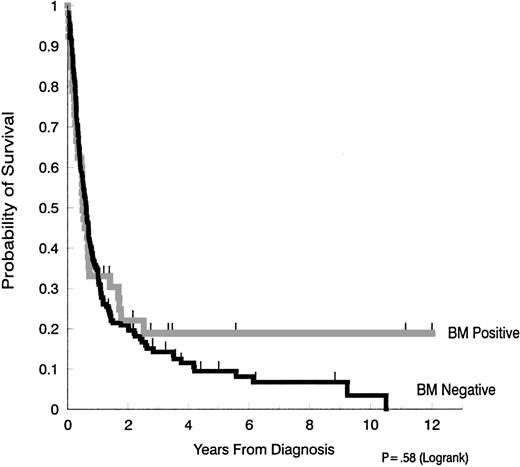
In univariate analysis, there was no difference in survival when patients with bone marrow involvement (median survival, 6.0 months) were compared with those without bone marrow involvement (median, 7.3 months) (Figure 1). Since the availability of antiretroviral and supportive therapy for HIV disease also varied throughout the time frame of this retrospective study, we also evaluated the median survival of those patients with and without marrow involvement, diagnosed before and after 1990. No significant differences were found. Thus, in patients whose AIDS-related NHL was diagnosed in 1990 or earlier, the median survival for those with marrow involvement was 4.1 months, versus 7.4 months in those with negative marrow (P = .43). In patients whose AIDS-related NHL was diagnosed after 1990, median survival was 8.6 months with marrow involvement and 7.1 months for those without lymphoma in the marrow (P = .18).
Survival in AIDS-NHL patients with and without bone marrow involvement.
The median duration of survival in patients with bone marrow involvement (6.0 months) was not significantly different from the median survival of patients without bone marrow involvement (7.3 months) (P = .58).
Survival in AIDS-NHL patients with and without bone marrow involvement.
The median duration of survival in patients with bone marrow involvement (6.0 months) was not significantly different from the median survival of patients without bone marrow involvement (7.3 months) (P = .58).
In our analysis of marrow-positive patients, factors considered for impact on survival in univariate analysis included pathologic type, B symptoms, CSF involvement, LDH, CD4 count, prior opportunistic infection, type of treatment (CHOP versus mBACOD), dose intensity of therapy (low versus standard dose), pattern of marrow involvement, extent of marrow involvement, WBC, hemoglobin level, and platelet count. On univariate analysis, B symptoms, a high percentage (greater than 50%) of marrow involvement, diffuse pattern of marrow involvement, LDH exceeding 300 U/dL, and high-grade pathologic type were all statistically associated with poorer survival.
Multivariate analysis revealed that systemic B symptoms (P = .008); greater than 50% marrow involvement (P = .002); and high-grade pathologic type (P = .035) were independently associated with shorter survival in marrow-positive patients.
An analysis of survival in terms of specific pathologic types, CD4 cells, and marrow involvement is presented in Table4.
Relationship between pathologic types, CD4 cells, and survival in marrow-positive and marrow-negative cases of systemic acquired immunodeficiency syndrome–related lymphoma
| . | Small noncleaved . | Immunoblastic . | Diffuse large cell . | |||
|---|---|---|---|---|---|---|
| Median . | P . | Median . | P . | Median . | P . | |
| BM+ | ||||||
| CD4, cells/μL (range) | 161 (12-819) | .16 | 100 (13-710) | .024 | 60 (1-579) | .61 |
| Survival, months | 5.36 | .13 | 5.95 | .45 | Not reached | .08 |
| BM− | ||||||
| CD4+, cells/μL (range) | 111 (0-920) | .16 | 52 (0-978) | .024 | 42 (12-503) | .61 |
| Survival, months | 6.05 | .13 | 7.60 | .45 | 7.63 | .08 |
| . | Small noncleaved . | Immunoblastic . | Diffuse large cell . | |||
|---|---|---|---|---|---|---|
| Median . | P . | Median . | P . | Median . | P . | |
| BM+ | ||||||
| CD4, cells/μL (range) | 161 (12-819) | .16 | 100 (13-710) | .024 | 60 (1-579) | .61 |
| Survival, months | 5.36 | .13 | 5.95 | .45 | Not reached | .08 |
| BM− | ||||||
| CD4+, cells/μL (range) | 111 (0-920) | .16 | 52 (0-978) | .024 | 42 (12-503) | .61 |
| Survival, months | 6.05 | .13 | 7.60 | .45 | 7.63 | .08 |
BM indicates bone marrow.
Discussion
Lymphomatous involvement of bone marrow is a relatively common finding in patients with NHL, reported in approximately 20% of HIV-negative patients with large-cell lymphoma, 20% to 50% of patients with all types of lymphoma,11-13,29-33 and 20% to 30% of patients with ARL.6-10 In the current large series, 19% of 291 HIV-positive patients were shown to have such morphologic involvement at diagnosis. Immunostains performed on a random sample of 10% of morphologically negative cases failed to detect occult lymphomatous involvement in any additional cases. High-grade, small noncleaved lymphoma was the most common pathologic type, observed in 55% of patients with bone marrow involvement versus 34% in marrow-negative patients (P = .008). The high incidence of marrow involvement in small noncleaved lymphoma has previously been reported in ARL.7
In HIV-negative patients with high-grade NHL, bone marrow involvement correlates with lymphomatous involvement of the central nervous system.34-37 The current report clearly establishes a similar relationship, with 24% of marrow-positive patients demonstrating NHL in the CNS versus 7% of those without marrow involvement. Bone marrow involvement has been a criterion for receipt of CNS prophylactic therapy in patients with HIV-negative lymphoma.35,37,38 On the basis of the current report, we would suggest a similar strategy in HIV-positive patients with marrow or bone involvement. Use of intrathecal cytosine arabinoside has been shown to be efficacious in this regard in patients with ARL.20
With the exception of anemia, relatively normal hemograms were observed in the majority of our patients with bone marrow involvement. These findings are consistent with prior reports of HIV-negative patients with NHL.12,33 Of interest, no significant difference was observed in the hemoglobin levels in our patients with and without marrow involvement. Nonetheless, the median hemoglobin level at 10.6 g/dL indicates significant anemia in the majority of patients and was lower overall than that seen in HIV-negative lymphoma patients, with or without marrow involvement.33 Median WBC in patients with marrow involvement was normal, at 5.0 × 103/μL, and leukopenia was uncommon. Although median platelet counts were similar in our HIV-infected patients with and without lymphoma in marrow, more severe thrombocytopenia was present in those with marrow involvement (P = .02 at a platelet count of fewer than 100 000/μL; P = .005 at a count of fewer than 50 000/μL).
Other laboratory abnormalities included elevated serum LDH levels in patients with bone marrow involvement, with a median of 479 U/dL; 65% had an elevated LDH, greater than 300 U/dL. On univariate analysis, elevated LDH (greater than 300 U/dL) was found to be a poor prognostic factor in patients with bone marrow involvement. However, the independent effect of LDH on prognosis was lost on multivariate analysis, perhaps secondary to the relatively small numbers of patients in the marrow-positive subset. Elevated serum LDH has been shown to be associated with shortened disease-free and overall survival in other reports.10,39 40
While the current study was retrospective, with inclusion of patients who received variable treatments over the years of evaluation, the majority of our patients (149 of 291 or 51%) received the mBACOD regimen,20-22 while the next largest group (29%) received CHOP.18,19 Approximately 80% of the whole group, then, received either mBACOD or CHOP, while 88% of treated patients received 1 of these 2 regimens. Large-scale studies have shown no difference in response rates between these 2 regimens,41 albeit those studies were performed in HIV-negative individuals. In terms of HIV-positive patients with NHL, the largest prospective trial (192 patients), randomized between low-dose and standard-dose mBACOD, reported no difference in these groups in terms of complete remission rate, duration of complete remission, or duration of survival.22 A recent small trial that looked at low-dose and then standard-dose CHOP in patients with ARL has also failed to demonstrate a difference between low-dose and standard-dose CHOP, and its results appear consistent with previous published data on survival after low-dose or standard-dose mBACOD.42 In another retrospective analysis of 369 patients with ARL, treated between 1982 and 1998 in one institution, no difference in survival was apparent when patients treated for various time intervals were compared.43 While the current study is retrospective and employed with variable treatment regimens, there still are no published data to suggest that any of the major regimens we employed was significantly different from the others in terms of outcome. With this background, despite differences in treatment regimen, we did not find significant differences in survival among patients with and without bone marrow involvement.
Of interest, the pattern of marrow involvement in patients with ARL was variable, with 31% demonstrating a diffuse pattern of marrow disease, 54% demonstrating a nodular pattern, and 10% an interstitial pattern. While diffuse involvement was associated with a statistically shorter survival than nodular cases (3.0 versus 16.8 months;P < .001), these differences were not significant on multivariate analysis. Multivariate analysis indicated that the degree of marrow involvement (greater than 50%), B symptoms, and presence of high-grade pathologic type were independently associated with shorter survival among marrow-positive cases.
CD4+ lymphocyte counts of fewer than 100 cells/μL have been shown to be a poor prognostic factor, associated with short survival and short duration of response in ARL patients.10 40 While the degree of immunosuppression seen in our patients is consistent with that observed in patients with advanced HIV infection, it is of interest that patients with bone marrow involvement had significantly higher median CD4+lymphocyte counts compared with those without marrow involvement (P = .005). However, no difference in survival was observed in marrow-positive patients with CD4+ lymphocyte counts greater than versus fewer than 100 cells/μL (P = .30).
In summary, the current report, comprising 291 patients whose diagnoses occurred during a 15-year period, indicates that marrow involvement is not an independent prognostic factor for survival in patients with ARL. The majority of patients with bone marrow involvement have relatively normal hemograms, except for anemia, which is a common finding in both the presence and absence of marrow involvement. Bone marrow involvement is statistically associated with involvement of the CSF and should be considered a criterion for CNS prophylaxis. Shorter survival is expected in marrow-positive patients with greater than 50% involvement, high-grade pathologic types, or presence of systemic B symptoms.
Supported in part by National Institutes of Health grants NO1-AI-62540 (from the National Institute of Allergy and Infectious Diseases); RO1-CA 50850; RO1-CA 55510; and UO1-CA 70072 (from the National Cancer Institute).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alexandra M. Levine, USC/Norris Cancer Hospital, 1441 Eastlake Ave, MS 34, Los Angeles, CA 90033; e-mail:hornor@hsc.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal