Thymic-dependent differentiation of bone marrow (BM)-derived progenitors and thymic-independent antigen-driven peripheral expansion of mature T cells represent the 2 primary pathways for T-cell regeneration. These pathways are interregulated such that peripheral T-cell expansion is increased in thymectomized versus thymus-bearing hosts after bone marrow transplantation (BMT). This study shows that this interregulation is due to competition between progeny of these 2 pathways because depletion of thymic progeny leads to increased peripheral expansion in thymus-bearing hosts. To test the hypothesis that competition for growth factors modulates the magnitude of antigen-driven peripheral expansion during immune reconstitution in vivo, a variety of T-cell active cytokines were administered after BMT. Of the cytokines (interleukins) tested (IL-3, IL-12, IL-6, IL-2, and IL-7), IL-2 modestly increased peripheral expansion in the face of increasing numbers of thymic emigrants, whereas IL-7 potently accomplished this. This report also demonstrates that the beneficial effect of IL-7 on immune reconstitution is related to both increases in thymopoiesis as well as a direct increase in the magnitude of antigen-driven peripheral expansion. Therefore, the administration of exogenous IL-7, and to a lesser extent IL-2, abrogates the down-regulation in antigen-driven peripheral expansion that occurs in thymus-bearing hosts after BMT. These results suggest that one mechanism by which T-cell–depleted hosts may support antigen-driven T-cell expansion in vivo is via an increased availability of T-cell–active cytokines to support clonal expansion.
Introduction
Current concepts hold that factors regulating the outcome of a T-cell activation event in vivo relate to the strength of the T-cell receptor-antigen (TCR/Ag) interaction, the quality and quantity of costimulatory interactions available at the time of TCR engagement, as well as the availability of growth factors required to support clonal expansion. In addition, it is generally appreciated that in T-cell–depleted hosts, a heretofore poorly understood mechanism results in the increased expansion of transferred mature T-cell populations.1,2 Several investigators have shown a requirement for major histocompatibility complex (MHC) interaction in the expansion of such transferred T-cell populations3-7and expansion is greatly increased in the presence of cognate antigen.4 We have shown previously that antigen-driven peripheral expansion is increased in athymic T-cell–depleted hosts compared to thymus-bearing T-cell–depleted mice, providing direct evidence that antigen-driven responses are modulated in vivo as a result of differences in host T-cell number.4 Despite these descriptive observations, the mechanisms by which T-cell depletion results in an increased capacity for antigen-driven clonal expansion in vivo remain undefined.
One model in which the role of T-cell depletion in modulating antigen-driven expansion in vivo can be studied involves the evaluation of antigen-driven peripheral expansion in thymus-bearing versus thymectomized hosts after T-cell depletion. In this model, thymectomized hosts reconstitute T cells primarily via antigen-driven peripheral expansion, whereas thymus-bearing mice, which have both thymic-dependent and thymic-independent pathways available, rely primarily on thymic-dependent pathways and down-regulate antigen-driven peripheral expansion. In this study, we used a murine model of congenic bone marrow transplantation (BMT) to assess the interregulation between thymic-dependent and thymic-independent T-cell regeneration in T-cell–depleted thymus-bearing versus thymectomized hosts. Our results show that the up-regulation of peripheral expansion in athymic hosts, which was previously observed in this model, is a result of the absence of recent thymic emigrants in athymic BMT recipients. Furthermore, we show that increasing the availability of T-cell growth cytokines (interleukin [IL]-7 and IL-2) can significantly modulate the relative size of peripheral T-cell pools following BMT and we identify IL-7 as uniquely capable of potently modulating both thymic-dependent and thymic-independent pathways of T-cell regeneration simultaneously. Together these results show that IL-7 can substantially modulate peripheral T-cell expansion after T-cell depletion and raise the possibility that the relative availability of endogenous IL-7 could contribute to the modulation of antigen-driven expansion that occurs as a result of variations in T-cell numbers in vivo.
Materials and methods
Mice
Mice were purchased from the Animal Production Unit, National Cancer Institute, Frederick, MD. C57BL/6 (B6/Thy 1.2)(H-2b) females were used as syngeneic bone marrow (BM) recipients and as Ld-nonexpressing hosts. B6D2F1(H-2b/d) mice were used as Ld-expressing hosts. BM donors and recipients were 8 to 12 weeks of age at the time of BMT. B6 PL Thy 1a (B6/Thy 1.1)(H-2b) females were used as lymph node (LN) donors at 12 to 16 weeks of age. Mice bearing the transgenic (Tg+) TCR 2C, which recognizes the MHC molecule Ld, were kindly provided by Dr Dennis Loh (Washington University, St Louis, MO).8
Thymectomies
At 4 to 6 weeks of age, mice were anesthetized with Xylazine 20 mg/mL (Mobay, Shawnee, KS) and ketamine 100 mg/mL (A. J. Buck, Owings Mills, MD) via intraperitoneal injection at approximately 0.20 mL/mouse. Suction thymectomy was performed through a sternal incision via approved protocol. Completeness of thymectomy was verified in each animal by visual inspection at the time of sacrifice.
BMT
Bone marrow was obtained by the passage of iced RPMI medium through the tibias and femurs of donor mice. Mature T cells were depleted by treatment with a mixture of anti–T-cell monoclonal antibodies (mAbs; HO-13-4, 83-12-5, C3PO)9-11 and guinea pig complement (Gibco BRL, Grand Island, NY). This was followed by rabbit antimouse brain antibody and complement as previously described.12 BM depleted of T cells was confirmed by flow cytometry to consistently contain less than 1% residual T cells. Recipient mice were lethally irradiated with 1000 cGy 137Cs gamma radiation (Gamma Cell 40) at a dose of 100 to 110 cGy/min and were injected within 8 hours with 10 × 106 BM cells. To obtain LN cells, axillary and inguinal nodes were harvested, minced with scissors in iced RPMI, filtered through nylon mesh, washed, and counted by hemocytometer. LN inocula were suspended in 0.2 mL RPMI and combined with the BM into one syringe for injection.
Treatment with mAbs
The anti-Thy 1.2 hybridoma 30-H-12 was obtained from American Tissue Culture Collection (Rockville, MD). Ascites was generated by injection of 1 × 106 hybridoma cells intraperitoneally into pristane-primed B10 nude mice. Ascites was harvested by repeated aspiration and clarified by centrifugation. Ascites (0.4 mL) was injected weekly into recipient mice; this was a dose shown to deplete more than 95% of Thy 1.2+ cells in vivo (data not shown).
Cyclosporin A treatment
Cyclosporin A (CsA) was purchased from Sandoz Pharmaceuticals (Hanover, NJ) and suspended in olive oil for injection. Sham-treated recipients received olive oil injections alone. CsA was administered at a dose of 15 mg/kg per day for 14 days after BMT followed by 15 mg/kg every other day from days 14 to 42 after BMT.
Immunofluorescence staining and flow cytometric analysis
Splenocytes from mice were studied at the times noted following BMT. Spleens from mice to be studied were removed and a single-cell suspension was prepared and passed through nylon gauze. Red blood cells (RBCs) were lysed with ammonium chloride lysing buffer and the cells were washed, counted, and suspended in Hanks balanced salt solution (HBSS) without phenol red with 0.2% human serum albumin (HSA; American Red Cross, Washington, DC) and 0.1% sodium azide. For 2-color direct immunofluorescence staining, 1 × 106 cells were incubated at 4°C for 10 minutes with rat antibody 2.4G2 to block Fc receptors followed by 20 minutes with biotin-conjugated antibody. The cells were then washed twice, incubated at 4°C for 20 minutes with fluorescein isothiocyanate (FITC)-conjugated antibody, then incubated at 4°C for 10 minutes with phycoerythrin (PE) streptavidin (Caltag, South San Francisco, CA). They were then washed, resuspended, and analyzed. In experiments where directly PE-conjugated antibodies were used, the FITC- and PE-conjugated antibodies were added simultaneously during the first step. The mAbs used for staining were FITC-conjugated Thy 1.1 (15.5.87, Serotec, Oxford, England); FITC- or biotin-conjugated Thy 1.2, anti-CD8 (Becton Dickinson, San Jose, CA); anti-CD4 (Gibco BRL); anti-CD45RB (23G2) and anti-CD44 (Pgp-1) (Pharmingen, San Diego, CA). Isotype control mAb were FITC- or biotin-conjugated Leu 4 (Becton Dickinson), which are murine mAbs specific for human cells and do not bind murine cells.
Two-color flow cytometry analyses were performed using a FACScan (Becton Dickinson). Fluorescence data were collected using 3-decade logarithmic amplification on 10 000 viable splenocytes as determined by forward and perpendicular light scatter intensity as previously described.13 The percentage of positive cells was calculated from contour diagrams with log intensities of green fluorescence on the x-axis and log intensities of red fluorescence on the y-axis.
Administration of cytokines
Cytokines were reconstituted according to manufacturer's instructions and resuspended in buffer containing 5% sucrose and 0.1% HSA in phosphate-buffered saline (PBS). Cytokines were administered as a continuous subcutaneous infusion via osmotic pumps (Alza, Mountain View, CA) for 14-day courses after BMT. Pump placement was performed under anesthesia according to approval protocols. In experiments where cytokines were administered for 28 days, the first pump was removed and a second pump implanted at the 14-day time point. The doses selected were based on tolerable doses noted in our laboratory for each cytokine as well as the availability of cytokine. In mice receiving IL-6 and IL-3, cytokines were administered just before BMT, from day −1 to day 27. IL-6 was administered at 20 μg/d and IL-3 at 2 μg/d. In the case of recombinant human IL-2 (rhIL-2) and recombinant murine (rmIL-12), cytokine administration was delayed because of previous evidence for toxicity when administered in the context of BMT. IL-2 was administered at 30 000 IU/d from days 14 to 28 after BMT in initial experiments and subsequently from days 28 to 42 after BMT where indicated. The rmIL-12 was administered from days 14 to 28 after BMT at a dose of 10 ng/d in initial experiments and subsequently at a dose of 100 ng/d where indicated. In the case of rhIL-7, availability of cytokine was limited and therefore it was administered for 14 days only on days 14 to 28 after BMT at a dose of 5 mg/day.
Results
Peripheral expansion is reduced in thymus-bearing hosts due to the emergence of recent thymic emigrants in the periphery
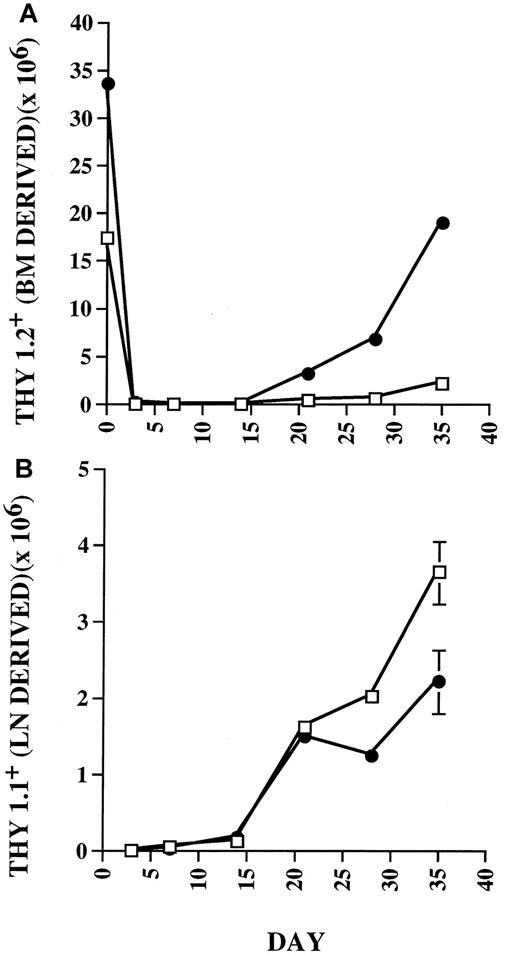
To compare the expansion of mature T cells following BMT in thymus-bearing versus thymectomized hosts, C57BL/6 Thy 1.2+mice underwent lethal irradiation and received 10 × 106syngeneic T-cell–depleted BM cells as a source of prethymic progenitors, and 5 × 105 B6/Thy 1.1+ LN cells as a source of postthymic progenitors. Analysis of reconstituting Thy+ cell populations using flow cytometry was carried out weekly during the first 5 weeks after BMT to study the relative contribution of cells reconstituted via thymic-dependent versus thymic-independent pathways of T-cell regeneration. As shown in Figure1, in thymus-bearing hosts, substantial numbers of BM-derived Thy 1.2+ cells appeared in the periphery after 21 days. In contrast, thymectomized mice showed minimal numbers of BM-derived progeny throughout the period of evaluation. With regard to LN-derived Thy 1.1+ cells, which represent progeny of postthymic progenitors, equivalent expansion was seen in both thymus-bearing and thymectomized recipients during the first 21 days. However, after this time point, thymus-bearing recipients showed diminished numbers of peripherally expanding cells compared to thymectomized hosts. These results suggested that the diminished expansion observed in thymus-bearing hosts was related to the thymic progeny.
Peripheral expansion in thymus-bearing hosts is down-regulated coincident with the appearance of recent thymic emigrants in the periphery.
Thymus-bearing (●) and thymectomized (□) C57BL/6 mice were lethally irradiated and given 10 × 106 Thy 1.2 T-cell–depleted BM cells and 5 × 105 Thy 1.1 congenic LN cells. Cohorts of 3 animals from each group were studied before BMT and at 3, 7, 14, 21, 28, and 35 days after BMT using flow cytometry to quantitate the absolute numbers of Thy 1.2+ (A) versus Thy 1.1+ (B) cells in the spleen. Statistical differences (P < .05) by Mann-Whitney test. SEs of the mean are shown by error bars. Statistically significant differences were observed at day 0, 3, 14, 21, 28, and 35 for Thy 1.2+ cell numbers and at day 7, 28, and 35 for Thy 1.1+ cell numbers.
Peripheral expansion in thymus-bearing hosts is down-regulated coincident with the appearance of recent thymic emigrants in the periphery.
Thymus-bearing (●) and thymectomized (□) C57BL/6 mice were lethally irradiated and given 10 × 106 Thy 1.2 T-cell–depleted BM cells and 5 × 105 Thy 1.1 congenic LN cells. Cohorts of 3 animals from each group were studied before BMT and at 3, 7, 14, 21, 28, and 35 days after BMT using flow cytometry to quantitate the absolute numbers of Thy 1.2+ (A) versus Thy 1.1+ (B) cells in the spleen. Statistical differences (P < .05) by Mann-Whitney test. SEs of the mean are shown by error bars. Statistically significant differences were observed at day 0, 3, 14, 21, 28, and 35 for Thy 1.2+ cell numbers and at day 7, 28, and 35 for Thy 1.1+ cell numbers.
Because down-regulation of peripheral expansion in thymus-bearing hosts could potentially relate to indirect effects of the thymus itself as opposed to effects of thymic emigrants, we sought to evaluate peripheral expansion in hosts in which the generation of recent thymic emigrants in thymus-bearing hosts was inhibited. To accomplish this, chimeras were generated using B6/Thy 1.2+ hosts, B6/Thy 1.2+ BM, and B6/Thy 1.1+ LN inocula and a depleting mAb directed toward Thy 1.2 was administered after BMT. As shown in Table 1, LN cell expansion was increased compared to sham-treated controls at a magnitude similar to that observed within thymectomized hosts. Furthermore, to provide evidence that the increased peripheral expansion in this experiment was related to inhibition of thymic emigrants rather than by nonspecific effects related to ascites, animals were treated in separate experiments with CyA. Although CyA therapy abrogated the development of thymic emigrants as previously described,14 this therapy did not abrogate peripheral expansion. Rather, peripheral expansion was increased approximately 2-fold in CyA-treated thymus-bearing hosts compared to sham-treated recipients, similar to the effects observed in anti-Thy–treated hosts (data not shown). Therefore, although a variety of nonspecific effects (eg, thymectomy, ascites therapy) in these various experimental models potentially prevent definitive conclusions from individual experiments, taken together, the data strongly suggest that the enhanced peripheral expansion observed in thymic-deficient hosts after BMT occurs in response to diminished numbers of thymic progeny. These results raised the possibility that competition for soluble or cell-associated factors might exist within the periphery, which results in a reduction in peripherally expanded cells in the presence of thymic emigrants. Furthermore, although delineation of the relative “fitness” of recent thymic emigrants versus peripheral cells was not the primary focus of these experiments, these results are in agreement with previous work that has directly shown enhanced competitiveness of thymic emigrants as compared to peripheral T cells.15 16
Depletion of thymic progeny via mAb treatment increases LN-derived progeny in thymus-bearing hosts after BMT
| Reconstituted cell type . | Treatment . | ||
|---|---|---|---|
| Sham (× 106) . | mAb (× 106) . | Pvalue . | |
| BM type (Thy 1.2) | 33.0 ± 2.2 | 0.22 ± 0.1 | .001 |
| LN derived (Thy 1.1) | 3.3 ± 0.5 | 6.7 ± 1.3 | .03 |
| Reconstituted cell type . | Treatment . | ||
|---|---|---|---|
| Sham (× 106) . | mAb (× 106) . | Pvalue . | |
| BM type (Thy 1.2) | 33.0 ± 2.2 | 0.22 ± 0.1 | .001 |
| LN derived (Thy 1.1) | 3.3 ± 0.5 | 6.7 ± 1.3 | .03 |
C57BL/6 mice underwent BMT as described in “Materials and methods.” Experimental animals received anti-Thy 1.2 mAb 0.4 mL intraperitoneally weekly × 4 beginning on day +7 after BMT (n = 5) and sham mice received a carrier over the same time period (n = 5). Mice were sacrificed and splenic populations were analyzed 5 weeks after BMT.
Cytokines modulate T-cell space following BMT
To investigate the possibility that competition for T-cell active growth factors may contribute to the diminished expansion of LN-derived cells observed in the presence of recent thymic emigrants, we supplied supraphysiologic doses of a variety of T-cell–active cytokines to analyze the effects on peripheral expansion during T-cell regeneration. As described in “Materials and methods,” the doses and treatment regimens chosen were based on expected tolerability of the cytokine therapy after BMT as well as the availability of the cytokine. IL-3 administered at 2 μg/d (day −1 to day 27) induced no significant effect on T-cell regeneration as measured by the overall numbers of reconstituting T cells or the source for T-cell regeneration (data not shown). Similarly, no significant effects were observed following administration of rmIL-12 at 10 ng/d or at a dose of 100 ng/d from day 14 to 28 after BMT. Subsequently, rhIL-6 (20 μg/d) was administered to mice for 28 days after BMT (day −1 to day 27). Although there was no significant effect on T-cell regeneration in thymectomized hosts, we observed diminished thymic size in rhIL-6 recipients (mean thymocyte number rhIL-6–treated 20.4 ± 6.6 × 106 versus 63.4 ± 16.2 × 106 control, P = .05) but no significant decline in thymic progeny in thymus-bearing recipients that received rhIL-6 (Figure 2). In addition, there was a modest increase in peripheral expansion in thymus-bearing IL-6 recipients (P = .05). These results are consistent with the observations made in recipients of anti-Thy 1.2 mAb therapy wherein diminution of thymic progeny led to a reciprocal increase in peripheral expansion. Indeed, the lack of an effect of IL-6 on peripheral expansion in athymic hosts suggested that the increased peripheral expansion observed in rhIL-6–treated thymus-bearing recipients was potentially related to the diminished thymic progeny rather than a primary effect on peripheral expansion. The animals treated with rhIL-6 also showed increased numbers of peripheral blood leukocytes and CD11c+ splenocytes compared to sham-treated controls (data not shown).
Effect of cytokine administration on T-cell regeneration after BMT.
Animals underwent BMT as described in “Materials and methods” and received the cytokines as a continuous subcutaneous infusion at the doses and times noted for each panel. Open boxes represent BM-type cells (Thy 1.2) and hatched boxes represent LN-derived (Thy 1.1+) cells as quantitated by flow cytometry. Shaded bar represents approximate range Thy+ cells in normal, non-BMT hosts. Unpaired comparisons between groups were performed using the Mann-Whitney test. (A) rhIL-6 20 μg/d day −1 to day 27 following BMT, analysis on day 28. Thymus-bearing IL-6–treated mice showed marginally statistically significant increases in LN-derived cells (P = .05) but no significant change in Thy 1.2+ cells (P = .14). No significant differences were noted in thymectomized mice with or without rhIL-6. (B) rhIL-2 30 000 IU/d days 28 to 42 after BMT, analysis on day 42. Thymus-bearing IL-2–treated mice showed marginally statistically significant increases in BM-type Thy 1.2+cells (P = .05), and an increase in Thy 1.1+LN-derived cells (P = .02). No significant differences were noted in thymectomized mice. (C) rhIL-7 5 μg/d days 14 to 28 after BMT, analysis on day 28. Thymus-bearing rhIL-7–treated mice showed statistically significant increases in BM-type Thy 1.2+ cells (P = .01) and LN-derived cells (P = .009). Thymectomized IL-7 recipients also had significant increases in Thy 1.1 LN-derived cells (P = .001). SEs of the mean are shown by error bars.
Effect of cytokine administration on T-cell regeneration after BMT.
Animals underwent BMT as described in “Materials and methods” and received the cytokines as a continuous subcutaneous infusion at the doses and times noted for each panel. Open boxes represent BM-type cells (Thy 1.2) and hatched boxes represent LN-derived (Thy 1.1+) cells as quantitated by flow cytometry. Shaded bar represents approximate range Thy+ cells in normal, non-BMT hosts. Unpaired comparisons between groups were performed using the Mann-Whitney test. (A) rhIL-6 20 μg/d day −1 to day 27 following BMT, analysis on day 28. Thymus-bearing IL-6–treated mice showed marginally statistically significant increases in LN-derived cells (P = .05) but no significant change in Thy 1.2+ cells (P = .14). No significant differences were noted in thymectomized mice with or without rhIL-6. (B) rhIL-2 30 000 IU/d days 28 to 42 after BMT, analysis on day 42. Thymus-bearing IL-2–treated mice showed marginally statistically significant increases in BM-type Thy 1.2+cells (P = .05), and an increase in Thy 1.1+LN-derived cells (P = .02). No significant differences were noted in thymectomized mice. (C) rhIL-7 5 μg/d days 14 to 28 after BMT, analysis on day 28. Thymus-bearing rhIL-7–treated mice showed statistically significant increases in BM-type Thy 1.2+ cells (P = .01) and LN-derived cells (P = .009). Thymectomized IL-7 recipients also had significant increases in Thy 1.1 LN-derived cells (P = .001). SEs of the mean are shown by error bars.
Subsequent experiments involved the administration of rhIL-2 to mice after BMT to evaluate the effects on the 2 primary pathways of T-cell regeneration. Initial studies using rhIL-2 at a dose of 30 000 U/d from days 14 to 28 revealed no significant effect on thymus-dependent or thymic-independent progeny after BMT (data not shown). As shown in Figure 3, however, there was an increase in thymus-dependent progeny following rhIL-2 administration (30 000 U/d) from days 28 to 42. With regard to peripheral expansion, rhIL-2 did not directly modulate this pathway, because no effect was seen in thymectomized mice. Interestingly, however, we observed a significant increase in peripheral expansion in thymus-bearing hosts treated with rhIL-2. Therefore, the down-modulation of peripheral expansion, which occurs as a result of the production of recent thymic emigrants, can be abrogated, at least in part, by supplying supraphysiologic doses of the T-cell–active cytokine IL-2, suggesting that IL-2 is limiting in this scenario.
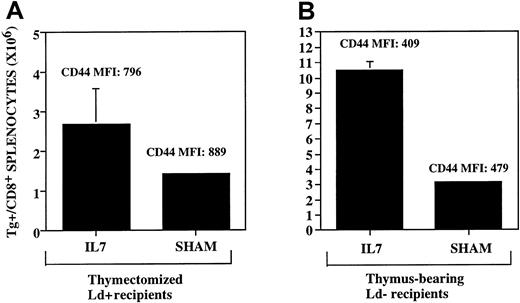
IL-7 up-regulates both antigen-driven peripheral expansion and thymic-dependent T-cell regeneration.
The rhIL-7 was administered where indicated via continuous infusion subcutaneous pump at a dose of 5 μg/d from days 14 to 28 after BMT. Both panels show the number of splenic TCR Tg+CD8+ cells present on day 28 as determined using flow cytometry. MFI denotes mean fluorescence intensity of CD44 expression on the TCR Tg+ cells. (A) Thymectomized B6D2F1 hosts (Ld+) underwent syngeneic BMT as described in “Materials and methods.” LNs derived from the 2C Tg+/TCR mice were administered on day 0 at a dose of .03 × 105 LN cells/mouse. (B) Thymus-bearing C57Bl/6 mice (Ld−) underwent BMT using T-cell–depleted BM from 2C Tg+/TCR mice as described in “Materials and methods.” No LN cells were administered. Mice treated with rhIL-7 show significant increases in the number of TCR Tg+ cells in both panels (P = .05, left panel, and P = .003, right panel). SEs of the mean are shown by error bars.
IL-7 up-regulates both antigen-driven peripheral expansion and thymic-dependent T-cell regeneration.
The rhIL-7 was administered where indicated via continuous infusion subcutaneous pump at a dose of 5 μg/d from days 14 to 28 after BMT. Both panels show the number of splenic TCR Tg+CD8+ cells present on day 28 as determined using flow cytometry. MFI denotes mean fluorescence intensity of CD44 expression on the TCR Tg+ cells. (A) Thymectomized B6D2F1 hosts (Ld+) underwent syngeneic BMT as described in “Materials and methods.” LNs derived from the 2C Tg+/TCR mice were administered on day 0 at a dose of .03 × 105 LN cells/mouse. (B) Thymus-bearing C57Bl/6 mice (Ld−) underwent BMT using T-cell–depleted BM from 2C Tg+/TCR mice as described in “Materials and methods.” No LN cells were administered. Mice treated with rhIL-7 show significant increases in the number of TCR Tg+ cells in both panels (P = .05, left panel, and P = .003, right panel). SEs of the mean are shown by error bars.
Similar experiments were undertaken using rhIL-7 after BMT. Because of a limited supply of this cytokine, it was administered only for 14 days from days 14 to 28. Nonetheless, we observed dramatic effects on both pathways of T-cell regeneration (Figure 2). In thymus-bearing hosts, there was a substantial increase in BM-derived, thymic-dependent progeny, similar to that reported previously after BMT.17-19 Increases were observed for both BM-derived CD4+ (13.5 ± 2.2 versus 3.48 ± 0.36 in IL-7 versus sham, respectively, P = .01) and CD8+(16.5 ± 4.3 versus 1.5 ± 0.16 in IL-7 versus sham, respectively,P = .01) populations. IL-7 therapy also increased B220+ class II+ B cells after BMT (145.5 ± 19.3 versus 24.1 ± 3.3 in IL-7 versus sham, respectively, P = .03). In addition, however, a dramatic increase in peripheral expansion was observed in both thymectomized and thymus-bearing hosts. Significant increases were observed in both peripherally expanded CD4+ (11.7 ± 3.1 versus 2.3 ± 0.35 in IL-7 versus sham, respectively, P = .03) and CD8+ (5.3 ± 0.8 versus 0.85 ± 0.09 in IL-7 versus sham, respectively, P = .03) populations. Therefore, similar to the effects observed with IL-2, the provision of supraphysiologic doses of a T-cell active cytokine is sufficient to abrogate the down-regulation in peripheral expansion observed in thymus-bearing hosts. Stated another way, increasing the availability of IL-7 was sufficient to increase T-cell space resulting in a simultaneous increase in both peripherally expanded and thymically derived progeny after BMT.
IL-7 increases antigen-driven expansion during immune reconstitution
Previous studies have shown that peripheral expansion during T-cell regeneration is antigen driven.4 To confirm that IL-7 was capable of enhancing antigen-driven peripheral expansion in vivo, we used LN cells from 2C TCR Tg+ mice that specifically recognize the MHC molecule Ld as LN inocula. These cells were administered to thymectomized, Ld-expressing B6D2F1 hosts in the context of a syngeneic BMT. In an attempt to avoid potentially lethal graft-versus-host disease (GVHD) in these experiments, relatively low numbers of TCR Tg+ LN inocula were used (1 × 105/kg). Evaluation of the Ld-directed, antigen-driven expansion with IL-7 administration is shown in Figure 3A wherein significant increases in antigen-driven expansion are shown following IL-7 therapy. Therefore, the provision of supraphysiologic doses of rhIL-7 in vivo leads to increases in antigen-driven peripheral expansion, providing evidence that this cytokine is limiting for antigen-driven expansion in vivo during a period of immune reconstitution. As expected, CD8+ T cells from this experiment displayed a memory phenotype as evidenced by high expression of CD44 with or without administration of rhIL-7 as shown in Figure 3A, reflecting the high level of activation that occurs during the process of cognate antigen-driven peripheral expansion.
IL-7 directly up-regulates thymic-dependent progeny
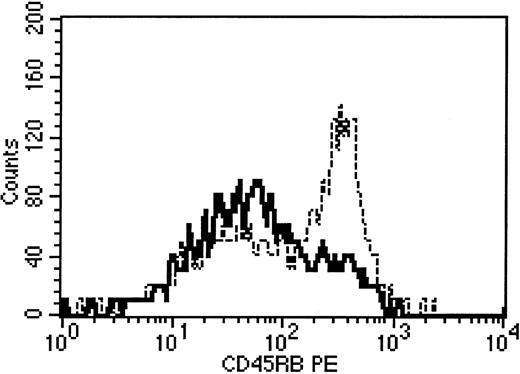
The observation that rhIL-7 was capable of increasing peripheral expansion raised the possibility that the increased numbers of thymic-dependent progeny observed in hosts treated with IL-7 merely reflected increased peripheral expansion of such progeny following exit from the thymus, rather than a direct effect on thymopoiesis itself. To address this possibility, we evaluated the CD45 isoform expression on thymic-dependent progeny from mice treated with IL-7. Previously we have shown that thymic-dependent progeny can be distinguished from thymic-independent progeny by differential CD45 isoform expression.20 Accordingly, in this study, whereas thymic progeny in sham-treated animals showed predominately high-molecular-weight CD45 isoform expression, we observed conversion of a substantial number of BM-derived progeny to a memory phenotype in animals treated with rhIL-7 (CD4+/Thy 1.1+ CD45 MFI 163.5 ± 10.2 fluorescent units with IL-7 versus 242.3 ± 9.4 without rhIL-7, P = .03; Figure4). These results were consistent with the hypothesis that IL-7 increased BM-derived progeny, at least in part, by increasing the peripheral expansion of such progeny. To address whether IL-7 could increase thymic-dependent progeny in the absence of cognate antigen-driven peripheral expansion, we administered TCR Tg+ 2C TCD BM to C57BL/6 mice, which lack the Ld antigen. Using this model, thymic-dependent progeny can be generated from the TCR Tg+ marrow inocula, but antigen-driven peripheral expansion is inhibited due to a lack of cognate antigen that is recognized by the Tg+TCR.4 In these experiments, IL-7 induced significant up-regulation of thymic progeny, despite inhibition of peripheral expansion as evidenced by a low mean fluorescence intensity for CD44 (Figure 3B). Therefore, administration of supraphysiologic doses of IL-7 during immune reconstitution up-regulates both thymic-dependent as well as thymic-independent peripheral expansion pathways of T-cell regeneration.
Alteration of CD45 isoform expression on BM-derived progeny in rhIL-7–treated hosts.
Thymus-bearing C57Bl/6 mice underwent syngeneic BMT as described in “Materials and methods.” No LN cells were administered. At 28 days after BMT animals were killed and analysis of CD45 expression on electronically gated Thy 1.2+/CD4+ splenocytes was performed. The panel shows results from 2 representative mice. Bold line indicates rhIL-7 recipient; dotted line, sham treated. Background isotype staining on gated Thy 1.2+/CD4+ cells was less than 101 for both animals.
Alteration of CD45 isoform expression on BM-derived progeny in rhIL-7–treated hosts.
Thymus-bearing C57Bl/6 mice underwent syngeneic BMT as described in “Materials and methods.” No LN cells were administered. At 28 days after BMT animals were killed and analysis of CD45 expression on electronically gated Thy 1.2+/CD4+ splenocytes was performed. The panel shows results from 2 representative mice. Bold line indicates rhIL-7 recipient; dotted line, sham treated. Background isotype staining on gated Thy 1.2+/CD4+ cells was less than 101 for both animals.
Discussion
Regulation of T-cell number occurs in large part by modulation of T-cell populations in the periphery. Although this conclusion has been drawn independently by several investigators across a variety of murine models,21-23 the precise factors and mechanisms by which peripheral T-cell homeostasis is regulated remain ill defined. To study this, we used a dramatic scenario wherein lymphocyte homeostasis is acutely disrupted after BMT. Using this model, we postulated that competition for T-cell active growth factors might contribute to the modulation of the magnitude of antigen-driven peripheral expansion known to occur in response to T-cell depletion. To study this, we analyzed the effects of supraphysiologic doses of multiple T-cell active cytokines administered after BMT. These results clearly show that IL-7 administration potently modulates the contribution of thymic-independent peripherally expanded progeny to T-cell regeneration in thymus-bearing hosts. Simultaneously, IL-7 also potently up-regulates thymic-dependent T-cell regeneration. The net result is that animals that were provided with an increased availability of IL-7 during the process of T-cell regeneration regenerated significantly larger peripheral T-cell pools. These results lend credence to the hypothesis that peripheral T-cell homeostasis is regulated, at least in part, by the availability of T-cell–active growth factors. Furthermore, the relative suppression of peripheral expansion by the presence of recent thymic emigrants can be largely overcome by supplying supraphyisologic doses of IL-7. Interestingly, similar evidence that competition for growth factors plays a role in the regulation of hematopoietic cell space has been generated as well.24
Interleukin 7 is a requisite and potent growth and survival factor for early T-cell development.25-28 Indeed, the possibility that therapeutic administration of IL-7 may restore thymic regenerative capacity in clinical scenarios associated with T-cell depletion has given rise to a great deal of enthusiasm for the clinical development of this agent. With regard to the effect of IL-7 on thymic-dependent pathways, the results presented here confirm and expand on previous reports in which increased thymopoiesis was observed when exogenous IL-7 was administered after BMT.17-19 Importantly, in our studies, this appears to be most significant for relatively young mice as studied here. When supraphysiologic doses of IL-7 were administered to aged mice (18-24 months) in similar experiments, significant increases in thymic-dependent progeny were not observed (data not shown). Therefore, although IL-7 clearly has potent effects on thymic function, evidence that IL-7 can completely reverse age-associated thymic atrophy is lacking.
The effects of IL-7 on mature T-cell populations are less well appreciated. Importantly, however, several investigators have previously shown that IL-7 acts to costimulate mature T cells29-31 and acts as a survival factor for mature T cells.32-34 These published reports, although consistent with the possibility that IL-7 might enhance antigen-driven peripheral expansion, would not necessarily lead to the prediction that IL-7 would induce such dramatic peripheral expansion in vivo as compared to IL-2. Clearly, the results shown here illustrate that therapy with IL-7 potently enhances antigen-driven expansion in vivo, whereas IL-2 accomplishes this only marginally and only in the face of high levels of thymic emigrants. These results raise the possibility that changes in the availability of endogenous levels of IL-7 throughout the normal host life span could dynamically modulate the capacity of endogenous T cells to expand in vivo. Furthermore, because unlike most interleukins, IL-7 is produced primarily by stromal cells rather than lymphoid cells, high levels of IL-7 could potentially be generated in the setting of T-cell depletion. Indeed, strong inverse relationships have been observed between circulating IL-7 levels and CD4 lymphopenia in diverse clinical settings associated with T-cell depletion35 (T. Fry, manuscript submitted). Together, these observations raise the possibility that IL-7 could potentially serve as an endogenous regulator of T-cell homeostasis.
Several further questions remain to be answered to better clarify the role of endogenous IL-7 in modulating peripheral T-cell homeostasis. First, although IL-7 appears sufficient to increase peripheral expansion, it remains unknown whether IL-7 is necessary for peripheral expansion. Because animals deficient in IL-7 have essentially no lymphocytes,25,27,28 experiments to address this issue will need to be designed such that IL-7 is neutralized in vivo during T-cell regeneration rather than by using hosts that have been genetically engineered to be deficient in IL-7. Second, the studies presented here do not rule out the likely possibility that other endogenous factors might also be required for and participate in the modulation of peripheral T-cell homeostasis and in the effects induced by exogenous IL-7. Third, the factors that regulate production of endogenous IL-7 remain completely unknown. Although there are no apparent upstream TATA or CAAT sequences typical of inducible genes,36 an interferon-inducible region containing an interferon-γ–specific response element has been identified37 and reciprocal regulation between transforming growth factor-β (TGF-β) and IL-7 has been described.38,39 Furthermore, evidence of a role for TGF-β in CD8+ T-cell homeostasis has recently been generated.40 If endogenous IL-7 levels play a role in T-cell homeostasis, delineation of the factors that regulate production of this molecule in vivo would be of great interest.
Based on the observations presented in this report, one would predict that IL-7 may prove useful as a vaccine adjuvant due to its potent capacity to enhance antigen-driven expansion in vivo. The previous observations that T-cell depletion leads to a propensity for increased antigen-driven expansion with skewing of the regenerating repertoire toward antigens present during the period of expansion4has led some investigators to consider the therapeutic use of T-cell depletion coincident with the administration of tumor vaccines in vivo41 (S. A. Rosenberg, written communication, June 2000). Concerns regarding this approach are that the reduced repertoire diversity induced by T-cell depletion might offset any improvement in expansion due to T-cell space. If, on the other hand, increased T-cell expansion can be induced by simply increasing the availability of IL-7 with a concomitant increase in antigen-driven peripheral expansion without T-cell depletion, then the therapeutic administration of this cytokine as a vaccine adjuvant would be logical.
In support of this concept, recent work in a murine model has shown that the administration of IL-7 in the context of immunization in T-cell–depleted hosts substantially improves host immune competence. We have observed significant improvements in the capacity for minor histocompatibility-mediated graft rejection in athymic mice reconstituted via peripheral expansion.42 Preliminary results also suggest that IL-7 therapy leads to an enhanced capacity to reject tumor grafts in T-cell–depleted hosts (T. Fry, manuscript in preparation). Therefore, emerging data from a variety of models suggest that the heretofore unemphasized effects of IL-7 on mature T-cell populations could prove to be of significant import not only for improving our understanding of the basic biology of T-cell homeostasis but also could provide new approaches for enhancing T-cell vaccine efficacy.
These results also have important implications for the potential clinical application of rhIL-7 in the setting of allogeneic BMT. Although the beneficial effects of IL-7 on thymopoiesis have fueled enthusiasm for the capacity for IL-7 to improve host immune competence after allogeneic BMT, the effects on mature T cells reported here raise the possibility that IL-7 might also result in an increased incidence or severity of GVHD. Indeed, preliminary results in our laboratory suggest that IL-7 administration following allogeneic BMT may increase GVHD in murine models (C.L.M., manuscript in preparation). Whether such increases in the incidence and severity of GVHD would be offset by improvements in immune reconstitution induced by the thymopoietic effects of this agent is an important area of ongoing study.
In summary, this report provides the novel observation that IL-7 administered following T-cell depletion potently enhances T-cell regeneration by increasing antigen-driven T-cell expansion while simultaneously enhancing thymic regenerative capacity. This observation supports the hypothesis that endogenous levels of IL-7 may contribute to the peripheral regulation of T-cell homeostasis. Furthermore, the potent effects of IL-7 on mature T cells raise the intriguing possibility that this cytokine may prove useful not only as a modulator of immune reconstitution but also potentially as a vaccine adjuvant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Crystal L. Mackall, Bldg 10, Rm 13N240, 10 Center Dr, MSC 1928, Bethesda, MD 20892-1928; e-mail:cm35c@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal