Abstract
Substantial numbers of human mast cells (MCs) were generated from umbilical cord blood (CB) and from adult peripheral blood (PB). A single CB progenitor produced 15 436 MCs, whereas a single PB progenitor produced 807 MCs on average. However, PB-derived MCs were far more active than CB-derived MCs in terms of high-affinity IgE receptor (FcεRI)-mediated reactions. One million sensitized PB-derived MCs released 3.6 μg histamine, 215 pg IL-5, and 14 ng granulocyte macrophage–colony-stimulating factor (GM-CSF), whereas 106 sensitized CB-derived MCs released only 0.8 μg histamine, 31 pg IL-5, and 0.58 ng GM-CSF on anti-IgE challenge. However, ionophore A23 187 released similar levels of histamine from the 2 MC types. PB-derived MCs highly expressed surface FcεRI α chain, and CB-derived MCs almost lacked it in the absence of IgE. PB-derived MCs expressed approximately 5 times higher levels of messenger RNA (mRNA) for FcεRI α chain than CB-derived MCs, but mRNAs for β and γ chains of the receptors were equally expressed. Among the approximately 5600 kinds of full-length human genes examined by using the high-density oligonucleotide probe-array system, FcεRIα was ranked the fifth most increased transcript in PB-derived MCs. The 4 other increased transcripts were unrelated to MC function. These results suggest that IgE-mediated reactions may be restricted during early infancy through the selective inhibition of FcεRIα transcription, which is probably committed at progenitor stages and is, at least in part, cytokine-insensitive.
Introduction
Mast cells (MCs)1 are multifunctional cells—that is, they not only evoke immediate allergic reaction through high-affinity IgE receptors (FcεRI), they also modulate innate and acquired immunity by releasing a variety of mediators and cytokines.1-3 Rodent MCs have been classified into 2 types, connective tissue MCs, preferentially located in tissues such as skin, and mucosal MCs, dominantly found in mucosa such as intestine.4-6 However, recent evidence suggests that both mouse MC subsets are closely related to each other and are interchangeable when it comes to environmental factors.7,8We have also recently demonstrated that all human MCs can produce chymase and that there are no discrete human MC subsets in terms of chymase concentrations, at least in culture.9
We have established a method for generating substantial numbers of human MCs from umbilical cord blood (CB)10-12 and from adult peripheral blood (PB).9,13 In this series of studies, others14,15 and we9,13 have found that human MCs proliferate more in serum-free conditions than in fetal calf serum-supplemented conditions. We are now able to generate more than 105 MCs from a single umbilical CB CD34+cell and can generate more than 105 MCs from 10 mL adult PB without cytokine priming for donors in vivo.9 13 During functional analysis of these MCs, we realized that PB-derived MCs often release a greater amount of mediators than CB-derived MCs in response to FcεRI-dependent stimuli.
In contrast to adult-type progenitors, it has been reported that CB-derived hematopoietic progenitors produce fetal hemoglobin-positive erythrocytes16 and nuclear factor of activated T cells 1 (NFAT1)-deficient T cells.17 Thus, we hypothesized that the properties of these cultured MCs may be different, depending on their origins; for instance, hematopoietic progenitors in CB and PB have capabilities that are distinct from each other. In the current study, we compared the characteristics of CB-derived MCs and PB-derived MCs by culturing them in the same culture conditions.
Using high-density oligonucleotide probe-arrays, the comparative screening of genes expressed in different cell types has become available.18 19 We report here that by using this newly developed technique, the level of FcεRI α gene expression was approximately 10 times higher in PB-derived MCs than in CB-derived MCs and that the difference was the fifth largest (PB greater than CB) among the approximately 5600 kinds of human genes analyzed.
Patients, materials, and methods
Subjects
Subjects provided written informed consent, which was approved by the ethical review board at each hospital. Nonphagocytic mononuclear cells were separated from PB or CB samples by density-gradient centrifugation using lymphocyte separation medium (Organon Teknika, Durham, NC) after phagocytes were depleted with silica (Immuno Biological Laboratories, Fujioka, Japan). The interface containing mononuclear cells was collected after density-gradient centrifugation. Lineage-negative (Lin−) cells were negatively selected from the mononuclear cells using a magnetic separation column (MACS II; Miltenyi Biotec, Bergisch Gladbach, Germany) and a mixture of magnetic microbead-conjugated antibodies against CD4, CD8, CD11b, CD14, and CD19 (Miltenyi Biotec) according to the manufacturer's instructions. In some experiments, CD34+ cells were positively selected from CB or bone marrow mononuclear cells using the CD34+ cell isolation kit (Miltenyi Biotec).
Cytokines and antibodies
The following cytokines were added to the cell suspension. Recombinant IL-3 was purchased from Intergen (Purchase, NY), and rIL-6 was kindly provided by Kirin Brewery (Maebashi, Japan). Bulk vials of rSCF were purchased from PeproTech EC (London, United Kingdom), and recombinant IL-4 was purchased from R&D Systems (Minneapolis, MN).
Cell culture
Cells were suspended in Iscove modified Dulbecco medium (IMDM; Gibco BRL, Grand Island, NY) supplemented with 1% insulin–transferrin–selenium (Gibco BRL), 50 μM 2-ME (Gibco BRL), 1% penicillin–streptomycin (Gibco BRL), and 0.1% bovine serum albumin (complete IMDM; Sigma, St Louis, MO). For methylcellulose culture, the Lin− 106 PB or 105 CB mononuclear cells or 103 CB or bone marrow CD34+ cells were suspended in 0.3 mL complete IMDM. The cells were mixed by shaking the tubes for more than 1 minute with 2.7 mL serum-free Iscove methylcellulose medium (MethoCult SFBIT; Stem Cell Technologies, Vancouver, Canada) supplemented with 200 ng/mL SCF, 50 ng/mL IL-6, and 1 ng/mL IL-3. IL-3 was added only at the beginning of culture because it efficiently enhances MC colony formation without inducing the other cell type colonies as has been reported.9 13 The cell suspension was inoculated at 0.3 mL per well in the 24-well plate (Iwaki Glass, Tokyo, Japan) at 37°C in 5% CO2. Every 2 weeks, 0.3 mL fresh methylcellulose medium containing 100 ng/mL SCF and 50 ng/mL IL-6 was layered over the methylcellulose cultures. At 6 weeks, whole cells were retrieved after methylcellulose medium was dissolved with PBS. They were then suspended and cultured in complete IMDM supplemented with 100 ng/mL SCF, 50 ng/mL IL-6, and 5% fetal calf serum (Cansera, Rexdale, Canada) in 25-cm2 flasks (Iwaki Glass) up to the 25th week. Although MCs cultured initially in methylcellulose were used in most of the experiments, we used some CB-derived MCs cultured initially in the cytokine-supplemented IMDM liquid medium in large-scale experiments (cytokine assay and GeneChip [Affymetrix, Santa Clara, CA] analysis) by mixing them with MCs cultured initially in methylcellulose. We confirmed each time that these MCs did not largely differ in terms of FcεRIα expression.
Staining
The differential count of cultured cells was determined based on 100 cells, unless smears had fewer cells, by using cultured samples centrifuged onto slides with Cytospin 2 (Shandon, Pittsburgh, PA). Cells were examined with May-Grünwald and Giemsa staining or with anti-tryptase immunostaining. Immunostaining for human mast cell tryptase was performed by using the method previously described.9-12 Briefly, the smears were fixed with Carnoy solution (60% ethanol, 30% chloroform, 0% glacial acetic acid) and stained for granular tryptase by the alkaline phosphatase anti-alkaline phosphatase (APAAP) method with the DAKO (Carpinteria, CA) APAAP kit according to the manufacturer's instructions.
Histamine release assay
MCs were sensitized with 1 μg/mL human myeloma IgE (a generous gift from Dr Kimishige Ishizaka, La Jolla, CA) at 37°C for 48 hours in the absence or presence of IL-4. After they were washed, cells were suspended at a density of 105 cells/mL in modified Tyrode solution (pH 7.4) containing 124 mM NaCl, 4 mM KCl, 0.64 mM NaH2PO4, 1 mM CaCl2, 0.6 mM MgCl2, 10 mM HEPES, and 0.03% human serum albumin. The cells were preincubated for 10 minutes and were challenged with 1.5 μg/mL rabbit anti-human IgE (DAKO, Glostrup, Denmark), ionophore A23187 (Sigma), or control Tyrode solution at 37°C for 30 minutes. Histamine was measured by using an automatic histamine analyzer with double high-performance liquid chromatography columns (Tosoh, Tokyo, Japan), as previously reported.10-12
Production of cytokines
Cultured MCs were sensitized with 1 μg/mL IgE in the presence or absence of 10 ng/mL IL-4 for 48 hours. These cells were suspended at a density of 1 × 106 cells/mL with 100 ng/mL SCF and 50 ng/mL IL-6, and they were challenged with 1.5 μg/mL anti-IgE antibody for 6 hours. The levels of IL-5 and GM-CSF were measured with sandwich enzyme-linked immunosorbent assay (Biotrak cytokine enzyme-linked immunosorbent assay; Amersham, Buckinghamshire, United Kingdom).11
Flow cytometric analyses
For surface staining, MCs were suspended in 1% bovine serum albumin and 0.1% NaN3 in phosphate-buffered saline (PBS; Gibco, Grand Island, NY). For intracytoplasmic staining, MCs were fixed with 4% paraformaldehyde for 20 minutes and permeabilized with 2% saponin solution in PBS for 60 minutes. After that, the cells were incubated with saturating concentrations of anti-human FcεRIα monoclonal antibody (CRA-1, IgG2b; Kyokuto Pharmaceutical, Tokyo, Japan) or an equivalent concentration of irrelevant mouse IgG2b (Coulter Immunology, Hialeah, FL) after pretreatment with 50 μg/mL human IgG (ICN Biomedical, Aurora, OH) for 30 minutes. CRA-1 has been shown to recognize an epitope that is not affected by FcεRI occupancy with IgE.11 The cells were then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Becton Dickinson, San Jose, CA) for 30 minutes at 4°C in the dark. After washing, the cells were analyzed using FACS and Cell Quest software. Mean fluorescence intensity (MFI) ratios of MCs stained with specific antibody to those stained with control antibody were obtained.
Messenger RNA expression of the α, β, and γ chains of FcεRI
Total RNA was isolated from CB-derived MCs and PB-derived MCs using Isogen (Japan Gene, Tokyo, Japan) according to the manufacturer's instructions and was reverse-transcribed to cDNA. FcεRI α, β, and γ gene segments from 10 ng of each cDNA sample were polymerase chain reaction (PCR)-amplified in the presence of specific sense and antisense primers (0.4 μM each; Table1), 200 μM dNTP, 0.5 U/mL AmpliTaq Gold (Perkin Elmer, Norwalk, CT), and PCR buffer (1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl, pH 8.3, 0.001% gelatin) in a final reaction volume of 50 μL. PCR was performed in a DNA Thermal Cycler (PE Biosystems, Foster City, CA) (α chain, 35 cycles; β and γ chains, 40 cycles). Each cycle included denaturation (94°C, 1 minute), annealing (α, β, and γ chains, 55°C, 1 minute), extension (72°C, 2 minutes), and final incubation (72°C, 10 minutes) after the last cycle. β-Actin cDNA was amplified as an internal control. PCR products were electrophoresed on 2% agarose gels (Takara, Tokyo, Japan). After they were stained with ethidium bromide, they were visualized under ultraviolet illumination so results could be seen. The density and area of each visualized PCR product was analyzed with NIH Image 1.62 (National Institutes of Health, Bethesda, MD).
GeneChip expression analysis
Mixtures of 3 different batches of CB-derived MCs or 4 different batches of PB-derived MCs at 12 weeks of culture were used. Cells were treated with or without IL-4 at 10 ng/mL for 6 hours. In a preliminary experiment, IL-4 increased FcεRIα mRNA to the highest level at 6 to 48 hours, and 48-hour priming with IL-4 often reduced the mRNA levels of other genes. The number of each sample exceeded 107cells. Gene expression was screened using the GeneChip Expression Array (HuGeneFL Array, Affymetrix), according to the manufacturer's protocol (expression analysis technical manual). We obtained 6.2 to 9.6 μg from each sample. The samples were treated for producing complementary RNA. The produced complementary RNA (62-83 μg) was adjusted at 10 μg (PB-derived MCs) or 20 μg (CB-derived MCs) and hybridized with HuGeneFL Array (Affymetrix) consisting of 7129 high-density oligonucleotide probe-arrays. Approximately 5600 full-length, nonredundant genes can be quantified by using the computer software, GeneChip Analysis Suite (Affymetrix). The expression level of a single mRNA was determined as the average fluorescent intensity among the intensities obtained by 16 to 20 paired (perfect-matched) and single (nucleotide-mismatched) primers consisting of 25-base oligonucleotides. If the intensities of mismatched primers are high, the gene expression is judged to be absent. Even high average fluorescence was obtained. Comparative analysis of the expression of 5600 genes was performed using specialized computer software with the GeneChip Analysis Suite (Affymetrix).
Statistical analysis
Statistical significance between the 2 independent groups was determined by the Mann-Whitney U test; results were considered significant at P < .05. Values are expressed as means ± SEM.
Results
Differentiation capacity to MCs of progenitors derived from CB and PB
Lin− PB, CD34+ CB, and Lin−CB cells formed tryptase-positive MC colonies at 5 to 6 weeks of culture, as has been described elsewhere.9 13 We were unable to generate MCs from Lin− PB cells when they were cultured initially in the cytokine-supplemented complete IMDM liquid medium (data not shown). The number of MC colonies in methylcellulose was counted at 5 to 6 weeks of culture, and the number of MCs per colony was calculated after retrieving the whole cells (more than 95% were MCs) by dissolving methylcellulose. Cell numbers per colony in PB-derived MCs (807 ± 115 cells/colony; n = 29) were approximately 20 times lower (P < .0001) than those in CB-derived MCs (15 436 ± 2938 cells/colony; n = 7). These results indicate that CB progenitors are far more capable of producing MCs than are PB progenitors.
Functional analyses of both MC types
Next, we examined the functional properties of CB-derived MCs and PB-derived MCs by using the cells at 12 to 14 weeks of culture. As shown in Table 2, CB-derived MCs sensitized with IgE and those treated with IgE + IL-4, respectively, released 2% and 9% of their histamine content (8.6 pg ± 0.7 pg/cell), whereas PB-derived MCs sensitized with IgE and those treated with IgE + IL-4, respectively, released 16% and 39% of their total histamine content (9.3 pg ± 2.2 pg/cell). On the other hand, ionophore A23187 induced similar amounts of histamine release from CB-derived MCs and PB-derived MCs.
We also measured IL-5 and GM-CSF production by the 2 MC types. One million PB-derived MCs sensitized with IgE produced 14.3 pg IL-5 and 1.94 ng GM-CSF, whereas 106 sensitized CB-derived MCs produced only 1.5 pg IL-5 and 0.06 ng GM-CSF on stimulation by anti-IgE. Similarly, 106 PB-derived MCs treated with IgE + IL-4 produced 215 pg IL-5 and 14 ng GM-CSF, whereas 106CB-derived MCs treated with IgE + IL-4 produced only 31 pg IL-5 and 0.58 ng GM-CSF (Table 2). These results suggest that PB-derived MCs are capable of releasing significantly greater amounts of mediators and cytokines than CB-derived MCs on IgE-dependent stimulation, but not on IgE-independent stimulus.
Protein expression of FcεRIα chain
The expression of surface FcεRI on these MCs was quantitatively determined by FACS. The results were expressed as an MFI ratio of cells stained with CRA-1 and those stained with irrelevant control antibody in Table 3. PB-derived MCs expressed significantly more surface FcεRIα chains than CB-derived MCs. CB-derived MCs expressed barely detectable amounts of cell surface FcεRIα unless MCs were treated with IgE. To clarify whether the time-course expression of FcεRIα on CB-derived MCs is different from that of PB-derived MCs, the cells at 8 to 10 weeks, 12 to 14 weeks, and 15 to 19 weeks of culture were primed with IgE + IL-4 for 48 hours. The MFI ratios of FcεRIα-to-control on PB-derived MCs at the above time points were, respectively 15.5 ± 1.2, 20.6 ± 12.7, and 10.4 ± 14.3, whereas those on CB-derived MCs were, respectively, 1.8 ± 0.1, 4.4 ± 2.1, and 2.4 ± 0.3 (n = 3-5). After 20 weeks of culture, the viability and the levels of various transcripts such as chymase mRNA of both cell types slowly decreased, as has been reported.9 Indeed, CB-derived MCs at 20 to 25 weeks of culture tended to show sometimes less FcεRIα than those at 12 to 14 weeks of culture (Figure 1). In any case, PB-derived MCs always expressed higher levels of FcεRIα than CB-derived MCs (see Figure 3).
Cell surface expression of FcεRI α chain.
The histograms represent 12-week-old PB-derived MCs (A, B, C), 12-week-old CB-derived MCs (D, E, F), and 22-week-old CB-derived MCs of the same batch with the 12-week-old MCs (G, H, I). These MCs were treated with SCF + IL-6–supplemented medium alone (A, D, G), IgE (B, E, H), and IgE + IL-4 (C, F, I) for 48 hours. CB-derived MCs at more than 20 weeks of culture showed significantly smaller levels of FcεRI α chain when compared with PB-derived MCs (MFI ratios, 1.00 ± 0.00, 1.22 ± 0.05, and 1.97 ± 0.09 when they were untreated, treated with IgE, and treated with IgE + IL-4, respectively; n = 3; Table 3).
Cell surface expression of FcεRI α chain.
The histograms represent 12-week-old PB-derived MCs (A, B, C), 12-week-old CB-derived MCs (D, E, F), and 22-week-old CB-derived MCs of the same batch with the 12-week-old MCs (G, H, I). These MCs were treated with SCF + IL-6–supplemented medium alone (A, D, G), IgE (B, E, H), and IgE + IL-4 (C, F, I) for 48 hours. CB-derived MCs at more than 20 weeks of culture showed significantly smaller levels of FcεRI α chain when compared with PB-derived MCs (MFI ratios, 1.00 ± 0.00, 1.22 ± 0.05, and 1.97 ± 0.09 when they were untreated, treated with IgE, and treated with IgE + IL-4, respectively; n = 3; Table 3).
To deny the possibility that the purity of progenitors (CD34+ cells or Lin− cells) might affect the expression of FcεRIα, we examined the expression of FcεRIα on MCs developed from adult bone marrow CD34+. FcεRIα expression was detected at high levels on 4 of 6 batches of bone marrow-derived MCs at 12 weeks of culture incubated without IgE and IL-4 (MFI ratio, 7.19 ± 2.04). The other 2 MC batches also highly expressed FcεRIα after IgE treatment (MFI ratio, 4.51 and 7.46).
Next, we permeabilized the cell membrane and stained cell surfaces and intracytoplasmic FcεRIα. As shown in Table 3, PB-derived MCs contained FcεRIα protein levels that were approximately 5 times higher than those of CB-derived MCs when treated with 1 μg/mL IgE in the presence or absence 10 ng/mL IL-4 for 48 hours. Surprisingly, the FcεRIα-to-control MFI ratio obtained by untreated PB-derived MCs was higher than that obtained by CB-derived MCs treated with 1 μg/mL IgE and 10 ng/mL IL-4 for 48 hours during the same culture period.
Analysis of the gene expression for FcεRI
To confirm whether the protein expression of FcεRI α chain reflects its gene expression, we analyzed the mRNA expression for α, β, and γ chains of FcεRI by reverse transcription (RT)-PCR. As shown in Figure 2, PB-derived MCs expressed 3 to 8 times more mRNA for the α chain but not for the β and γ chains of FcεRI than CB-derived MCs did. Because IL-4 up-regulated the expression of the α chain, we examined the endogenous production of IL-4 by MCs. We could not detect IL-4 mRNA in CB- and PB-derived MCs, even after pretreatment with IgE + IL-4 and challenge with anti-human IgE (data not shown).
Expression for α, β, and γ chains of FcεRI mRNA by RT-PCR.
Representative results of RT-PCR analysis for α, β, and γ chains of FcεRI and β-actin mRNA are shown. CB-derived MCs (A) expressed less α but not β or γ chain than PB-derived MCs (B). The density and area of these blots were quantitatively determined by using NIH Imagesoftware version 1.62. The expression ratio of FcεRIα to β-actin in CB-derived MCs was 0.22 ± 0.03 (n = 6), whereas that in PB-derived MCs was 1.76 ± 0.31 (n = 3; P < .05), when the cells were pretreated for 48 hours with IgE but not with IL-4. The ratios of FcεRIβ to β-actin and FcRγ to β-actin in CB-derived MCs were, respectively, 0.16 ± 0.05 and 0.21 ± 0.03 (n = 6). Those in PB-derived MCs were 0.21 ± 0.03 and 0.29 ± 0.04 (n = 3; not significant). Similar results were obtained in IL-4–primed MCs, though FcεRIα to β-actin ratio was enhanced by the cytokine in CB-derived MCs (0.55 ± 0.15; n = 6;P < .05).
Expression for α, β, and γ chains of FcεRI mRNA by RT-PCR.
Representative results of RT-PCR analysis for α, β, and γ chains of FcεRI and β-actin mRNA are shown. CB-derived MCs (A) expressed less α but not β or γ chain than PB-derived MCs (B). The density and area of these blots were quantitatively determined by using NIH Imagesoftware version 1.62. The expression ratio of FcεRIα to β-actin in CB-derived MCs was 0.22 ± 0.03 (n = 6), whereas that in PB-derived MCs was 1.76 ± 0.31 (n = 3; P < .05), when the cells were pretreated for 48 hours with IgE but not with IL-4. The ratios of FcεRIβ to β-actin and FcRγ to β-actin in CB-derived MCs were, respectively, 0.16 ± 0.05 and 0.21 ± 0.03 (n = 6). Those in PB-derived MCs were 0.21 ± 0.03 and 0.29 ± 0.04 (n = 3; not significant). Similar results were obtained in IL-4–primed MCs, though FcεRIα to β-actin ratio was enhanced by the cytokine in CB-derived MCs (0.55 ± 0.15; n = 6;P < .05).
Analysis of transcriptome (whole transcript) screening of MCs by GeneChip
To investigate whether the transcription of FcεRIα gene is selectively down-regulated in CB-derived MCs, we screened 5600 kinds of mRNA expression by using the newly developed GeneChip (Affymetrix) technology. Of the 5600 full-length human genes, 2037 to 2368 probe-arrays indicated that the transcripts were present in MCs by using Absolute Analysis software (Affymetrix). Then we compared the expression of these transcripts between PB-derived MCs and CB-derived MCs with or without IL-4 priming (6 hours) using Comparison Analysis software (Affymetrix). In the IL-4 priming experiment, 166 transcripts were judged to be increased in CB-derived MCs compared to PB-derived MCs, whereas 85 transcripts were increased in PB-derived MCs. Similar results were obtained using those cells without IL-4 priming. Of the 85 transcripts increased in PB-derived MCs, FcεRI α chain was ranked the fifth most increased transcript (Figure3). Only 8 transcripts were found to be increased at more than twice the levels in PB-derived MCs and expressed at more than 10% the β-actin levels as shown in Table4. Table 4 also shows 6 representative MC-specific genes. CB-derived MCs and PB-derived MCs almost equally expressed these MC-specific mRNAs. Among these proteins, the mRNA level of chymase was higher in PB-derived MCs and was unaffected by IL-4 incubation (data not shown), as has been reported.9Cyclin-dependent kinase inhibitor, p27kip1,20 21 was the most increased transcript in PB-derived MCs. However, the p27 protein was almost equally expressed in both PB- and CB-derived MCs (data not shown). In contrast to the results obtained by the RT-PCR experiment shown in Figure 2, the β-chain transcript of FcεRI was down-regulated in CB-derived MCs. This might have resulted from the number of primers used in the 2 systems. Both primers were used in RT-PCR, and 20 perfect-match primers were used in GeneChip (Affymetrix) study. We did not find that the genes were expressed more in CB-derived MCs because some of the transcripts that were increased in CB-derived MCs appeared to be macrophage-specific, and we found that approximately 1% to 2% of the macrophages were contaminated in CB-derived MCs but not in PB-derived MCs.
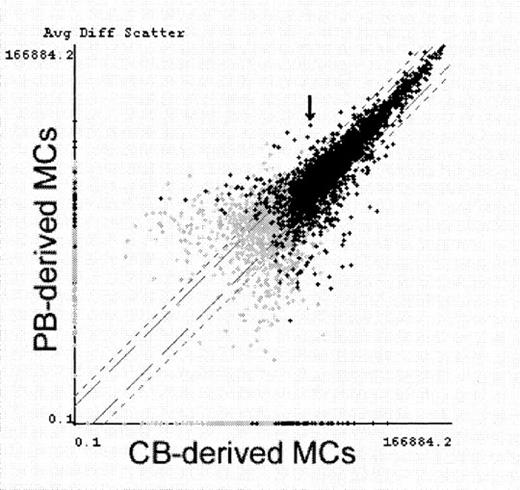
Comparative analysis of gene expression in PB-derived MCs and CB-derived MCs.
CB-derived MCs and PB-derived MCs were primed with IL-4 for 6 hours and examined for gene expression screening using GeneChip. Average fluorescence differences of approximately 5600 genes (7129 probe-arrays) were scattered on the logarithmic graph according to the values obtained from PB-derived MCs (vertical) and CB-derived MCs (horizontal). Black crosses indicate transcripts judged to be present at least in either MC type, and gray crosses indicate transcripts judged to be absent or marginal in both MC types. FcεRIα mRNA (indicated with an arrow) was determined with Comparison Analysis software to be the fifth most increased transcript in PB-derived MCs. Similar results were obtained in another experiment using IL-4–unprimed MCs.
Comparative analysis of gene expression in PB-derived MCs and CB-derived MCs.
CB-derived MCs and PB-derived MCs were primed with IL-4 for 6 hours and examined for gene expression screening using GeneChip. Average fluorescence differences of approximately 5600 genes (7129 probe-arrays) were scattered on the logarithmic graph according to the values obtained from PB-derived MCs (vertical) and CB-derived MCs (horizontal). Black crosses indicate transcripts judged to be present at least in either MC type, and gray crosses indicate transcripts judged to be absent or marginal in both MC types. FcεRIα mRNA (indicated with an arrow) was determined with Comparison Analysis software to be the fifth most increased transcript in PB-derived MCs. Similar results were obtained in another experiment using IL-4–unprimed MCs.
Discussion
We succeeded in efficiently generating MCs in serum-deprived cultures from PB and CB progenitors without injection of the progenitor-mobilizing cytokine, G-CSF.9,13 In the comparative functional studies on these cultured MCs, we found that PB-derived MCs released more histamine, IL-5, and GM-CSF than CB-derived MCs in response to anti-IgE challenge, even though they developed under the same culture conditions. The increased response to anti-IgE was related to the increased expression of surface FcεRI α chain on PB-derived MCs. By intracytoplasmic staining, it was revealed that the level of FcεRIα protein was also increased in PB-derived MCs. As expected, the level of FcεRIα mRNA was increased similarly in the protein of PB-derived MCs and CB-derived MCs, indicating that the mRNA level of FcεRIα directly regulates its protein level. On the other hand, receptor-bound IgE molecules mainly controlled the ratio of surface-to-intracytoplasmic FcεRIα protein expression, as has been reported.22-24
It may be claimed that the decreased level of FcεRIα in CB-derived MCs simply reflects the immaturity of the cells. Thus, we examined the time-course expression of FcεRIα on CB-derived MCs and PB-derived MCs in the same cytokine combination. The expression of FcεRIα peaked at 12 to 14 weeks of culture on both MC types, suggesting that the levels of various transcripts, such as chymase RNA, decreased, accompanied by aging of the cells, especially after 20 weeks, as been reported.9 Nevertheless, these results suggest that the down-regulation of FcεRIα is already determined at the CB progenitor stage and is, at least in part, cytokine-insensitive.
These results are consistent with the findings of Nilsson et al25 that SCF-dependent fetal liver-derived cultured MCs express little FcεRIα compared with dispersed MCs in adult tissue. They concluded that additional growth factor(s) might be required for full expression of the receptor in vitro.25 After this report, IL-4 was found to enhance the expression of FcεRIα protein by enhancing its mRNA expression.3 26 In the current study, IL-4 did enhance the mRNA level and protein expression of the receptor. However, it should be noted that IL-4 failed to compensate the total mRNA or protein levels of FcεRIα in CB-derived MCs up to those levels in PB-derived MCs (Figure 2, Table 3).
Next, we tested the hypothesis that IL-4, which is only one molecule known to up-regulate the expression of FcεRIα mRNA,23may be produced by PB-derived MCs. However, IL-4 mRNA expression was not detected in these cultured CB-derived MCs10 and PB-derived MCs in the current study, even after pretreatment with IgE and IL-4 and challenge with anti-human IgE.
To find out the responsible molecule for down-regulating the levels of FcεRIα in CB-derived MCs and to determine whether down-regulation of the FcεRIα mRNA is selective, we used the newly developed technology with high-density oligonucleotide probe-arrays, the GeneChip (Affymetrix) system. We found the down-regulation of FcεRIα was mostly selective among the whole gene expression. Of 5600 kinds of genes analyzed, FcεRIα mRNA was the fifth most increased transcript in PB-derived MCs. The 4 other strikingly increased transcripts were ubiquitously distributed proteins, not MC-specific, and their expression levels were less than 10% of β-actin mRNA except the cyclin-dependent kinase inhibitor, p27kip1protein.20 21 We quantitatively determined for the first time that CB-derived progenitors were capable of producing 20 times the number of MCs than PB-derived progenitors. Because the p27 protein inhibits cell division, we expected that the different expression level of p27 might cause different proliferation capacities of the 2 MC types. However, in spite of the decrease in mRNA levels, CB-derived MCs clearly expressed a similar protein level of p27 compared with PB-derived MCs. We are now searching responsible molecules for the down-regulation of FcεRIα mRNA in CB-derived MCs. However, we should be cautious to interpret the results of mRNA expression while investigating the level of protein expression.
In addition to the results obtained by using the GeneChip system, a nonimmunologic stimulus, ionophore A23187, induced histamine release from CB-derived MCs to an extent similar to that from PB-derived MCs. We conclude, therefore, that the down-regulation of FcεRIα in CB-derived MCs is nearly selective among whole events compared with PB-derived MCs. It should be noted that the down-regulation of FcεRIα in MCs results from transcriptional regulation in their progenitors, because these MCs were cultured under the same culture conditions. CB-derived T cells are known to be less affected by antigens27 and to produce less tumor necrosis factor-α and less interferon-γ, probably because of down-regulated NFAT1 transcription,17 though we failed to detect NFAT1 protein in human MCs by intracytoplasmic staining with flow cytometry (data not shown).
Nevertheless, we found that the expression of FcεRIα mRNA was almost exclusively down-regulated in CB-derived MCs developed from their progenitors. IgE is impaired in cord blood, and the levels increase with age.28 IgE-mediated reactions may be restricted during early infancy through the down-regulation of receptor expression in MCs and the impairment of IgE production. This observation is almost consistent with the report that basophils circulating in cord blood have sparse levels of FcεRIα.29 In addition to their conclusion that only IgE levels regulate basophil receptor expression,29however, we propose the possibility that the down-regulation of FcεRIα gene transcription in MCs may be determined at the progenitor levels.
Acknowledgments
We thank Dr Kiyoshi Kawashima, Dr Shigenobu Shoda, and the staff of the Department of Obstetrics, Gyoda Chuo Hospital, for their continued support in generously providing the umbilical cord blood. We thank Dr Kentaro Matsuda, Ms Noriko Hashimoto, and Mr Keisuke Yuki of the National Children's Medical Research Center for their technical assistance. We also thank Dr Jun Miyauchi of the Department of Pathology, National Children's Hospital, for his efforts to stain p27 protein in mast cells.
Supported in part by grants from the Japanese Ministry of Health and Welfare (pediatric research grant 9-04/1999) and from the Japan Health Science Foundation (grant 21045/1999).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hirohisa Saito, Department of Allergy, National Children's Medical Research Center, 3-35-31 Taishido, Setagaya-ku, Tokyo 154-8509, Japan; e-mail: hsaito@nch.go.jp.