Abstract
The key player for adaptation to reduced oxygen availability is the transcription factor hypoxia-inducible factor 1 (HIF-1), composed of the redox-sensitive HIF-1α and the constitutively expressed HIF-1β subunits. Under normoxic conditions, HIF-1α is rapidly degraded, whereas hypoxia, CoCl2, or desferroxamine promote protein stabilization, thus evoking its transcriptional activity. Because HIF-1 is regulated by reactive oxygen species, investigation of the impact of reactive nitrogen species was intended. By using different nitric oxide (NO) donors, dose- and time-dependent HIF-1α accumulation in close correlation with the release of NO from chemically distinct NO donors was established. Intriguingly, small NO concentrations induced a faster but transient HIF-1α accumulation than higher doses of the same NO donor. In contrast, NO attenuated up-regulation of HIF-1α evoked by CoCl2 in a concentration- and time-dependent manner, whereas the desferroxamine-elicited HIF-1α signal remained unaltered. To demonstrate an autocrine or paracrine signaling function of NO, we overexpressed the inducible NO synthase and used a coculture system of activated macrophages and tubular cells. Expression of the NO synthase induced HIF-1α accumulation, which underscored the role of NO as an intracellular activator for HIF-1. In addition, macrophage-derived NO triggered HIF-1α up-regulation in LLC-PK1 target cells, which points to intercellular signaling properties of NO in achieving HIF-1 accumulation. Our results show that NO does not only modulate the HIF-1 response under hypoxic conditions, but it also functions as a HIF-1 inducer. We conclude that accumulation of HIF-1 occurs during hypoxia but also under inflammatory conditions that are characterized by sustained NO formation.
Introduction
Aerobic organisms adapted life to conditions of well-defined oxygen tension. Under diverse physiologic settings such as at high altitude or during organ development as well as pathophysiologic situations such as stroke, ischemic heart disease, organ transplantation, or tumor development, the partial pressure of oxygen within an organ or the tissue varies. These changes are sensed by a still poorly characterized oxygen sensor, thus allowing the system to adapt to the new situation among other mechanisms by altered gene expression.1 Well-known candidates that respond to decreased oxygen pressure are erythropoietin, which stimulates proliferation and differentiation of erythrocytic progenitors in bone marrow, resulting in a greater oxygen-transporting capacity of the blood; and vascular endothelial growth factor, which promotes angiogenesis.2-4 Activation of these genes is achieved by an oxygen-sensitive transcription factor known as hypoxia-inducible factor 1 (HIF-1).5,6 HIF-1 is a heterodimer composed of the basic helix-loop-helix-PAS domain containing proteins HIF-1α and the aryl hydrocarbon receptor nuclear translocator (ARNT, also known as HIF-1β). The availability of HIF-1 is mainly determined by HIF-1α, which is regulated in an oxygen-sensitive manner, in contrast to HIF-1β. HIF-1β may also increase in response to hypoxic stress, but its abundance is more constitutive than that of HIF-1α. Under normoxia, HIF-1α is efficiently degraded by the proteasomal system, thus keeping its protein level extremely low.7-9 Under hypoxia, the messenger RNA of HIF-1α appears unchanged, but HIF-1α protein accumulates, dimerizes with HIF-1β, translocates to the nucleus, and binds to the target DNA sequence within the hypoxia-responsive element found in the promotor region of different genes. However, signaling mechanisms that culminate in HIF-1β protein accumulation are poorly defined, although intracellular redox changes elicited by the oxygen sensor and phosphorylation events are postulated.10-12
In addition to hypoxia, HIF-1 can be activated by the transition metal Co2+ as well as by the iron chelator desferroxamine (DFX). Although underlying mechanisms of action are unknown, there is evidence that they decrease the level of reactive oxygen species, which may serve as signaling molecules.5
Besides oxygen or its reduced species, another reactive molecule, nitric oxide (NO), affects physiologic and pathophysiologic signal transmission. NO serves as an intracellular or intercellular mediator, produced by NO synthases (NOS), that use L-arginine and O2to generate NO and citrulline.13 NOS isoenzymes are roughly distinguished as constitutively expressed versus cytokine-inducible (iNOS). The latter generates large amounts of NO over an extended time. Physiologically, NO binds to the heme of guanylyl cyclase, which causes cyclic GMP (cGMP) formation and downstream phosphorylation events.14 The impact of NO on the redox-sensitive target HIF-1 is controversially discussed. Some authors reported an inhibitory function of NO donors on hypoxia- or CoCl2-induced HIF-1 action,15-17 whereas Kimura et al18 described enhanced HIF-1 activity in response to NO. The existing diversity of NO in stimulating or blocking HIF-1 signaling prompted us to study the action of NO in greater detail and to question the role of endogenously generated NO. During our experiments we carefully controlled the time- and concentration-dependent effects of NO that was delivered to the system by using chemically distinct NO donors that have different half-lives. Our results show that low and high NO concentrations evoke a transient and delayed HIF-1α accumulation in a cGMP-independent manner, while only low NO concentrations suppress CoCl2-induced HIF-1α accumulation within a short time. However, in tubular LLC-PK1 cells, iNOS-generated NO or NO delivered by activated macrophages in a coculture system triggered HIF-1α accumulation. We conclude that NO not only modulates a HIF-1 response under hypoxic conditions but also functions as an inducer of HIF-1 under inflammatory conditions.
Materials and methods
L-NAME, carboxy-PTIO, spermine-NO, Dea-NO, Deta-NO, and NS 2028 were purchased from Alexis, Grünberg, Germany. Lipopolysaccharide (LPS) and DFX were bought from Sigma, Deisenhofen, Germany. All other chemicals were of the highest grade of purity commercially available.
Cell culture
Proximal tubular LLC-PK1 cells were cultured in Dulbecco modified Eagle medium (DMEM) with low glucose, supplemented with 2 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin (Biochrom, Berlin, Germany), and 10% fetal calf serum (Life Technologies, Berlin, Germany). Cells were transferred 2 times a week, and medium was changed before the experiments. Cells were kept in a humidified atmosphere of 5% CO2 in air at 37°C. For hypoxic stimulation, dishes were placed for 4 hours in an incubator with 0% O2, 5% CO2, and 95% N2in a humidified atmosphere.
S-nitrosoglutathione synthesis
S-nitrosoglutathione (GSNO) was synthesized as described previously.19
Western blot analysis
HIF-1α or iNOS were quantified by Western blot analysis. Briefly, 1 × 106 cells were incubated for the times indicated, scraped off, lysed in 150 μL lysis buffer (50 mM Tris-HCl, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, pH 8.0), and sonicated. After centrifugation (17 000g, 15 minutes), the protein content in the supernatants was analyzed. Finally, 100 or 200 μg protein was added to the same volume of 2 × sample buffer (125 mM Tris-HCl, 2% sodium dodecyl sulfate, 10% glycerin, 1 mM dithiothreitol, 0.002% bromphenol blue, pH 6.9) and boiled for 5 minutes. Proteins were resolved on 7.5% sodium dodecyl sulfate–polyacrylamide gels and blotted onto nitrocellulose (Amersham, Freiburg, Germany). Molecular weights were calibrated in proportion to the running distance of rainbow markers. Transblots were washed twice with tris(hydroxymethyl)aminomethane-buffered saline (TBS) (140 mM NaCl, 50 mM Tris-HCl, pH 7.2) containing 0.1% Tween 20 before blocking unspecific binding with TBS/5% skim milk for 1 hour. The HIF-1α (1:250 in TBS/0.5% milk) antibody (Transduction Laboratories, Heidelberg, Germany) or iNOS (1:1500 in TBS/0.5% milk) antibody (Transduction Laboratories) were added and incubated overnight at 4°C. Afterward, nitrocellulose membranes were washed 5 times for 15 minutes with TBS containing 0.1% Tween 20. For protein detection, blots were incubated with goat antimouse secondary antibodies or, in case of iNOS, goat antirabbit secondary antibodies conjugated with peroxidase (1:10 000 in TBS/0.2% milk) (Promega, Mannheim, Germany) for 45 minutes, followed by enhanced chemiluminescence detection (Amersham).
NO measurement
The release of NO from GSNO was measured using an NO-sensitve electrode (World Precision Instruments Inc, Sarasota, FL) calibrated in a stirred 1 mL chamber thermostated at 37°C by addition of known amounts of authentic NO to nitrogen-sparged water. NO was synthesized by the reaction of KNO2 with FeSO4 in H2SO4 exactly as described by the manufacturer. To take into account that NO release from GSNO may be affected by components in the culture medium or metabolism of the cells, generation of NO was measured in culture dishes in the supernatant of cells growing exactly as under experimental conditions.
Overexpression of iNOS
LLC-PK1 cells were grown to 60% to 80% confluence prior to transfection with the calcium-phosphate method. Briefly, 10 μg DNA (human iNOS plasmid or control plasmid), 657 μL dH2O, 91.5 μL 2 M CaCl2, and 750 μL 2 × HBS buffer (2.3 M NaCl, 12 mM Na2HPO4, 202 mM HEPES, pH 7.1) were mixed and preincubated at room temperature for 5 minutes. Afterward, the mixture was added dropwise to 2 × 106 cells/6 mL DMEM/10% fetal calf serum medium containing 25 μM chloroquin. Seven hours later, the medium was changed, and iNOS and HIF-1α content was analyzed by Western blot analysis 28 hours after transfection. The pCIS-human-iNOS plasmid was kindly provided by T. Billiar (Pittsburgh, PA).
Coculture of RAW 264.7 and LLC-PK1 cells
The mouse macrophage cell line RAW 264.7 was cultured in RPMI medium supplemented with 2 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin (Biochrom), and 10% fetal calf serum (Life Technologies). Cells were transferred 3 times a week and were kept in nonadherent dishes. For stimulation, RAW 264.7 was seeded in new dishes and stimulated with LPS and interferon (IFN)-γ in the absence or presence of L-NAME or carboxy-PTIO for 18 hours. Afterward, macrophages were collected, centrifuged (37°C, 570g, 7 minutes), counted, and resuspended in fresh DMEM medium (see LLC-PK1culturing conditions) without LPS and IFN-γ but with the readdition of L-NAME or carboxy-PTIO. A total of 1.6 × 106 RAW 264.7 cells were transferred to LLC-PK1 cells. For these experiments, 8 × 105 LLC-PK1 cells were seeded in 10 cm dishes one day prior to coculture with macrophages. Cocultures were terminated after times indicated, and the medium was collected to analyze nitrite formation. Cells (RAW 264.7 and LLC-PK1together) were used for Western blot analysis as described.
Nitrite determination
Nitrite, a stable NO oxidation product, was determined using the Griess reaction. Cell-free culture supernatants were collected (200 μL), adjusted to 4°C, and mixed with 20 μL sulphanilamide (dissolved in 1.2 M HCl) and 20 μLN-naphthylethylenediamine dihydrochloride. After 5 minutes at room temperature, the absorbance was measured at 560 nm with a reference wavelength at 690 nm. Nitrite concentrations were calculated using a NaNO2 standard.
Statistical analysis
Each experiment was performed at least 3 times, and representative pictures or data are shown.
Results
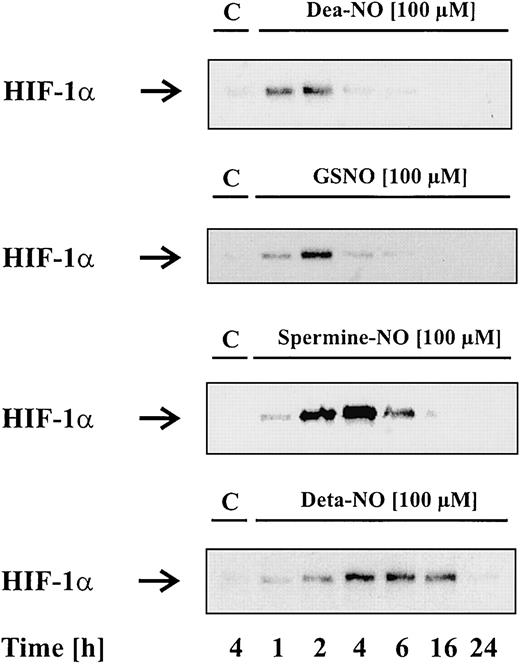
To study the effect of NO on HIF-1α accumulation, we tested several NO donors. These chemically distinct compounds spontaneously release NO—however, with remarkably different kinetics. Dea-NO (2-[N,N-diethylamino]-diazenolate-2-oxide · Na) decomposes fast compared with GSNO or spermine-NO, while Deta-NO is considered a slow NO releaser, showing the longest half-life of all tested agents. In the first set of experiments, we stimulated LLC-PK1 cells for 1 to 24 hours with vehicle (control) or 100 μM of the indicated NO donors, followed by Western blot analysis of HIF-1α. In the absence of NO donors, LLC-PK1 cells lacked HIF-1α protein, whereas Dea-NO, GSNO, spermine-NO, or Deta-NO induced a transient protein accumulation. We noticed some correlation between the kinetics of NO release and HIF-1α accumulation. With the fast NO releaser Dea-NO, HIF-1α accumulation occurred within 1 to 2 hours and declined afterward. Similar effects were achieved with 100 μM GSNO, which evoked the strongest HIF-1α signal after 2 hours. In contrast, HIF-1α appeared more stable after stimulation with spermine-NO or Deta-NO. Spermine-NO evoked maximal HIF-1α accumulation after 2 to 4 hours, whereas Deta-NO triggered a delayed but 12- to 14-hour–lasting HIF-1α response.
Figure 1 shows that NO donors induce HIF-1α accumulation. In contrast to the other NO donors, sodium nitroprusside (SNP) was unable to provoke HIF-1α accumulation (data not shown). We further examined the effect of glutathione and spermine, which resemble cleavage products of GSNO and spermine-NO, in evoking a HIF-1α response (data not shown). As assumed, they were uneffective, thus indicating that the NO moiety of the compounds is needed for HIF-1α stabilization.
NO donors induce HIF-1α accumulation.
LLC-PK1 cells were stimulated with 100 μM Dea-NO, GSNO, spermine-NO, Deta-NO, or vehicle for the times indicated. Accumulation of HIF-1α was determined by Western blot analysis as outlined in “Materials and methods.” Blots are representative for at least 3 independent experiments.
NO donors induce HIF-1α accumulation.
LLC-PK1 cells were stimulated with 100 μM Dea-NO, GSNO, spermine-NO, Deta-NO, or vehicle for the times indicated. Accumulation of HIF-1α was determined by Western blot analysis as outlined in “Materials and methods.” Blots are representative for at least 3 independent experiments.
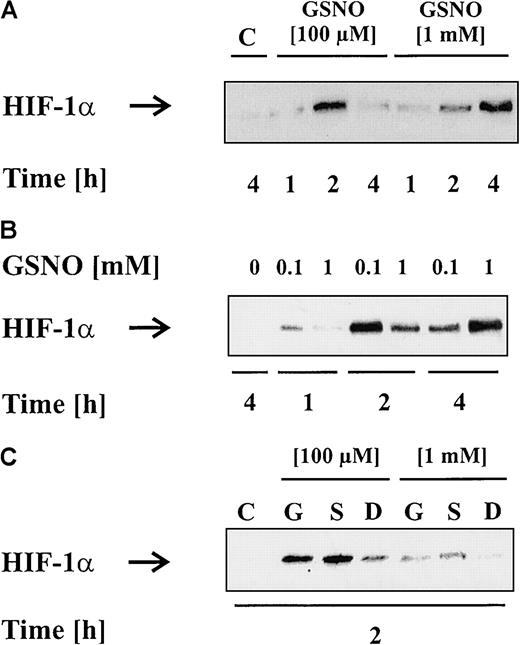
In the next set of experiments, we evaluated the concentration dependency of nontoxic NO doses on HIF-1α accumulation. As shown before, 100 μM GSNO elicited a rapid but transient accumulation of HIF-1α whereas 1 mM GSNO evoked a delayed HIF-1α signal (Figure2A). Herein, HIF-1α increased between 2 and 4 hours and remained elevated for 6 to 8 hours (data not shown). For a more detailed analysis on the question of whether a lower concentration initiated a faster response than a higher concentration, we loaded representative, time-matched samples next to each other on the gel. Western blot analysis (Figure 2B) clearly showed that HIF-1α started to accumulate after 1 hour, reached the maximum at 2 hours, and decreased at 4 hours when activated by 100 μM GSNO. In contrast, when 1 mM GSNO was employed, HIF-1α was absent after 1 hour, increased slightly after 2 hours—albeit to a lesser extent compared with stimulation with 100 μM GSNO—and revealed the maximum expression after 4 hours. Irrespective of the concentration of GSNO being used, the amount of HIF-1α protein being expressed appeared equal, although the time point of maximal expression varied significantly.
HIF-1α accumulation patterns induced by low versus high NO donor concentrations.
LLC-PK1 cells were treated with vehicle, 100 μM GSNO, or 1 mM GSNO (A,B). After 1, 2, or 4 hours, HIF-1α accumulation was detected by Western blot analysis. To directly compare HIF-1α accumulation induced by 100 μM or 1 mM GSNO, samples of corresponding time points were loaded next to each other (B). (C) LLC-PK1cells were exposed to 100 μM or 1 mM GSNO (“G”), spermine-NO (“S”), or Dea-NO (“D”) for 2 hours, and HIF-1α accumulation is shown by Western blot analysis. Blots are representative for at least 3 independent experiments.
HIF-1α accumulation patterns induced by low versus high NO donor concentrations.
LLC-PK1 cells were treated with vehicle, 100 μM GSNO, or 1 mM GSNO (A,B). After 1, 2, or 4 hours, HIF-1α accumulation was detected by Western blot analysis. To directly compare HIF-1α accumulation induced by 100 μM or 1 mM GSNO, samples of corresponding time points were loaded next to each other (B). (C) LLC-PK1cells were exposed to 100 μM or 1 mM GSNO (“G”), spermine-NO (“S”), or Dea-NO (“D”) for 2 hours, and HIF-1α accumulation is shown by Western blot analysis. Blots are representative for at least 3 independent experiments.
To confirm that the timely distinct HIF-1α accumulation response was not restricted to high versus low GSNO concentrations, we repeated these experiments with 100 μM and 1 mM spermine-NO as well as Dea-NO (Figure 2C). Herein, we obtained similar results, when focusing on the 2-hour time point only. In conclusion, low NO concentrations induced a faster HIF-1α response than higher amounts of the individual NO donors. Having that in mind, we continued and measured the NO release over time (Table 1). The question arose whether the NO concentration generated by high amounts of GSNO had fallen back into a stimulatory range at later time points. This was excluded because 1 mM GSNO still released 2.5-fold to 3.5-fold (1620 ± 560 nM) more NO at 4 hours than the amount of NO generated by 100 μM at 1 hour (420 ± 20 nM). Therefore, a simple matter of NO concentration is unlikely to explain the timely diverse HIF-1α accumulation pattern. Whether this points to different signal transduction pathways is subject to speculation.
NO often evokes activation of soluble guanylyl cyclase, and subsequent intracellular signaling is cGMP-dependent. To check for the possible involvement of cGMP, we used NS 2028, which is a specific inhibitor of guanylyl cyclase. LLC-PK1 cells were preincubated with 5 μM NS 2028 followed by the addition of 100 μM (Figure3A) or 1 mM GSNO (Figure 3B) for 1, 2, and 4 hours. As shown in Figure 3, NO-induced HIF-1α accumulation was unaltered in the presence of NS 2028. In additional experiments, which turned out to be negative, we applied lipophilic cGMP analogues and looked for HIF-1α accumulation (data not shown). Obviously, NO triggered HIF-1α stabilization in a cGMP-independent manner.
NO-induced HIF-1α accumulation is unrelated to cGMP action.
LLC-PK1 cells were exposed to 100 μM (A) or 1 mM (B) GSNO in the absence or presence of 5 μM of the guanylyl cyclase inhibitor NS 2028, preincubated for 30 minutes. HIF-1α protein was analyzed after 1, 2, or 4 hours by Western blot analysis. Blots are representative for at least 3 different experiments.
NO-induced HIF-1α accumulation is unrelated to cGMP action.
LLC-PK1 cells were exposed to 100 μM (A) or 1 mM (B) GSNO in the absence or presence of 5 μM of the guanylyl cyclase inhibitor NS 2028, preincubated for 30 minutes. HIF-1α protein was analyzed after 1, 2, or 4 hours by Western blot analysis. Blots are representative for at least 3 different experiments.
In some analogy to our examinations, Kimura and coworkers18 underscored the potency of NO donors to provoke HIF-1 induction. However, this is in contrast with some other publications describing an inhibitory activity of NO on hypoxia- and CoCl2-induced HIF-1 activation.15-17 This prompted us to study the time- and concentration-dependent actions of NO in modulating CoCl2-, the iron chelator DFX-, or hypoxia-evoked HIF-1α responses. First, we treated LLC-PK1 cells with 100 μM CoCl2 and confirmed HIF-1α accumulation between 2 and 4 hours. Second, we costimulated cells with 100 μM CoCl2 in the presence of 100 μM (Figure 4A) or 1 mM GSNO (Figure 4B). At 4 hours, 100 μM GSNO attenuated CoCl2-evoked HIF-1α stabilization, while no difference emerged at 2 hours. Overall, protein expression of HIF-1α closely resembles the time response noticed with 100 μM GSNO. In contrast to low NO concentrations, 1 mM GSNO did not alter the CoCl2 response at all. Experiments were repeated in the presence of NS 2028 or by adding lipophilic cGMP analogues, but with no indications for a role of cGMP (data not shown).
Impact of NO on CoCl2, DFX, or hypoxia-induced HIF-1α accumulation.
LLC-PK1 cells were stimulated with vehicle, 100 μM (A,C,E) or 1 mM (B,D) GSNO, 100 μM CoCl2 (A,B), 250 μM DFX (C,D), or hypoxia (E), either alone or in different combinations. Cells were lysed and prepared for HIF-1α Western blot analysis after times indicated. Results are representative for at least 5 independent experiments.
Impact of NO on CoCl2, DFX, or hypoxia-induced HIF-1α accumulation.
LLC-PK1 cells were stimulated with vehicle, 100 μM (A,C,E) or 1 mM (B,D) GSNO, 100 μM CoCl2 (A,B), 250 μM DFX (C,D), or hypoxia (E), either alone or in different combinations. Cells were lysed and prepared for HIF-1α Western blot analysis after times indicated. Results are representative for at least 5 independent experiments.
Similar to the experiments with CoCl2, we used 250 μM DFX to achieve HIF-1α accumulation between 2 and 4 hours (Figure 4C). We went on to stimulate cells with DFX in combination with 100 μM GSNO, applied for 1, 2, and 4 hours, but noticed no variations in HIF-1α protein accumulation compared with the DFX signal. In some analogy, 1 mM GSNO left the DFX response at the 4-hour time point unaltered (Figure 4D). As described in the literature and similar to CoCl2 (Figure 4A), 100 μM GSNO also suppressed the HIF-1α signal induced by hypoxia. This is shown for the 4-hour time point only (Figure 4E).
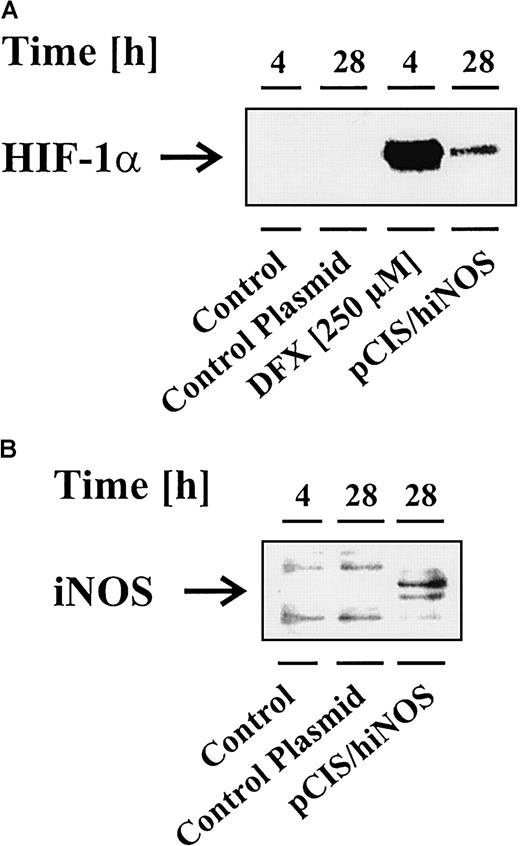
After having established the modulatory impact of NO donors in achieving variations in HIF-1α accumulation, the question of its physiologic or pathophysiologic importance was raised. To demonstrate the importance of endogenously generated NO in affecting HIF-1α accumulation, we set up 2 different experiments to prove the role of NO as an intracellular and intercellular messenger molecule. First, we overexpressed the human iNOS in LLC-PK1 cells to study any contribution of endogenously generated NO in achieving HIF-1α accumulation. This experimental approach avoids side effects of cytokines, which would be required in order to express iNOS, in modulating HIF-1α expression. Successful iNOS expression was confirmed by Western blot analysis against the iNOS protein and by measuring nitrite, which resembles one stable oxidation end product of NO. Figure 5B shows iNOS expression in transfected LLC-PK1 cells compared with unstimulated cells or transfections with a control plasmid. Nitrite values amounted to 2 μM for pCIS/hiNOS-transfected LLC-PK1 cells, compared with controls with nitrite values below the detection limit of the Griess assay. In this experiment, HIF-1α expression was noticed in correlation with an active iNOS only (Figure 5A). The response of untransfected LLC-PK1 cells toward 250 μM DFX, incubated for 4 hours, served as a positive HIF-1α control.
Overexpression of iNOS promotes HIF-1α accumulation.
LLC-PK1 cells were transfected with the pCIS plasmid containing the human iNOS sequence (pCIS/hiNOS) or a control plasmid as described in “Materials and methods.” Twenty-eight hours after transfection, cells were prepared for Western blots to analyze HIF-1α accumulation (A) and iNOS expression (B). For control reasons, untransfected LLC-PK1 cells were exposed to vehicle (control) or 250 μM DFX for 4 hours. Blots are representative for 3 independent experiments.
Overexpression of iNOS promotes HIF-1α accumulation.
LLC-PK1 cells were transfected with the pCIS plasmid containing the human iNOS sequence (pCIS/hiNOS) or a control plasmid as described in “Materials and methods.” Twenty-eight hours after transfection, cells were prepared for Western blots to analyze HIF-1α accumulation (A) and iNOS expression (B). For control reasons, untransfected LLC-PK1 cells were exposed to vehicle (control) or 250 μM DFX for 4 hours. Blots are representative for 3 independent experiments.
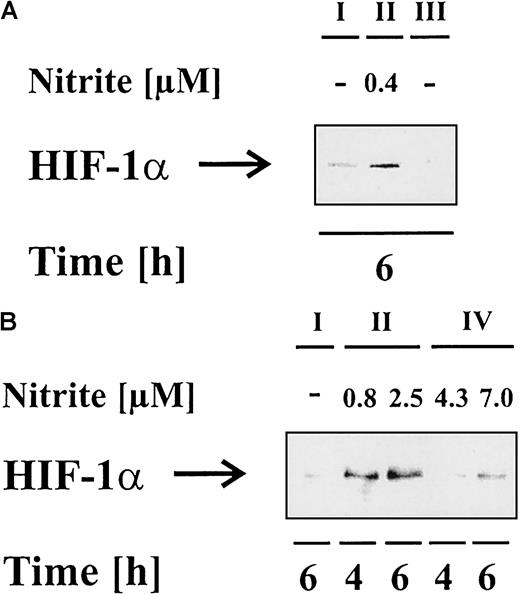
In complex, multicellular organisms, the expression of iNOS and the formation of NO normally is found in close association with inflammatory settings, when competent immune cells such as macrophages fight pathogens or infections, which often has an impact on bystander host cells as well. Therefore, our second approach mimicked an inflammatory situation by coculturing activated macrophages with LLC-PK1 cells. Herein, RAW 264.7 macrophages were stimulated with vehicle (Figure 6, I) or 1 μg/mL LPS and 100 U/mL IFN-γ (Figure 6, II) for 18 hours, which resulted in up-regulation and activation of macrophage iNOS. NO production was determined by nitrite formation (data not shown). To interfere in NO formation or action, we either supplied 1 mM L-NAME (NG-nitro-L-arginine-methyl ester) (Figure 6, III) in combination with LPS/IFN-γ to attenuate NO production, or we added 100 μM carboxy-PTIO (carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) (Figure 6, IV) to specifically scavenge NO. Following macrophage activation, cells were collected and added to LLC-PK1 cells as described in “Materials and methods.” Cocultures were terminated after 4 or 6 hours, and HIF-1α accumulation and nitrite formation in the coculture supernatant were analyzed. In case of carboxy-PTIO, it rapidly reacts with NO to yield nitrite without concomitant production of nitrate. Therefore, nitrite values can be 4- to 5-fold higher, which does not interfere with the NO-specific scavenging capacity.20 21
Activated RAW 264.7 macrophages provoke HIF-1α accumulation in LLC-PK1 cells under coculture conditions.
RAW 264.7 macrophages were stimulated with vehicle (I), 1 μg/mL LPS in combination with 100 U/mL IFN-γ (II), LPS/IFN-γ plus 1 mM L-NAME (III), or LPS/IFN-γ plus 100 μM carboxy-PTIO (IV) for 18 hours. Afterward, macrophages were resuspended in fresh medium as described in “Materials and methods” and added to LLC-PK1 cells in a ratio of 1:1 (A) or 1:2 (B) (LLC-PK1:RAW 264.7). Cells were cocultured for 4 or 6 hours in the absence or presence of 1 mM L-NAME (III) or 100 μM carboxy-PTIO (IV). Cells were prepared for HIF-1α Western blot analysis, while medium was used for nitrite measurements. Results are representative for 3 different experiments.
Activated RAW 264.7 macrophages provoke HIF-1α accumulation in LLC-PK1 cells under coculture conditions.
RAW 264.7 macrophages were stimulated with vehicle (I), 1 μg/mL LPS in combination with 100 U/mL IFN-γ (II), LPS/IFN-γ plus 1 mM L-NAME (III), or LPS/IFN-γ plus 100 μM carboxy-PTIO (IV) for 18 hours. Afterward, macrophages were resuspended in fresh medium as described in “Materials and methods” and added to LLC-PK1 cells in a ratio of 1:1 (A) or 1:2 (B) (LLC-PK1:RAW 264.7). Cells were cocultured for 4 or 6 hours in the absence or presence of 1 mM L-NAME (III) or 100 μM carboxy-PTIO (IV). Cells were prepared for HIF-1α Western blot analysis, while medium was used for nitrite measurements. Results are representative for 3 different experiments.
As seen in Figure 6A,B, activated RAW 264.7 macrophages induced HIF-1α accumulation after 4 and 6 hours, which correlated with NO production. RAW 264.7 macrophages cultured alone did not up-regulate HIF-1α within 18 to 24 hours after LPS/IFN-α stimulation (data not shown) and, therefore, the HIF-1α signal must have been generated in LLC-PK1 cells. L-NAME significantly suppressed nitrite formation and attenuated HIF-1α accumulation (Figure 6A). In analogy, carboxy-PTIO attenuated accumulation of HIF-1α as well (Figure 6B). We conclude that activated macrophages evoke accumulation of HIF-1α in LLC-PK1 cells via the formation of NO.
Discussion
HIF-1 represents the most important transcription factor regulating gene expression under hypoxic conditions. The concept has been put forward that the intracellular level of reactive oxygen species and phosphorylation events determine HIF-1 activation, but detailed mechanisms await clarification. Because oxygen species promote HIF-1α degradation,5 it was predicted that another reactive molecule, NO, would negatively affect HIF-1α stabilization. Indeed, several papers describe an inhibitory effect of NO donors on hypoxia- or CoCl2-induced HIF-1 activation, thus supporting radical-evoked HIF-1α degradation.7-9 However, this simple idea missed including the diverse effects of NO that appear as a result of time- and concentration-dependent delivery as well as the interaction with other biomolecules. Under normoxic conditons, only Kimura et al18 described stimulating properties of NO donors on HIF-1 activity, which is contrasted by reports showing inhibitory actions of NO in combination with hypoxia and CoCl2.15-17 To explain these discrepancies, we tested several NO donors with diverse chemical structures and NO-releasing half-lives. Herein, we concur with Kimura et al18 in showing NO donor-induced HIF-1α protein accumulation. Our study extends these finding by establishing a correlation between the NO-releasing capacity of the NO donor and HIF-1α regulation. The rapid decomposition of Dea-NO induced the faster HIF-1α response, while the slow decomposing Deta-NO elicited a delayed HIF-1α accumulation (Figure 1). Although we put the focus on HIF-1α accumulation, protein appearance and DNA binding activity correlated (data not shown). In addition, several aspects of NO-elicited HIF-1α accumulation were reproduced in HepG2 and HEK 293 cells (data not shown).
SNP, often chosen in earlier studies to show the inhibitory effect of NO,16 17 was the only NO donor that was unable to provoke HIF-1α accumulation (data not shown). However, because SNP releases cyanide prior to NO, it is far from being an ideal NO donor.
Irrespective of the NO donor with the exception of SNP, we noticed a cGMP-independent HIF-1α accumulation. Interestingly, a low NO concentration induced a more rapid and, at that time point, more pronounced HIF-1α stabilization compared with a higher concentration, which was followed by fast degradation (Figure 2). The simple explanation that high NO concentrations block HIF-1α accumulation whereas low concentrations promote its accumulation was excluded by measuring actual NO values with a NO electrode (Table 1). However, early HIF-1α degradation by low NO concentrations could also be explained by used NO donors, but it is not an explanation for the more rapid HIF-1α accumulation. Thus, the possibility remains that 2 different signal transduction pathways are involved such as phosphorylation events, which are activated by low NO concentrations and inhibited with higher amounts or vice versa.
An established target gene under the control of HIF-1 is vascular endothelial growth factor (VEGF).22 Our study may shed light on 2 independent in vitro studies performed in mesangial cells and smooth muscle cells describing increased VEGF expression after NO generation. In glomerular mesangial cells, GSNO was used as a NO donor23 and vascular smooth muscle cells were transfected with the endothelial NOS or stimulated with SNP.24 GSNO and endothelial NOS overexpression up-regulated VEGF, although HIF-1 involvement was not examined. Intriguingly, application of SNP attenuated VEGF synthesis, an activity that was mimicked by cyanide. These findings can be explained by our experiments showing that NO promotes HIF-1α accumulation. However, the role of NO on in vivo VEGF expression and angiogenesis or tumor growth is controversial. Inhibition of angiogenesis in the chick chorioallantoic membrane25 or attenuated growth and metastatic properties of the Lewis lung tumor in mice26 are described as well as a positive correlation between NOS activity, tumor growth, and vascular density.27-29 It should be kept in mind that most of these examples combine hypoxia and NO effects.
Erythropoietin (Epo), another well-studied HIF-1 target gene, has mainly been examined in response to hypoxia or CoCl2. Therefore, the impact of NO on Epo expression remains unclear, whereas hypoxia-induced Epo synthesis was suppressed by NO donors.30,31 Based on our studies, we provide detailed analysis that the inhibitory effect of NO on HIF-1α accumulation was concentration- and time-dependent. Inhibition was only achieved as long as we supplied low NO concentrations, eg, 100 μM GSNO or Dea-NO, that induced a faster and more transient HIF-1α occurrence than CoCl2. As seen in Figure 4A, the HIF-1α accumulation pattern elicited by 100 μM GSNO/CoCl2 more or less resembled the one that was induced by 100 μM GSNO alone. We assume that under the influence of NO we first stimulate mechanisms that cause HIF-1α up-regulation followed by activation of degradation processes. We propose that moderate stress induces degradation by the proteasomal system whereas enhanced stress may inhibit the 26S proteasome.32 33 As a result of an active degradation process, the CoCl2/HIF-1α signal was only suppressed when the accumulation pattern of HIF-1α evoked by NO preceded that of CoCl2. That was not the case with higher NO concentrations, eg, with 1 mM GSNO (Figure 4B). Here we assume that NO directly blocks HIF-1α degradation. In contrast to CoCl2, the DFX signal was not altered by NO under the conditions tested (Figure 4C,D) whereas, similar to CoCl2, NO also suppressed the hypoxia-induced response (Figure 4E).
NO donors are valuable tools for mechanistic studies, but they appear unsuited to answer the question of the physiologic or pathophysiologic impact of NO formation. Therefore, we overexpressed the human iNOS to demonstrate the importance of NO as an intracellular messenger molecule, at the same time avoiding known side effects of cytokines on HIF-1α accumulation.34 Evidently (Figure 5), successful iNOS expression triggered HIF-1α accumulation, supporting our notion that NO, as an autocrine or paracrine factor, causes HIF-1α accumulation. Considering a moderate transfection efficiency in these experiments, one may conclude that our overall effect has even been underestimated.
In a second set of experiments, an inflammatory setting and NO-producing system was mimicked by coculturing activated macrophages with resting tubular LLC-PK1 cells. These conditions provoked HIF-1α accumulation in LLC-PK1. The HIF-1α response correlated with NO formation and administration of an iNOS inhibitor or carboxy-PTIO to scavenge NO-suppressed HIF-1α accumulation. So far, we conclude that macrophage-derived NO stimulates HIF-1α in tissue, ie, target cells.
Our results imply that an immunoregulator and inflammatory molecule such as NO acts as an intracellular and intercellular HIF-1 modulator. The action of NO is defined by its concentration- and time-dependency. Inflammatory conditions that are associated with NO formation may require our further attention in evoking an HIF-1 response under normoxic conditions.
Acknowledgments
We thank C. Blechner and S. Liehner for excellent technical assistance, and we are also grateful to Dr T. Billiar for providing the hiNOS plasmid.
Supported by the Deutsche Forschungsgemeinschaft (SFB 423/A5).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernhard Brüne, University of Erlangen-Nürnberg, Faculty of Medicine, Loschgestrasse 8, 91054 Erlangen, Germany; e-mail:mfm423@rzmail.uni-erlangen.de.