Indinavir (IDV) is a potent and selective human immunodeficiency virus type 1 (HIV-1) protease inhibitor (PI) widely used in antiretroviral therapy for suppression of HIV, but its effects on the immune system are relatively unknown. Recently, it has been reported that PIs inhibit lymphocyte apoptosis. In the present study we have investigated the effects of ex vivo addition of IDV on lymphocyte activation and apoptosis in cells from HIV-infected children (n = 18) and from healthy uninfected individuals (controls, n = 5) as well as in Jurkat and PM1 T-cell lines. Pretreatment of control peripheral blood mononuclear cell (PBMC) cultures with IDV resulted in a dose-dependent inhibition of lymphoproliferative responses to different activation stimuli. Additionally, this treatment led to cell-cycle arrest in G0/G1 phase in anti-CD3 monoclonal antibody–stimulated PBMC cultures in controls and in 15 of 18 HIV-infected children. Spontaneous- or activation-induced apoptosis of PBMCs from HIV-infected or uninfected individuals or of Fas-induced apoptosis in Jurkat and PM1 T cell lines were not inhibited by IDV. Moreover, IDV did not inhibit activation of caspases-1, -3, -4, -5, -9, and -8 in lysates of Jurkat T cells undergoing Fas-induced apoptosis. The findings indicate that IDV interferes with cell-cycle progression in primary cells but does not directly affect apoptosis. It is concluded that IDV may prolong cell survival indirectly by inhibiting their entry into cell cycle. In individuals on PI therapy, PI-mediated effects could potentially modulate immunologic responses independently of antiviral activity against HIV.
Introduction
Immunodeficiency is a hallmark of human immunodeficiency virus type 1 (HIV-1) disease and is characterized by a progressive decrease in CD4 T cells. The introduction of combination therapies (eg, with drugs targeting the viral-specific enzymes reverse transcriptase and protease1-3) have had a dramatic effect in slowing disease progression. In the majority of patients, drug regimens containing HIV-1 protease inhibitors (PIs) and reverse transcriptase inhibitors (RTIs) are effective in reducing plasma viral burden, with concomitant increase in CD4 lymphocyte counts.4,5 When therapy fails, the virologic rebound is associated with a decline in CD4 T cells. Curiously, in a subset of patients, the immunologic benefit persists despite virologic failure.6 7 The mechanism of this “discordant” response is not clearly understood, and the observed effects have been ascribed to virologic factors as well as to direct effects of the PIs on the immune system, distinct from their antiviral effects.
Mechanisms of CD4 T-cell loss in HIV disease have been under debate for several years. Loss of CD4 T cells by apoptosis has been put forth as one mechanism for the immune deficiency in HIV infection. Increased lymphocyte apoptosis is recognized as a feature of HIV infection and has been attributed to direct and indirect effects of HIV on the immune system.8,9 Therapy-induced decrease in plasma HIV RNA has, in fact, been shown to result in reduction in lymphocyte apoptosis.10,11 The antiviral effect of PI drugs is mediated by the inhibition of the HIV protease, the enzyme that is required for the processing of HIV polypeptides into smaller peptides and maturation of viral particles.12-14 It has been argued that the similarity between HIV protease (an aspartyl protease) and the cellular caspases (cysteine proteases) involved in apoptosis could result in PI-mediated anticaspase activity.13-16 Some recent studies with the PI Ritonavir have implicated a direct antiapoptotic effect of the drug on T cells17-19 as a possible mechanism for CD4 T-cell increase following therapy. However, in another study, the in vitro addition of the PI Indinavir (IDV) to cells of HIV-infected patients did not result in antiapoptotic effects.20 The mechanism by which PI therapy results in immunologic benefit independently of its antiviral effects thus remains unclear.
A characteristic feature of PIs is their ability to modulate proteosomes21,22 that are involved in a variety of cellular functions and constitute an important component of the biological system. It has been shown that this effect of PIs on proteosomes impairs antigen presentation and processing by antigen-presenting cells.21 In addition, PIs have also been shown to influence lymphocyte proliferation20 23; the reported effects, however, have been contradictory, presumably because the experimental systems have been different as have the doses of drugs used, which have varied widely in these studies.
In the present study we have tested the hypothesis that PIs mediate immunomodulatory effects that can influence host immunity in a manner distinct from the benefits attributable to control of viral replication. Here we have examined the effect of therapeutic concentrations of IDV on lymphocyte activation and on spontaneous and activation-induced lymphocyte apoptosis in peripheral blood mononuclear cell (PBMC) cultures of healthy volunteers and HIV-infected patients and in T cell lines.
Materials and methods
Donors
This study was conducted in healthy volunteers (n = 5) and in HIV-infected children (n = 18) during regularly scheduled visits to the Pediatric Immunology Clinic at North Shore University Hospital (Manhasset, NY) by protocols approved by the hospital's institutional review board. The children ranged in age from 0.4 to 13.9 years (median, 8.7) and had absolute CD4 counts ranging from 17 to 2371 cells/μL (median, 595). Plasma virus load ranged from 1.6 to 5.5 log10 HIV RNA copies/mL (median, 3 log10). Most infected children were on antiretroviral therapy that included the RTIs Zidovudine, Stavudine, Zalcitabine, and Didanosine and the PIs Nelfinavir or Saquinavir plus Ritonavir. Nine of 18 patients were on one or 2 PIs in combination with 2 RTIs, whereas the others were on 2 (n = 4) or 3 RTIs (n = 5).
Isolation of cells
Peripheral venous blood was collected by venipuncture into tubes containing acid citrate dextrose as an anticoagulant. PBMCs were isolated by Ficoll-Hypaque (Lymphoprep; Nycomed, Oslo, Norway) density gradient centrifugation and suspended at a density of 1 × 106 cells/mL in RPMI 1640 (Whittaker Biproducts, Walkersville, MD), 10% fetal calf serum (Hyclone Laboratories, Logan, UT), 2 mM L-glutamine (Whittaker Biproducts), penicillin G (100 U/mL), and streptomycin (100 μg/mL) (Whittaker Biproducts).
Cell lines and other reagents
Two different T-cell lines, Jurkat (clone E6-1; ATCC, American Type Culture Collection, Manassas, VA) and PM124 (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) were maintained and cultured in RPMI 1640, 10% fetal bovine serum, 2 mM L-glutamine, and antibiotics penicillin and streptomycin. Antihuman Fas (CD95) monoclonal antibody (mAb) CH11 and fluorogenic substrates Ac-YVAD-AFC, Ac-DEVD-AFC, Ac-LEHD-AFC, and Ac-IETD-AFC of caspase-1; caspase-3; caspases-4, -5, and -9; and caspase-8, respectively, and their specific inhibitors (Ac-YVAD-CHO, Z-DEVD-FMK, Z-LEHD-FMK, and Z-IETD-FMK, respectively) were purchased from Kamiya Biomedical Company (Seattle, WA). Purified mouse antihuman CD3 mAb was purchased from BD Pharmingen (San Diego, CA). A gift of IDV sulfate powder was generously given by Merck Research Laboratories (Rahway, NJ). RNAse, phytohemagglutinin (PHA), concanavalin A (Con A), phorbol 12-myristate 13-acetate (PMA), and Ionomycin were purchased from Sigma (St Louis, MO), and propidium iodide was obtained from Molecular Probes (Eugene, OR).
Culture conditions and measurement of lymphocyte apoptosis and cell-cycle profiles
Test PBMCs were cultured for 3 days with anti-CD3 mAb (0.5 μg/mL) or with control isotype-matched antibody to study spontaneous- and activation-induced lymphocyte apoptosis. To study the effect of IDV, the drug was added ex vivo to PBMC cultures for 18 hours in 24-well culture plates (Becton Dickinson Labware, Franklin Lakes, NJ). Different concentrations of IDV were prepared from IDV powder dissolved in dimethyl sulfoxide (DMSO) and added to the cultures at a final dilution of 1 μL/mL. Thereafter, the cells were cultured with anti-CD3 mAb (0.5 μg/mL) or with control isotype-matched antibody for an additional 48 hours. At termination of cultures, cells were harvested by centrifugation, washed, and fixed in 70% ethanol for 1 hour and incubated with propidium iodide (50 μg/mL) and RNAse (100 μg/ml). Samples were kept in the dark at room temperature for 30 minutes and stored at 4°C until analyzed for cell-cycle profile and apoptosis. In healthy volunteers, for the study of IDV effect on lymphocyte apoptosis, additional experiments were performed to simulate the state of in vivo lymphocyte activation, which is a characteristic feature of the HIV-infected state. PBMCs were first activated with PHA (2 μg/mL) for 4 days, washed, and then treated as described above with different concentrations of IDV for 18 hours, followed by activation with anti-CD3 mAb for 48 hours.
Apoptosis in Jurkat and PM1 T cells was induced through Fas death pathway by culturing the cells with anti-Fas mAb CH11 (100 ng/mL) in the presence of different concentrations of IDV for 2, 6, and 18 hours. In other experiments, Jurkat and PM1 T cells were precultured with different concentrations of IDV for 18 hours followed by treatment with CH11 for 6 and 18 hours. To investigate the effect of IDV on the cell cycle, Jurkat and PM1 T cells were cultured with different concentrations of IDV over periods of 24, 48, and 72 hours and stained with propidium iodide.
Lymphocyte apoptosis was investigated as described in propidium iodide-stained cells using the Coulter Epics Elite ESP (Coulter, Hialeah, FL) instrument after careful gating for lymphocytes.25 For cell-cycle studies, DNA content was determined by using listmode files with the multicycle flow cytometry software (Phoenix Flow System, San Diego, CA). In healthy volunteers, results of cycling cells (in S + G2/M phase) or of cells undergoing apoptosis were expressed as a percentage of total cells for that culture condition. In patients, cycling cells and apoptotic cells in IDV-treated cultures are expressed as a percentage of cycling cells or apoptotic cells in cultures without IDV (ie, cells precultured with 1 μL/mL DMSO).
Lymphoproliferation studies
PBMCs from healthy human volunteers were precultured in medium alone or with different concentrations of IDV for 18 hours in 96-well plates (Becton Dickinson Labware), followed by the addition of stimuli in triplicate [viz, anti-CD3 mAb (0.5 μg/mL), PHA (2 μg/mL), Con A (2 μg/mL), PMA (10 ng/mL) plus Ionomycin (1 μM)], isotype-matched antibodies, or DMSO (control) for an additional 48 hours. Cultures were pulsed with [14C]-thymidine (1 μCi/well) for the last 18 hours, and uptake of [14C]-thymidine in cells was quantitated by scintillation counting.26Lymphoproliferative responses of IDV-treated cultures are expressed as the percentage of [14C]-thymidine incorporated in cultures without IDV treatment.
Study of caspase activity
Cell lysates from CH11-treated Jurkat CD4 T cells was used to quantitate activity of caspase-1, -3, -4, -5, -9, and -8. In brief, 1 × 106 Jurkat CD4 T cells/mL were cultured with 100 ng/mL anti-Fas antibody CH11 for 3 hours. Cells were washed once with ice-cold Hanks balanced salt solution, followed by suspension in cell lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mg/mL leupeptin, and 20 mg/mL aprotinin] for 20 minutes. Cells were incubated on ice for 20 minutes and then disrupted by passing through a 20-gauge needle for 10 times. Cell extracts were clarified by centrifugation at 16 000g for 15 minutes at 4°C. To study the effect of IDV on caspase activity, cell extracts were incubated with different concentrations of IDV (12.5 μM to 100 μM) or with specific caspase inhibitors (Ac-YVAD-CHO, Z-DEVD-FMK, Z-LEHD-FMK, and Z-IETD-FMK) followed by addition of caspase-specific fluorogenic substrates (Ac-YVAD-AFC, Ac-DEVD-AFC, Ac-LEHD-AFC, and Ac-IETD-AFC) for caspase-1; caspase-3; caspases-4, -5, and -9; and caspase-8, respectively. Activity of each caspase was estimated as a release of 7-amino-4-trifluoromethyl coumarin (AFC) from the caspase-specific substrate and measured by dual scanning microplate spectrofluorometer (SPECTRAmax Gemini XS; Molecular Devices, Sunnyvale, CA). Kinetics of individual reactions were monitored for 2 hours at 37°C in the microplate spectrofluorometer.
Statistical analysis
SigmaStat 2.0 statistical software (Jandel Scientific Software, San Rafel, CA) was used for all statistical analysis.
Results
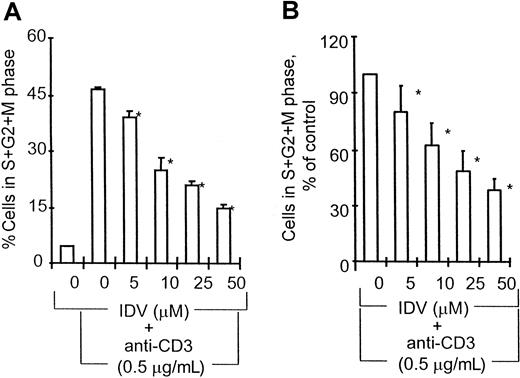
IDV blocks lymphocyte cell cycle in G0/G1 phase in cells from healthy volunteers and HIV-infected children
PBMCs from healthy volunteers and from HIV-infected children were incubated in the absence or presence of different concentrations of IDV for 18 hours followed by stimulation with anti-CD3 or control antibodies for an additional 48 hours. The effect of IDV on anti-CD3 mAb–induced cell-cycle progression in healthy volunteers is shown in Figure 1A. Preincubation of PBMCs with IDV blocked anti-CD3–induced cell-cycle progression in a dose-dependent manner. IDV was found to block cells in the G0/G1 phase, prior to their entry into synthesis phase (S). At the lowest concentration of IDV (5 μM), 36.2% ± 3.5% cells were in cell cycle compared with 47.2 ± 1.2 cycling cells in PBMCs cultured with anti-CD3 mAb without prior exposure to IDV (P < .05). At the highest concentration of IDV (50 μM), only 15% ± 1.3% cells were in cycling phase. The effect of IDV on anti-CD3–induced cell-cycle progression in PBMCs of HIV-infected children is shown in Figure 1B. Pretreatment of PBMCs with IDV was found to inhibit anti-CD3–induced cell entry into S phase in a dose-dependent manner in 15 of 18 HIV-infected children. The ex vivo blocking effect of IDV on anti-CD3–induced cell cycling was noted to occur in patients regardless of whether they were receiving PI therapy or not. Addition of IDV at the same time as anti-CD3 mAb did not inhibit cell-cycle progression. Viability of cells was equivalent in cultures with or without IDV pretreatment.
Effect of IDV on anti-CD3–induced cell-cycle progression.
(A) PBMCs from control volunteers (n = 5). *, P < .005. (B) PBMCs from HIV-infected children (n = 15). PBMCs from healthy volunteers and HIV-infected children were preincubated without or with different concentrations of IDV for 18 hours followed by activation with anti-CD3 mAb (0.5 μg/mL) for an additional 48 hours, and cells were stained with propidium iodide for cell-cycle profile analysis by multicycle flow cytometry software. In healthy volunteers, cycling cells in S + G2/M phase are represented as percentage (mean ± SD) of total cells for each test condition. For patients, cells in S + G2/M phase in IDV-treated cultures are expressed as a percentage (mean ± SD) of cells in S + G2/M phase in cultures without IDV treatment. *, P < .05.
Effect of IDV on anti-CD3–induced cell-cycle progression.
(A) PBMCs from control volunteers (n = 5). *, P < .005. (B) PBMCs from HIV-infected children (n = 15). PBMCs from healthy volunteers and HIV-infected children were preincubated without or with different concentrations of IDV for 18 hours followed by activation with anti-CD3 mAb (0.5 μg/mL) for an additional 48 hours, and cells were stained with propidium iodide for cell-cycle profile analysis by multicycle flow cytometry software. In healthy volunteers, cycling cells in S + G2/M phase are represented as percentage (mean ± SD) of total cells for each test condition. For patients, cells in S + G2/M phase in IDV-treated cultures are expressed as a percentage (mean ± SD) of cells in S + G2/M phase in cultures without IDV treatment. *, P < .05.
IDV impairs lymphoproliferative responses
Pretreatment of PBMC cultures of healthy volunteers with IDV (0-50 μM) for 18 hours, followed by stimulation with anti-CD3 mAb (0.5 μg/mL) for 48 hours resulted in dose-dependent reduction of lymphoproliferative responses (Figure 2). This antiproliferative effect of IDV was also noted for cultures stimulated with PHA, PMA plus Ionomycin, or Con A. A similar pattern of dose-dependent inhibition was noted in cultures of unstimulated cells, with more than 50% inhibition of [14C]-thymidine uptake at a 5 to 10 μM dose range of IDV. At 18 hours after preincubation of PBMCs with IDV, no significant difference in [14C]-thymidine uptake and cell viability was noted between cells cultured with and without different concentrations of IDV (data not shown).
Effect of IDV on lymphoproliferation.
PBMCs from healthy volunteers were preincubated without or with different concentrations of IDV for 18 hours followed by activation with different stimuli for an additional 48 hours. Proliferation was measured with [14C]-thymidine incorporation added at 1 μCi/well for the last 48 hours. Results of [14C]-thymidine incorporation in IDV-treated cultures are expressed as the percentage of responses of cells cultured without IDV. Data represents mean ± SD for 1 of 5 representative experiments: ♦, medium alone; ●, anti-CD3 (0.5 μg/mL); ▴, PHA (2 μg/mL); ▪, PMA (10 ng/mL); + Ionomycin (1 μM); and ○, Con A (2 μg/mL).
Effect of IDV on lymphoproliferation.
PBMCs from healthy volunteers were preincubated without or with different concentrations of IDV for 18 hours followed by activation with different stimuli for an additional 48 hours. Proliferation was measured with [14C]-thymidine incorporation added at 1 μCi/well for the last 48 hours. Results of [14C]-thymidine incorporation in IDV-treated cultures are expressed as the percentage of responses of cells cultured without IDV. Data represents mean ± SD for 1 of 5 representative experiments: ♦, medium alone; ●, anti-CD3 (0.5 μg/mL); ▴, PHA (2 μg/mL); ▪, PMA (10 ng/mL); + Ionomycin (1 μM); and ○, Con A (2 μg/mL).
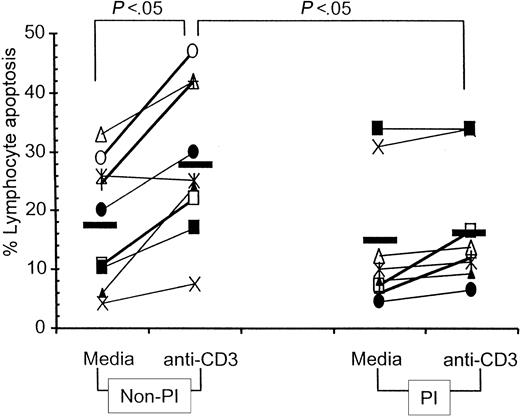
Spontaneous and anti-CD3–induced lymphocyte apoptosis in HIV-infected children
Spontaneous lymphocyte apoptosis was investigated in 3-day PBMC cultures of HIV-infected children. As shown in Figure3, spontaneous lymphocyte apoptosis was not significantly different in HIV-infected children who were on PI drug regimens (range, 4.4% to 34%; median, 10%) from those not on PI-containing drug regimens (range, 4.3% to 33%; median, 20%). Activation-induced lymphocyte apoptosis following anti-CD3 stimulation, however, was significantly increased over spontaneous apoptosis only in children on non-PI therapies, whereas the percentage of lymphocyte apoptosis remained unaffected following anti-CD3 activation in children who were on PI regimens. Anti-CD3–induced lymphocyte apoptosis was also significantly higher in children on non-PI drug regimens (range, 7.6% to 47%; median, 25.1%) as compared with those on PI-containing drug regimens (range, 6.5% to 34%; median, 12.2%;P < .05).
Spontaneous and anti-CD3–induced apoptosis in PBMCs from HIV-infected children.
PBMCs of HIV-infected children were cultured in medium for 3 days without anti-CD3 mAb to determine spontaneous lymphocyte apoptosis and with anti-CD3 mAb (0.5 μg/mL) for 48 hours to determine activation-induced lymphocyte apoptosis. Data shown represents percentage of lymphocyte apoptosis, estimated by propidium iodide staining. Symbols represent individual patients who were on non-PI– (left) or on PI- (right) containing antiretroviral therapy regimens. Mean value in each data set is represented by a solid line.
Spontaneous and anti-CD3–induced apoptosis in PBMCs from HIV-infected children.
PBMCs of HIV-infected children were cultured in medium for 3 days without anti-CD3 mAb to determine spontaneous lymphocyte apoptosis and with anti-CD3 mAb (0.5 μg/mL) for 48 hours to determine activation-induced lymphocyte apoptosis. Data shown represents percentage of lymphocyte apoptosis, estimated by propidium iodide staining. Symbols represent individual patients who were on non-PI– (left) or on PI- (right) containing antiretroviral therapy regimens. Mean value in each data set is represented by a solid line.
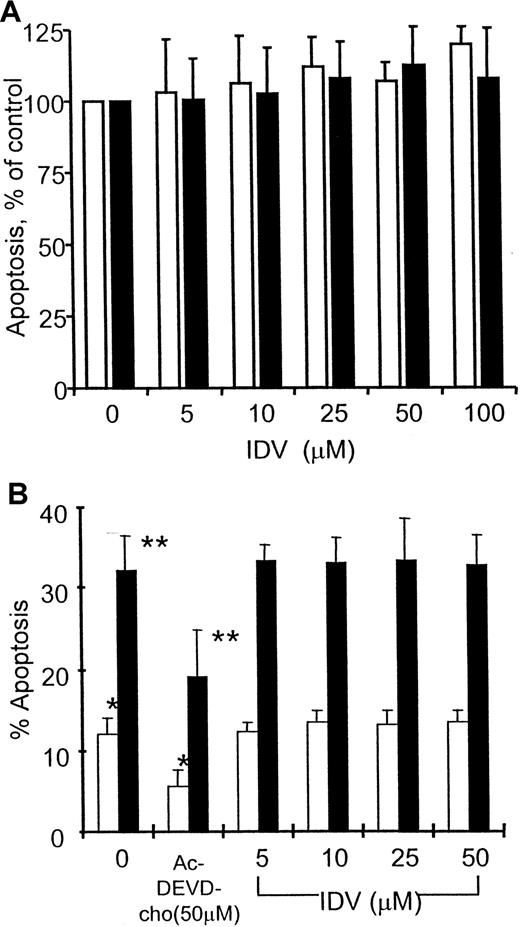
IDV does not inhibit lymphocyte apoptosis
Preincubation of PBMCs from HIV-infected children with IDV (5-50 μM) did not influence spontaneous or anti-CD3–induced apoptosis (Figure 4A); this response was not different between patients receiving PI- or non–PI-containing antiretroviral therapies (data not shown).
Effect of IDV on spontaneous and anti-CD3–induced apoptosis in PBMCs.
The PBMCs were from HIV-infected children (A, n = 15) and from healthy volunteers (B). PBMCs were preincubated without and with different concentrations of IDV followed by culture in the absence (■) or presence (▪) of anti-CD3 mAb (0.5 μg/mL) for an additional 48 hours. Lymphocyte apoptosis was estimated by propidium iodide staining. In healthy volunteers, cultures with 50 μM caspase inhibitor Ac-DEVD-CHO were also established. In patients, apoptosis in IDV-treated cultures are expressed as a percentage of cells undergoing apoptosis in cultures without IDV pretreatment. In healthy volunteers, lymphocytes undergoing apoptosis are expressed as a percentage of total cells for each condition. * and **, P < .05.
Effect of IDV on spontaneous and anti-CD3–induced apoptosis in PBMCs.
The PBMCs were from HIV-infected children (A, n = 15) and from healthy volunteers (B). PBMCs were preincubated without and with different concentrations of IDV followed by culture in the absence (■) or presence (▪) of anti-CD3 mAb (0.5 μg/mL) for an additional 48 hours. Lymphocyte apoptosis was estimated by propidium iodide staining. In healthy volunteers, cultures with 50 μM caspase inhibitor Ac-DEVD-CHO were also established. In patients, apoptosis in IDV-treated cultures are expressed as a percentage of cells undergoing apoptosis in cultures without IDV pretreatment. In healthy volunteers, lymphocytes undergoing apoptosis are expressed as a percentage of total cells for each condition. * and **, P < .05.
In PBMCs of healthy individuals, the above protocol of 18 hours' preincubation with IDV followed by anti-CD3 activation yielded very few cells undergoing apoptosis. These studies were extended to examine the effect of IDV in apoptosis of PBMC cultures following prior activation with PHA (Figure 4B). PBMCs from healthy volunteers were cultured with PHA (2 μg/mL) for 4 days followed by incubation with different concentrations of IDV for 18 hours. These activated cells were cultured with anti-CD3 or control mAb (0.5 μg/mL) for an additional 48 hours to study activation-induced apoptosis. No significant difference in apoptosis was noted in PBMC cultures that were treated or not treated with different concentrations of IDV, whereas anti-CD3–induced apoptosis was significantly blocked by Ac-DEVD-CHO, a known inhibitor of caspases.
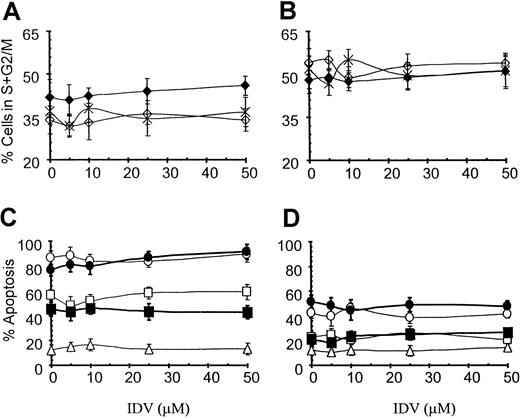
IDV does not influence cell cycle or apoptosis of Jurkat and PM1 T-cell lines
IDV at concentrations of 5 to 50 μM did not influence the cell-cycle profile of Jurkat (Figure 5A) or PM1 (Figure 5B) T-cell lines over incubation periods of 24, 48, or 72 hours. Fas-induced apoptosis was not inhibited in cells precultured with different concentrations of IDV or in cultures simultaneously incubated with different concentrations of IDV and anti-Fas antibody CH11, either in Jurkat (Figure 5C) or PM1 T cells (Figure 5D) over a period of 2 to 18 hours.
Effect of IDV on cell-cycle progression.
Cell-cycle progression of Jurkat (A) and PM1 T (B) cells and apoptosis in Jurkat (C) and PM1 (D) T-cell lines. For cell-cycle analysis Jurkat and PM1 T cells were cultured with different concentrations of IDV for 24 (⋄), 48 (♦), and 72 (×) hours. Cell-cycle progression was determined by propidium iodide staining and is represented as the percentage of cells in S + G2/M phase by using multicycle flow cytometry software. To study the effect of IDV on anti-Fas–induced apoptosis in Jurkat and PM1 T cells, cells were cultured without or with anti-Fas mAb CH11 (100 ng/mL) in presence of different concentrations of IDV, and apoptosis was determined at 2 (▵), 6 (■), and 18 (●) hours. Cells were also precultured with different concentrations of IDV for 18 hours, and apoptosis was induced by culturing these cells with CH11 for 6 (▪) and 18 (●) hours. Data are shown for mean ± SD of the percentage of cells undergoing apoptosis as estimated by propidium iodide staining.
Effect of IDV on cell-cycle progression.
Cell-cycle progression of Jurkat (A) and PM1 T (B) cells and apoptosis in Jurkat (C) and PM1 (D) T-cell lines. For cell-cycle analysis Jurkat and PM1 T cells were cultured with different concentrations of IDV for 24 (⋄), 48 (♦), and 72 (×) hours. Cell-cycle progression was determined by propidium iodide staining and is represented as the percentage of cells in S + G2/M phase by using multicycle flow cytometry software. To study the effect of IDV on anti-Fas–induced apoptosis in Jurkat and PM1 T cells, cells were cultured without or with anti-Fas mAb CH11 (100 ng/mL) in presence of different concentrations of IDV, and apoptosis was determined at 2 (▵), 6 (■), and 18 (●) hours. Cells were also precultured with different concentrations of IDV for 18 hours, and apoptosis was induced by culturing these cells with CH11 for 6 (▪) and 18 (●) hours. Data are shown for mean ± SD of the percentage of cells undergoing apoptosis as estimated by propidium iodide staining.
IDV does not inhibit caspase activity
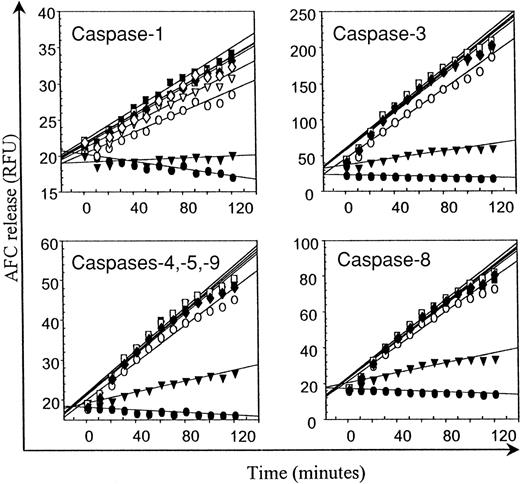
The activity of caspases-1, -3, -4, -5, -9, and -8 over 120 minutes in lysates of CH11 mAb-treated Jurkat T cells as demonstrated by release of AFC from caspase-specific fluorogenic substrates is illustrated in Figure 6. IDV at concentrations of 12.5 to 100 μM did not inhibit the activity of any of the caspases, whereas addition of caspase-specific inhibitors resulted in the expected inhibition of caspase activity.
Effect of IDV on caspases-1, -3, -4, -5, -8, and -9.
Cell lysates from apoptotic Jurkat T cells were preincubated with different concentrations of IDV (▿, 12.5 μM; ■, 25 μM; ▪, 50 μM; and ♦, 100 μM) or with 100 μM of specific caspase inhibitors (▾) followed by assay for caspase activity as described in the “Materials and methods” section by the release of 7-amino-4-trifluoromethyl coumarin, expressed as relative fluorescence units (RFU), from caspase-specific fluorogenic substrates over a 2-hour period. Controls consisted of wells without lysates (●) and of lysates without inhibitors (○).
Effect of IDV on caspases-1, -3, -4, -5, -8, and -9.
Cell lysates from apoptotic Jurkat T cells were preincubated with different concentrations of IDV (▿, 12.5 μM; ■, 25 μM; ▪, 50 μM; and ♦, 100 μM) or with 100 μM of specific caspase inhibitors (▾) followed by assay for caspase activity as described in the “Materials and methods” section by the release of 7-amino-4-trifluoromethyl coumarin, expressed as relative fluorescence units (RFU), from caspase-specific fluorogenic substrates over a 2-hour period. Controls consisted of wells without lysates (●) and of lysates without inhibitors (○).
Discussion
Combination therapies with HIV PIs have had a dramatic effect on the survival of HIV-infected patients.27,28 An intriguing observation is that, in a subset of patients, the increase in CD4 T cells and the decrease in activation status of T cells can be sustained, despite virologic relapse after development of drug resistance.6,7,29 It has recently been suggested that PIs can directly inhibit lymphocyte apoptosis and that this effect may contribute to an immunologic benefit independently of an antiviral effect.17-19 In the present study we demonstrate that the effects of the PI, IDV, are broad ranging and that at therapeutic concentrations IDV can inhibit entry of cells into cell cycle. The drug was found to inhibit cell proliferation but had no direct antiapoptotic activity nor did it inhibit cellular caspases involved in apoptosis. These findings, and the known effects of HIV proteases and HIV PIs on cellular proteases and proteasomes,21,22 30-32 implicate PIs in potentially modulating a wide range of immunologic responses and provide insight into a mechanism for promoting cell survival, without directly influencing cell death.
A novel finding in this study was that IDV blocks cells from entering into cell cycle. This effect was reflected in the inhibition of cell proliferation following activation stimuli. In fact, IDV was found to inhibit 14C-thymidine uptake in cells activated through 3 different pathways: direct T-cell receptor cross-linking using anti-CD3 mAbs, indirect T-cell activation induced by mitogens such as PHA and ConA, as well as activation induced by pharmacologic means using PMA plus Ionomycin. These findings are in agreement with the previously reported antiproliferative effects of IDV reported for myeloblastic and promyelocytic leukemia cell lines.23 The broad-based antiproliferative effect favors the likelihood of inhibition of proteasomal activity,21,22 or modulation of cytokine production.33-36 Proteasomes are the main proteolytic complexes operating in the cytosol and nucleus and are involved in many biological and degradative processes.37,38 The PI, Ritonavir, has been shown to block the chymotrypsin-like activity of murine 20S proteasome.21,22 Proteasomes control the cell cycle by proteolytic degradation of several cell-cycle regulatory proteins such as cyclins, cyclin-dependent kinases, and their inhibitors.37,38 Additionally, PIs have been reported to influence cytokines that regulate lymphocyte proliferation.33-36,39,40 It has been shown that the serum levels of interleukin 16 (IL-16) are increased in patients on IDV-containing drug regimens.35,36 IL-16 is known to inhibit anti-CD3–induced lymphocyte activation and lymphoproliferation.39 40 These observations, together with our findings, suggest that IDV may be blocking cell-cycle progression in lymphocytes, possibly by enhancing the levels of IL-16 production in association with the blocking of proteasomal activity.
Recent clinical investigations, including our own studies and those of several other groups, have shown that following potent antiretroviral therapy a decrease in virus load is accompanied by an increase in CD4 T-cell counts and decreased T-cell apoptosis.10,11,41-43Accelerated lymphocyte apoptosis is a well-known feature of HIV disease.8,9,44,45 The increased apoptosis involving CD4 and CD8 T cells in HIV infection has been explained on the basis of host and viral factors.9,44 Host factors implicated include imbalance in cytokine production and immune activation following HIV infection that results in increased expression of death receptors and ligands.45 Viral factors include viral proteins (gp120, vpr, tat, nef, and protease), all of which have been shown to influence lymphocyte apoptosis in vitro.46-50Thus, the decrease in lymphocyte apoptosis in patients on antiretroviral therapy has been attributed to the rescue of cells from the direct and indirect effects of HIV. Additionally, the PIs themselves have been ascribed to have antiapoptotic properties. Although the activity of PIs is aimed at viral proteases, it has been hypothesized that they can influence host cellular proteases (viz, caspases) because of the similarity shared by these proteases at their active sites. Ritonavir has been reported to inhibit apoptosis in PBMCs cultured from HIV− donors,19 from HIV+ donors,17 and in CD34 cells18 and also has been shown to inhibit caspase activity.17-19 In our studies reported herein, the in vitro addition of IDV did not inhibit the spontaneous or anti-CD3–induced apoptosis in PBMCs of healthy volunteers and of HIV-infected children or the apoptosis induced through Fas pathway in Jurkat and PM1 T-cell lines. We also did not find evidence for inhibition of specific caspases involved in the apoptosis cascade. In agreement with these findings, Lu and Andrieu20 have reported that neither T-cell receptor or CD3 ligation nor Fas-triggered apoptosis was affected by IDV in PBMCs from healthy volunteers or HIV-infected donors.
The differences in our findings from the reported inhibition of lymphocyte apoptosis and of caspase activity by PIs17-19can be explained on the basis of the difference in experimental systems and in data interpretation. A major difference is that we used short-term exposure of lymphocytes to IDV (18 hours), whereas others17-19 have used longer culture periods (3-15 days) with Ritonavir prior to lymphocyte activation. Thus, in cells cultured for longer duration with a PI, fewer cells would be cycling because PIs block their entry into cell cycle. It has been shown that cells that have undergone multiple rounds of cell division are more susceptible to apoptosis51 (Chavan et al, unpublished observations, 2000). Thus, cells cultured for prolonged periods with a PI would be expected to exhibit apparently reduced apoptosis as compared with cells cultured in the absence of the PI. These cells would also be expected to show less caspase activity, as reported by Sloand et al17,18 and Weichold et al19 even without a direct effect of the PI on caspases, as shown in our study. The cell-cycle inhibitory effect of PIs could also be a factor responsible for the smaller increase of apoptosis following anti-CD3 stimulation in cells of patients on PI therapy as compared with patients not on PI therapy. Collectively, these studies indicate that IDV may not have a direct effect on apoptosis but may prolong cell survival by inhibition of cell-cycle progression. The difference in the molecular structure of IDV and Ritonavir is a less likely explanation for differential effects on lymphocyte apoptosis. In agreement with this contention we have found that another PI, Nelfinavir, has effects similar to IDV on cell-cycle progression without direct antiapoptotic activity (Chavan et al, unpublished observations, 2000).
The inhibition of cell-cycle progression and antiproliferative property of IDV could affect the host/virus relationship in various ways, as well as modulate immune responses in general. Potential adverse effects of IDV include inhibition of clonal expansion of the cells of the immune system.52 PIs are known to have unexplained toxicities such as abnormal fat distribution and lipodystrophy, insulin-resistant diabetes, and hypercholesteremia.53-56Inhibition of cell-cycle progression by IDV may be a potential factor contributing to the increased differentiation of lipid-producing adipose tissues,53,54 as cell-cycle blocking is essential for differentiation of adipose cells. Interestingly, the effect of IDV on cell cycle was seen only in primary PBMC cultures, and IDV was unable to block continuously cycling cells such as Jurkat and PM1. Thus, inhibition of immune activation may be restricted to newly recruited cells. This property of PIs may in fact be beneficial to the host in terms of host/virus interaction; a decrease in activation of the immune system may prevent the spread of the virus and T-cell–mediated immunopathogenesis in HIV infection. It has been well documented that HIV replication is supported mainly in cycling cells.57,58 HIV-specific proteins such as vpr block the cells in G2 phase that the virus uses to its advantage to increase the synthesis of viral proteins, and thereby increases its replication.59 Blocking of the cells in G0/G1 phase by PIs could result in reduced viral production of wild-type as well as drug-resistant mutant virus strains, thereby contributing to persistent benefit despite development of viral resistance mutations observed in different clinical cohorts.31 Additionally, as reported by Ikezoe et al,23 this property might be useful for treatment of patients with acute promyelocytic leukemia with PIs. Elucidation of mechanisms by which PIs block cell-cycle progression and exert antiproliferative activity will provide insight into the full effect of PI therapy on the immunopathogenesis of HIV disease and open new vistas for disease targets for this class of agents.
Supported by grants AI28281 and DA05161 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Savita Pahwa, Division of Allergy/Immunology, North Shore University Hospital, NYU School of Medicine, 350 Community Dr, Research Bldg #303, Manhasset, NY 11030; e-mail: spahwa@nshs.edu.

![Fig. 2. Effect of IDV on lymphoproliferation. / PBMCs from healthy volunteers were preincubated without or with different concentrations of IDV for 18 hours followed by activation with different stimuli for an additional 48 hours. Proliferation was measured with [14C]-thymidine incorporation added at 1 μCi/well for the last 48 hours. Results of [14C]-thymidine incorporation in IDV-treated cultures are expressed as the percentage of responses of cells cultured without IDV. Data represents mean ± SD for 1 of 5 representative experiments: ♦, medium alone; ●, anti-CD3 (0.5 μg/mL); ▴, PHA (2 μg/mL); ▪, PMA (10 ng/mL); + Ionomycin (1 μM); and ○, Con A (2 μg/mL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.383/5/m_h81411295002.jpeg?Expires=1765903781&Signature=wGnD8-TH~3~HL2zY8zKzpcPh0e02v8jDuqDWmHYg~~jHLwEepfKI~133f~z9F4GzKCpXivMlP~9Ec3jHsd4k0Myar764eaknWVRQwIQ1W3XJb-rl35h6CP-40hrZlV2K384mf5nDt-r0cGjbEeibUFkWrEm76LlvPQbrCoV9UnAZ4WUZQQ4naWjwuYZmaN1DYpe5kgtgJO65zw15pTbOSIDGPXVnPS27G6CdH9vy~W~pQqxOIOpY8nJdZRn92X2iIGZE2Os1SpeOoCPvWxA1QIAaIpzCg1fzqPED0vRBWmPUtDjpuwfLn1Fj0n-w1YasWKFfOsdV~ZPVRQGrfCRZBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal