Patients undergoing full haplotype-mismatched hematopoietic transplantations may experience severe intractable invasive fungal infections. To verify whether an imbalanced production of T-helper 1 (TH1) and TH2 cytokines may be responsible for susceptibility to fungal infections, C3H/HeJ (H-2k) recipient mice were lethally irradiated, received transplantations with T-cell–depleted allogeneic bone marrow (BM) cells from mice ofH-2d haplotype, and were infected withCandida albicans. At different time-points after transplantation, mice were assessed for pattern of TH cytokine production and susceptibility to infection. The results show that a long-term, donor-type chimerism was achieved as early as 2 weeks after BM transplantation (BMT), at the time when high-level production of TH2 cytokines (interleukin-4 [IL-4] and IL-10) and impaired production of TH1 cytokines (interferon-γ [IFN-γ] and IL-12] were observed. At this time, mice were highly susceptible to both disseminated and mucosal infections, as indicated by decreased survival, uncontrolled fungal growth, and failure to develop protective TH1 immunity. However, a predominant production of TH1 cytokines was observed by week 5 after BMT, at the time when mice developed donor-type protective TH1 responses and were resistant to infections. Therapeutic ablation of IL-4 or IL-10 greatly increased resistance to candidiasis. These results indicate that a dysregulated production of TH cytokines occurs in mice undergoing T-cell–depleted allogeneic BMT. The transient predominant production of TH2 cytokines over that of IL-12 impaired the ability of mice to develop antifungal TH1 resistance, an activity that could be efficiently restored upon treatment with TH2 cytokine antagonists.
Introduction
Invasive fungal infections remain a major problem in bone marrow transplantation (BMT) recipients.1,2Infections may occur in a bimodal distribution, which implies that host defects predisposing to fungal infections are different in the different phases of BMT.1 2
Neutropenia is acknowledged as one of the most important predisposing factors to invasive fungal infections,1,2 as highlighted by the efficacy of immunotherapies aimed at restoring neutrophil antifungal activity.3 However, the occurrence of fungal infections in patients with adequate or even normal neutrophil counts4,5 suggests that factors other than neutropenia contribute to fungal susceptibility in the BMT setting. These observations, together with the notion that the occurrence of graft-versus-host disease (GVHD) and its therapy is associated with increased risk of fungal infections,2 suggest that a defective T-helper (TH)–dependent immunity may also contribute to the susceptibility to fungal infections in BMT.
Clinical evidence and experimental data indicate that both the innate and the adaptive immune systems regulate resistance to Candidainfections.6-8 In murine experimental models of infection, it has been demonstrated that TH-cell reactivity plays a central role in regulating immune responses to the fungus: TH1 reactivity is responsible for resistance, and TH2 reactivity is associated with susceptibility.8-10 Among parameters influencing antifungal CD4+ TH-cell development, both the initial handling of fungal pathogen by cells of the innate immune system and cytokine production play an important role.8-10 Indeed, quantitative or qualitative defects of antifungal effector and immunoregulatory functions of phagocytic cells result in the development of anticandidal TH2, rather than TH1, cell responses.7 Moreover, the development of protective anticandidal TH1 responses requires the concerted actions of several cytokines, including interferon-γ (IFN-γ) and interleukin-12 (IL-12), in the relative absence of TH2 cytokines, such as IL-4 and IL-10, which inhibit development of TH1 responses.8-10Early in infection, neutralization of TH1 cytokines (IFN-γ and IL-12) leads to the onset of TH2 rather than TH1 responses, whereas neutralization of TH2 cytokines (IL-4 and IL-10) allows for the development of TH1 rather than TH2 responses.8-10
Full haplotype-mismatched hematopoietic transplantations are employed for treatment of bad-risk leukemia patients who lack a matched donor.11-13 However, also due to delayed immunoreconstitution, patients may experience severe, often fatal infections caused by Candida and Aspergillusspp.14
To understand whether an imbalanced production of TH1 and TH2 cytokines may be responsible for the observed susceptibility to fungal infections in this BMT setting, we resorted to a well-characterized mouse model of T-cell–depleted allogeneic BMT.15,16 In this model, autologous reconstitution of host stem cells is greatly reduced to the benefit of a long-term, donor-type chimerism in more than 95% of the mice.15,16 Moreover, the low incidence of GVHD12,13,16 allowed us to directly assess the pattern of antifungal TH-cell development in the relative absence of a biased TH reactivity eventually superimposed by the occurrence of GVHD17 and its prophylaxis.
To address the issue, lethally irradiated C3H/HeJ mice, reconstituted with T-cell–depleted allogenic BMT, were assessed for susceptibility to C albicans and parameters of antifungal TH immunity. At different time-points following BMT, production of TH1 and TH2 cytokines was different, and this correlated with the pattern of resistance and susceptibility to infection. The dysfunctional production of TH cytokines contributed to the impaired ability of mice to develop antifungal protective TH1 immunity, an activity that could be efficiently restored upon treatment with TH2 cytokine antagonists.
Materials and methods
Mice
We used 8- to 10-week-old inbred C3H/HeJ, BALB/c, and hybrid (BALB/c × DBA/2)F1 (CD2F1) female mice (Charles River Breeding Laboratories, Calco, Italy). All mice were kept in small sterile cages (5 animals in each cage) and fed with sterile food and water. Breeding pairs of homozygous IL-4–deficient (IL-4 KO)7 and IL-12p40–deficient (IL-12 KO) mice,7 raised on BALB/c background, were bred under specific pathogen-free conditions at the breeding facilities of the University of Perugia, Perugia, Italy. Mice of both sexes, 8- to 10-weeks old, were used for the experiments. Procedures involving animals and their care were conducted in conformity with national and international laws and policies.
Irradiation
C3H/HeJ mice were exposed to a single lethal dose of 9 Gy from an 18-mV photon beam linear accelerator (Clinac 600/C Varian; Cernusco, Milano, Italy) with focus to skin distance of 75 cm and dose per rate of 0.7 Gy per minute.15 Without BMT, the mice died within 14 days.
Preparation of T-cell–depleted BM cells
BM cells were prepared as described elsewhere, with minor modifications.15 Donor BM cells were collected into phosphate-buffered saline (PBS) by flushing the shafts of the femur and tibia. The cells were suspended, and clumps of debris were allowed to settle out. The cells were washed 3 times with PBS and resuspended at a final concentration of 3 × 108 cells per mL. The cells were then fractionated by differential agglutination with soybean agglutinin, as described elsewhere.15 Briefly, the BM cell suspension was incubated in polystyrene tubes with 2 mg/mL soybean agglutinin for 5 minutes at room temperature. The cells were gently layered on top of 5% bovine serum albumin (BSA) solution in 8 mL PBS in 15-mL conical tubes. After 15 minutes at room temperature, the cells remaining on the surface of the albumin were removed, whereas the sedimented cells were washed in 1% BSA solution in 10 mL PBS and then suspended in 0.2 M D-galactose in 10 mL PBS.
After 10 minutes at room temperature the cells were collected by centrifugation at 200g for 5 minutes and were washed twice with D-galactose to dissociate all aggregates into single cells. Finally, the T-cell–depleted soybean agglutinin–positive cells, containing less than 1% contaminating T cells on fluorescence-activated cell sorter (FACS) analysis, were washed twice with PBS and resuspended at the final concentration of at least 4 × 106 cells per mL saline. The cells were injected into recipient mice intravenously via the lateral tail vein in a volume of 0.5 mL. According to previous studies,16 more than 95% of the mice survived, showing stable donor-type hematopoietic chimerism, as revealed by donor-type major histocompatability complex (MHC) class I antigen expression on cells from spleens.
Fungi and infections
The origin and characteristics of the low-virulence live vaccine strain of C albicans PCA-2 and the highly virulent CA-6 strain used in this study have been previously described.18,19 For primary disseminated C albicans infection, 106 PCA-2 or CA-6 cells were injected intravenously in a volume of 0.5 mL saline per mouse. Fourteen days later, PCA-2–infected mice were assessed for resistance to secondary infection by intravenous injection of 106 CA-6 cells. For gastrointestinal infection 108 CA-6 cells were injected intragastrically via an 18-gauge 4-cm-long plastic catheter in a volume of 0.2 mL saline per mouse, as described elsewhere.19 Quantification of fungal growth in the organs of infected mice (4-6 mice per group) was performed by plating serial dilutions of homogenized organs in Sabouraud dextrose agar, and the results (mean ± SEM) were expressed as colony forming units (CFUs) per organ. Mice succumbing to fungal challenge were routinely necropsied for histopathological confirmation of candidiasis.
In vivo analysis and treatments
Total and differential white blood cell counts were done by hemocytometry and by staining blood smears from mice that received transplantations with May-Grünwald-Giemsa reagents (Sigma Chemical, St Louis, MO) before analysis. Treatments with cytokine antagonists were completed either the day of BMT or the day of primary PCA-2 infection and then 1, 3, and 5 days later. According to preliminary experiments indicating the optimal dose and schedule for each treatment, mice were treated intraperitoneally with 80 μg per dose of soluble IL-4 receptor (sIL-4R) (Immunex, Seattle, WA) or 200 μg per dose of anti–IL-10R monoclonal antibody (mAb) 1B1.3A.20 Control mice received vehicle alone.
Candidacidal activity
For the candidacidal assay, peripheral blood neutrophils, obtained by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation, were plated at 1 × 106 cells per 100 μL per well in 96-well flat-bottomed microtiter plates with 1 × 105C albicans yeasts per 100 μL per well for one hour, as described elsewhere.21 The percentage of CFU inhibition (mean ± SE) was determined as a percentage of colony formation inhibition equal to 100 − (CFU experimental group/CFU control cultures) × 100.
Flow cytometry
For chimerism analysis, erythrocyte-depleted spleen cells were reacted with fluorescein isothiocyanate (FITC)–conjugated mAb reacting to donor H-2Dd MHC class I antigen or to hostH-2Kk MHC class I antigen, and donor-type chimera were recorded when more than 30% of the cells were positive with anti–H-2Dd antibody, as described elsewhere.16 For lymphocyte analysis, splenocytes were reacted with FITC-conjugated anti-CD3 (clone 145-2C11), anti-CD45R/B220 (clone RA3-6B2), anti-CD4 (L3T4, clone RM4-5), or anti-CD8α (Ly-2, clone 53-6.7) mAbs (all from PharMingen, San Diego, CA). For double-staining, splenocytes were sequentially reacted with saturating amounts of FITC-conjugated anti-CD4 mAb and phycoerythrin (PE)-conjugated anti-CD44 (Pgp-1, clone IM7) or PE-conjugated anti–L-selectin (clone MEL-14) (PharMingen). Cells were analyzed with a FACScan flow cytofluorometer (Becton Dickinson, Mountain View, CA). Nonviable cells were excluded from analysis by accepted procedures involving propidium iodide and narrow forward-angle light scatter gating. Control staining of cells with irrelevant antibody was used to obtain background fluorescence values. Data are expressed as a percentage of positive cells over total cells analyzed.
Collection of fecal samples
Fecal samples were obtained from the mice infected intragastrically with C albicans, as described elsewhere.19 Briefly, 0.1 g fecal sample was incubated with 1 mL PBS at room temperature for 15 minutes, vortexed, left for 15 minutes more, revortexed until all material was suspended, and centrifuged at 2000 rpm for 10 minutes. Supernatants were removed, filtered throughout a 0.2-μm filter, and stored at −80°C until assayed for cytokine contents.
Culture of cells
Unfractionated splenocytes (5 × 106 cells per mL) were cultured in complete medium (Roswell Park Memorial Institute [RPMI] medium containing 10% fetal calf serum, 50 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 10 mM HEPES [4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid], and 50 μg/mL gentamycin) in the presence of 10 μg/mL Concanavalin A (Sigma) to determine IFN-γ, IL-4, IL-10, and IL-12p70 production. After a 48-hour culture, supernatants were harvested, and cytokine production was determined by specific enzyme-linked immunosorbent assays (ELISAs).
Cytokine assays
The levels of IFN-γ, IL-4, and IL-10 were determined by cytokine-specific ELISAs using pairs of anticytokine mAbs, as described elsewhere.18,19 The mAb pairs (PharMingen) used are listed by capture/biotinilated detection as follows: IFN-γ and R4-6A2/XMG1.2; IL-4 and BVD4-1D11/BVD6-24G2; and IL-10 and JES5-2A5/SXC-1. For IL-12p70, the rat antimouse IL-12p70 mAb, clone 48110.111, was used as the capture reagent, and a goat biotinylated antimouse IL-12 antibody (R&D Systems, Minneapolis, MN) was used as the detecting reagent.22 Cytokine titers were calculated by reference to standard curves, constructed with known amounts of recombinant cytokines (PharMingen; IL-12p70, R&D Systems).
RNA preparation and reverse transcriptase–polymerase chain reaction
RNA extraction and amplification of synthesized complementary DNA (cDNA) from spleen cells were performed as previously described.21,22 For hypoxanthine-guanine phosphoribosyl transferase (HPRT), IFN-γ, IL-4, IL-10, IL-12p40, IL-12Rβ1 and IL-12Rβ2 chains, the primers, positive controls, cycles, and temperature are described elsewhere.21 The HPRT primers were used as a control for both reverse transcriptase (RT) and the polymerase chain reaction (PCR) itself and also for comparing the amount of products from samples obtained with the same primer. The PCR fragments were analyzed by 1.5% agarose gel electrophoresis and visualized by ethidium-bromide staining. PCR-assisted messenger RNA (mRNA) amplification was repeated at least twice for at least 2 separately prepared cDNA samples for each experiment. Data are representative of at least 3 different experiments.
Statistical analysis
Survival data were analyzed using the Mann-Whitney test; significance was defined as P < .05. The Studentt test was used to determine statistical significance between organ clearance, antifungal activity, or cytokine production. In vivo groups consisted of 4-6 animals. The data reported were pooled from 3-5 experiments.
Results
Leukocyte recovery following T-cell–depleted allogeneic BMT
Recipient C3H/HeJ mice were lethally irradiated the day before injection of at least 2 × 106 T-cell–depleted BM cells from donor CD2F1 mice. Total and differential counts of blood leukocytes and phenotypic analysis of splenic lymphocytes were completed at different weeks after transplantation. The results indicate that the absolute number of circulating lymphocytes progressively increased after BMT to a complete recovery at week 5, whereas the number of neutrophils increased progressively during the first 2 weeks and then dropped to the levels of donor mice by week 5. The number of monocytes did not show significant variations throughout the experimental period (data not shown).
FACS analysis of lymphocytes from spleens indicates that 2 weeks after BMT, more than 60% of splenic lymphocytes were B220+cells, while the percentages of CD3+CD4+ and CD3+CD8+ cells were very low. However, the cellular proportions of the donor were almost re-established by week 5, at the time when the percentage of B220+ cells decreased and the percentage of CD3+CD4+ and CD3+CD8+ cells increased. On assaying levels of CD44 and MEL-14 expression on CD4+ T cells, it was found that 2 weeks after BMT, most of the cells expressed the activated phenotype, ie, CD44high and MEL-14low, whereas the nonactivated phenotype of the donor was reestablished at 5 weeks (Figure 1).
Phenotypic analysis of splenic lymphocytes following T-cell–depleted allogeneic BMT.
Lethally irradiated C3H/HeJ mice each received a transplantation with ≥ 2 × 106 T-cell–depleted allogenic BM cells from CD2F1 mice a number of weeks before phenotypic analysis. Splenocytes were reacted with FITC-conjugated anti-CD3, anti-CD45R/B220, anti-CD4, or anti-CD8 mAbs or, for double-staining, with FITC-conjugated anti-CD4 mAb followed by PE-conjugated anti-CD44 or anti–L-selectin (clone MEL-14) mAb. Cells were analyzed with a FACScan flow cytofluorometer, and the data were expressed as percentage of positive cells over total cells analyzed.
Phenotypic analysis of splenic lymphocytes following T-cell–depleted allogeneic BMT.
Lethally irradiated C3H/HeJ mice each received a transplantation with ≥ 2 × 106 T-cell–depleted allogenic BM cells from CD2F1 mice a number of weeks before phenotypic analysis. Splenocytes were reacted with FITC-conjugated anti-CD3, anti-CD45R/B220, anti-CD4, or anti-CD8 mAbs or, for double-staining, with FITC-conjugated anti-CD4 mAb followed by PE-conjugated anti-CD44 or anti–L-selectin (clone MEL-14) mAb. Cells were analyzed with a FACScan flow cytofluorometer, and the data were expressed as percentage of positive cells over total cells analyzed.
Susceptibility to C albicans infection following T-cell–depleted allogeneic BMT
To assess susceptibility to disseminated candidiasis following T-cell–depleted allogeneic BMT, recipient mice were lethally irradiated and infused with T-cell–depleted BM cells from donor CD2F1 mice, a strain known to be able to develop either an antifungal TH1 or TH2 type of response depending on the experimental conditions.7 Mice were intravenously infected at different weeks after transplantation, with either the low-virulent strain ofC albicans PCA-2 or the virulent C albicans CA-6. For secondary infection, PCA-2–infected mice were reinfected with virulent C albicans CA-6 2 weeks later. Resistance or susceptibility to reinfection in donor mice reflects TH1 or TH2 immune reactivity, respectively.7
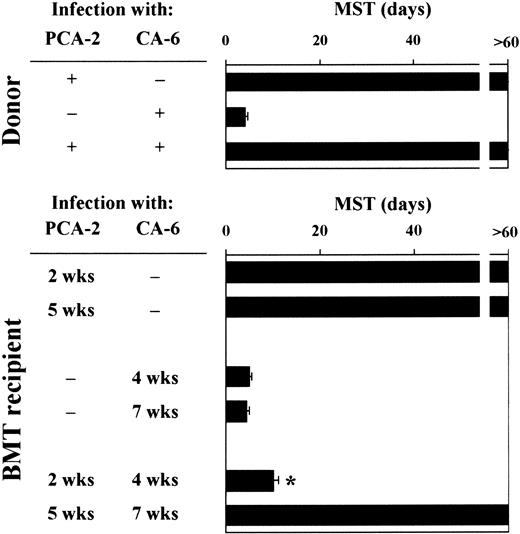
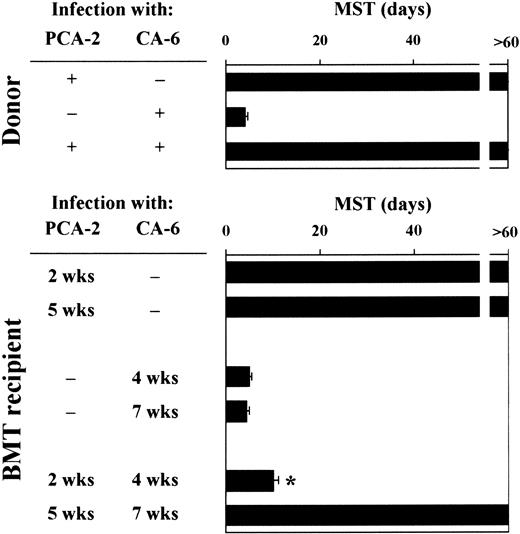
Mice were monitored for survival to primary and secondary infections. Similar to donor mice, animals that received a transplantation survived the primary infection with PCA-2 and succumbed to the primary infection with CA-6, thus suggesting that the susceptibility to primary infections with either low-virulent or virulent Candidacells was not modified following T-cell–depleted allogeneic BMT (Figure 2). Upon re-infection, however, recipient mice who had received the primary PCA-2 infection 2 weeks after BMT did not resist reinfection with CA-6. In contrast, similar to donors, recipient mice who had received the primary PCA-2 infection 5 weeks after BMT resisted reinfection (Figure 2). Therefore, susceptibility or resistance to reinfection in T-cell–depleted allogeneic BMT varies at different time-points after transplantation. Because mice were equally susceptible to virulent CA-6 at the same time-points, these findings suggest that the different susceptibility to virulent fungal challenge seen between uninfected and PCA-2–infected mice are not due to a primary difference between effector cells at different times after transplantation. Thus, immunoregulatory circuits may exist that exert control over the expression of antifungal resistance.
Susceptibility to primary or secondary disseminatedC albicans infection following T-cell–depleted allogeneic BMT.
For primary infection, BMT recipient mice were infected intravenously with 106 low-virulent PCA-2 C albicans or high-virulent CA-6 at different weeks after transplantation. For secondary infection, PCA-2–infected mice were reinfected with 106 virulent CA-6 C albicans 2 weeks after the primary infection. MST indicates median survival time in days. *Indicates P < .05 (BMT mice vs donor mice).
Susceptibility to primary or secondary disseminatedC albicans infection following T-cell–depleted allogeneic BMT.
For primary infection, BMT recipient mice were infected intravenously with 106 low-virulent PCA-2 C albicans or high-virulent CA-6 at different weeks after transplantation. For secondary infection, PCA-2–infected mice were reinfected with 106 virulent CA-6 C albicans 2 weeks after the primary infection. MST indicates median survival time in days. *Indicates P < .05 (BMT mice vs donor mice).
TH1 and TH2 cytokine production following T-cell–depleted allogeneic BMT
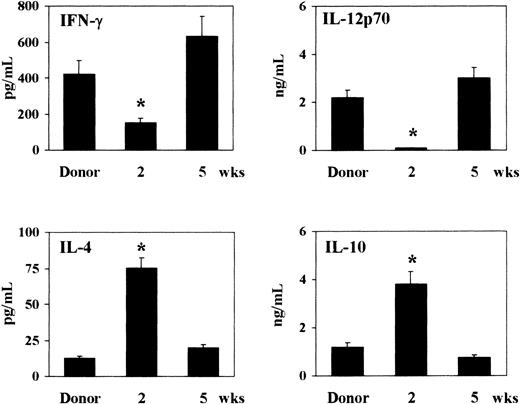
As the cytokine milieu greatly influences the pattern of susceptibility and resistance to murine candidiasis,8,17 23 we assessed the pattern of TH1 (IFN-γ and IL-12p70) and TH2 (IL-4 and IL-10) cytokine production following T-cell–depleted allogeneic BMT. To this purpose, 2 or 5 weeks after BMT, total splenocytes from recipient or donor mice were mitogen-stimulated in vitro for 48 hours before assessing cytokine contents by specific ELISAs. Two weeks after transplantation, high levels of IL-4 and IL-10 and low levels of IFN-γ and IL-12p70 were observed in mice that received transplantations as opposed to donor mice. On the contrary, 5 weeks after transplantation, production of IFN-γ and IL-12p70 was increased, and production of IL-4 and IL-10 decreased, reaching the levels of donor mice (Figure3). Thus, a predominant production of TH2 cytokines occurs 2 weeks after T-cell–depleted allogeneic BMT.
Pattern of TH1 (IFN-γ and IL-12p70) and TH2 (IL-4 and IL-10) cytokine production following T-cell–depleted allogeneic BMT.
At 2 and 5 weeks after transplantation, recipient C3H/HeJ mice, irradiated and reconstituted as in Figure 1 legend, were assessed for cytokine production by means of specific ELISAs. Cytokines were assessed in culture supernatants of splenocytes stimulated with mitogen for 48 hours. *Indicates P < .05 (BMT mice vs donor mice).
Pattern of TH1 (IFN-γ and IL-12p70) and TH2 (IL-4 and IL-10) cytokine production following T-cell–depleted allogeneic BMT.
At 2 and 5 weeks after transplantation, recipient C3H/HeJ mice, irradiated and reconstituted as in Figure 1 legend, were assessed for cytokine production by means of specific ELISAs. Cytokines were assessed in culture supernatants of splenocytes stimulated with mitogen for 48 hours. *Indicates P < .05 (BMT mice vs donor mice).
Antifungal TH-cell development following T-cell–depleted allogeneic BMT
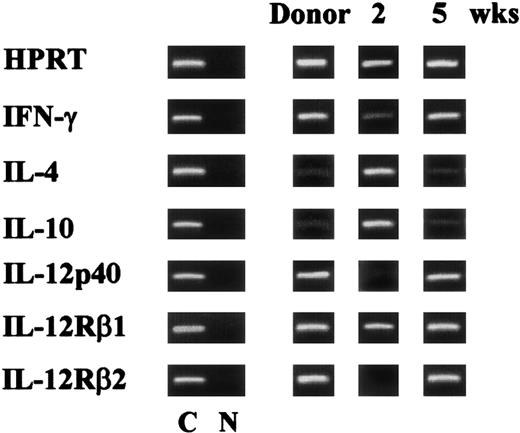
Protective and nonprotective acquired immunity to C albicans correlates with the induction of CD4+ TH1 and TH2 responses, respectively.7 To assess the pattern of antifungal TH1 and TH2 reactivity in T-cell–depleted allogeneic BMT, recipient mice were assessed for TH cytokine gene expression upon reinfection. As the expression of the IL-12Rβ2 chain correlates with TH1 reactivity in mice with fungal infections,7 the IL-12Rβ2 mRNA was also assessed. The results clearly indicate that the increased susceptibility to reinfection of mice infected with PCA-2 at 2 weeks after BMT correlates with a decreased expression of both IFN-γ and IL-12 and an increased expression of IL4 and IL10 genes. Moreover, the IL-12Rβ2 chain RNA message was undetectable (Figure 4). An opposite pattern of cytokine and cytokine receptor gene expression was observed upon reinfection of recipient mice receiving the primary infection at 5 weeks after BMT. Similar to what was observed in donor mice, mRNAs of TH1 cytokines and IL-12Rβ2 were present, while those of TH2 cytokines were barely detectable (Figure 4).
Pattern of antifungal TH reactivity following T-cell–depleted allogeneic BMT.
Cytokine and cytokine receptor genes expression in splenocytes from recipient C3H/HeJ mice, irradiated and reconstituted as in Figure 1legend, intravenously infected with PCA-2 at different weeks after transplantation, and reinfected with CA-6 as in Figure 2 legend. At 3 days after reinfection, mice were assessed for cytokine gene expression by RT-PCR. C indicates HPRT- or cytokine-specific control. N indicates no DNA added to the amplification mix during PCR.
Pattern of antifungal TH reactivity following T-cell–depleted allogeneic BMT.
Cytokine and cytokine receptor genes expression in splenocytes from recipient C3H/HeJ mice, irradiated and reconstituted as in Figure 1legend, intravenously infected with PCA-2 at different weeks after transplantation, and reinfected with CA-6 as in Figure 2 legend. At 3 days after reinfection, mice were assessed for cytokine gene expression by RT-PCR. C indicates HPRT- or cytokine-specific control. N indicates no DNA added to the amplification mix during PCR.
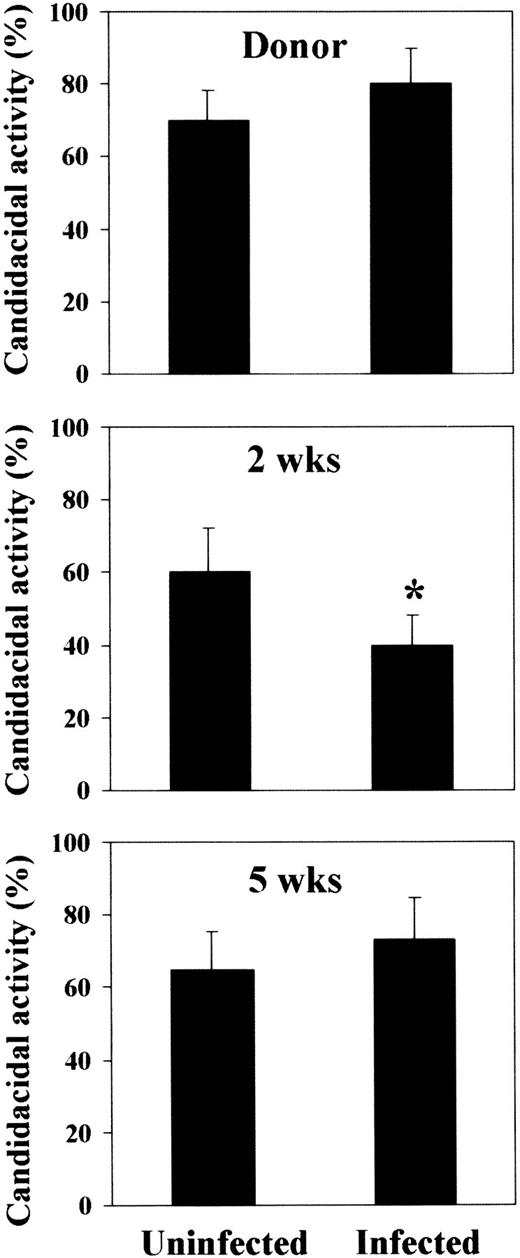
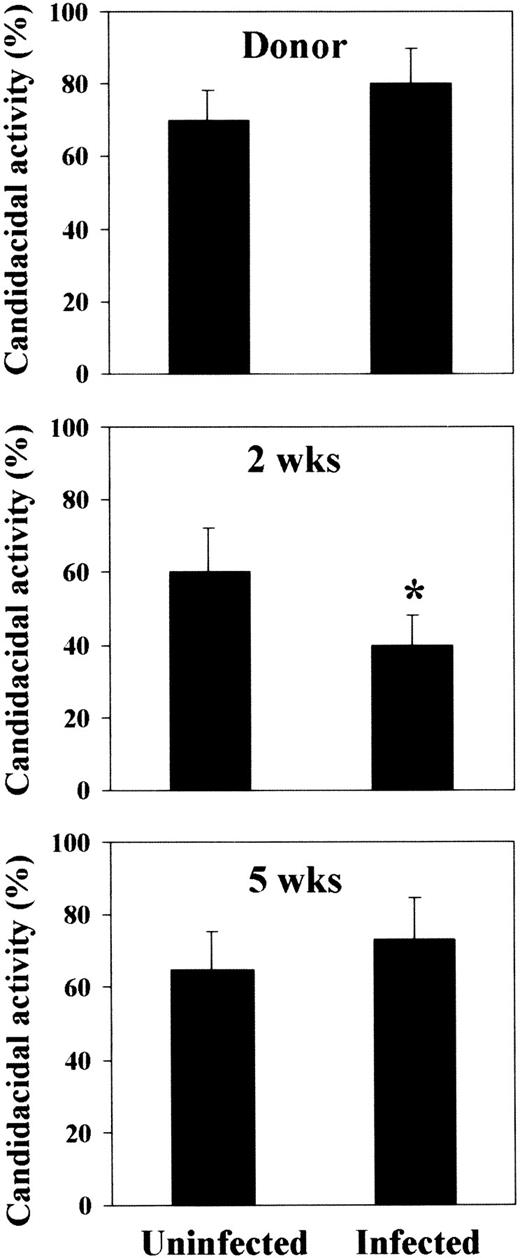
As the delivery of activating signals by TH1 cells results in the activation and mobilization of fungicidal phagocytes,7 23the effector antifungal activity of neutrophils was also assessed. It was found that this activity was constantly elevated in recipient mice at any time-point after BMT, a finding in line with the observed resistance to PCA-2 infection. However, the candidacidal activity was impaired in mice susceptible to reinfection, but not in mice resistant to it (Figure 5). These results indicate that susceptibility and resistance to infection following T-cell–depleted allogeneic BMT are associated with the occurrence of TH1 or TH2 cell responses, respectively, that oppositely regulate the antifungal activity of effector cells.
Candidacidal activity of neutrophils following T-cell–depleted allogeneic BMT.
Peripheral blood neutrophils were obtained from donor CD2F1 or recipient C3H/HeJ mice and irradiated and reconstituted as in Figure 1legend. Mice were either uninfected or infected with PCA-2 at different weeks after transplantation and reinfected with CA-6 as in Figure 2legend. Three days after reinfection, neutrophils were assessed for ability to kill yeast cells, as described in “Material and methods.” *Indicates P < .05 (BMT mice vs donor mice).
Candidacidal activity of neutrophils following T-cell–depleted allogeneic BMT.
Peripheral blood neutrophils were obtained from donor CD2F1 or recipient C3H/HeJ mice and irradiated and reconstituted as in Figure 1legend. Mice were either uninfected or infected with PCA-2 at different weeks after transplantation and reinfected with CA-6 as in Figure 2legend. Three days after reinfection, neutrophils were assessed for ability to kill yeast cells, as described in “Material and methods.” *Indicates P < .05 (BMT mice vs donor mice).
Susceptibility or resistance to mucosal candidiasis is considered to reflect the pattern of locally induced TH reactivity.19Therefore, we evaluated the course of gastrointestinal candidiasis together with the pattern of cytokine production in recipient mice at 2 and 5 weeks after BMT. Resistance to mucosal candidiasis was greatly impaired at 2 weeks after transplantation, at the time when fungal colonization in the stomach was significantly higher than in donor mice. Five weeks after transplantation, reconstituted mice were as resistant as donor mice to the infection (Figure6). Susceptibility to infection correlated with low levels of IFN-γ/IL-12 and high levels of IL-10, which were both significantly different from those observed in either donor or resistant recipient mice (Figure 6). Therefore, reconstituted mice are more susceptible to mucosal candidiasis at the time when protective TH1-mediated responses are impaired.
Fungal growth and pattern of TH cytokine production in BMT mice with gastrointestinal candidiasis.
At 2 and 5 weeks after transplantation, recipient C3H/HeJ mice, irradiated and reconstituted as in Figure 1 legend, were injected intragastrically with 108C albicans cells. CFUs from the stomach of C albicans–infected mice and cytokine contents in fecal samples were measured one week after infection. *Indicates P < .05 (BMT mice vs donor mice).
Fungal growth and pattern of TH cytokine production in BMT mice with gastrointestinal candidiasis.
At 2 and 5 weeks after transplantation, recipient C3H/HeJ mice, irradiated and reconstituted as in Figure 1 legend, were injected intragastrically with 108C albicans cells. CFUs from the stomach of C albicans–infected mice and cytokine contents in fecal samples were measured one week after infection. *Indicates P < .05 (BMT mice vs donor mice).
Antifungal resistance following T-cell–depleted allogeneic BMT is sensitive to modulation by TH2 cytokine antagonists
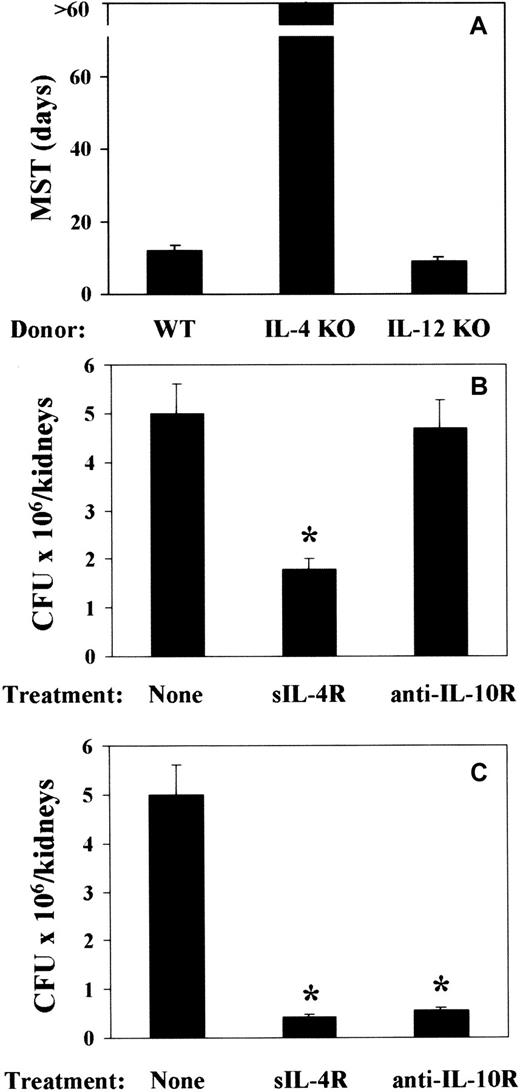
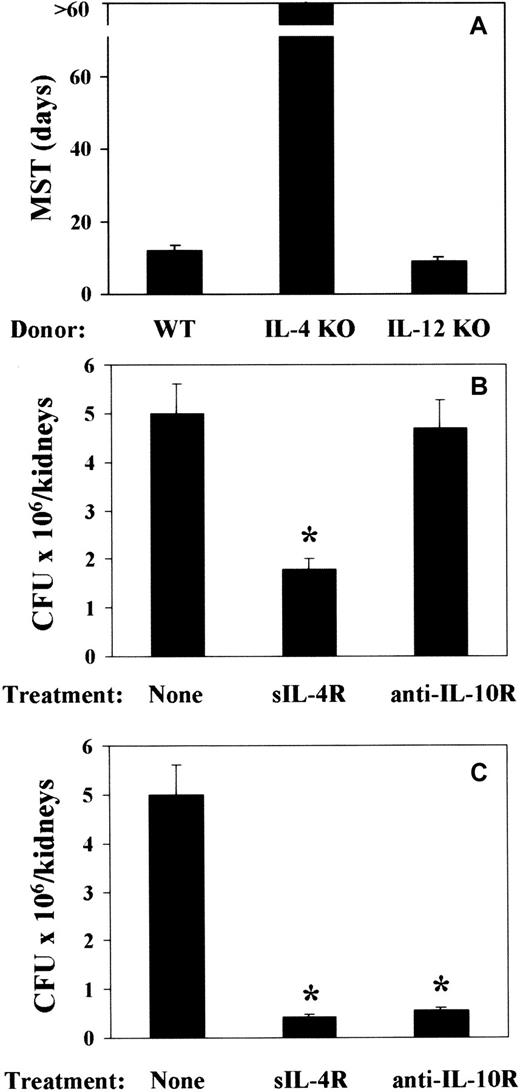
To address the issue of a cause-and-effect relationship between TH cytokine production and pattern of susceptibility and resistance observed in mice that received a transplantation, 2 experimental approaches were taken. Mice received a transplantation either with T-cell–depleted allogeneic BM from IL-4– or IL-12p40–deficient mice or with T-cell–depleted allogeneic BM from cytokine-sufficient mice and were treated with sIL-4R or anti–IL-10R mAb either around the time of BMT or infection. At 2 weeks after BMT, mice were infected with PCA-2 and reinfected with CA-6. The results show that infusion of BM cells from IL-4–deficient mice greatly increased resistance to the infection, as judged by the long-term survival upon reinfection (Figure7A). As expected, fungal resistance was not increased upon infusion of BM cells from IL-12–deficient mice (Figure 7A). Actually, about 30% of the mice did not survive the primary infection (data not shown). Both IL-4 or IL-10 neutralization increased resistance to the infection, as judged by the reduced fungal burden in the kidneys of mice upon reinfection (Figure 7B,C). rIL-12 administration only partially increased resistance to the infection (data not shown). Interestingly, treatment with sIL-4R was effective both at the time of BMT (Figure 7B) and infection (Figure 7C). In contrast, IL-10 neutralization was effective at the time of infection (Figure 7C) and not at the time of BMT (Figure 7B). Therefore, the ablation of the TH2 cytokines, IL-4 and IL-10, more than the administration of TH1-promoting IL-12, is associated with an increased resistance to fungal infection in T-cell–depleted allogeneic BMT recipients.
Effect of ablation of TH2 cytokines on resistance toC albicans in mice with T-cell–depleted allogeneic BMT.
Recipient C3H/HeJ mice were irradiated and reconstituted with T-cell–depleted BM from IL-4– or IL-12p40–deficient (IL-4 KO and IL-12 KO, respectively) as well as (A) wild-type (WT) mice or as in Figure 1 legend. Two weeks later, mice were intravenously infected with low-virulent PCA-2 C albicans and reinfected with 106 virulent CA-6 C albicans cells 2 weeks after the primary infection. Treatments with sIL-4R or anti–IL-10R mAb were done at the time of (B) BMT or (C) primary infection. MST indicates median survival time in days; CFUs, a week after reinfection; none, vehicle alone. *Indicates P < .05 (BMT mice vs donor mice).
Effect of ablation of TH2 cytokines on resistance toC albicans in mice with T-cell–depleted allogeneic BMT.
Recipient C3H/HeJ mice were irradiated and reconstituted with T-cell–depleted BM from IL-4– or IL-12p40–deficient (IL-4 KO and IL-12 KO, respectively) as well as (A) wild-type (WT) mice or as in Figure 1 legend. Two weeks later, mice were intravenously infected with low-virulent PCA-2 C albicans and reinfected with 106 virulent CA-6 C albicans cells 2 weeks after the primary infection. Treatments with sIL-4R or anti–IL-10R mAb were done at the time of (B) BMT or (C) primary infection. MST indicates median survival time in days; CFUs, a week after reinfection; none, vehicle alone. *Indicates P < .05 (BMT mice vs donor mice).
Discussion
The results of the present study show that in a murine model of T-cell–depleted allogeneic BMT mice, susceptibility and resistance to fungal infections vary with time following transplantation and correlate with the occurrence of distinct patterns of antifungal TH reactivity that are sensitive to modulation by treatments with selected cytokine antagonists.
Clinical and experimental evidence indicates that tolerance to donor graft following T-cell–depleted allogeneic BMT could be achieved by escalating the dose of stem cells infused, possibly through suppression of persisting host cell reactivity mediating graft rejection.12,13,16 In the experimental model adopted in the present study, the infusion of a huge number of BM stem cells in lethally irradiated mice resulted in a successful engraftment in the majority of mice without the need of additional conditioning regimens, as previously described.16 In this BMT setting, we found that susceptibility to fungal infections vary with time following transplantation. Mice were more susceptible to both mucosal and disseminated candidiasis soon after BMT rather than later.
The increased susceptibility observed early after BMT was not due to a primary defect of host innate antifungal resistance. Indeed, the recovery of the antifungal effector activity of neutrophils was not impaired in uninfected recipients after BMT. However, this activity was greatly impaired upon reinfection of mice that had received the primary infection 2 weeks after transplantation, at the time when failure to develop antifungal TH1 responses occurred. These findings indicate that the adaptive TH immunity exerts a regulatory control over the innate antifungal resistance.
In terms of the nature of the immunoregulatory mechanisms through which TH1 development is impaired to the benefit of nonprotective TH2 responses, we found that production of IL-12 was decreased and production of IL-4/IL-10 was increased 2 weeks after transplantation. Because neutrophils24 and dendritic cells22produce IL-12 in response to C albicans, it is likely that both types of cells may contribute to the antifungal TH-cell repertoire following T-cell–depleted allogeneic BMT. However, the presence of CD4+ cells of the activated phenotype at 2 weeks after infection, together with the occurrence of a population of natural killer cells during the first week after BMT (data not shown), suggest that production of inhibitory cytokines by these cells25may affect the qualitative development of anticandidal TH-cell responses. Rapid recovery of natural killer cells in recipients of T-cell–depleted allogeneic BMT has been described.26
Patients receiving T-cell–depleted BMT are unable to develop antigen-specific T-cell responses soon after transplantation.27 It has been demonstrated that T-cell depletion of allogeneic BMT is associated with a slow recovery of CD4+ and CD8+ T cells.28 However, functional recovery of the T-cell system after T-cell–depleted allogeneic BMT has been demonstrated, although little is known about the mechanisms whereby the T-cell repertoire is reconstituted and the factors that influence the extent of reconstitution.29,30The reconstitution of the normal T-cell repertoire is thought to occur by thymic-dependent and/or thymic-independent pathways that lead to the generation of a new repertoire of naive T cells from hematopoietic progenitors and/or to proliferation of residual mature T cells present in the BM graft.31,32 In this regard, it has been reported that patients who received transplantations with antigen-experienced T cells usually have a prompt memory-type response at early times after transplantation.33-35 Interestingly, it has been reported that T-cell reactivity to Candida readily recovered after T-cell–depleted allogeneic BMT, and the fact that recovery was related to the absence of antifungal prophylactic measures indicates that anticandidal reactivity of residual T cells could be maintained through continuous exposure to the fungus.36
However, it is worth mentioning that while serving as a prompt antifungal defense, the presence of memory anticandidal reactivity in thymectomized mice was insufficient to induce development of antigen-specific TH1 responses.25 CD4+ cells of the naive phenotype were indeed required for the expression of fungal-specific TH1 reactivity. This suggests that the insufficiency of the repertoire of naive T cells may underlie the inability of patients receiving T-cell–depleted allogeneic BMT to develop antigen-specific T-cell responses soon after transplantation. The data of the present study support this observation. Indeed, an effective antigen-specific TH1 response only occurred at 5 weeks after BMT and was concomitant with the recovery of CD4+ cells of the nonactivated phenotype. As optimal activation and mobilization of antifungal effector phagocytes requires the presence of TH1 cells,7 10 this implies that reconstitution of the T-cell repertoire diversity is a prerequisite for resistance to fungal infections in patients with T-cell–depleted allogeneic BMT.
It has recently been shown that the occurrence of immunological memory relies on the induction of 2 types of T cells, namely the central memory and the effector memory T cells. Central memory cells differentiate rapidly to effector memory cells that provide immediate protection, particularly at epithelial surfaces.37 Whether a dysfunctional immunological memory occurs during immunoreconstitution in T-cell–depleted allogeneic BMT is not known, although it is an attractive working hypothesis. As the 2 memory subsets are phenotypically distinguishable, it will be interesting to address this important issue in further studies.
The immunoreconstitution occurs as a balance between quantitative lymphocyte reconstitution and qualitative lymphocyte functions and is associated with changes in cytokine patterns.38 That the occurrence of GVHD in allogeneic BMT is associated with discrete patterns of cytokine production is well known.39 TH2 cytokine-producing cells may serve to down-regulate GVHD in both human40,41 and experimental42,43 BMT settings, although opposite results have also been reported.44 45 The data of the present study indicate that neutralization of TH2 cytokines, particularly IL-4, has a beneficial effect in recipients of T-cell–depleted allogeneic BMT. Neutralization of IL-4 at the time of BMT, as well as infusion of BM cells from IL-4–deficient donor, did not adversely affect immunoreconstitution, as judged by the occurrence of donor-type antifungal TH responses.
In contrast, TH1 reactivity was not restored in recipient mice upon infusion of BM cells from IL-12–deficient donor. However, neutralization of TH2 cytokines, more than admininistration of IL-12, at the time of fungal exposure rendered susceptible recipient mice highly resistant to infection, a finding suggesting that antifungal TH responses are highly sensitive to cytokine modulation during immunoreconstitution following T-cell–depleted allogeneic BMT. Thus, through a combined activity on immunoreconstitution and development of antifungal TH reactivity, selected cytokines or cytokine antagonists may represent promising strategies of immunointervention and management of refractory opportunistic fungal infections. In addition, as the absence of IL-4 in donor BM cells positively affected reconstitution of the antifungal TH repertoire, this may be taken to indicate that the measurement of IL-4 reactivity in donors can be a valuable strategy for optimizing donor selection in unrelated BMT.
It is becoming clear that the incorporation of new antifungal agents into practice is needed to further improve outcome of patients with hematological malignancies. In addition, the identification of nosocomial transmission as an unexpected means for the spread of fungal infections in BMT units has emphasized the need for infection control monitoring. The results of the present study suggest that finding cells and cytokines that are important in effective antifungal TH development during immunoreconstitution in T-cell–depleted allogeneic BMT could be a further strategy to improve the management of refractory opportunistic fungal infections.
We thank Andrea Casagrande and Sabrina Fiorucci for their dedicated work in the laboratory, and Jo-Anne Rowe for editorial assistance.
Supported by grant 50B.30 from the National Research Project on AIDS, Rome and by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Luigina Romani, Microbiology Section, Department of Experimental Medicine and Biochemical Sciences, University of Perugia, Via del Giochetto, 06122 Perugia, Italy; e-mail:lromani@unipg.it.