Experimental allogeneic bone marrow transplantation (BMT) models using cytotoxic single-deficient (perforin/granzyme or Fas ligand [FasL]) and cytotoxic double-deficient (cdd) CD4+donor T cells have previously demonstrated roles for both effector pathways in graft-versus-host disease (GVHD). In the present study, the role of CD4-mediated antihost cytotoxicity in a GVH response is further examined across a complete major histocompatibility complex class I/II mismatch. As predicted, a double cytotoxic deficiency resulted in a clear delay in GVH-associated weight loss, clinical changes, and mortality. Interestingly, analysis of donor T-cell presence in 5.5-Gy recipients soon after BMT demonstrated that the double cytotoxic deficiency resulted in a marked decrease in donor CD4 numbers. Transplantation of singularly perforin- or FasL-deficient donor CD4+ T cells demonstrated that the absence of FasL was responsible for the markedly diminished CD4 number in recipient lymph nodes and spleens soon after BMT. However, increasing recipient total body irradiation conditioning (11.0 Gy) abrogated the decrease in FasL-defective B6-cdd and B6-gld CD4 numbers. Thus, the decrease was not a result of inherent CD4 defects, but was probably attributable to host resistance. Consistent with these observations, transplantation into 11.0-Gy recipients resulted in identical GVH lethality by equal numbers of B6 wild-type, B6-cdd, and B6-gld CD4+ T-cell inoculum. In total, the findings indicate that aggressive host conditioning lessens the requirement for donor CD4+ cytotoxic function in GVH responses soon after BMT. The present results thus support the notion of a role for cytotoxic effector function in donor CD4+ T cells prior to GVH-induced tissue injury.
Introduction
Donor antihost responses following allogeneic bone marrow transplantation (BMT) can lead to acute and/or chronic graft-versus-host disease (GVHD), a complex characterized by multiorgan involvement that is often life threatening.1,2 The principal targeted tissues, ie, lymphohematopoietic, liver, skin, and gastrointestinal tract, exhibit a number of histologically identifiable alterations, including mononuclear infiltrates, architectural disruption, and cell death.1 T cells play a central role in the development of this disorder. It is therefore important to elucidate the molecular pathways responsible for GVHD-induced injury to enable attempts to minimize/abrogate the tissue damage in order to design approaches that selectively favor antitumor activity. A number of laboratories, including our own, have been investigating the involvement of donor-mediated cytotoxicity in the GVH process.3-7 Previous work has shown that the absence of granule-dependent cytotoxicity impairs the ability of donor T cells to effect GVHD-induced weight loss, mortality, and histological damage.3,4,6,7 Notably, the impairment has been manifested by delayed kinetics of onset in comparison with equal numbers of cytotoxically normal donor T cells.3,6,7 The use of cytotoxically deficient T-cell subsets subsequently confirmed the importance of perforin for optimal GVHD induction.7,8Additionally, depending on the model system employed for transplantation, the absence of CD95L-dependent cytotoxicity can diminish GVH responses in the liver, skin, and marrow.3,5 9
Several reports have examined the GVH capacity of donor cells that are simultaneously impaired in their ability to mediate granule- and CD95L-dependent cytotoxic killing.8,10-12 The results have been conflicting in that both the inability and the ability of cytotoxically doubly impaired cells to mediate GVHD have been reported.8,10-12 It is clear from such studies that cytotoxic double-deficient (cdd) T cells can mediate GVHD when recipients are aggressively prepared with lethal total body irradiation (TBI) prior to transplantation.11 12 The present experiments were designed to investigate the importance of cytotoxic effector function early in the GVH process induced by CD4+T cells in complete major histocompatibility complex (MHC) class I/II–mismatched recipients. The results show that soon after transplantation, host conditioning dictates the need for cytotoxic function by these donor T cells. In summary, the present findings demonstrate that in addition to participation in the tissue injury occurring during GVHD, donor CD4 T-cell cytotoxic function plays an immediate and crucial role after transplantation—prior to pathogenesis. We speculate that under nonmyeloablative conditioning, this cytotoxic function is required by these donor T cells to overcome host resistance.
Materials and methods
Mice
C57BL/6 cytotoxic double-deficient (perforin and Fas) mice (B6-cdd) were generated from the breeding of B6-pfp−/+Faslgld/gld pairings as described previously13 (also in M. Komatsu et al, unpublished data, 2000). The offspring were then screened for homozygous perforin deficiency by means of polymerase chain reaction as previously reported14 15 to select for B6-pfp−/−Faslgld/gld(ie, B6-cdd). Six- to 8-week-old B6-cdd and B6-pfp−/− (ie, B6-perforin knockout [pko]) mice maintained in pathogen-free conditions in the Department of Microbiology and Immunology at the University of Miami School of Medicine were used as donors for all experiments. Six- to 8-week-old female BALB/c, B6-Ly5.1, C57BL/6 (B6), and B6.SmnC3H-Faslgld (ie, B6–FasL-deficient [B6-gld], H-2b) mice were purchased from Jackson Laboratories (Bar Harbor, ME).
Bone marrow transplantation
An MHC class I/II–mismatched GVHD model was employed with BALB/c mice (H-2d) as recipients. At 1 day before transplantation, BALB/c (or syngeneic H-2b) recipients were conditioned with 8.5-Gy TBI from a 60Co source at a dose rate of 44.5 cGy/min. Bone marrow cells obtained by flushing donor B6-Ly5.1 or B6 femurs and tibias were resuspended at a concentration of 2.5 × 107/mL in RPMI-1640 medium and incubated with anti-Thy1.2 monoclonal antibody (mAb) (HO13.2) at 4°C for 30 minutes. The cells were then washed and incubated at 37°C for 45 minutes in the presence of complement (rabbit Low Tox-M; Cedarlane Laboratories, Hornby, Ontario, Canada). T-cell–depleted (TCD) bone marrow cells were collected after washing with RPMI-1640 medium. The viable cells were counted by means of trypan blue staining. We then injected 5 × 106 TCD bone marrow cells from MHC class I/II–mismatched B6-Ly5.1 or B6 donors intravenously into BALB/c recipients alone (no GVHD control) or together with selected numbers of B6 or B6-cdd CD4+ T cells.
CD4+ T-cell preparation
Highly enriched CD4+ T cells were obtained by positive selection by means of the Miltenyi Macs System (Miltenyi Biotec, Auburn, CA). Donor spleen and lymph node cells were suspended in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) at 1 × 108/mL and labeled with anti-CD4–conjugated magnetic beads by incubating for 15 minutes at 4°C. The cells were then washed and resuspended in PBS/0.5% BSA and loaded onto a Macs separation column in a magnetic field. The unlabeled cells were removed by 3 washes with PBS/0.5% BSA, and CD4+cells were then eluted from the column by the use of the same buffer outside the magnetic field. In some experiments, CD4+ T cells were positively enriched with Dynal Beads (Dynal Biotech, Oslo, Norway). Briefly, donor spleen and lymph node cells were incubated with anti-CD4–conjugated Dynal magnetic beads for 20 minutes. The cells binding to Dynal beads were then precipitated on a magnetic rack, and the cells in suspension were discarded. After 3 washes using PBS/0.5% BSA, the cells attached to the beads were freed by means of the releasing reagent. Enriched CD4+ cells were stained with phycoerythrin anti-CD4 (Pharmingen, San Diego, CA) and fluorescein isothiocyanate anti-CD8 (Pharmingen) mAbs and examined for purity by flow cytometric analysis with a FACScan (Becton Dickinson, Mountain View, CA). The percentage CD4+CD8− cells obtained following either enrichment protocol was always greater than 95%.
Identification and determination of donor CD4+ T-cell numbers after BMT
At 5 days following BMT, the recipients were killed and the mesenteric lymph node cells were harvested. The cells were then counted and stained with anti–H-2Kb, anti-Ly5.1, and anti-CD4 mAbs (Pharmingen). Donor CD4+ T-cells were identified as the H-2Kb+Ly5.1−CD4+ population by flow cytometry analysis with FACScan flow cytometer (Becton Dickinson). The percentage of H-2Kb+Ly5.1−CD4+cells × the total number of nucleated lymph node cells was used to determine the number of donor CD4+ T cells.
GVHD assessment
Recipients were monitored for changes in total body weight and overall survival. The clinical signs of GVHD were recorded for individual mice by means of a clinical GVHD scoring system modified from Cooke et al.16 Briefly, recipients were scored on a scale of from 0 to 2 for 5 clinical parameters: weight loss (0, less than 10%; 1, 10% to 25%; 2, greater than 25%); diarrhea (0, not detectable; 1, mild; 2, severe); fur texture (0, unremarkable; 1, slight back ruffling; 2, entire body ruffling); posture (0, normal; 1, hunching noted only at rest; 2, severe hunching and impaired movement); alopecia (0, unremarkable; 1, mild to moderate; 2, severe with obvious areas of denuded skin). The clinical score for a BMT group represents the average of at least 5 mice per group.
Statistics
Comparison of the survival rates of BMT groups were assessed by means of the Mantel-Cox log-rank test. Groups of transplant recipients were analyzed for weight change, cell numbers, and clinical scores by means of a Student paired t test. A P < .05 was considered significant.
Results
GVH induced weight loss in complete MHC-mismatched recipients with the use of B6 wild-type and B6-cdd T cells
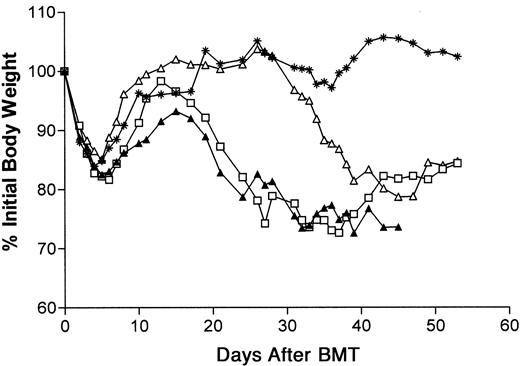
To study the ability of T cells lacking functional FasL and perforin to induce GVHD, an MHC class I/II–mismatched acute GVHD model using BALB/c mice as recipients was employed. In this model, BALB/c recipients (H-2d) were conditioned with lethal TBI (8.5 Gy) 1 day before receiving TCD B6 (H-2b) bone marrow cells. BMT of 5 × 106 TCD B6 allogeneic bone marrow cells only resulted in the complete recovery of recipient body weight within the first month after BMT (Figure 1). T cells from B6-cdd or wild-type B6 donors were also infused together with the B6 marrow. As previously observed, unfractionated B6-cdd T cells, as well as B6 wild-type T cells, resulted in GVHD-induced weight loss in the model (Figure 1).12 Although significantly greater numbers of B6-cdd than B6 T cells were required to induce weight loss with comparable kinetics, lower numbers of cdd T cells also induced weight loss albeit with delayed (10 to 12 days) onset.
Injection of unfractionated B6 or B6-cdd surface Ig−CD3+ T cells together with B6 bone marrow–induced weight loss in BALB/c recipients.
BALB/c recipients (5 per group) were prepared with 8.5-Gy TBI at 1 day before transplantation; 5 × 106 B6 TCD–bone marrow cells were injected intravenously together with T cells from B6 or B6-cdd donors. ∗, no donor T cells; ▴, 0.5 × 106 B6 T cells; ■, 5.0 × 106 B6-cdd T cells; ▵, 0.5 × 106 B6-cdd T cells.
Injection of unfractionated B6 or B6-cdd surface Ig−CD3+ T cells together with B6 bone marrow–induced weight loss in BALB/c recipients.
BALB/c recipients (5 per group) were prepared with 8.5-Gy TBI at 1 day before transplantation; 5 × 106 B6 TCD–bone marrow cells were injected intravenously together with T cells from B6 or B6-cdd donors. ∗, no donor T cells; ▴, 0.5 × 106 B6 T cells; ■, 5.0 × 106 B6-cdd T cells; ▵, 0.5 × 106 B6-cdd T cells.
Comparison of capacity of B6 normal and cytotoxically impaired CD4+ T cells to induce GVHD weight loss and lethality in BALB/c recipients
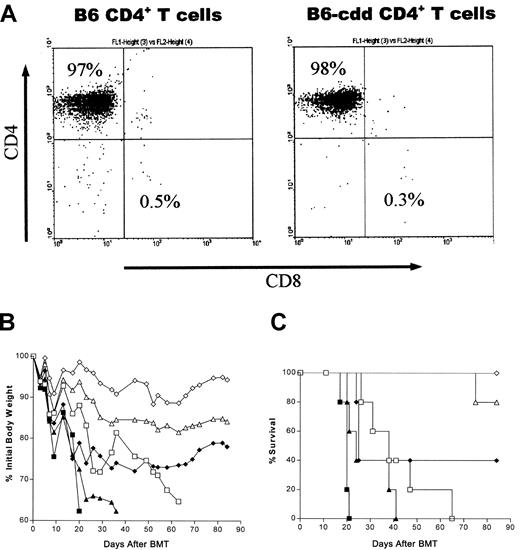
Both CD4+ and CD8+ T-cell subsets have been shown to contribute to the GVHD process in class I/II–mismatched models.17 18 Since CD4+ T cells can secrete a diverse array of cytokines that contribute to GVHD, we examined the impact of a cytotoxic double deficiency on the capacity of CD4+ T cells to induce GVHD. Donor CD4+ T cells were produced by positive selection as described in “Materials and methods.” Highly enriched CD4+ T-cell populations were prepared from suspensions of donor spleen and lymph node cells. A representative population from the enrichment procedure that uses the Macs system illustrates the purity of the CD4+ T-cell populations determined by staining with anti-CD4 and anti-CD8 directly conjugated fluorescent mAbs (Figure 2). Routinely, greater than 95% of the selected cells were CD4+ T cells, whereas typically fewer than 1% were contaminating CD8+ cells. Similar results were obtained with populations prepared by means of Dynal beads (data not shown).
Allogeneic TCD marrow plus highly enriched B6-cdd donor CD4+ T cells induce GVHD-associated weight loss and lethality in BALB/c recipients.
(A) Enrichment of B6 and B6-cdd CD4+ T cells used for BMT. Spleen and lymph node cells were positively selected by means of the Macs system. The enriched T-cell population was stained with anti-CD4 and anti-CD8 mAbs and analyzed with a FACScan. The percentage of contaminating CD8+ cells in each experiment ranged from 0.3% to 1.1%. Comparable results (data not shown) were obtained with the use of Dynal magnetic beads. (B) CD4+ T cells from B6-cdd donors induced weight loss in allogeneic BALB/c BMT recipients. BALB/c recipients (5 per group) irradiated with 8.5 Gy were transplanted with 5 × 106 B6-Ly5.1 TCD–bone marrow cells by means of varying numbers of CD4+ T cells from either wild-type B6 or B6-cdd donors as indicated. Total body weights of recipients were monitored following BMT. Compared with wild-type B6 CD4+ T cells, transplantation of equivalent numbers of B6-cdd CD4+ T cells induced delayed and milder weight loss in BALB/c recipients. (C) CD4+ T cells from B6-cdd donors can affect lethality in BALB/c BMT recipients. The BMT recipient groups described in Figure 2B were examined for survival after transplantation. Recipients of 2.5 × 106 and 1 × 106 B6-cdd CD4+ T cells exhibited mortality. However, there was a significant delay in these groups compared with recipients of wild-type B6 CD4+ T cells (P = .0002). ■, 2.5 × 106 B6-cdd; ▵, 1.0 × 106 B6-cdd; ◊, 2.5 × 106 B6-cdd; ■, 2.5 × 106 B6; ▴, 1.0 × 106 B6-wt; and ⧫, 0.25 × 106 B6 donor CD4+ T cells.
Allogeneic TCD marrow plus highly enriched B6-cdd donor CD4+ T cells induce GVHD-associated weight loss and lethality in BALB/c recipients.
(A) Enrichment of B6 and B6-cdd CD4+ T cells used for BMT. Spleen and lymph node cells were positively selected by means of the Macs system. The enriched T-cell population was stained with anti-CD4 and anti-CD8 mAbs and analyzed with a FACScan. The percentage of contaminating CD8+ cells in each experiment ranged from 0.3% to 1.1%. Comparable results (data not shown) were obtained with the use of Dynal magnetic beads. (B) CD4+ T cells from B6-cdd donors induced weight loss in allogeneic BALB/c BMT recipients. BALB/c recipients (5 per group) irradiated with 8.5 Gy were transplanted with 5 × 106 B6-Ly5.1 TCD–bone marrow cells by means of varying numbers of CD4+ T cells from either wild-type B6 or B6-cdd donors as indicated. Total body weights of recipients were monitored following BMT. Compared with wild-type B6 CD4+ T cells, transplantation of equivalent numbers of B6-cdd CD4+ T cells induced delayed and milder weight loss in BALB/c recipients. (C) CD4+ T cells from B6-cdd donors can affect lethality in BALB/c BMT recipients. The BMT recipient groups described in Figure 2B were examined for survival after transplantation. Recipients of 2.5 × 106 and 1 × 106 B6-cdd CD4+ T cells exhibited mortality. However, there was a significant delay in these groups compared with recipients of wild-type B6 CD4+ T cells (P = .0002). ■, 2.5 × 106 B6-cdd; ▵, 1.0 × 106 B6-cdd; ◊, 2.5 × 106 B6-cdd; ■, 2.5 × 106 B6; ▴, 1.0 × 106 B6-wt; and ⧫, 0.25 × 106 B6 donor CD4+ T cells.
To investigate the GVHD-inducing capacities of B6-cdd and wild-type B6 CD4+ T cells, varying doses of enriched CD4+ T cells were transplanted together with TCD B6-Ly5.1 bone marrow cells. Addition of 2.5 × 106 and 1 × 106 B6-cdd CD4+ cells to the marrow inoculum induced significant weight loss in BALB/c BMT recipients (Figure 2B). However, recipients of B6-cdd CD4+ T cells (ie, 2.5 × 106 and 1 × 106) exhibited a clearly delayed and less severe loss of weight compared with recipients of equal numbers of B6 wild-type (B6) CD4+ cells (Figure 2B).
B6-cdd CD4+ T cells were also capable of inducing lethal GVHD (Figure 2C). All BALB/c recipients died within 9 weeks following BMT that was done by means of 2.5 × 106 B6-cdd CD4+ T cells (mean survival time [MST] = 41.1). However, the mortality induced by B6-cdd CD4+ T cells was significantly delayed (P = .0002) compared with mice that received equivalent numbers of wild-type B6 CD4+ T cells (MST = 19.6). Lower doses of B6-cdd CD4+ T cells induced much lower mortality. For example, the majority (80%) of recipients survived 80 days following injection of 1 × 106 B6-cdd CD4+ T cells, and all recipients survived transplantations with 0.5 × 106 of these cytotoxically defective T cells. In total, CD4+ T cells unable to mediate perforin- and CD95L-dependent cytotoxicity could induce both GVH-associated weight loss and lethality following BMT across complete MHC class I/II disparities. However, as previously observed with the use of unfractionated B6-cdd spleen cells, considerably greater numbers of cytotoxically impaired than normal CD4+ T cells were required for these effects.12 These findings demonstrate that CD4+ T cells lacking cytotoxic function possess a diminished capacity to mediate a class I/II–disparate GVHD.
To more carefully compare the numbers of cytotoxically double-deficient and normal CD4+ T cells necessary for GVH-induced weight loss and mortality, 2 T-cell doses were selected: a lethal (2.5 × 106) and a sublethal (0.25 × 106) number (Figure 3A). These doses enabled analysis of a 10-fold difference in cell numbers between transplantation groups. BALB/c BMT recipients of 2.5 × 106 or 0.25 × 106 B6-cdd T cells induced significantly delayed (P < .0001) weight loss compared with the equivalent numbers of B6 CD4+ T cells (Figure 3A). Notably, 2.5 × 106 B6-cdd CD4+T cells resulted in weight loss almost indistinguishable in onset and severity from the weight loss that resulted from 10-fold fewer (0.25 × 106) normal B6 CD4+ T cells. Similarly, the lethality curves comparing recipients of 2.5 × 106 B6-cdd and 0.25 × 106 B6 donor inocula were not statistically different (Figure 3B,P = .1369). In total, these findings demonstrated that (1) B6-cdd CD4+ T cells can induce GVHD weight loss and lethality and (2) greater numbers (approximately 10-fold) of B6-cdd versus B6 CD4+ T cells were required to induce comparable GVH-associated effects.
Titration of donor CD4+ T-cell dose and induction of GVHD.
(A) CD4+ T cells from B6-cdd donors exhibit significant but delayed weight loss after BMT. BALB/c recipients were transplanted with 5 × 106 B6-Ly5.1 TCD–bone marrow cells by means of 2.5 × 106 and 0.25 × 106 CD4+ T cells from either wild-type B6 or B6-cdd donors (5 mice per group). The kinetic onset of weight loss showed that higher numbers of B6-cdd than of B6 CD4+ T cells were needed to induce a similar pattern. (B) CD4+ T cells from B6-cdd donors were less efficient in inducing lethality of recipients. Recipients of BMT described in Figure3A were monitored for survival rate. B6-cdd CD4+ T cells induced a delayed and lower mortality when recipients of the same numbers of wild-type B6 CD4+ T cells were compared. It was found that 5 × 106 B6-cdd CD4+ T cells induced 100% lethality (data not shown, n = 5). The lethality curves of 2.5 × 106 B6-cdd (■) versus 0.25 × 106 B6 (▾) were not significantly different (P = .1369). ■, 2.5 × 106 B6-cdd; ▿, 0.25 × 106 B6-cdd; ■, 2.5 × 106 B6; and ▾, 0.25 × 106 B6 donor CD4+ T cells.
Titration of donor CD4+ T-cell dose and induction of GVHD.
(A) CD4+ T cells from B6-cdd donors exhibit significant but delayed weight loss after BMT. BALB/c recipients were transplanted with 5 × 106 B6-Ly5.1 TCD–bone marrow cells by means of 2.5 × 106 and 0.25 × 106 CD4+ T cells from either wild-type B6 or B6-cdd donors (5 mice per group). The kinetic onset of weight loss showed that higher numbers of B6-cdd than of B6 CD4+ T cells were needed to induce a similar pattern. (B) CD4+ T cells from B6-cdd donors were less efficient in inducing lethality of recipients. Recipients of BMT described in Figure3A were monitored for survival rate. B6-cdd CD4+ T cells induced a delayed and lower mortality when recipients of the same numbers of wild-type B6 CD4+ T cells were compared. It was found that 5 × 106 B6-cdd CD4+ T cells induced 100% lethality (data not shown, n = 5). The lethality curves of 2.5 × 106 B6-cdd (■) versus 0.25 × 106 B6 (▾) were not significantly different (P = .1369). ■, 2.5 × 106 B6-cdd; ▿, 0.25 × 106 B6-cdd; ■, 2.5 × 106 B6; and ▾, 0.25 × 106 B6 donor CD4+ T cells.
GVHD-associated clinical changes in BALB/c recipients of B6 and B6-cdd T cells
GVHD-associated clinical changes of recipients following BMT by means of highly enriched CD4+ T cells were also examined. Clinical changes associated with experimental GVHD include diarrhea, skin changes, hair loss, and kyphosis.2 In this BMT model, B6-cdd CD4+ T cells were able to induce all of these clinical signs, although with delayed kinetics compared with recipients of cytotoxically normal CD4+ T cells. Both B6-cdd and B6 CD4+ T cells caused changes in the skin of recipients. Skin changes (ruffled fur, tail scaling), haunched posture, and hair loss were observed in both high- and low-dose experimental groups with the use of B6 or B6-cdd T cells. The recipients of high doses of B6 and B6-cdd CD4+ T cells (2.5 × 106) started to develop these changes at approximately the same time (Table 1). However, comparison of recipients of low doses (0.25 × 106) of CD4+ T cells demonstrated a greater delay in the onset of ruffled fur, haunched posture, and hair loss. In contrast, no difference in onset times of diarrhea between recipients of B6 and B6-cdd T cells were detected (Table 1).
We assessed 5 clinical changes to compare BALB/c recipient groups of different CD4+ T-cell doses (Figure4). During the first 2 weeks after BMT, the clinical signs of groups that received equivalent doses (2.5 × 106 or 0.25 × 106) of either B6 or B6-cdd CD4+ T cells exhibited essentially similar kinetic onset. However, following this time period, the recipients of both high-dose (2.5 × 106) and low-dose (0.25 × 106) B6-cdd groups displayed a slower development in the pathological process. In the recipients of low, but not high, T-cell doses, the severity of clinical changes decreased somewhat during the second week following BMT and then began to re-emerge during the third week. In total, these findings demonstrated that B6-cdd CD4+ T cells were able to induce clinical changes with a pattern similar to that of normal B6 CD4+ T cells, but with delayed kinetics.
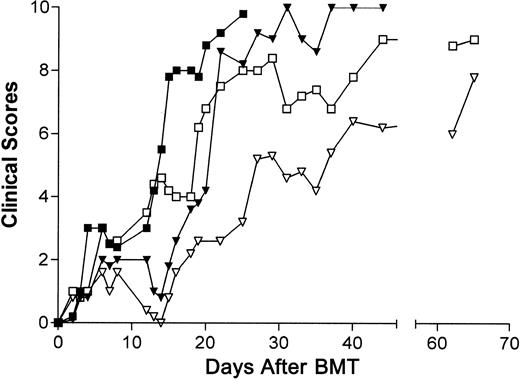
Clinical signs of GVD in recipients of B6-cdd CD4 T cells.
Recipients of B6-cdd CD4 T cells exhibited clinical signs of GVHD (weight loss, diarrhea, fur texture, posture, and alopecia). Clinical changes (maximum score, 10) associated with GVHD were assessed by means of a scoring system described in “Materials and methods.” Recipients of 5 × 106 B6-cdd CD4+ T cells exhibited a clinical score of 8 within 21 days of BMT (data not shown). ▪, 2.5 × 106 B6; ▾, 0.25 × 106 B6; ■, 2.5 × 106 B6-cdd; ▿, 0.25 × 106B6-cdd donor CD4+ T cells.
Clinical signs of GVD in recipients of B6-cdd CD4 T cells.
Recipients of B6-cdd CD4 T cells exhibited clinical signs of GVHD (weight loss, diarrhea, fur texture, posture, and alopecia). Clinical changes (maximum score, 10) associated with GVHD were assessed by means of a scoring system described in “Materials and methods.” Recipients of 5 × 106 B6-cdd CD4+ T cells exhibited a clinical score of 8 within 21 days of BMT (data not shown). ▪, 2.5 × 106 B6; ▾, 0.25 × 106 B6; ■, 2.5 × 106 B6-cdd; ▿, 0.25 × 106B6-cdd donor CD4+ T cells.
The importance of cytotoxic function by donor CD4+ T cells early after BMT
Since 10-fold greater numbers of B6-cdd CD4+ T cells were required to induce GVH effects at kinetics comparable to that of wild-type B6 T cells, the numbers of donor cells present in recipient lymphoid tissues soon after BMT of normal and cytotoxically defective T cells were examined. Spleens and lymph nodes from BALB/c recipients of 1 × 106 highly enriched CD4+ donor T cells were collected, and the mean numbers of nucleated cells per tissue obtained. Aliquots of spleen or lymph node cells from groups receiving cytotoxically impaired or normal donor CD4+ T cells were stained for H-2b and Ly5.2 (donor) and CD4 expression to determine the numbers of these cells (Table2). The total numbers of B6-cdd CD4+ T cells calculated (percentage of H-2Kb+Ly5.1−CD4+ × total cell number) were at least 3- to 5-fold lower compared with the numbers of wild-type CD4+ T cells in recipient spleens and the mesenteric lymph nodes at both time points examined during the first week after BMT. These findings indicate that as early as 120 hours following transplantation of equivalent cell numbers, there were significantly lower numbers of donor B6-cdd CD4+ T cells present in the spleens and lymph nodes of recipients who subsequently demonstrate a markedly delayed onset of GVHD.
In order to compare the capacity of B6-cdd to B6 T cells to proliferate in response to BALB/c alloantigens, a mixed lymphocyte response (MLR) was performed with unfractionated spleen cells or purified (greater than 95%) CD4+ T cells (Figure5). No differences were detected in the proliferative responses by cultures containing B6-cdd versus B6 responding cells. Thus, the diminished numbers of donor B6-cdd T cells detected soon after BMT was not a result of their inherently diminished capacity to expand in response to recipient BALB/c antigen stimulation. Since donor B6-cdd CD4+ T-cell numbers were diminished compared with B6 CD4+ T cells in 8.5-Gy–TBI BALB/c recipients (Table 2), groups of BALB/c mice received lower (5.5 Gy) and higher (11.0 Gy) doses of TBI to investigate the effect of conditioning on the observed differences (Table 3). At 5 days after BMT of highly purified donor CD4+ T cells transplanted into 5.5-Gy recipients, the percentage and numbers (6-fold) of B6-cdd versus B6 CD4+ T cells were again markedly diminished in the mesenteric lymph nodes (Table 3). In contrast, the numbers of B6-cdd CD4+ T cells at this time after BMT were not found to be diminished in 11.0-Gy–conditioned BALB/c recipients. These findings indicate that under the latter conditions, B6-cdd CD4+ T cells can efficiently expand in recipients after BMT.
MLR by B6 and B6-cdd T cells against BALB/c (host) alloantigens.
B6 (░) or B6-cdd (▪) spleen and lymph node CD4+ T cells were isolated and enriched by means of the Macs column. Then 20-Gy–irradiated BALB/c spleen cells or bone marrow–derived (granulocyte-macrophage colony-stimulating factor plus interleukin-4 (IL-4) dendritic cells (data not shown) were used to stimulate responses. The cultures were pulsed with [3H]thymidine for the final 24 hours, and cpm was measured after 96 hours. BALB/c dendritic cells also induced equivalent responses by B6-cdd– and B6–responding T cells (data not shown).
MLR by B6 and B6-cdd T cells against BALB/c (host) alloantigens.
B6 (░) or B6-cdd (▪) spleen and lymph node CD4+ T cells were isolated and enriched by means of the Macs column. Then 20-Gy–irradiated BALB/c spleen cells or bone marrow–derived (granulocyte-macrophage colony-stimulating factor plus interleukin-4 (IL-4) dendritic cells (data not shown) were used to stimulate responses. The cultures were pulsed with [3H]thymidine for the final 24 hours, and cpm was measured after 96 hours. BALB/c dendritic cells also induced equivalent responses by B6-cdd– and B6–responding T cells (data not shown).
The lack of FasL function results in diminished CD4+donor T-cell presence soon after BMT
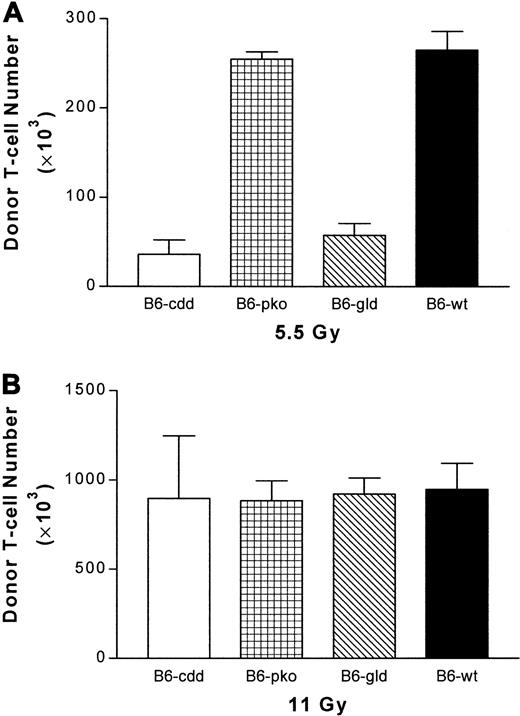
The lack of functional FasL and perforin resulted in lower numbers of donor CD4+ T cells in host lymph node and spleen soon after transplantation. To investigate the importance of each cytotoxic pathway during early donor CD4+ T-cell expansion, 5 × 106 TCD B6-Ly5.1 bone marrow cells were injected together with highly enriched CD4+T cells from B6-cdd, B6-gld, B6-pko, and B6 donors into BALB/c mice conditioned with nonmyeloablative TBI (5.5 Gy) 24 hours before transplantation. The numbers of donor cells in host lymph nodes were assessed 5 days following BMT. Compared with recipients of wild-type B6 CD4+ T cells, donor CD4+ cell numbers in recipients of B6-cdd and B6-gld CD4+ were both significantly reduced. In contrast, the donor CD4+ T-cell numbers in recipients of B6-pko donors were similar to those receiving B6 cells (Figure 6A). However, when a lethal dose of irradiation (11 Gy) was used to condition recipients, donor CD4+ T-cell numbers in each recipient group increased, and the difference in donor CD4+ numbers between each recipient group was essentially eliminated (Figure 6B). To confirm the correlation between early donor T-cell numbers in the host lymph node and the development of GVHD, weight changes and survival of recipient groups were monitored following BMT. High-dose irradiation (Figure 7) effectively resulted in the diminishment of differences in GVHD onset time of weight loss and lethality between recipients of B6 and B6-cdd CD4+ T cells. Additionally, recipients of FasL-defective B6-gld donor cells also exhibited the same GVHD onset. In total, the data indicate the FasL-dependent function is important during the process of early donor CD4+ expansion after transplantation.
Early expansion of CD4+ donor T cells depends on FasL function.
Highly enriched CD4+ T cells were obtained from spleens of B6, B6-pko, B6-gld, and B6-cdd mice by means of magnetic beads on Macs column (see “Materials and methods”). Then, 1 × 106donor CD4+ T cells were combined with 5 × 106 T-cell–depleted B6-Ly5.1 bone marrow cells and injected into BALB/c recipients (2 per group) irradiated at 5.5-Gy (A) or 11-Gy (B) TBI 24 hours earlier. At day 5, the mesenteric lymph nodes were harvested from individual recipient mice and counted. Donor T cells were identified by staining with anti-CD4, anti–H-2Kb, and anti-Ly5.1 mAbs. The percentage of CD4+H-2Kb+Ly5.1− cells × the total number of nucleated lymph node cells was used to determine donor cell numbers. (A) B6-cdd and B6-gld versus B6: P < .0014. B6-pko versus B6: P > .5. (B) B6-cdd, B6-gld, and B6-pko versus B6-wt: P > .5.
Early expansion of CD4+ donor T cells depends on FasL function.
Highly enriched CD4+ T cells were obtained from spleens of B6, B6-pko, B6-gld, and B6-cdd mice by means of magnetic beads on Macs column (see “Materials and methods”). Then, 1 × 106donor CD4+ T cells were combined with 5 × 106 T-cell–depleted B6-Ly5.1 bone marrow cells and injected into BALB/c recipients (2 per group) irradiated at 5.5-Gy (A) or 11-Gy (B) TBI 24 hours earlier. At day 5, the mesenteric lymph nodes were harvested from individual recipient mice and counted. Donor T cells were identified by staining with anti-CD4, anti–H-2Kb, and anti-Ly5.1 mAbs. The percentage of CD4+H-2Kb+Ly5.1− cells × the total number of nucleated lymph node cells was used to determine donor cell numbers. (A) B6-cdd and B6-gld versus B6: P < .0014. B6-pko versus B6: P > .5. (B) B6-cdd, B6-gld, and B6-pko versus B6-wt: P > .5.
Effect of high-dose irradiation.
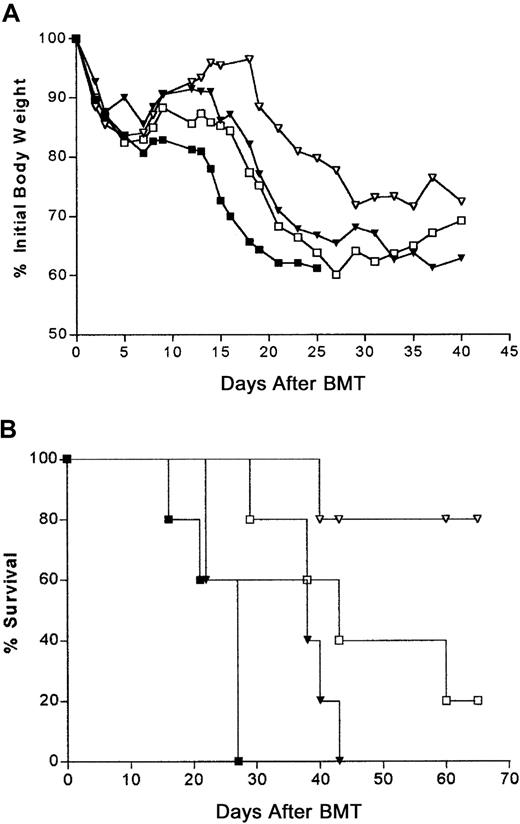
High-dose irradiation conditioning abolished the delay of GVHD-induced weight loss and lethality by B6-cdd compared with B6 donor CD4+ T cells. Highly enriched CD4+ cells were obtained as described in Figure 6. Then, 1 × 106 donor CD4+ T cells from B6 (♦), B6-pko (▿), B6-gld (▵), and B6-cdd (⋄) donors were combined with 5 × 106 TCD B6-Ly5.1 bone marrow cells and injected into BALB/c recipients (5 per group) that had been irradiated at 11-Gy TBI 24 hours earlier. Weight loss (A) and lethality (B) were monitored. ∗ indicates BM only.
Effect of high-dose irradiation.
High-dose irradiation conditioning abolished the delay of GVHD-induced weight loss and lethality by B6-cdd compared with B6 donor CD4+ T cells. Highly enriched CD4+ cells were obtained as described in Figure 6. Then, 1 × 106 donor CD4+ T cells from B6 (♦), B6-pko (▿), B6-gld (▵), and B6-cdd (⋄) donors were combined with 5 × 106 TCD B6-Ly5.1 bone marrow cells and injected into BALB/c recipients (5 per group) that had been irradiated at 11-Gy TBI 24 hours earlier. Weight loss (A) and lethality (B) were monitored. ∗ indicates BM only.
Discussion
Alloreactive donor T cells rapidly expand following recognition of antigen in the host after BMT, a process required for the subsequent development of GVHD. The present studies have examined the involvement of cytotoxic pathways in CD4+ donor T cells following a BMT across a full donor/recipient MHC class I/II disparity. The results demonstrate that CD4+ T cells lacking the ability to effect both perforin/granzyme– and FasL-dependent cytotoxicity are severely impaired in their ability to induce GVHD. The demonstration that increased numbers of cytotoxically impaired B6-cdd compared with wild-type CD4+ T cells are necessary to induce comparable GVHD-associated weight loss and lethality supports previous findings that donor T-cell–mediated cytotoxicity provides an important GVH component after transplantation.3-7 The complex nature of GVHD makes it difficult to define precisely when and how following BMT donor-mediated cytotoxicity is involved. Notably, examination of donor CD4+ T cells soon after transplantation revealed that their inability to express functional FasL resulted in markedly diminished numbers compared with cytotoxically normal CD4+ donor cells. Therefore, the present findings have demonstrated another role for CD4+ donor-mediated cytotoxic function, ie, prior to GVHD-associated tissue pathogenesis.
CD4+ T cells possess the capacity to produce a diverse array of cytokines. Since many of these, including IL-1, interferon-γ, and tumor necrosis factor-α are known to contribute to the induction and inflammation processes that underlie GVHD,19,20 removal of the principal cytotoxic effector pathways in such a population could enable further insight concerning when and how such modalities participate in the GVHD process. Previous studies reported a delay in mortality with the use of donor B6-cdd CD4+ cells in a GVH model involving only an MHC class II donor/recipient genetic disparity.8 The present findings comparing transplantation of highly enriched cytotoxic double-deficient and wild-type CD4+ cells across a complete class I/II disparity also detected a significant delay in GVHD mortality as well as weight loss and clinical changes when equal numbers of donor cells were infused. These observations are consistent with our previous findings after transplantation of unfractionated H-2bB6-cdd spleen cells into H-2d recipients.12 It was noteworthy that increasing the number of B6-cdd CD4+cells (5- to 10-fold) resulted in comparable GVHD onset, demonstrating that CD4+ T cells deficient in the ability to mediate the 2 major pathways of cell-mediated cytotoxicity can induce GVHD-associated weight loss, lethality, and tissue damage following transplantation across MHC class I and II genetic disparities. The most straightforward interpretation of these findings is that under certain conditions, use of the 2 principal cytotoxic pathways by CD4+ donor T cells can be circumvented in the induction of GVHD. Accordingly, other cytotoxic molecules and/or cytokines must be sufficient to effect most GVHD-associated pathogenesis.
Interestingly, a prior investigation reported that unfractionated B6-cdd spleen cells together with normal B6 marrow failed to induce GVHD lethality in the identical full MHC class I/II–mismatched (B6-cdd→BALB/c) model examined in the present studies.10Importantly, the BALB/c recipients in that study were administered sublethal TBI (ie, 5.0 Gy) conditioning. The inability of cytotoxically impaired T cells to survive after BMT owing to vigorous host-versus-graft (HVG) responses obscures the opportunity to evaluate the capacity of such cells to induce GVHD. These results emphasize that donor cell survival after transplantation is a prerequisite for accurately determining if the transferred population possesses the functional ability to effect GVH responses. An important observation in the present studies was the finding that donor B6-cdd CD4+T-cell numbers in host spleen and lymph nodes were significantly diminished early after BMT when 8.5 Gy or lower levels of TBI were used to condition recipients. The induction of GVHD is critically dependent on the numbers of immunocompetent T cells contained in the donor inoculum.21-23 One possibility that could account for the delayed onset of GVHD observed after equivalent numbers of B6-cdd and wild-type CD4+ donor T-cells were transplanted may be the diminished ability of the cytotoxically impaired cells to survive in recipients following BMT. The subsequent finding that B6-cdd cells failed to induce GVHD with kinetics comparable to that by cytotoxically normal T cells suggests that events in the immediate posttransplantation period probably contributed to the delay in GVHD onset. These findings demonstrate a role for donor T-cell cytotoxic function in the CD4+ subset soon after BMT, well prior to development of GVHD-associated weight loss and pathogenesis in target tissues. Such a role is consistent with, and not exclusive of, previous observations (1) identifying participation of these cytotoxic pathways—when present—in the tissue damage ensuing during the disease process and (2) demonstrating that the absence of donor perforin and FasL function does not preclude development of GVHD after transplantation of sufficient donor cell numbers and/or appropriate host conditioning.3-9,11 12
To more carefully assess the requirement of functional cytotoxic effector pathways for successful expansion of donor T cells soon after transplantation, CD4+ populations with single cytotoxic deficiencies were examined. The numbers of CD4+ B6-gld, but not B6-pko, T cells were also diminished following transplantation into low- (ie, 5.5 Gy), but not high- (11.0 Gy), conditioned BALB/c recipients. These results support the notion that under conditions when host resistance is intact, a cytotoxic impairment in CD4+donor T cells is responsible for their diminished numbers early after transplantation. Moreover, the diminished numbers were associated with defective FasL, not perforin, function. A defect in the ability of B6-cdd or B6-gld cells to expand could also be proposed as contributing to the diminished numbers of these cells detected. A defect in the ability of FasL-defective CD4+ donor T cells to expand after BMT was noted in a P→F1 GVHD model.24 However, the defect in expansion was detected several weeks following transplantation of allogeneic parental C3H-Faslgld CD4+ T cells into F1 recipients. The authors postulated that the defect occurred in the expansion of memory/activated gld cells in the F1 recipients, resulting in their impaired ability to induce GVHD.24 However, no difference between wild-type versus FasL-defective donor cell numbers were observed in these F1 recipients soon after transplantation. In contrast, diminished numbers of FasL-defective B6-cdd and B6-gld CD4+ T cells were detected soon after BMT (5 to 7 days) in the present study. Under conditions of high host TBI, both B6-cdd and B6-gld CD4+ T cells expanded equivalently to the expansion by their B6 counterparts. Moreover, together with the observation of equivalent proliferative responses by B6-cdd and B6 CD4+ T cells against BALB/c “host” alloantigen during in vitro MLR responses, it is unlikely that the cytotoxic-impaired donor populations possessed any inherent inability to expand after transplantation.
The early elimination/deletion of transplanted donor T cells would have effects similar to decreasing the size of the donor inoculum and would therefore be expected to reduce the GVH potential. Strong radio-resistant host antigraft responses are mediated against donor cells following BMT across complete MHC class I and II disparities.25-27 Both natural killer and CD8+ T cells function as a barrier that inhibits engraftment by donor progenitor populations contained in the inoculum and eliminates T cells contained in the transplant.27-30Martin and colleagues11,31 have convincingly demonstrated that donor-mediated cytotoxicity is crucial for overcoming allogeneic host resistance leading to successful engraftment. It was demonstrated that perforin-deficient CD8+ T cells, including B6-cdd T cells, were greatly diminished in their capacity to overcome host resistance.11 Although CD4+ donor cells cannot directly recognize class II–negative host resistance elements, FasL-impaired CD4+ T cells may be particularly unable to defend themselves from HVG effector attack. Thus, under BMT conditions that permit significant HVG responses, ie, low conditioning (5.5 Gy) (Braun et al10 and the present study) and/or that involve the addition of HVG activity (eg, inclusion of syngeneic, ie, recipient bone marrow) at time of transplantation,8 it may not be surprising that diminished numbers of B6-cdd and B6-gld, compared with wild-type T cells, survive the early period after BMT. In contrast, under conditions of weak/diminished HVG resistance (P→F1, high recipient TBI),11,12 24 donor cell survival after transplantation regardless of cytotoxic impairment would be predicted—and was observed in this study to increase. The heightened susceptibility of B6-cdd and B6-gld CD4+ T cells to host resistance was probably a consequence of their FasL-impaired cytotoxic function because perforin-deficient CD4+ T-cell numbers were not diminished. Studies are currently examining whether veto activity may be involved in this process.
We thank Dr Dingding Xiong for his help with screening of B6-cdd mice; Mrs Emma Weaver for animal care and technical assistance; Monica Jones for overseeing the breeding colonies of the mice used in this study; and the Sylvester Cancer Center for support of the Flow Cytometry Facility for the phenotypic analysis of cell populations used in these experiments.
Supported by National Institutes of Health (NIH) grants 1RO1 RR11576 and 5RO1 HL52461 (R.B.L.) and by NIH grants CA39201, CA57904, and CA80228, US Department of Defense grant DAMD17-98-1-8317 (E.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert B. Levy, University of Miami School of Medicine, Department of Microbiology and Immunology, PO Box 016960 (R-138), Miami, FL 33101; e-mail: rlevy@med.miami.edu.

![Fig. 5. MLR by B6 and B6-cdd T cells against BALB/c (host) alloantigens. / B6 (░) or B6-cdd (▪) spleen and lymph node CD4+ T cells were isolated and enriched by means of the Macs column. Then 20-Gy–irradiated BALB/c spleen cells or bone marrow–derived (granulocyte-macrophage colony-stimulating factor plus interleukin-4 (IL-4) dendritic cells (data not shown) were used to stimulate responses. The cultures were pulsed with [3H]thymidine for the final 24 hours, and cpm was measured after 96 hours. BALB/c dendritic cells also induced equivalent responses by B6-cdd– and B6–responding T cells (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.390/5/m_h81411292005.jpeg?Expires=1769493851&Signature=CnhgDsALay~TcCbnMFWtYrgPzBeDRoT-OHfcshjnUrhWnDxnkUyfmJn8TLP53jkYg4A3KF2OKfe1pj0k8dsKQYW3Aje5cozBsN50Qpp0aZiv6nQD2ZX-ziASiZzh3yg~T-tfiHDZVWbhb5jQyfC3qyd-2ODz34SIfCeQSvolMK7RTKYHAg-Xspj54Of~~cihBtj9GqIU~uUZWYndcAZYIyREZgpkiI8sXwpXDUuDNOfUKP~YMfsZgi5x23J5Tu6g1MJqFkoG1MkFNrHCdqL9Wb3DL2v-FYn5OAHAMlDsFayJ~y1UmkQebtDSdEGyS7xjNp1mnFFclAFaALOgM~sz0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)