Abstract
Adoptive immunotherapy using receptor-modified T lymphocytes has shown promise in preclinical studies for the treatment of infectious and malignant diseases. These modified T cells express chimeric receptors that link ligand recognition and signal transduction domains in a single gene product. Typically, a single chain Fv fragment is genetically attached to the cytoplasmic domain of the T-cell receptor (TCR) ζ chain. Modulating the signaling characteristics of chimeric receptors will be important for their application to human immunotherapy. It was hypothesized that linking coreceptor and costimulatory signaling motifs together with the ζ signaling domain will enhance receptor function. The present study compares signaling characteristics of 9 single-chain receptors consisting of the H-2Kb extracellular and transmembrane domains and various combinations of T cell signal transduction domains. Signal transduction regions studied include the TCR ζ chain, the CD4 coreceptor, the lck protein tyrosine kinase, and the CD28 costimulatory receptor. Biochemical characteristics of the receptors, analyzed using calcium flux, receptor, and ZAP-70 phosphorylation, and lck association may be predicted from the known functions of receptor constituents. The combination of ζ together with coreceptor and costimulatory function in a single receptor maximizes chimeric receptor sensitivity and potency. Combining ζ with either the costimulatory or coreceptor function independently also enhances receptor function, though to a lesser extent. It is therefore possible to link TCR, coreceptor, and costimulatory activities in a single functional entity using modular domains. Such receptors demonstrate distinct signaling properties and should prove useful in the development of chimeric receptors for therapeutic purposes.
Introduction
Cellular immunotherapy using autologous or host-compatible antigen-specific T lymphocytes has shown significant potential in the treatment of malignant and infectious diseases.1-3 As an alternative to using conventional T lymphocytes, preclinical and limited clinical studies support the therapeutic use of receptor-modified T lymphocytes. These receptor-modified cells express single-chain chimeric receptors that contain both antigen recognition and signal transduction domains.4-8 Most frequently, antigen recognition occurs through single-chain Fv (scFv) fragments, although other recognition domains may be used.9 Ligand engagement stimulates the T lymphocyte through the receptor's integrated signal transduction domain, most commonly the cytoplasmic regions of the T-cell receptor (TCR) ζ chain or the FcRγ chain.
Despite their therapeutic potential, there have been only limited efforts to develop modified receptors with improved signal transduction characteristics. Because of data showing that the limited TCR signaling domains present within chimeric receptors are inadequate to fully activate T lymphocytes, such efforts may be essential to develop clinically effective receptors.10 Although the chimeric receptors serve as surrogates for the TCR, they differ from native TCRs in important aspects. Only a restricted subset of the TCR's signal transduction domains are present in the chimeric receptor. The TCR signals through the cytoplasmic domains of the invariant γ, δ, ε, and ζ chains.11 Specific motifs (immune tyrosine activation motifs [ITAMs]) within these receptor subunits are phosphorylated upon receptor crosslinking.12 These then serve as docking sites for tyrosine kinases as well as other proteins important in early T cell signal transduction. Although single-chain chimeric receptors containing limited numbers of ITAMs can signal T lymphocytes, the sensitivity of receptors signaling through only a restricted complement of ITAMs is unclear. We have found that increasing the numbers of ITAMs within a chimeric receptor beyond those contained in a single ζ cytoplasmic domain does not result in enhanced chimeric receptor signaling (T.L.G. and P.N., unpublished data, May 2000). This suggests that it may not be possible to modulate chimeric receptor function merely by altering the ITAM number.
An alternative is to qualitatively modify the functional characteristics of chimeric receptors. The interaction of receptor-modified T lymphocytes with their targets is fundamentally different from that of a TCR with major histocompatibility complex (MHC)–peptide. When TCR interacts with MHC-peptide, the CD4 or CD8 coreceptors are recruited to the signaling complex via their interactions with class II or class I MHC ligand.13 These coreceptors escort the src kinase lck to the TCR, promoting phosphorylation of the TCR's ITAMs as well as other components of the multiprotein signaling complex that forms around the TCR.14 The presence of coreceptor is estimated to enhance T-cell sensitivity to antigen by a factor of 100. When chimeric receptors on receptor-modified T lymphocytes encounter MHC unrestricted ligands, coreceptor will not colocalize with receptor. Indeed, crosslinking a chimeric receptor in which the IAsclass II MHC is linked to the ζ cytoplasmic tail results in only weak calcium flux and receptor phosphorylation.15 This can be significantly enhanced by co-crosslinking CD4 or CD8 together with the chimeric receptor.
We hypothesized that incorporating coreceptor activity may increase the sensitivity and potency of chimeric receptors. We therefore developed chimeric receptors in which the src family kinase lck or the CD4 cytoplasmic tail is directly linked to the cytoplasmic tail of ζ chain and the MHC Kb extracellular and transmembrane domains. The class I Kb molecule was selected so as to allow the receptors to target alloreactive T lymphocytes involved in graft rejection or graft-versus-host disease. Previous reports have shown that chimeric receptors linking the CD28 cytoplasmic tail to ζ enhances receptor potency.16 We find that combining either CD28-tail, lck, or CD4-tail with ζ in a single-chain chimeric receptor provides similar increases in receptor function as measured by interleukin-2 production after stimulation with antibody. The costimulatory functions of the CD28-tail, however, synergize with the coreceptor activity of lck in single-chain receptors containing CD28-tail, ζ, and lck.
Materials and methods
Construct synthesis
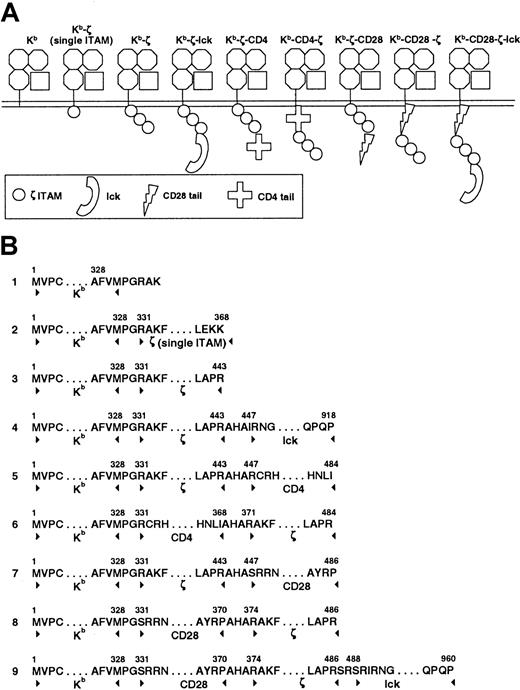
Chimeric constructs (Figure 1) were prepared using a cassette-based approach. Genetic fragments were isolated by polymerase chain reaction (PCR). Flanking oligonucleotides introduced restriction enzyme sites to permit the ligation of different genetic elements. PCR for murine lck, CD4, and CD28 fragments were performed using mouse spleen complementary DNA (cDNA). PCR for ζ and H-2 Kb was performed on cloned cDNA constructs. Sequences of all subclones were confirmed prior to further assembly of constructs. As a final step, assembled constructs were subcloned into the pJZ442 retroviral expression vector (gift of H. Yu, Yale University, New Haven, CT). Oligonucleotides were produced at the Keck Biotechnology Resources Laboratory at Yale University and the Hartwell Center for Bioinformatics and Biotechnology at St Jude Children's Research Hospital.
Chimeric construct structure.
Constructs were created by linking components in a cassette fashion (A). Amino acid sequence at the intersection of each component is noted (B). Additional amino acids added at the junction between components result from the insertion of restriction enzyme sites required for construct synthesis. Extracellular and transmembrane domains of all constructs are derived from the MHC class I H-2 Kbmolecule. The cytoplasmic domains are as listed. Construct 2 truncates the ζ cytoplasmic domain after the first ITAM.
Chimeric construct structure.
Constructs were created by linking components in a cassette fashion (A). Amino acid sequence at the intersection of each component is noted (B). Additional amino acids added at the junction between components result from the insertion of restriction enzyme sites required for construct synthesis. Extracellular and transmembrane domains of all constructs are derived from the MHC class I H-2 Kbmolecule. The cytoplasmic domains are as listed. Construct 2 truncates the ζ cytoplasmic domain after the first ITAM.
Transfections
Ten μg chimeric receptor constructs and 10 μg retrovirus helper DNA construct PEQPAM (gift of J. Cleveland) were cotransfected into 293-T cells using calcium phosphate precipitation as has been described.17 At 16 hours the cells were washed with phosphate buffered saline and incubated in Dulbecco modified Eagle medium (DMEM)/10% fetal calf serum (FCS) for 48 hours. Supernatant was collected twice daily and used to infect the GP+E86 retroviral producer cells in the presence of polybrene as has been described.17 Transduced GP+E86 cells were flow-cytometrically sorted for the presence of green fluorescent protein, expanded, and supernatant used to infect murine surface TCR-deficient 4G4 T hybridoma cells18 19 (gift of C. Janeway, Yale University, New Haven, CT) as above.
Antibodies and flow cytometry
Sources of antibodies not otherwise listed are as follows: monoclonal anti–H-2Kb-PE AF6-88.5 (Pharmingen); AF6-88.5 supernatant (hybridoma provided by M. Blackman, St Jude Children's Research Hospital, Memphis, TN); goat antimouse IgG (Jackson Immunoresearch, Westgrove, PA). Flow cytometric sorting was performed on a FACs Vantage SE high speed cell sorter (Becton Dickinson, San Jose, CA). Flow cytometric analyses were performed on FACs Vantage, FACs Calibur, and FACs Scan instruments using Cellquest software (Becton Dickinson).
Immunoprecipitation and Western blot
Chimeric receptor tyrosine phosphorylation was assayed as previously described.20 Briefly, 107 4G4 transfectants were incubated with AF6-88.5 (anti– H-2Kb) supernatant on ice for 20 minutes, washed with phosphate-buffered saline (PBS), and warmed briefly to 37°C prior to the addition of goat antimouse IgG at 37°C. Cells were incubated at 37°C for 2 to 5 minutes then lysed for 0.5 to 1 hour with buffer containing 1% Brij97 (Sigma, St Louis, MO) or 1% NP-40 and protease and phosphatase inhibitors (10 mM Na4P2O7.10H2O, 1 mM Na3VO4, 50 mM NaF, 1 mM phenylmethanesulfonyl fluoride (PMSF), 10 μg/mL leupeptin, and 10 μg/mL aprotinin). Insoluble material was removed by centrifugation at 15 000g for 15 minutes. Lysate was precleared with protein A-sepharose, and immunoprecipitated with AF6-88.5 anti-Kb, H146 anti–CD3-ζ, or anti–ZAP-70 and protein A-sepharose (Amersham Pharmacia Biotech). Proteins were eluted by boiling under reducing conditions in loading buffer, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose or polyvinylidenefluoride (PVDF) membrane. Tyrosine phosphorylation was analyzed by Western blotting with biotinylated or nonbiotinylated 4G10 monoclonal primary antibody (Upstate Biotechnology, Lake Placid, NY) followed by streptavidin-horseradish peroxidase (HRP) (Amersham) or goat antimouse HRP (Biorad, Hercules, CA). Blots were alternatively stained with anti–CD3-ζ (H146), lck, or ZAP-70 specific antibodies followed by protein A-HRP or goat antimouse HRP. Detection used enhanced chemiluminescence (Amersham). When blots were stained with more than one antibody they were stripped by incubation in 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl (pH 6.7) at 50°C for 30 minutes prior to reprobing. ZAP-70 and lck blotting was performed with polyclonal rabbit antisera produced in the laboratory of one of the authors (D.L.).
Calcium flux
We loaded 1.5 × 106 cells with 10 μg indo-1 (Molecular Probes) for 45 minutes at room temperature, washed the cells once and incubated them with 0.5 mL AF6-88.5 (anti–H-2Kb) supernatant at 4°C for 20 minutes. Cells were washed and kept at 4°C until immediately prior to analysis, at which time they were warmed to 37°C. They were then analyzed on a FACS Vantage SE flow cytometer (Becton Dickinson) at a rate of 1000 cells per second. Baseline fluorescence ratio at 530 and 295 nm was measured for 60 seconds, 50 μg goat antimouse IgG was added (Affinipure), and cells were analyzed for 7 additional minutes.
Interleukin-2 production
We stimulated 105 T cell hybridomas with varying dilutions of AF6-88.5 supernatant loaded onto goat antimouse IgG–coated wells in 96-well plates. After 24 hours, or the designated time after stimulation, supernatant was harvested, and interleukin-2 (IL-2) production was quantitated by bioassay as has been previously described.21 Absolute IL-2 concentrations were determined by interpolation of results with those obtained using dilutions of recombinant IL-2 (R&D Systems).
CD69 up-regulation
Transduced 4G4 cell lines were incubated with 50 μg/mL ovalbumin 257-264 (SIINFEKL; Hartwell Center for Bioinformatics and Biotechnology, St Jude Children's Research Hospital) for 16 hours, washed 3 times with Hanks balanced salt solution (HBSS), then irradiated with 20 000 rad. A quantity of 5 × 104 of the peptide-pulsed 4G4 cells were added to 4 × 105 splenocytes from OT-1 transgenic mice (Jackson Laboratory, Bar Harbor, ME) and incubated for 20 hours. Cells were stained with CD69- and CD8-specific antibodies in the presence of Fc block (Pharmingen) and analyzed by flow cytometry.
Intracellular cytokine staining
We added 5 × 104 transduced 4G4 cells to 106 OT-1 transgenic lymph node cells in the presence of 50 μg/mL ovalbumin 257-264 peptide and 10 μg/mL Brefeldin A (Epicenter Biotechnologies). After 6 hours incubation at 37°C, cells were fixed with 1% paraformaldehyde (Ted Pella) for 15 minutes at room temperature, permeabilized with 0.5% saponin (Sigma) and stained with phycoerythrin-conjugated anti–IL-2 (Pharmingen) for 30 minutes at 4°C. Cells were washed and analyzed by flow cytometry.
Results
Expression of chimeric receptor constructs
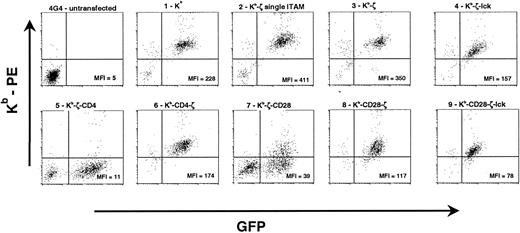
The chimeric constructs and their constituent components are shown in Figure 1. Each receptor includes the same extracellular and transmembrane domains derived from the murine H-2 Kbmolecule and signaling domains derived from the cytoplamsic domains of ζ, CD4, CD28, and/or lck. Constructs incorporating lck include all catalytic and regulatory domains of lck except the membrane proximal region, which is involved in lck fatty acylation and membrane association. Receptors were subcloned into the pJZ442 Moloney murine leukemia virus (MMLV)–driven retroviral vector upstream from an internal ribosomal entry site linked to green fluorescent protein (GFP). Retroviral supernatant was used to transduce the murine surface–TCR-deficient T cell hybridoma 4G4.18 19Transduced cells were anlayzed by flow cytometry with H-2Kb–specific antibody. Good correlation between GFP levels and H-2Kb expression was observed in most primary transductants. However, receptors containing CD4 distal to the ζ cytoplasmic domain (Kb-ζ-CD4) failed to express surface receptor, even in the presence of high levels of GFP (Figure2). Similarly, receptors expressing the CD28 tail distal to ζ showed only low levels of surface receptor (Kb-ζ-CD28). Unlike the placement of the CD4 or CD28 tail distal to ζ, placement of these domains proximal to the ζ chain and immediately distal to the transmembrane domain resulted in good surface expression. Transductants were sorted for expression of GFP and H-2Kb (Figure 2). Western blot analysis of receptors immunoprecipitated with ζ-specific antibody confirmed the predicted receptor sizes (data not shown).
Flow cytometric analysis of 4G4 transductants.
4G4 T cells were transduced with retroviral supernatants. Data for construct 5 (Kb-ζ-CD4) and construct 7 (Kb-ζ-CD28) show unsorted primary transductants. Because of the poor surface expression of these constructs, they were not further analyzed. Data for other constructs show GFP-sorted populations. Mean fluorescent intensities for chimeric receptor (Kb) expression among GFP-positive cells (total cells for 4G4-untransfected) are listed within each plot. Relative expression levels were consistently observed with multiple transductions of each construct.
Flow cytometric analysis of 4G4 transductants.
4G4 T cells were transduced with retroviral supernatants. Data for construct 5 (Kb-ζ-CD4) and construct 7 (Kb-ζ-CD28) show unsorted primary transductants. Because of the poor surface expression of these constructs, they were not further analyzed. Data for other constructs show GFP-sorted populations. Mean fluorescent intensities for chimeric receptor (Kb) expression among GFP-positive cells (total cells for 4G4-untransfected) are listed within each plot. Relative expression levels were consistently observed with multiple transductions of each construct.
Biochemical responsiveness of chimeric receptors
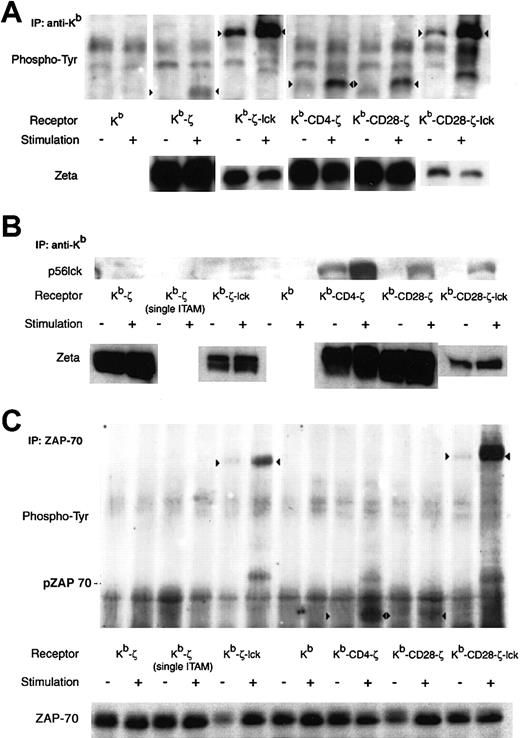
To determine whether provision of costimulatory or coreceptor function could enhance chimeric receptor signaling, basal and activation-induced tyrosine phosphorylation were analyzed. Phosphorylation patterns differ with the different receptors (Figure3A). The Kb-ζ, Kb-CD4-ζ, and Kb-CD28-ζ receptors show no significant basal phosphorylation but substantial stimulation-induced phosphorylation. When normalized to total ζ chain present, the stimulation-induced phosphorylation of the Kb-CD4-ζ and Kb-CD28-ζ receptors are modestly increased relative to that of the Kb-ζ receptor. This demonstrates that coreceptor or costimulatory domains can modulate receptor sensitivity.
Biochemical changes after chimeric receptor stimulation.
Crosslinking induced chimeric receptor phosphorylation (A), lck association (B), and ZAP-70 phosphorylation (C) are shown. A quantity of 107 transduced 4G4 cells were incubated with H-2Kb–specific antibody followed by crosslinking with goat antimouse IgG. Lysate was immunoprecipitated with a Kb, or ZAP-70–specific monoclonal antibody. Western blots were probed with antiphosphotyrosine (A, C) or anti-lck (B), stripped, and then probed with ζ-specific (A,B) or ZAP-70–specific (C) antibody to control for gel loading and membrane transfer. Representative analyses are shown. Arrowheads in (A) and (C) indicate tyrosine phosphorylated chimeric receptors. The position of phosphorylated ZAP-70 is shown in (C). As noted in the text, staining of the Kb-ζ-lck and Kb-CD28-ζ–lck receptors with ζ-specfic antibody was consistently poor, and likely reflects a diminished affinity of antibody for these chimeric receptors. In (A), the faint diffuse band present in the unstimulated lane of the phosphotyrosine analysis of Kb-CD4-ζ and migrating slightly lower than the Kb-CD4-ζ construct was not observed in 2 additional experiments and therefore likely represents crossreactive material.
Biochemical changes after chimeric receptor stimulation.
Crosslinking induced chimeric receptor phosphorylation (A), lck association (B), and ZAP-70 phosphorylation (C) are shown. A quantity of 107 transduced 4G4 cells were incubated with H-2Kb–specific antibody followed by crosslinking with goat antimouse IgG. Lysate was immunoprecipitated with a Kb, or ZAP-70–specific monoclonal antibody. Western blots were probed with antiphosphotyrosine (A, C) or anti-lck (B), stripped, and then probed with ζ-specific (A,B) or ZAP-70–specific (C) antibody to control for gel loading and membrane transfer. Representative analyses are shown. Arrowheads in (A) and (C) indicate tyrosine phosphorylated chimeric receptors. The position of phosphorylated ZAP-70 is shown in (C). As noted in the text, staining of the Kb-ζ-lck and Kb-CD28-ζ–lck receptors with ζ-specfic antibody was consistently poor, and likely reflects a diminished affinity of antibody for these chimeric receptors. In (A), the faint diffuse band present in the unstimulated lane of the phosphotyrosine analysis of Kb-CD4-ζ and migrating slightly lower than the Kb-CD4-ζ construct was not observed in 2 additional experiments and therefore likely represents crossreactive material.
Tyrosine phosphorylation of the receptors including the src kinase lck cannot be compared with those receptors lacking lck. First, lck contains tyrosine phosphorylation sites that cannot be distinguished from sites within ζ by phospho-tyrosine blotting. Lck is constitutively phosphorylated at Y509, inhibiting its kinase activity.14 With activation, it is dephosphorylated at this site, and autophosphorylates at Y394. Second, despite immunoprecipitating lysates from equivalent numbers of cells with either Kb or ζ-specific antibodies, the ζ-specific antibody used to control for total receptor loading showed diminished reactivity for chimeric receptors including lck linked C-terminal to ζ compared with receptors lacking lck (Figure 3A bottom panel and data not shown). This altered reactivity prohibits receptor normalization when comparing different receptors with or without lck. Nevertheless, analysis of tyrosine phosphorylation in the Kb-ζ-lck and Kb-CD28-ζ–lck receptors provides insight into their functions. First, these receptors demonstrate significant basal phosphorylation, likely of lck. It is unlikely that the basal phosphorylation reflects ITAM tyrosine phosphorylation and receptor activation. IL-2 was not produced by unstimulated cells and no spontaneous cell death was apparent as might be expected if the hybridoma cells were undergoing activation-induced cell death from chronic stimulation (data not shown). The significantly enhanced level of phosphorylation after stimulation of the Kb-ζ-lck and Kb-CD28-ζ–lck receptors (approximately 4-fold by densitometry) implies that ζ ITAMs, potentially in addition to the lck Y394, are phosphorylated upon receptor crosslinking.
Whereas receptors including lck would be expected to enhance receptor phosphorylation directly through the enzymatic activity of the lck, the CD4 tail has no intrinsic kinase activity. The CD4 component of the Kb-CD4-ζ receptor may however enhance chimeric receptor phosphorylation by recruiting p56lck to the chimeric receptor in a manner analogous to CD4 coreceptor recruitment of lck to the TCR. Figure 3B shows this recruitment. Even in the absence of receptor crosslinking, p56lck coimmunoprecipitates with the Kb-CD4-ζ receptor but not the other chimeric receptors. With stimulation, p56lck binding to this receptor is significantly enhanced. Notably, studies by others have shown that p56lck association with CD8 is increased after T-cell activation.22 This suggests that lck association with the Kb-CD4-ζ receptor is regulated by T-cell activation in a similar manner.
Weak lck association is also observed after stimulation of receptors including the CD28 costimulatory tail (Kb-CD28-ζ and Kb-CD28-ζ–lck). This implies that the CD28 tail can likewise promote the association of lck with chimeric receptors. Although direct association of CD28 with lck has not been shown, several studies have demonstrated that lck is required for CD28 signaling, and an association between the SH3 domain of lck and a PXXP motif in CD28 has been suggested.23 24
Subsequent to receptor crosslinking, among the earliest detectable events in TCR signal transduction is phosphorylation and activation of the ζ associated protein-70 (ZAP-70) protein tyrosine kinase. ZAP-70 binds to phosphorylated ζ ITAMs and is then phosphorylated by src kinases, such as lck. It would be anticipated that chimeric receptors including or binding lck (ie, Kb-CD4-ζ, Kb-ζ−lck, and Kb-CD28-ζ–lck) would more readily phosphorylate and trigger ZAP-70 then those receptors that do not bring src kinase into the receptor complex. Indeed, immunoprecipitation and Western blot analysis of ZAP-70 (Figure 3C) shows that ZAP-70 phosphorylation is enhanced at early time points only after crosslinking those receptors containing or associated with lck (Kb-CD4-ζ, Kb-ζ−lck, and Kb-CD28-ζ–lck). Other receptor types, including Kb-ζ and Kb-CD28-ζ, fail to show this increased phosphorylation. This demonstrates that integral or associated lck in chimeric receptors promotes ZAP-70 phosphorylation, and may thereby facilitate early signaling events.
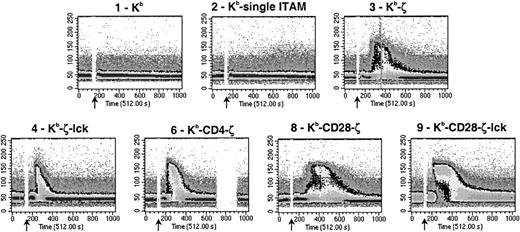
To correlate receptor phosphorylation with downstream signaling events, the T-cell lines were loaded with the calcium-binding fluorochrome indo-1 and stimulation-induced calcium flux was measured by flow cytometry. Figure 4 demonstrates that total calcium flux is similar in cells expressing the Kb-ζ-lck or Kb-CD4-ζ receptors when compared with the Kb-ζ receptor. The presence of CD4-tail or lck however results in a steeper upslope in the calcium flux after stimulation, possibly reflecting a more rapid rate of signal transduction due to increased coreceptor activity. In a similar fashion we have previously observed that cocrosslinking CD4 with either CD3 or a chimeric receptor composed of the class II MHC IAsmolecule linked to ζ cytoplasmic domain results in a more rapid rise in calcium compared with crosslinking CD3 or the chimeric receptor alone.15 Calcium flux with these chimeric receptors approximates in magnitude that seen with a 4G4 clone transfected with the D10 TCR and crosslinked with the CD3ε–specific Ab 2C11 (data not shown).
Chimeric receptors stimulate calcium mobilization.
4G4 T cells were loaded with the calcium sensitive fluorochrome, indo-1, stained with H-2Kb–specific antibody at 4°C, warmed to 37°C, and stimulated by antibody crosslinking with goat antimouse IgG at the timepoint indicated by the arrow. Changes in intracellular calcium were monitored by flow cytometry. Graphs represent calcium mobilization as a function of time. Addition of ionomycin resulted in marked calcium fluxes in all cell lines (data not shown). Data are representative of 2 experiments.
Chimeric receptors stimulate calcium mobilization.
4G4 T cells were loaded with the calcium sensitive fluorochrome, indo-1, stained with H-2Kb–specific antibody at 4°C, warmed to 37°C, and stimulated by antibody crosslinking with goat antimouse IgG at the timepoint indicated by the arrow. Changes in intracellular calcium were monitored by flow cytometry. Graphs represent calcium mobilization as a function of time. Addition of ionomycin resulted in marked calcium fluxes in all cell lines (data not shown). Data are representative of 2 experiments.
Stimulation of the Kb-CD28-ζ receptor results in an increased duration of calcium flux compared with Kb-ζ, Kb-CD4-ζ, or Kb-ζ-lck (Figure 4). However, the rate of rise in calcium is similar to Kb-ζ and not as great as the CD4- or lck-containing receptors. Therefore, a dissociation is observed between flux rate and sustenance of calcium flux, likely reflecting differences in the mechanism of the activity of the CD28 or CD4 and lck incorporated into chimeric receptors.
In contrast to cells expressing chimeric receptors containing coreceptor or costimulatory functions independently, cells including both of these functions (Kb-CD28-ζ–lck) demonstrate both the rapid rise observed with the Kb-CD4-ζ or Kb-ζ-lck receptors, as well as the sustained response seen with the Kb-CD28-ζ receptor.
Functional responsiveness of chimeric receptors
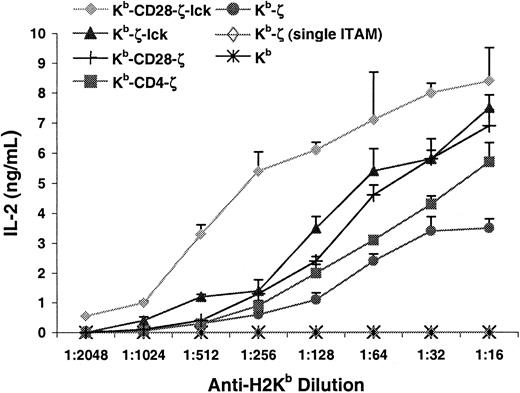
To analyze the influence of the inclusion of coreceptor and costimulatory domains in chimeric receptors on T-cell function, IL-2 production was measured after receptor stimulation with plate-bound anti–H-2Kb. Figure 5demonstrates that the addition of either CD28-tail, CD4-tail, or lck to the ζ chain reduced by approximately 2- to 4-fold the antibody concentration required to stimulate equivalent IL-2 production. Furthermore, cells expressing the Kb-CD28-ζ, Kb-CD4-ζ, or Kb-ζ-lck receptors produced consistently greater total quantities of IL-2 at optimal stimulation conditions compared with those expressing the Kb-ζ receptor. Including both coreceptor and costimulatory functions in a single-chain chimeric receptor had a more substantial effect. Peak IL-2 production by cells expressing the Kb-CD28-ζ–lck receptor was approximately 2- to 3-fold greater than those expressing the Kb-ζ receptor. Further, these cells showed an 8- to 12-fold increase in sensitivity based on antibody titration. Therefore, coreceptor and costimulatory activities synergize when linked with ζ, resulting in a single-chain chimeric receptor superior at transducing stimulatory signals and inducing functional response compared with receptors including these functions independently.
Addition of coreceptor or costimulatory function to chimeric receptors enhances interleukin-2 production.
Indicated cell lines were incubated for 24 hours in 96-well plates coated with H-2Kb–specific antibody. Interleukin-2 production was measured as described in “Materials and methods.” Data are representative of 5 experiments.
Addition of coreceptor or costimulatory function to chimeric receptors enhances interleukin-2 production.
Indicated cell lines were incubated for 24 hours in 96-well plates coated with H-2Kb–specific antibody. Interleukin-2 production was measured as described in “Materials and methods.” Data are representative of 5 experiments.
Chimeric receptor response to low-affinity ligand
The above data involve receptor crosslinking with a high-affinity antibody ligand. This would be anticipated to mimic the interaction that occurs with chimeric receptors containing scFv receptor domains. However, this high-affinity interaction may not reflect all types of chimeric receptors and ligands. Chimeric receptors containing scTCR or MHC ligand recognition domains would be expected to have low affinities for ligand. Response to low-affinity ligand will be necessary if the Kb-containing receptors described here are to target alloreactive T lymphocytes. To assess for response after low-affinity engagement of the chimeric receptors OT-1 TCR transgenic T cells specific for an H-2Kb–restricted ovalbumin peptide were used to stimulate the transduced 4G4 cell lines. After binding of antigenic peptide to the extracellular (H-2Kb) domain of the chimeric receptor, the OT-1 TCR can engage and stimulate the chimeric receptor–ovalbumin peptide complex. The dissociation constant (Kd) of the transgenic TCR for this peptide-MHC is approximately 13 μM, 100- to 1000-fold lower than a typical antibody-antigen interaction.25 Only a subset of chimeric receptor molecules would be anticipated to incorporate the ovalbumin peptide, further limiting the avidity of the TCR–chimeric receptor interaction in this analysis.
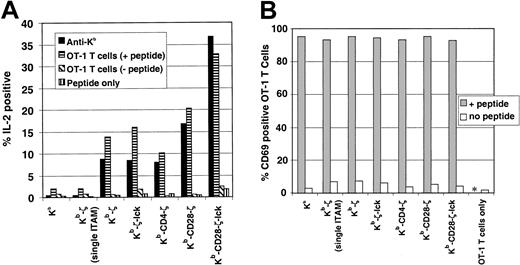
Initial studies measuring IL-2 production after coincubation of peptide-pulsed 4G4 cells with OT-1 T cells demonstrated significant background due to the production of IL-2 by the OT-1 T lymphocytes. Intracytoplasmic cytokine staining was therefore used to distinguish IL-2 production by stimulator and responder cells. At analysis, transduced 4G4 cells were segregated by flow cytometry from transgenic T cells by their expression of GFP and by cell size. Response to stimulation of chimeric receptors with anti–H-2Kb antibody was also assayed to provide correlation with the IL-2 production study shown in Figure 5. Figure 6A shows that anti–H-2Kb antibody stimulates IL-2 production in a fraction of receptor-modified 4G4 cells. The hierarchy of this production is similar though distinct from that observed in Figure 5. Whereas Figure 5 shows that cells expressing receptors with coreceptor function (Kb-ζ-lck and Kb-CD4-ζ) produce more IL-2 than the cells expressing Kb-ζ, the proportion of Kb-ζ-lck, Kb-CD4-ζ, and Kb-ζ expressing cells that stain for intracytoplasmic IL-2 is similar. This may reflect differences in assay conditions (6 hours vs 24 hours; presence of brefeldin A) or differences in IL-2 production on a per cell basis. The Kb-CD28-ζ and Kb-CD28-ζ–lck receptors, however, stimulate a greater number of cells to produce IL-2 than does the Kb-ζ receptor. Approximately twice the number of cells expressing the Kb-CD28-ζ receptor and more than 4 times the number of cells expressing the Kb-CD28-ζ–lck receptor stain for IL-2 when compared with the Kb-ζ–expressing cells. This is true despite the diminished surface levels of these receptors when compared with the Kb-ζ receptor (Figure 2).
Chimeric receptor response to low-affinity ligand.
(A) Transduced 4G4 cells were incubated with 50 μg/mL ovalbumin p257-264 and OT-1 TCR transgenic lymph node T cells in the presence of brefeldin A. Alternatively, 4G4-transduced cells were added to 96-well plates coated with H-2Kb–specific antibody, also with brefeldin A. After 6 hours incubation, cells were fixed, permeabilized, and stained for intracytoplasmic interleukin-2. The 4G4 cells could be readily distinguished from the transgenic lymph node cells during flow cytometric analysis based on cell size and due to their expression of GFP. Comparable results were obtained by preincubating 4G4 T cells with ovalbumin peptide, and washing unbound peptide prior to coincubation with OT-1 cells (data not shown). (B) Transduced 4G4 T cells were preincubated with p257-264, washed, and coincubated for 20 hours with OT-1 T cells. Surface expression of the early activation marker CD69 was determined by staining with CD69 and CD8 followed by flow cytometric analysis with gating for CD8+ (OT-1) cells. * indicates not tested.
Chimeric receptor response to low-affinity ligand.
(A) Transduced 4G4 cells were incubated with 50 μg/mL ovalbumin p257-264 and OT-1 TCR transgenic lymph node T cells in the presence of brefeldin A. Alternatively, 4G4-transduced cells were added to 96-well plates coated with H-2Kb–specific antibody, also with brefeldin A. After 6 hours incubation, cells were fixed, permeabilized, and stained for intracytoplasmic interleukin-2. The 4G4 cells could be readily distinguished from the transgenic lymph node cells during flow cytometric analysis based on cell size and due to their expression of GFP. Comparable results were obtained by preincubating 4G4 T cells with ovalbumin peptide, and washing unbound peptide prior to coincubation with OT-1 cells (data not shown). (B) Transduced 4G4 T cells were preincubated with p257-264, washed, and coincubated for 20 hours with OT-1 T cells. Surface expression of the early activation marker CD69 was determined by staining with CD69 and CD8 followed by flow cytometric analysis with gating for CD8+ (OT-1) cells. * indicates not tested.
Stimulation of the transduced 4G4 cells with OT-1 T cells shows a similar pattern of responsiveness as anti-Kb stimulation. All cell lines capable of signaling in response to antibody crosslinking were capable of responding to low-affinity TCR and peptide–mediated stimulation. The Kb-CD28-ζ–lck receptor showed the strongest response followed by the Kb-CD28-ζ receptor. The Kb-ζ-lck, Kb-CD4-ζ, and Kb-ζ receptors showed similar responses. No response was observed with the control Kb or Kb-ζ (single ITAM) receptors. Likewise, no response was observed in controls lacking either peptide or OT-1 T cells, demonstrating specificity in the OT-1 T-cell interaction with chimeric receptor/peptide. These results show that cells can be activated with low-affinity ligand through the chimeric receptors and reaffirms our finding that inclusion of both coreceptor and costimulatory activities enhances this responsiveness.
One caveat to this analysis is that different chimeric receptors may present the ovalbumin peptide and engage the OT-1 cells with variable efficiency. To control for this, after engaging the 4G4 cell lines, OT-1 T cells were stained for upregulation of the CD69 activation marker. OT-1 T-cell activation would indicate that an adequate density of chimeric receptor-peptide ligand is present on the 4G4 cells for effective ligand-receptor engagement. When each of the different 4G4 T-cell lines were pulsed with peptide and then coincubated with OT-1 T cells, more than 90% of OT-1 T cells up-regulated the early T-cell activation marker CD69, showing that antigen was effectively presented by all the cell lines (Figure 6B).
Discussion
Current understanding of T-cell signal transduction would suggest that the limited TCR signal transduction domains most commonly used in single-chain chimeric receptors of receptor-modified T lymphocytes will not provide an optimal signal. The requirements for effective signal transduction by ζ-containing chimeric receptors are likely similar to those of native TCR.12 26
Sufficient induction of src kinase activity is important for the initiation of T-cell receptor signal transduction and may affect the balance between positive and negative regulatory elements. Mice deficient in the src kinase lck show severely impaired signaling through the TCR.27 Likewise, T-cell signaling is dramatically reduced in the absence of coreceptor, which ushers lck to the TCR after TCR engagement.13 Agonist ligands in the presence of coreceptor may act as antagonists in its absence.28,29 Partial phosphorylation of TCR ITAMs, possibly reflecting inadequate src kinase activity, has been associated with negative signaling.30 It would be expected that inadequate stimulation through chimeric receptors may likewise generate downmodulatory signals into receptor-modified T cells.
Costimulatory function is likewise crucial for adequate T-cell activation. CD28 is the primary costimulatory receptor. It promotes T-cell proliferation and cytokine production, and inhibits apoptosis.31 Costimulatory stimulation also prevents the induction of anergy and significantly diminishes the TCR signal intensity required to activate T lymphocytes.
We compared 9 related single-chain chimeric receptors transfected into the 4G4 T cell hybridoma. 4G4 lacks surface TCR as a result of the absence of expressed TCR α and β chains.18 Outcome measures for studies with these cells therefore reflect the chimeric receptor alone, without interference from any association of the chimeric receptor with the TCR or its components. Further, because the constructs used here contain the transmembrane domain from H-2Kb, association of the chimeric receptor with ζ or other similar signal-transducing molecules would not be expected.32 Because of this we believe these studies stratify signaling by the chimeric receptors solely based on the capabilities of their signal transduction domains.
Several conclusions can be made (Table1). Most significantly, these studies demonstrate that coreceptor and costimulatory activities are both important for chimeric receptor signal transduction and that receptors can be designed that incorporate these activities. Linking lck, the tail of CD4, or the tail of CD28 to ζ-containing chimeric receptors increases single-chain chimeric receptor response. The addition of both lck and CD28-tail synergizes to further enhance receptor function, as measured here both biochemically and functionally. It is of interest that receptors containing coreceptor and costimulatory domains show not only increased sensitivity compared with those only containing ζ, but increased potency under maximal stimulatory conditions. This increased potency implies that even with optimal stimulation these receptors promote formation of a qualitatively superior signal-transducing complex compared with receptors that only contain ζ. This may increase the proportion of receptor-modified cells that are activated by a particular stimulus (Figure 6A) or increase the magnitude of the effector response by the receptor-modified lymphocyte (Figure 5).
Synopsis of receptor phenotypes
| Receptor . | Tyrosine phosphorylation (basal/stimulated) . | lck association (basal/stimulated) . | ZAP-70 phosphorylation (stimulated) . | Calcium flux (stimulated) . | IL-2 production (sensitivity) . | IL-2 production (potency) . | IL-2 production (low-affinity ligand) . |
|---|---|---|---|---|---|---|---|
| Kb-ζ | −/+ | −/− | − | + | + | + | + |
| Kb-CD4-ζ | −/++ | +/+++ | ++ | + | ++ | ++ | + |
| (rapid rise) | |||||||
| Kb-ζ-lck | +/+++ | −/− | +++ | + | ++ | ++ | + |
| (rapid rise) | |||||||
| Kb-CD28-ζ | −/++ | −/+ | − | + | ++ | ++ | ++ |
| (protracted flux) | |||||||
| Kb-CD28-ζ-lck | +/+++ | −/+ | +++ | ++ | +++ | +++ | +++ |
| (rapid rise and protracted flux) |
| Receptor . | Tyrosine phosphorylation (basal/stimulated) . | lck association (basal/stimulated) . | ZAP-70 phosphorylation (stimulated) . | Calcium flux (stimulated) . | IL-2 production (sensitivity) . | IL-2 production (potency) . | IL-2 production (low-affinity ligand) . |
|---|---|---|---|---|---|---|---|
| Kb-ζ | −/+ | −/− | − | + | + | + | + |
| Kb-CD4-ζ | −/++ | +/+++ | ++ | + | ++ | ++ | + |
| (rapid rise) | |||||||
| Kb-ζ-lck | +/+++ | −/− | +++ | + | ++ | ++ | + |
| (rapid rise) | |||||||
| Kb-CD28-ζ | −/++ | −/+ | − | + | ++ | ++ | ++ |
| (protracted flux) | |||||||
| Kb-CD28-ζ-lck | +/+++ | −/+ | +++ | ++ | +++ | +++ | +++ |
| (rapid rise and protracted flux) |
There is significant overlap between the signaling pathways used by the TCR and CD28. Because these receptors are functionally complementary, their signaling mechanisms may be partially distinct. Nevertheless, whether CD28 provides a qualitatively distinct signal from that transmitted by the TCR or merely provides a quantitative enhancement is not established. If signaling mechanisms are distinct then receptors with integrated coreceptor and costimulatory activities would be expected to synergize, enhancing cytokine production through at least partially nonredundant pathways. This expectation is supported by our finding that a receptor including TCR, coreceptor, and costimulatory domains (Kb-CD28-ζ–lck) has markedly increased sensitivity to stimulation as determined biochemically, as well as significantly increased IL-2 production at maximal stimulation compared with receptors containing these domains singly or in dual combinations. This enhanced functional response is present despite significantly lower cell surface levels of the Kb-CD28-ζ–lck receptor (Figure 2).
It is of interest that T-cell signaling subunits can be linked in a modular fashion. There are limits, however, in receptor engineering. Thus we found that placement of CD28 or CD4-tail distal to the membrane resulted in significantly diminished or abolished receptor expression (Figure 2). A similar result with CD28-tail was observed by others.16 Although we have not examined the mechanism, it likely results from clathrin-dependent receptor uptake and degradation as a result of exposure of dileucine motifs in the receptor tails. Further studies on the mechanism of chimeric receptor internalization may permit formation of receptors with increased stability and surface expression.
These studies describe novel chimeric receptors with potential therapeutic application. Figure 6 demonstrates that recognition of the extracellular class I MHC domain by antigen-specific T cells activates the genetically modified T cell. It is anticipated that similar recognition of alloreactive T cells will occur, permitting genetically modified T cells expressing these receptors to target allo-MHC–specific T cells involved in graft rejection or graft-versus-host disease. However, validation of the clinical usefulness of these receptors will require additional analyses. It is possible that some receptors uniting multiple components of the TCR signal transduction machinery may be too sensitive, resulting in unacceptable levels of nonspecific stimulation. Different therapeutic applications and therapeutic cell types may require distinct receptor types depending on the receptor affinity for ligand, ligand density, presence of costimulatory and adhesion molecules on target cells, and desired sensitivity thresholds. The results presented, however, demonstrate that by varying the constituents of chimeric receptors using a modular design it will be possible to synthesize single-chain receptors with predetermined sensitivity, potency, and function for cellular immunotherapy.
We thank Dario Vignali for helpful discussions; Richard Cross, Dick Ashmun, and Mahnaz Paktinat for their assistance with flow cytometric sorting and analysis; and Dan Dan Lu for assistance with Western blot analyses.
Supported by the American Lebanese Syrian Affiliated Charities (T.L.G., P.N.), National Institutes of Health (NIH) grant K08 AI01480 and P30 CA21765 (T.L.G.), NIH grant AI42963 (D.L.), the Arthritis Foundation (D.L.), and the Howard Hughes Medical Institute (R.A.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Terrence L. Geiger, Department of Pathology, St Jude Children's Research Hospital, 332 N Lauderdale St, DT-4047E, Memphis, TN 38105; e-mail: terrence.geiger@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal