To determine whether immune stimulation could reduce acute myelogenous leukemia (AML) lethality, dendritic cells (DCs) were pulsed with AML antigens and used as vaccines or generated in vivo by Flt3 ligand (Flt3L), a potent stimulator of DC and natural killer (NK) cell generation. Mice were then challenged with AML cells. The total number of splenic anti-AML cytotoxic T-lymphocyte precursors (CTLPs) present at the time of challenge was increased 1.9-fold and 16.4-fold by Flt3L or DC tumor vaccines, respectively. As compared with the 0% survival of controls, 63% or more of recipients of pulsed DCs or Flt3L survived long term. Mice given AML cells prior to DC vaccines or Flt3L had only a slight survival advantage versus non-treated controls. NK cells or NK cells and T cells were found to be involved in the antitumor responses of Flt3L or DCs, respectively. DC vaccines lead to long-term memory responses but Flt3L does not. Syngeneic bone marrow transplantation (BMT) recipients were analyzed beginning 2 months post-BMT. In contrast to the uniform lethality in BMT controls given AML cells, recipients of either Flt3L or DC vaccines had a significant increase in survival. The total number of splenic anti-AML CTLPs at the time of AML challenge in BMT controls was 40% of concurrently analyzed non-BMT controls. Flt3L or DC vaccines increased the total anti-AML CTLPs 1.4-fold and 6.8-fold, respectively. Neither approach was successful when initiated after AML challenge. It was concluded that DC vaccines and Flt3L administration can enhance an AML response in non-transplanted or syngeneic BMT mice but only when initiated prior to AML progression.
Introduction
Acute myeloid leukemia (AML) requires myelosuppresive therapy for achieving remission and long-term survival.1 Intensification of chemotherapy has led to remission in 70% to 85% of patients. However, increased morbidity and mortality complicate this approach, and post-remission relapses are frequent.2,3 Myelo-ablative bone marrow transplantation (BMT) is an important way to improve long-term survival in AML patients.4 Relapse remains a significant barrier to success, with the risk of relapse varying between 30% and 70% for autologous BMT, depending on remission status and preparative regimen.5 6 For AML patients failing to respond or relapsing, new approaches are needed.
AML is susceptible to T-cell effectors. There is a well-documented graft-vs-leukemia (GVL) effect seen in AML patients experiencing graft-versus-host disease.7 AML patients who relapse post-BMT can be treated with donor lymphocyte infusions.8 For patients not undergoing BMT or those receiving autologous BMT, allogeneic responses as a means to achieving remission is not an option. Since endogenous T-cell responses are not sufficiently potent to protect against AML recurrence, alternative approaches are needed. The generation of tumor-specific immune responses requires that either the tumor cell or an antigen-presenting cell (APC) present tumor antigens to immune-competent cells. Tumors are usually poor stimulators of immune responses. Reasons cited are poor APC capacity of tumor cells due to low/no expression of major histocompatibility complex (MHC) antigens, defects in antigen-processing pathways, poor adhesion between tumors and immune effector cells, and lack of costimulatory molecules.
DCs are potent APCs that stimulate naive and memory T-cell immune responses.9 DCs express high levels of MHC antigens, provide costimulation to amplify T-cell responses, and secrete cytokines necessary to initiate and sustain immune effectors' functions.10 DCs pulsed with antigens have been used successfully to treat mice with solid tumors and to generate immune responses in humans.10-19 DCs generated after culturing peripheral blood of AML patients can stimulate anti-AML cytotoxic T-lymphocyte (CTL) responses in vitro,20 although no studies have reported on the efficacy of DCs in facilitating an anti-AML immune response in vivo.
Although DCs are present in low frequency in the periphery,17,19,21 DCs can be expanded from immature precursors present in bone marrow (BM) or blood.17,19,21Ex vivo–propagated DCs can be loaded with tumor antigens/peptides to generate immune effectors.13-19,21-23 An alternative would be to increase DC number or function in vivo, which would provide APCs with access to antigens as tumor cells undergo cell death in vivo. High concentrations of granulocyte-macrophage colony–stimulating factor (GM-CSF), known to drive DC maturation,17,21,24,25 can facilitate antitumor responses in vivo. When administered as an adjuvant at the local site of antigen delivery26 or as a transduced cell vaccine,27-29 GM-CSF is associated with DCs' recruitment into the tumor sites, suggesting that DCs generated can support an antitumor immune response. Flt3 ligand (Flt3L) is a hematopoietic stem- and progenitor-cell growth factor30,31that has a profound effect on the generation of functionally mature DCs in multiple organs in mice.32 In addition to its effects on stimulators of the immune system such as DC cells, Flt3L has been shown to stimulate the generation and function of murine and human natural killer (NK) effector cells.33-38
The relative efficacy of DC vaccines and Flt3L for therapy of murine AML has not been previously investigated. We now show that significant protection against AML challenge in naive or BMT mice was provided by either in vitro tumor-lysate–pulsed DCs or Flt3L but only when these interventions were initiated prior to AML challenge. DC vaccines but not Flt3L resulted in the generation of anti-AML memory cells. These data have important implications for human clinical trials in AML patients, including those who have undergone autologous BMT.
Materials and methods
Mice
C57BL/6 (H2b) (termed B6) mice, 6 to 12 weeks old at study, were obtained from the National Institutes of Health (Bethesda, MD). Mice were housed in micro-isolator cages under specific pathogen-free conditions and were fed ad libitum.
Tumor cell line
C1498 was derived as a spontaneous myeloid tumor line from a B6 mouse and adapted to tissue culture. C1498, obtained from the American Type Culture Collection (ATCC) (Rockville, MD), was grown in RPMI (Life Technologies, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (HyClone, Ogden, UT), 2 mM L-glutamine, minimal essential media amino acids, 1 mM sodium pyruvate, 50 mM 2-ME, and penicillin/streptomycin (referred to as complete medium).39
BM-derived DC isolation and antigen pulsing
BM-derived DC generation was adapted from Inaba et al.25 BM, treated with AKC lysis buffer to remove red cells, was depleted of T cells, B cells, granulocytes, and IA+ cells by means of a mixture of monoclonal antibodies (mAbs) followed by complement. The mAbs included anti-Thy 1.2 (30-H-12), anti-B220 (RA3-6B2), anti-Gr-1 (RA-8C5), and anti-IAb (AF6-120.1.2) (ATCC). BM cells were plated in 6-well culture plates (106 cells per milliliter, 3 mL per well) with murine GM-CSF (5 ng/mL) (R&D Systems, Minneapolis, MN) and murine interleukin (IL)–4 (1000 IU/mL) (Immunex, Seattle, WA) in RPMI complete medium (described above). On day 3, floating cells were removed and fresh medium was added. After 5 days, cells were washed and replated with IL-4 and murine tumor necrosis factor α (4 ng/mL) (R&D Systems). After 7 to 10 days, cultures showed morphology consistent with DC appearance and had high levels of MHC class I and II antigens, adhesion molecules, B7 ligands, and CD11c (not shown). At 18 hours prior to vaccinations, DCs were pulsed with C1498 lysates obtained by subjecting C1498 to 4 freeze-and-thaw cycles in liquid nitrogen followed by 37°C exposure in a water bath. No viable cells were present. The tumor lysate was centrifuged at 1200 rpm for 10 minutes, and supernatants were added to DCs at a 3:1 ratio in a 6-well plate containing 106 DCs. Lysate-pulsed DCs were washed and injected intravenously at 0.5 × 106 cells per mouse. Increasing the DC vaccine dose by 3-fold did not result in additional survival benefit (not shown).
AML challenge
Leukemia was induced by intravenous injection of C1498 (1 to 2 × 105 cells per mouse) on day 0. Cohorts were immunized with 2 or 3 weekly doses of C1498-lysate–pulsed DCs intravenously, either prior to leukemia challenge or beginning 1 day after. Other cohorts were injected subcutaneously once daily with Chinese hamster ovary cell–derived human Flt3L (Immunex) at 30 μg per injection from days −10 to +11 or days 1 to 21, with the leukemia challenge occurring on day 0. In studies comparing Flt3L at 10 μg per dose with 30 μg per dose for 21 days, the higher dose enhanced the antitumor response of Flt3L.40 The schedule was chosen so that the day 0 C1498 injection would occur at peak DC availability for antigen loading.41 To determine the efficacy of irradiated cellular vaccines in preventing AML lethality or treating established disease, 2 weekly doses (107 cells per dose) of irradiated (100 Gy) C1498 cellular vaccines were injected subcutaneously at various periods of time relative to C1498 challenge.
To determine whether the anti-C1498 immune responses were due to T cells or NK cells, Flt3L-treated recipients were given anti-Thy 1.2 mAb (30-H-12), anti-NK1.1 mAb (PK136), or irrelevant rat immunoglobulin G (IgG) (400 μg per injection) weekly from days −2 to +54 post-challenge. For DC vaccination, recipients of C1498-lysate–pulsed DCs were challenged on day 0 and given irrelevant rat IgG, anti-NK1.1, or anti-CD4 (GK1.5) plus anti-CD8 (2.43) mAbs (400 μg each) weekly from days −1 to +27 post-challenge.
To determine if Flt3L recipients had an anti-AML memory response, cohorts of Flt3L-treated, AML-challenged mice were rechallenged on day 60 with the same AML cell dose used for the initial challenge. Initial challenge of Flt3L recipients was necessary to provide a source of AML antigens in vivo in an attempt to generate memory effector cells. To determine if recipients of C1498-lysate–pulsed DCs had a memory cell response, recipients were given 3 separate weekly DC vaccines and then challenged 71 days after the last DC vaccine with a lethal dose of AML cells. Nonmanipulated B6 mice were challenged with AML cells to serve as a concurrent comparison group for rechallenge.
Syngeneic BMT studies
B6 mice undergoing BMT were conditioned with 800-centigray irradiation from an x-ray source (day −1) and infused with 5 × 106 syngeneic B6 BM cells on day 0. Leukemia cells were injected on day 80 post-BMT. We administered 3 weekly doses of tumor-pulsed DC vaccines or Flt3L before leukemia challenge on days 59, 66, and 73 or days 70 through 91, respectively. Additional cohorts were first given leukemia cells and then either tumor-pulsed DC vaccines on days 81, 88, and 95 or Flt3L on days 81 through 102. A control group received BMT only.
Anti-AML cytotoxic T-lymphocyte precursors
Cytotoxic T-lymphocyte precursor (CTLP) frequency was analyzed by limiting dilution as previously described.39Splenocytes from control, DC-immunized, or Flt3L-treated mice were plated in complete medium at 6 dilutions (30 replicates per dilution) in 96-well microplates and incubated with irradiated C1498 cells (104 per well) and IL-2 (10 IU/mL) (Amgen, Thousand Oaks, CA) for 7 days. After the addition of thymidine-labeled C1498 (1000 per well), cytotoxicity was assessed by a 4-hour JAM assay,42 which measures the retention of tritiated thymidine in target cells not undergoing cytolysis. Wells were scored as positive if the counts per minute exceeded 3 SDs below the mean spontaneous thymidine release in wells containing labeled C1498 with no effector cells. By means of Poisson distribution statistics,43 the likelihood of a single hit was confirmed, and a frequency estimate calculated. Comparisons were made only for frequency estimations performed at the same time under the same conditions.
Statistical analysis
The Kaplan-Meier product-limit method was used to calculate survival rates. Log-rank statistics were used to test differences between groups.
Results
AML-lysate–pulsed DC vaccinations or Flt3L injections mediate significant protection against AML in non-BMT mice
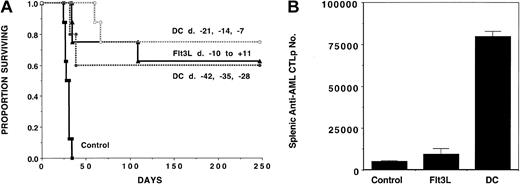
To evaluate the role of DC vaccines and Flt3L in generating an immune response against AML cells, we administered AML-lysate–pulsed DCs or Flt3L before mice were challenged with C1498 cells. A cohort was given 3 weekly intravenous doses of lysate-pulsed DCs (5 × 105 per mouse) followed by a lethal dose of C1498 (105) on day 0 (Figure 1A). To determine whether immunizations had to be given shortly before AML challenge, 2 schedules were used, with the last immunization being completed either 1 week (days −21, −14, and −7) or 4 weeks (day −42, −35, and −28) prior to live AML challenge. A separate cohort received daily Flt3L injections on days −10 through +11. Mice that received AML without immunization succumbed to AML prior to day 35 post-challenge. As compared with non-treated controls, recipients immunized with AML-pulsed DCs given according to either injection schedule had a significantly (P < .003) higher survival, with 60% to 75% surviving long term (day 250 post-challenge). Recipients administered Flt3L had a long-term survival (63%) comparable to recipients of DC vaccines.
AML-lysate–pulsed DC vaccines or Flt3L injections mediate potent antileukemia effect when given prior to a lethal dose of AML cells in nontransplanted animals.
Cohorts of naive B6 mice (n = 8-10) received either 3 weekly doses of tumor-lysate–pulsed DCs (beginning on day −42 or −21), or 21 daily subcutaneous Flt3L (30 μg per dose) injections (days −10 to +11), or no treatment (controls) followed by a lethal leukemia dose (105 cells per mouse) on day 0. Eight to 10 mice per group were analyzed. (A) A Kaplan-Meier survival plot shows days post–lethal C1498 challenge (day 0) on the x-axis and the proportion of mice surviving on the y-axis. The actuarial survival rate of either DC-vaccinated or Flt3L-treated recipients was significantly higher (P < .001) than the control animals. (B) On day 0, 2 mice in each group were killed for splenic C1498 leukemia-reactive CTLP frequency estimation. The mean total splenic anti-AML CTLP number ± SD is shown on the y-axis for each of the 3 groups. A significant increase in CTLP number was present in both DC-vaccinated (P = .01) and Flt3L-treated (P = .03) animals, although DC-treated mice showed a significantly higher CTLP expansion than mice receiving Flt3L (P = .02).
AML-lysate–pulsed DC vaccines or Flt3L injections mediate potent antileukemia effect when given prior to a lethal dose of AML cells in nontransplanted animals.
Cohorts of naive B6 mice (n = 8-10) received either 3 weekly doses of tumor-lysate–pulsed DCs (beginning on day −42 or −21), or 21 daily subcutaneous Flt3L (30 μg per dose) injections (days −10 to +11), or no treatment (controls) followed by a lethal leukemia dose (105 cells per mouse) on day 0. Eight to 10 mice per group were analyzed. (A) A Kaplan-Meier survival plot shows days post–lethal C1498 challenge (day 0) on the x-axis and the proportion of mice surviving on the y-axis. The actuarial survival rate of either DC-vaccinated or Flt3L-treated recipients was significantly higher (P < .001) than the control animals. (B) On day 0, 2 mice in each group were killed for splenic C1498 leukemia-reactive CTLP frequency estimation. The mean total splenic anti-AML CTLP number ± SD is shown on the y-axis for each of the 3 groups. A significant increase in CTLP number was present in both DC-vaccinated (P = .01) and Flt3L-treated (P = .03) animals, although DC-treated mice showed a significantly higher CTLP expansion than mice receiving Flt3L (P = .02).
The potential influence of DC vaccines and Flt3L treatments on splenic anti-AML CTL responses was examined. CTLs were not detected from DC vaccine–treated or Flt3L-treated recipients without in vitro restimulation (not shown). After a 6-day restimulation with C1498 cells, splenic anti-C1498 CTLs obtained from DC-vaccinated but not Flt3L-treated mice were increased by approximately 10-fold (not shown). To obtain comparative data on the frequency of anti-C1498 CTL responses in the various groups, we chose to use a CTLP assay. CTLP data were used to calculate the total number of anti-C1498–reactive CTLP cells in the spleen of mice from these various groups. Therefore, we could correct for differences in both total splenocyte number (which is 2- to 4-fold higher in Flt3L-treated mice vs the other 2 groups) and T-cell content (which is proportionately lower in the Flt3L-treated group owing to expansion of non–T-cell populations by Flt3L administration). Results in nonmanipulated controls were compared with mice that received either 3 weekly DC vaccines ending 1 week earlier or Flt3L given for 10 days (Figure 1B). As compared with non-treated controls, the total number of splenic anti-AML CTLPs was increased 16.4-fold or 1.9-fold in recipients of DC vaccines (P = .01 vs control;P = .02 vs Flt3L group) or Flt3L (P = .03 vs control), respectively, consistent with the in vivo generation of anti-AML CTL effector cells.
Although both AML DC vaccinations and Flt3L induce an NK-mediated immune response, only DC vaccinations generate anti-AML memory effector cells
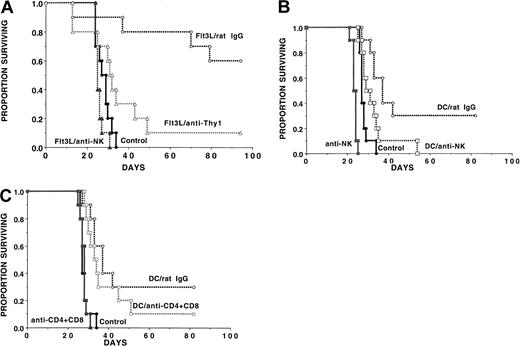
Long-term survival outcome after AML challenge was similar in DC-vaccinated as compared with Flt3L-treated mice even though DC vaccinations induced a greater increase in splenic anti-AML CTLPs than Flt3L when analyzed on the day of AML challenge. We hypothesized that CTLP analysis may not be reflective of the anti-AML effect because NK cells may be the major effector cells in the Flt3L-treated recipients and may therefore not be adequately represented by this assay. Alternatively, CTLPs were higher in the DC-vaccinated than the Flt3L-treated mice since T cells in the DC-treated group but not the Flt3L group had had prior exposure to AML antigens in vivo and were therefore reprimed in vitro during the CTLP assay. To determine whether NK cells or T cells were responsible for the anti-AML effect observed in Flt3L-treated recipients, cohorts were given no treatment or anti-NK1.1 mAb to deplete NK cells (Figure2A). Because Flt3L-generated DCs express CD8 antigens,41 anti-Thy1.2 mAb was used to deplete T cells, along with a proportion of splenic NK cells. All mice were challenged with a lethal AML cell dose (2 × 105 cells). Mice given Flt3L and irrelevant rat IgG had a 60% long-term survival vs 0% in non-treated controls. In marked contrast, recipients given Flt3L and anti-NK1.1 mAb had no survival benefit, indicating that NK cells were probably the major effector cells involved in the immune effect against this NK-sensitive AML cell line under these conditions. Anti-Thy1.2 mAb reduced the anti-AML protective effect of Flt3L with the result that only 10% of mice survived long term (Figure 2A), consistent with the fact that Thy1.2 depletion eliminates about 50% of splenic NK cells along with T cells.44
Flt3L administration augments an anti-AML immune resistance via the generation of NK effector cells while DC vaccinations generate both NK and T-cell effector cells.
(A) Flt3L (30 μg per dose) was administered from days −10 to +11. A separate cohort of mice was untreated. On day 0, mice were challenged with C1498 cells (2 × 105 cells per mouse). Flt3L-treated mice were injected with no mAb (n = 20) or either irrelevant rat IgG mAb, anti-NK1.1 mAb, or anti-Thy1.2 mAb (n = 10 per group) beginning on day −2 and continuing weekly until day 54 as described in “Materials and methods.” The actuarial survival rate of Flt3L-treated recipients was significantly higher than non-treated controls (P = .00069). The actuarial survival rate of Flt3L-treated recipients given irrelevant rat IgG mAb was significantly higher than those given anti-Thy1.2 (P = .005) or anti-NK1.1 (P = .0077). NK depletion eliminated all of the protective effect of Flt3L treatment (P = .065 vs non–Flt3L-treated controls). (B) (C) In a separate experiment, recipients were vaccinated with DC cells (0.5 × 106cells per mouse) on days −21, −14, and −7 or were left untreated. Mice then were randomized to receive irrelevant (panels B, C), anti-NK1.1 (panel B), or anti-CD4 plus anti-CD8 (panel C) mAb from days −1 to +27 post-BMT. NK depletion resulted in a lower survival rates in non-treated control recipients (P = .0005) and in DC-vaccinated recipients (P = .016). DC-vaccinated mice depleted of NK cells had a higher survival rate than NK-depleted controls (P = .0095) and nonvaccinated, nondepleted controls (P = .036), indicating that NK cells were not the only cell population responsible for C1498 resistance. Depletion of T cells reduced survival rates in DC-vaccinated mice from 30% to 10% although these differences did not reach statistical significance (P = .15).
Flt3L administration augments an anti-AML immune resistance via the generation of NK effector cells while DC vaccinations generate both NK and T-cell effector cells.
(A) Flt3L (30 μg per dose) was administered from days −10 to +11. A separate cohort of mice was untreated. On day 0, mice were challenged with C1498 cells (2 × 105 cells per mouse). Flt3L-treated mice were injected with no mAb (n = 20) or either irrelevant rat IgG mAb, anti-NK1.1 mAb, or anti-Thy1.2 mAb (n = 10 per group) beginning on day −2 and continuing weekly until day 54 as described in “Materials and methods.” The actuarial survival rate of Flt3L-treated recipients was significantly higher than non-treated controls (P = .00069). The actuarial survival rate of Flt3L-treated recipients given irrelevant rat IgG mAb was significantly higher than those given anti-Thy1.2 (P = .005) or anti-NK1.1 (P = .0077). NK depletion eliminated all of the protective effect of Flt3L treatment (P = .065 vs non–Flt3L-treated controls). (B) (C) In a separate experiment, recipients were vaccinated with DC cells (0.5 × 106cells per mouse) on days −21, −14, and −7 or were left untreated. Mice then were randomized to receive irrelevant (panels B, C), anti-NK1.1 (panel B), or anti-CD4 plus anti-CD8 (panel C) mAb from days −1 to +27 post-BMT. NK depletion resulted in a lower survival rates in non-treated control recipients (P = .0005) and in DC-vaccinated recipients (P = .016). DC-vaccinated mice depleted of NK cells had a higher survival rate than NK-depleted controls (P = .0095) and nonvaccinated, nondepleted controls (P = .036), indicating that NK cells were not the only cell population responsible for C1498 resistance. Depletion of T cells reduced survival rates in DC-vaccinated mice from 30% to 10% although these differences did not reach statistical significance (P = .15).
In an analogous study, DC-vaccinated mice were treated with irrelevant or anti-NK1.1 mAb. Treated DC-vaccinated mice had a better survival rate than non–DC-vaccinated controls, with 30% of mice surviving long term as compared with 0% of controls (P = .0017) (Figure2B). The reason for the lower degree of DC vaccine protection in this experiment may relate, in part, to the 2-fold higher number of AML cells used for day 0 challenge as compared with the other 2 experiments (Figures 1, 4). Nonetheless, NK depletion of DC-vaccinated mice diminished the protective effect of the vaccines (P = .016 vs irrelevant mAb). DC-vaccinated mice depleted of NK cells survived longer than controls not receiving DC vaccines (P = .036), indicating that only part of the effect of DC vaccination was dependent upon NK cells. DC-vaccinated mice that were NK-depleted survived significantly longer than NK-depleted controls (P < .001), providing evidence that the protective effect of DC vaccines was not entirely dependent upon NK cells. In the DC-vaccinated groups, combined T-cell and NK-cell depletion resulted in a lower survival than NK depletion alone (P = .0026), while combined T- and NK-cell depletion did not lower survival rates in nonvaccinated controls (P = .34) (not shown). These data suggest that T cells facilitated C1498 resistance in DC-vaccinated recipients. Depletion of CD4+ and CD8+ T cells in DC-vaccinated mice reduced survival from 30% to 10% (P = .15) (Figure 2C), albeit statistical significance was not reached in this experiment. Collectively, these data are most consistent with a contribution of both NK cells and T cells to DC-mediated protection against AML challenge.
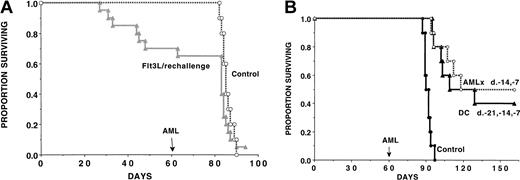
To determine whether we could uncover anti-AML T-cell immune response in Flt3L-treated mice, a separate cohort of recipients from studies shown in Figure 2A was designated to be used for memory cell (rechallenge) experiments at the onset of the study. Mice were treated with Flt3L (days −10 to +11) and then challenged with live AML cells (2 × 105) on day 0 to provide a source of AML antigen for in vivo DC loading (Figure 3A). All surviving Flt3L-treated mice were rechallenged with live AML cells (2 × 105) on day 60 after initial challenge. A separate cohort of non-treated concurrent controls were given live AML cells. Flt3L-treated recipients previously protected from a lethal AML dose that were rechallenged on day 60 after initial inoculation had no evidence of a long-lived anti-AML memory response. The deaths observed in Flt3L-treated mice rechallenged on day 60 had the same kinetics course as concurrently challenged controls, suggesting that deaths after rechallenge were more likely from the AML used for rechallenge than from the initial challenge. In aggregate, the data are most consistent with a dominant role of NK cells in generating the anti-AML response in Flt3L-treated recipients.
DC vaccines but not Flt3L administration generate an anti-AML memory cell response.
(A) A cohort of Flt3L-treated mice (dose and schedule as in Figure 2) that were challenged with C1498 cells on day 0 were rechallenged with the same dose of C1498 cells (2 × 105 per mouse) on day 60. As a control, a cohort of concurrent, nonmanipulated B6 controls (n = 10) were given C1498 AML cells (2 × 105 per mouse) on the same day used for rechallenge. There was no significant difference in these groups when comparing survival rates after day 60 (day of rechallenge). (B) In a separate experiment, recipients were vaccinated with C1498-lysate–pulsed DCs (as in Figure 2) or irradiated C1498 cellular vaccines (107 cells per mouse) given on days −14 and −7 or left untreated. On day 60, mice were challenged with C1498 cells (2 × 105). Mice given AML-lysate–loaded DC vaccines or irradiated whole cellular AML vaccines 71 days earlier had a memory cell response since 40% and 50% of mice challenged with a supralethal AML cell dose (2 × 105 cells) survived long term as compared with 0% of nonvaccinated controls (P = .00055; P = .00037 vs control, respectively). There was no significant difference (P = .32) between these 2 vaccination approaches in terms of anti-AML memory responses.
DC vaccines but not Flt3L administration generate an anti-AML memory cell response.
(A) A cohort of Flt3L-treated mice (dose and schedule as in Figure 2) that were challenged with C1498 cells on day 0 were rechallenged with the same dose of C1498 cells (2 × 105 per mouse) on day 60. As a control, a cohort of concurrent, nonmanipulated B6 controls (n = 10) were given C1498 AML cells (2 × 105 per mouse) on the same day used for rechallenge. There was no significant difference in these groups when comparing survival rates after day 60 (day of rechallenge). (B) In a separate experiment, recipients were vaccinated with C1498-lysate–pulsed DCs (as in Figure 2) or irradiated C1498 cellular vaccines (107 cells per mouse) given on days −14 and −7 or left untreated. On day 60, mice were challenged with C1498 cells (2 × 105). Mice given AML-lysate–loaded DC vaccines or irradiated whole cellular AML vaccines 71 days earlier had a memory cell response since 40% and 50% of mice challenged with a supralethal AML cell dose (2 × 105 cells) survived long term as compared with 0% of nonvaccinated controls (P = .00055; P = .00037 vs control, respectively). There was no significant difference (P = .32) between these 2 vaccination approaches in terms of anti-AML memory responses.
To determine whether DC vaccinations could result in a memory cell response against AML cells, DC-vaccinated mice were challenged on day 60 (71 days after the last DC vaccine), the same time point as for Flt3L (Figure 3B). As a control for memory cell generation, a cohort was given irradiated AML cellular vaccines (107 cells per injection) subcutaneously on days −14 and −7, which has been found to be a highly effective means of generating splenic anti-AML CTLPs and resistance to AML challenge (Boyer et al39; see also Figure 5). Mice given AML-lysate–loaded DC vaccines or irradiated cellular AML vaccines 71 days earlier had a memory cell response since 40% or 50%, respectively, of mice challenged with a supralethal AML cell dose (2 × 105 cells) survived long term as compared with 0% of nonvaccinated controls (P = .00055;P = .00037 vs control, respectively) (Figure 3B). There was no significant difference in survival (P = .32) between these 2 vaccination approaches in terms of anti-AML memory responses. These data are most consistent with the induction of memory T cells by DC vaccines.
Neither AML DCs, Flt3L, nor irradiated cellular vaccines confer protection against AML challenge in most naive animals with established disease
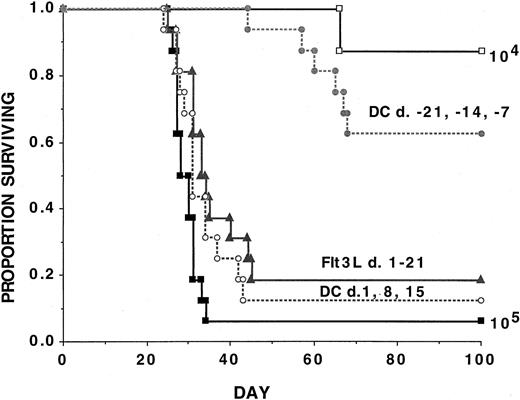
To define whether DC vaccines or Flt3L were effective in treating established disease, experiments were performed by infusing 3 consecutive weekly vaccinations with AML-lysate–pulsed DC vaccines or daily Flt3L for 3 weeks beginning 1 day after AML challenge (Figure4). As a positive control, a cohort received DC vaccines prior to AML challenge according to the above schedule. An additional group received a lower AML cell dose (104) to permit quantification of the degree of reduction of tumorigenicity. Recipients of DC vaccines initiated prior to AML challenge had a 63% long-term survival rate, consistent with an approximate 10-fold reduction in the number of live AML cells that escaped immune-mediated elimination. In contrast, recipients given either DC vaccines initiated post–AML-challenge or Flt3L had survival rates of 13% and 19%, respectively, versus 6% in non-treated controls (Figure 4). Although these approaches were significantly (P ≤ .05) superior to no treatment, in both instances, the vast majority of mice could not withstand live AML challenge, indicating only a very modest survival advantage.
AML-lysate–pulsed DC vaccines initiated prior to AML challenge reduce AML tumorigenicity by approximately 10-fold, but neither DC vaccines nor Flt3L will protect mice from AML tumorigenicity if initiated as late as 1 day after AML challenge.
Cohorts of naive B6 mice received either 3 weekly doses of tumor-lysate–pulsed DCs (beginning on day −21 or +1, as indicated) or 21 daily subcutaneous Flt3L (30 μg per dose) injections (days +1 to +21) or no treatment followed by a lethal leukemia dose (105 per mouse) on day 0. Results are pooled from 2 independent experiments with similar results (n = 16 mice per group). An additional cohort of control mice was given a lower leukemia cell dose (104 per mouse) (n = 8 mice). A Kaplan-Meier survival plot shows days post–lethal C1498 challenge (day 0) on thex-axis and the proportion of mice surviving on they-axis. The actuarial survival rate of recipients given DC vaccines initiated 3 weeks prior to AML challenge was significantly (P = 000021) higher than controls but not significantly different (P ≥ .1) from recipients of a 10-fold lower AML cell dose (104 per mouse). The actuarial survival rate of recipients of either DC-vaccinated or Flt3L-treated recipients initiated 1 day after live AML challenge was statistically significantly higher (P ≤ .05) than the control animals although the vast majority of animals succumbed to leukemia.
AML-lysate–pulsed DC vaccines initiated prior to AML challenge reduce AML tumorigenicity by approximately 10-fold, but neither DC vaccines nor Flt3L will protect mice from AML tumorigenicity if initiated as late as 1 day after AML challenge.
Cohorts of naive B6 mice received either 3 weekly doses of tumor-lysate–pulsed DCs (beginning on day −21 or +1, as indicated) or 21 daily subcutaneous Flt3L (30 μg per dose) injections (days +1 to +21) or no treatment followed by a lethal leukemia dose (105 per mouse) on day 0. Results are pooled from 2 independent experiments with similar results (n = 16 mice per group). An additional cohort of control mice was given a lower leukemia cell dose (104 per mouse) (n = 8 mice). A Kaplan-Meier survival plot shows days post–lethal C1498 challenge (day 0) on thex-axis and the proportion of mice surviving on they-axis. The actuarial survival rate of recipients given DC vaccines initiated 3 weeks prior to AML challenge was significantly (P = 000021) higher than controls but not significantly different (P ≥ .1) from recipients of a 10-fold lower AML cell dose (104 per mouse). The actuarial survival rate of recipients of either DC-vaccinated or Flt3L-treated recipients initiated 1 day after live AML challenge was statistically significantly higher (P ≤ .05) than the control animals although the vast majority of animals succumbed to leukemia.
Our previous studies indicated that irradiated AML whole cellular vaccines could augment anti-AML CTLP by 6- to 28-fold as compared with nonimmunized mice, which resulted in a high proportion of mice surviving challenge with live AML cells.39 To determine whether irradiated AML cellular vaccines would be effective under conditions in which neither DC vaccines nor Flt3L are effective, cohorts received 2 consecutive weekly irradiated AML cellular vaccines at various time periods (beginning on day −21 until up to day +14) relative to live AML challenge (Figure5). Recipients given irradiated AML cellular vaccines beginning on day −21 were fully protected against live AML challenge. Recipients given irradiated AML cellular vaccines initiated up until the day of live AML challenge had a significant (P ≤ .0025) survival advantage as compared with nonimmunized controls. There was no survival advantage for recipients of irradiated AML cellular vaccines unless these were initiated prior to or on the day of live AML challenge. These data collectively indicate that DC vaccines and Flt3L must be given prior to AML challenge to provide protection to the majority of mice.
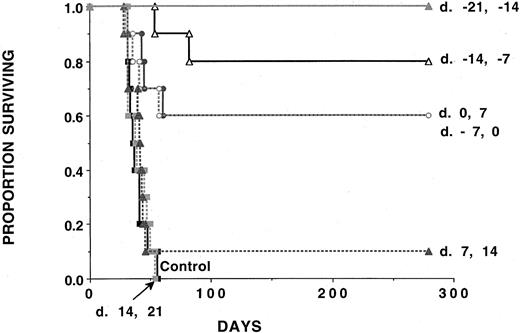
Irradiated AML cellular vaccines are ineffective against AML challenge if initiated after challenge with a lethal dose of AML cells.
Cohorts of naive B6 mice (n = 10 per group) received either 2 doses of irradiated AML cells (107 per mouse) administered subcutaneously 1 week apart beginning at time periods ranging from day −21 to day +14 relative to C1498 challenge (105 cells per mouse) on day 0. In each group, 10 mice were analyzed. A Kaplan-Meier survival plot shows days post–lethal C1498 challenge (day 0) on thex-axis and the proportion of mice surviving on they-axis. The actuarial survival rate of AML-vaccinated recipients that had AML vaccines initiated at time periods ranging from days −21 to +0 was significantly higher (P ≤ .0025) than the control animals or animals receiving AML vaccines initiated after this time. There was no survival advantage to mice that were given C1498 vaccines initiated on days +7 or +14 relative to C1498 challenge (P ≥ .27).
Irradiated AML cellular vaccines are ineffective against AML challenge if initiated after challenge with a lethal dose of AML cells.
Cohorts of naive B6 mice (n = 10 per group) received either 2 doses of irradiated AML cells (107 per mouse) administered subcutaneously 1 week apart beginning at time periods ranging from day −21 to day +14 relative to C1498 challenge (105 cells per mouse) on day 0. In each group, 10 mice were analyzed. A Kaplan-Meier survival plot shows days post–lethal C1498 challenge (day 0) on thex-axis and the proportion of mice surviving on they-axis. The actuarial survival rate of AML-vaccinated recipients that had AML vaccines initiated at time periods ranging from days −21 to +0 was significantly higher (P ≤ .0025) than the control animals or animals receiving AML vaccines initiated after this time. There was no survival advantage to mice that were given C1498 vaccines initiated on days +7 or +14 relative to C1498 challenge (P ≥ .27).
AML-lysate–pulsed DCs or Flt3L treatment can induce a potent anti-AML immune response in syngeneic BMT recipients when initiated prior to AML challenge
To evaluate the effects of DC vaccines or Flt3L on generating an anti-AML immune response in syngeneic BMT recipients, B6 mice were lethally irradiated on day −1 and infused with unmanipulated syngeneic BM on day 0. Our previous studies indicated that central and peripheral T-cell recovery in B6 syngeneic BMT mice occurs by 2 months post-BMT as assessed by absolute cell numbers and by proliferative responses.45 Therefore, cohorts received either AML-lysate–pulsed DCs on days 59, 66, and 72 or daily Flt3L injections on days 70 to 91 (Figure 6A). A separate cohort of mice received no treatment and served as controls. On day 80 post-BMT, mice were challenged with a lethal dose of AML. As compared with controls, which succumbed to leukemia by day 120 post-BMT, survival was higher in recipients given either DC vaccines (P < .001) or Flt3L (P = .01); 25% of recipients in the latter two groups survived 200 days post-transplant (Figure 6A). Evaluation of splenic anti-AML CTLP expansion was conducted on the day of the AML challenge (day 80). The absolute number of anti-AML–reactive CTLPs/spleen was calculated (Figure 6B). As compared with BMT controls, significant (P = .01) anti-AML–reactive CTLP expansion was 6.8-fold higher in the DC-vaccinated group. Splenic anti-AML–reactive CTLPs were only modestly increased in Flt3L-treated mice (1.4-fold) as compared with BMT controls. As compared with the non-BMT group, recipients of syngeneic BMT without any additional treatment had significantly (P = .05) lower numbers of splenic anti-AML–reactive CTLPs (44% of control numbers). Thus, despite the lower baseline splenic anti-AML CTLPs in BMT as compared with non-transplanted mice, DC vaccines were able to markedly increase anti-AML CTLPs to a sufficiently high level that 25% of mice were able to survive an otherwise lethal AML dose.
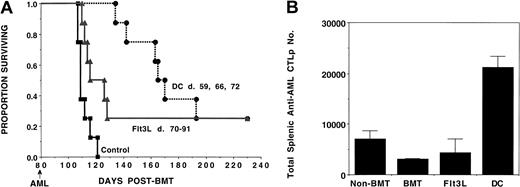
Tumor-pulsed DCs or Flt3L treatment induces antileukemia immunity in syngeneic BMT recipients.
B6 recipients were lethally irradiated on day −1 and infused with syngeneic B6 BM on day 0. Cohorts of recipients (n = 8-10) received either 3 weekly doses of tumor-pulsed DCs (days 59, 66, and 72), or daily Flt3L injections (day 70 to 91) or no treatment (controls) with lethal (105) C1498 challenge on day 80. (A) A Kaplan-Meier survival plot shows days post-BMT on the x-axis and the proportion surviving on the y-axis. The actuarial survival rate of tumor-pulsed DC or Flt3L recipients was significantly higher (P < .001, P = .01, respectively) than control animals receiving BMT only. (B) On day 80, 2 mice in each group were killed for splenic C1498 leukemia-reactive CTLP frequency estimation. The mean absolute splenic CTLP number ± SD for each group is shown on the y-axis. Significant (P = .01) CTLP increase was detected in the DC-treated group as compared with BMT-only controls or Flt3L-treated mice. Recipients of only syngeneic BMT had significantly (P = .05) lower CTLP frequency than naive animals.
Tumor-pulsed DCs or Flt3L treatment induces antileukemia immunity in syngeneic BMT recipients.
B6 recipients were lethally irradiated on day −1 and infused with syngeneic B6 BM on day 0. Cohorts of recipients (n = 8-10) received either 3 weekly doses of tumor-pulsed DCs (days 59, 66, and 72), or daily Flt3L injections (day 70 to 91) or no treatment (controls) with lethal (105) C1498 challenge on day 80. (A) A Kaplan-Meier survival plot shows days post-BMT on the x-axis and the proportion surviving on the y-axis. The actuarial survival rate of tumor-pulsed DC or Flt3L recipients was significantly higher (P < .001, P = .01, respectively) than control animals receiving BMT only. (B) On day 80, 2 mice in each group were killed for splenic C1498 leukemia-reactive CTLP frequency estimation. The mean absolute splenic CTLP number ± SD for each group is shown on the y-axis. Significant (P = .01) CTLP increase was detected in the DC-treated group as compared with BMT-only controls or Flt3L-treated mice. Recipients of only syngeneic BMT had significantly (P = .05) lower CTLP frequency than naive animals.
We have previously reported that vaccination with irradiated C1498 was a highly potent means of inducing anti-AML CTLPs and conferring protection against AML challenge (Boyer et al39; Figures3B, 5). To determine whether DC vaccines are more potent than irradiated AML vaccines under these BMT conditions, syngeneic BMT recipients were given weekly AML-lysate–pulsed DCs or irradiated AML vaccines according to established vaccination doses and schedules39 (Figure 7). A cohort was vaccinated with unpulsed DCs as a specificity control, and a separate cohort of BMT recipients was given neither AML cells nor vaccinations. Recipients given either AML-lysate–pulsed DC vaccines or irradiated cellular AML vaccines had a 30% long-term survival rate, significantly higher than the 0% long-term survival rate for recipients given either no vaccines or unpulsed DCs.
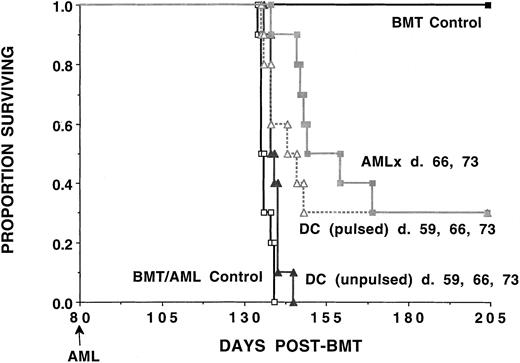
Immunization of syngeneic BMT recipients with either AML-lysate–pulsed DCs or irradiated AML cellular vaccines results in a significant decrease in leukemia-related mortality.
B6 recipients were lethally irradiated on day −1 and infused with syngeneic B6 BM on day 0. Cohorts of recipients (n = 10 per group) received either no manipulation (BMT/AML control) or 3 weekly doses of tumor-pulsed or nonpulsed DCs (days 59, 66, and 72) or irradiated AML vaccines (107 per dose) given subcutaneously (days 66 and 73). Mice were challenged with a lethal dose of C1498 cells (105) on day 80 post-BMT. A separate group of mice received syngeneic BMT but were not challenged with C1498 cells. A Kaplan-Meier survival plot shows days post-BMT listed on the x-axis and the proportion surviving on the y-axis. The actuarial survival rate of recipients of tumor-pulsed DCs or irradiated AML cellular vaccines was significantly higher (P < .006) than control animals receiving BMT only or recipients receiving unpulsed DCs.
Immunization of syngeneic BMT recipients with either AML-lysate–pulsed DCs or irradiated AML cellular vaccines results in a significant decrease in leukemia-related mortality.
B6 recipients were lethally irradiated on day −1 and infused with syngeneic B6 BM on day 0. Cohorts of recipients (n = 10 per group) received either no manipulation (BMT/AML control) or 3 weekly doses of tumor-pulsed or nonpulsed DCs (days 59, 66, and 72) or irradiated AML vaccines (107 per dose) given subcutaneously (days 66 and 73). Mice were challenged with a lethal dose of C1498 cells (105) on day 80 post-BMT. A separate group of mice received syngeneic BMT but were not challenged with C1498 cells. A Kaplan-Meier survival plot shows days post-BMT listed on the x-axis and the proportion surviving on the y-axis. The actuarial survival rate of recipients of tumor-pulsed DCs or irradiated AML cellular vaccines was significantly higher (P < .006) than control animals receiving BMT only or recipients receiving unpulsed DCs.
To determine whether DC vaccines or Flt3L can support an anti-AML immune response in the presence of the established disease post-BMT, DC or Flt3L was administered beginning 1 day after a lethal dose of AML cells (day 80 post-BMT). Cohorts of BMT animals received either 3 weekly doses of tumor-pulsed DCs (days 81, 87, and 94), daily Flt3L injections (days 81 to 101), or no treatment (controls). Both treatments failed to eradicate established AML post-syngeneic BMT, with all mice succumbing to AML between days 112 and 121 post-BMT (not shown). To achieve long-term survival under these syngeneic BMT conditions, immune stimulation via DC vaccines or Flt3L needs to be initiated prior to AML challenge.
Discussion
Our initial goal was to compare the efficacy of 2 approaches directed toward harnessing the antitumor benefits of APCs: (1) the in vivo infusion of tumor-lysate–pulsed DCs generated ex vivo from BM–derived DC precursors and (2) Flt3L, which generates DCs from precursors present in vivo. Our major findings are that AML-lysate–pulsed DC vaccines and Flt3L provide substantial protection against subsequent AML challenge but not against established disease. Despite the known immune-deficiency state associated with BMT and the lower number of splenic anti-AML CTLPs present in transplanted as compared with nontransplanted controls, syngeneic BMT recipients challenged 80 days post-BMT could mount a sufficient anti-AML immune response, resulting in a significant prolongation in survival after DC vaccination. Flt3L given post–syngeneic BMT resulted in a comparable level of protection against an otherwise lethal AML dose, as compared with non-treated BMT controls. However, both treatments had to be initiated prior to AML challenge since no protective effect was observed with either treatment if therapy was initiated after AML challenge. Nonetheless, our data suggest that autologous BMT recipients may derive benefit from either AML-lysate–pulsed DC vaccines or Flt3L initiated at approximately 2 months post-BMT at a time when disease is at a minimum and disease progression is not sufficiently rapid to preclude the generation of a potent anti-AML immune response.
Studies by others indicated the role of DC vaccines in solid tumor eradication in rodents (eg, Boczkowski et al,9 Zitvogel et al,10 Ashley et al,11 and Young and Inaba22). In this report, we provide evidence for in vivo efficacy of BM-derived DC-based vaccination against a widely metastatic systemic hematological malignancy, AML. We found that DC vaccines were more effective than Flt3L in facilitating the development of splenic anti-AML CTLPs despite the fact that DCs produced by Flt3L are known to be effective in inducing T-cell responses both in vitro and in vivo.46 47 In subsequent studies, we have been able to show that even one DC vaccine given on day −7 prior to AML cell challenge could protect 20% of mice from a supralethal AML challenge dose of 2 × 106 cells, consistent with a greater than 10-fold reduction in AML cell burden (not shown). AML-lysate pulsing of DCs was required for optimal resistance since recipients of unpulsed DC vaccines all succumbed to AML. However, unpulsed DCs did induce a nonspecific response to AML since these recipients survived 1.5 weeks longer than nonvaccinated controls. Although the prolonged survival effect of unpulsed DCs is presumably due to fetal calf serum components used to propagate the DC and AML cells, this effect was not sufficient to result in long-term survival after supralethal AML challenge in non-BMT or in syngeneic BMT recipients (see Figure 7).
In contrast, splenic anti-AML CTLPs were increased far less with Flt3L as compared with DC vaccines. While NK depletion completely eliminated the protective effect of Flt3L, only part of the effect of DC vaccines was NK dependent. Flt3L expands the absolute number and function of NK cells in multiple tissue compartments.33 Flt3L induction of NK cells has been shown to have antitumor effects in a murine liver-metastasis model.35 Lynch46 has demonstrated that there is a substantial decrease in the rate of fibrosarcoma growth in T-cell–deficient severe combined immune–deficient mice receiving Flt3L when compared with non-treated controls. For NK-sensitive tumor cells, Flt3L may be a useful therapeutic agent by directly stimulating NK effector cell expansion and function. NK cells also may contribute to the antitumor effect of DC vaccines, as shown in Figure 2B. Recent data indicate that DCs can directly trigger NK cell function.48
Consistent with findings that NK cells were major and perhaps sole effectors of primary resistance in Flt3L-treated recipients, rechallenge experiments in Flt3L-treated mice indicated no detectable memory cell response. These data would appear to differ somewhat from those of Wang et al,47 who have shown that a T-cell memory response could be induced to a bcr/abl gene–transfected cell line that was transduced with a retroviral vector that produced Flt3L. Important differences may relate to the use of transduced cells, which may produce Flt3L longer than the 21 days administered here, as well as potential differences in tumor cell lines and mouse strains. In contrast, DC-vaccinated mice had a memory cell response, consistent with an induction of anti-AML CTLPs, as assessed 2.5 months after vaccination. Antigen-loaded DCs have been shown to be able to stimulate CD4+ T-cell responses and can support the generation of anti-tumor–reactive CTLs without the requirement for CD4+T-cell help.49-51 Despite the fact that DC vaccines and Flt3L may stimulate different effector cell types, our results were similar in terms of in vivo resistance to initial AML challenge. Flt3L would be logistically simpler than the propagation and infusion of large numbers of tumor-membrane–pulsed DCs. For patients with myeloid leukemias, consideration must be given to the observation that a high proportion of blasts from these patients, particularly those with AML, express functional Flt3 receptors.52-57 In these circumstances, DC vaccines rather than Flt3L would avoid the issue of whether Flt3L would have a beneficial or detrimental effect on AML elimination and recurrence. This was not an issue as the AML line we used does not respond to Flt3L as assessed by in vitro proliferation (unpublished observations).
An important aspect of our studies was the finding that syngeneic BMT recipients could benefit from an immune-based anti-AML strategy initiated post-BMT. Although we have previously shown that the infusion of donor splenocytes given early post-allogeneic BMT could provide a potent GVL effect, in the present studies only syngeneic effector cells would be available to mediate an antitumor effect. T cells generated post-BMT may not be fully immune-competent owing to defects in the thymic micro-environment and other types of injury.58 At the time of AML challenge post-BMT, the splenic anti-AML CTLP frequency was significantly lower than in normal controls. Our study has shown that despite the lower CTLP frequency post-BMT, DC vaccines and Flt3L were each able to confer a degree of resistance against AML. A recent report demonstrates proof in principle that multiple myeloma patients who had received autologous peripheral blood stem cells months earlier could be induced to mount an antigen-specific immune response to nominal antigen (keyhole limpet hemocyanin) or tumor antigen (idiotypic protein).15 Our data in syngeneic BMT recipients given AML-lysate–pulsed DCs indicate that such responses could lead to a long-term survival benefit.
In syngeneic BMT recipients, Flt3L administration also resulted in a long-term survival benefit, probably owing to the augmentation of NK cell–mediated elimination of AML cells as observed in non-transplanted recipients. NK cell recovery post-BMT is rapid while T-cell recovery typically requires a longer period of time owing to the requirements for thymic education.59-61 Notably, NK cells present early post-BMT have been shown to be potent effectors against myeloid leukemias.61 Thus, Flt3L administered to humans may permit the development of an immune response against myeloid leukemias. Since T-cell defects have been reported in humans for prolonged periods of time post-BMT and since NK activity is restored far earlier than T-cell function post-BMT, it is conceivable that Flt3L would be a particularly advantageous approach at this time as Flt3L appears to be a potent inducer of NK effects against AML cells. However, since there is no memory cell response with Flt3L, DC vaccines may be more advantageous at time periods when T-cell reconstitution has occurred since T cells appear to contribute more to the DC vaccine than the Flt3L-mediated anti-AML effect.
In contrast to reports by others in solid tumor models (reviewed in Lotze et al19), both AML-pulsed DC and Flt3L do not provide substanial protection in animals with established AML. C1498, the AML line used in the present studies, is injected systemically and grows as a widespread metastatic disease. The failure of DC vaccines or Flt3L to treat the minimal residual disease in mice given AML cells only 1 day prior to therapy could be related to either the fast growth rate of AML cells in vivo, which outstrips the ability of the immune system to resist the AML cells, or the immunosuppression conferred by an expanding tumor burden. Our previous studies have shown that splenic anti-AML CTLP generation was not suppressed in mice given high doses of AML cells (106) and analyzed 3 weeks later at a time when AML cells were not detectable by flow cytometry.39 Thus, we favor the former explanation. Therefore, these approaches are likely to be most effective in patients with more indolent leukemias.
In conclusion, we have shown that DC vaccines and Flt3L can be used as the potent inducers of an anti-AML immunity with comparable efficacy in non-transplanted and syngeneic BMT recipients. They appear to play critical roles in generating anti-AML responses in both mature and developing (regenerating) immune systems. Flt3L is a potent inducer of NK-mediated AML resistance, while DC vaccines elicit NK and T-cell responses against AML cells. Biological efficacy will probably be realized only in settings in which AML cells are not expanding at a rate that overwhelms the immune response. The potential therapeutic implications of our work are substantial.
Supported in part by the Children's Cancer Research Fund and National Institutes of Health grants R01 CA72669 and T32 CA71340.
A.P. and S.H. contributed equally to this work.
H.M. is an employee of Immunex Corporation, which provided the Flt3L protein.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce R. Blazar, University of Minnesota, Department of Pediatrics, Division of Bone Marrow Transplantation, Box 109 UMHC, 420 Delaware St SE, Minneapolis, MN 55455 e-mail:blaza001@tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal