Abstract
Leukocyte adhesion deficiency type I (LAD-1) is a disorder associated with severe and recurrent bacterial infections, impaired extravascular targeting and accumulation of myeloid leukocytes, altered wound healing, and significant morbidity that is caused by absent or greatly diminished surface expression of integrins of the β2 class. We report clinical features and analysis of functions of cells from a patient with a myelodysplastic syndrome and infectious complications similar to those in the severe form of LAD-1, but whose circulating neutrophils displayed normal levels of β2 integrins. Analysis of adhesion of these cells to immobilized ligands and to endothelial cells and assays of cell-cell aggregation and chemotaxis demonstrated a profound defect in adhesion mediated by β2 integrins indicative of a variant form of LAD-1. A novel cell line established from Epstein-Barr virus–transformed lymphoblasts from the subject demonstrated deficient β2 integrin–dependent adhesive function similar to that of the primary leukocytes. In addition, these cells had markedly impaired β1 integrin–dependent adhesion. Sequence analysis and electrophoretic mobility of β1 and β2 proteins from the cell line demonstrated that the defects were not a result of structural abnormalities in the integrin subunit chains themselves and suggest that the adhesive phenotype of these cells is due to one or more abnormalities of inside-out signaling mechanisms that regulate the activity of integrins of these classes. These features define a unique LAD-1 variant syndrome that may reveal important insights that are generally relevant to inside-out signaling of integrins, a molecular process that is as yet incompletely understood.
Introduction
To eliminate invasive microrganisms, leukocytes must adhere to endothelial cells and migrate from the blood through the endothelial barrier and the extracellular matrix to sites of infection and tissue injury. The ability of neutrophils (polymorphonuclear leukocytes [PMNs]) and other classes of leukocytes to reach the extravascular milieu is dependent on the coordinate actions of adhesion molecules on their surfaces and on endothelial cells.1-4These critical tethering molecules include integrins.5 The principal class of integrins on neutrophils and other leukocytes is composed of a common β2 protein subunit that is noncovalently paired with α polypeptide chains, termed αL, αM, αX, and αD, to yield functional αβ heterodimers. These have been termed the “β2 or leukocyte integrins” because of ubiquitous expression of one or more members of the family on all types of leukocytes.6,7 Integrins of the β1, β3, and other classes are also variably expressed on individual leukocyte subtypes and mediate specific adhesive functions.7-9
Neutrophils and other leukocytes circulate in a quiescent nonadhesive state but can rapidly become adhesive when activated by signaling molecules that include bacterial and complement peptides, chemokines, lipid factors, and other inflammatory mediators.7,10-12Cellular activation causes heterodimers to become competent to recognize their ligands on other cells and in matrix structures, a process that is termed “inside-out signaling” or “integrin activation”1,13,14 and that involves altered conformation and/or clustering of the integrins.6,7,15,16 Cytoskeletal proteins and intracellular transduction molecules interact directly or indirectly with the cytoplasmic domains of integrins of different classes and are involved in their conversion from the quiescent to the adhesive state.13,14,17 The specific biochemical mechanisms that mediate inside-out signaling of integrins are, however, not completely defined.14,17 In addition to their adhesive functions, integrins can modulate cellular behavior by transmitting signals from the exterior to the inside of the cell, a process referred to as “outside-in signaling.”13,18 19
Human syndromes resulting from deficiency or impaired function of integrins and other adhesion molecules have contributed critical insights into their biologic roles.7 They are also important, albeit rare, clinical problems. Leukocyte adhesion deficiency type I (LAD-1) is an autosomal recessive disorder characterized by recurrent infections that are frequently life-threatening, impaired extravascular accumulation of PMNs and monocytes that is manifested clinically as lack of pus formation, and dysregulated wound healing that causes late separation of the umbilical cord and other abnormalities of tissue repair in many subjects.6,20,21 LAD-1 is distinguished from LAD-2, which is caused by a defect in synthesis of fucosylated glycoconjugates that are required for the functions of selectin ligands,22-24by impaired β2 integrin–dependent adhesion in the former syndrome and deficient selectin-dependent adhesion in the latter disorder.25,26 The impaired adhesive function in LAD-1 is secondary to defects in the common β2 subunit of the leukocyte integrins imposed by a variety of types of mutations.6 The aberrant β2 integrin is either undetectable or is unable to properly associate with the α subunits. Monocytes and neutrophils from these patients have profound defects in adhesion-related defensive functions and fail to adhere and migrate to extravascular sites of acute infection and inflammation despite the chronic leukocytosis. In severe deficiency (less than 1% of the normal amount of cell surface expression of the β2integrin heterodimers) patients often die within the first year.6,20,21 Moderately deficient β2expression (5%-10% of the normal level) results in a milder form of the syndrome. In both the severe and milder forms of LAD-1, the infectious complications are predominantly bacterial rather than viral or fungal, indicating that the adhesive behavior of neutrophils and monocytes is impaired to a greater extent than is that of lymphocytes.6,20,27 The relative preservation of lymphocyte-dependent functions in vivo6,27 has been attributed to normal expression of β1integrins,28-30 which mediate the adhesive functions and signaling of lymphocytes,8,31 and to compensatory mechanisms in other molecular systems.20
Recently 2 subjects with “variant” forms of LAD-1 have been reported.29,30 The surface levels of β2integrins on leukocytes from these patients were sufficient to mediate adhesion and transmigration, but the ability of the integrin heterodimers to recognize ligands was markedly impaired. Here we report clinical features and studies of primary neutrophils and a cell line from a subject with a variant LAD syndrome different from either of those previously reported. Primary neutrophils from the patient were defective in adhesive function, and Epstein-Barr virus (EBV)–transformed lymphoblasts from the patient, who had a myelodysplastic stem cell disorder, exhibited defects in inside-out signaling of β1, as well as β2, adhesive function. Although it is now known that human PMNs display members of the β1 family of integrins7 deficiencies in β1 function have not been found in the 2 other examples of variant LAD identified to date or in other human syndromes that have been reported. Our findings provide new evidence that the activation mechanisms of β1 and β2 integrins share common regulatory features.
Patient, materials, and methods
Antibodies
The monoclonal antibodies (mAbs) 60.1 (anti-αM) and 60.3 (anti-β2) were provided by Patrick Beatty of the University of Utah and Bristol-Myers Squibb Pharmaceutical Research Institute (Seattle, WA), the β2-stimulating mAb KIM 185 by Dan Simon (Brigham and Women's Hospital, Boston, MA), and S12 (anti–P-selectin) and PL-2 (anti–PSGL-2) by Rodger McEver (University of Oklahoma, Oklahoma City, OK). The anti–L-selectin mAbs Dreg 200 and Dreg 56 were gifts from Thomas Tedder (Duke University). The B-D15 (anti-β1) and H130 (anti-CD45) were purchased from Biosource International (Camarillo, CA), and MAB 13 (anti-β1) from Becton Dickinson (Bedford, MA). The β1-stimulating mAb TS2/16-producing hybridoma cell line was purchased from American Tissue Culture Collection (ATTC, Rockville, MD) and used as a hybridoma supernatant. The mAbs P4C2 (anti-α4) and P1D6 (anti-α5) were purchased from Life Technologies (Gaithersburg, MD). The anti-αL(CD11a) mAb BCA1 was purchased from R&D Systems (Minneapolis, MN) and the anti-CD14 mAb TÜK4 from Dako (Glostrup, Denmark). The primary isotype control antibodies anti-IgG2a,κ and anti-IgG1a,κ were purchased from Becton Dickinson (San Jose, CA) and FITC-conjugated secondary goat antimouse IgG (heavy and light chain specific) was purchased from Southern Biotechnology (Birmingham, AL).
Assay of surface expression of cell adhesion molecules
Assays of neutrophil β2 integrin function
Blood samples from the subject and from healthy adult controls were collected using procedures and consent forms approved by the Institutional Review Board of the University of Utah. We measured β2 function on stimulated neutrophils with assays of adhesion, aggregation, and chemotaxis. The β2integrin–dependent adhesion to human umbilical vein endothelial cells (HUVECs) and to immobilized matrix proteins was assayed as previously described.33-35 Matrix proteins were added to wells at concentrations from 30 to 60 μg/mL, allowed to adhere at 4°C overnight, blocked with 1% human serum albumin (HSA) for 2 hours at 37°C, and washed with Hanks Balanced Salt Solution (HBSS; Biowhittaker; Walkersville, MD) before use. HUVECs were isolated with collagenase and grown in a primary culture on gelatin-treated wells.36 Neutrophils were isolated and labeled with 0.0185 MBq (0.5 μCi) 111Indium per 106cells.36 Adhesion was induced with activating agonists that trigger inside-out signaling of β2integrins34 or with divalent cation substitution.37-39 We have previously shown that adhesion under these conditions is specifically mediated by β2integrins.34,35 For divalent cation substitution, neutrophils were suspended in HBSS, free of Ca++ and Mg2+, containing 1 mM EDTA, washed one time, and then resuspended in 1 mM Mn2+.38 The mAb KIM 185 (10 μg/mL) was also used to stimulate β2-dependent adhesion of PMNs.40,41 Aggregation assays were performed with 5.5 × 106 neutrophils per milliliter and were measured by the increase in light transmission in an aggregometer (Payton Scientific, Buffalo, NY).36 Chemotaxis was measured as previously described.42
Analysis of other leukocyte functions
Oxygen radical generation.
Superoxide anion generation was measured as the fraction of cytochrome c reduction inhibited by superoxide dismutase.33 Briefly, 2.2 × 106 neutrophils were added to the well of a 96-well plate in a final volume of 20 mL HBSS containing 1% HSA and cytochrome c (0.5 mg/mL) with or without superoxide dismutase (0.1 mg/mL). Neutrophils were stimulated with phorbol myristate acetate (PMA, 10−7 M) (Sigma Chemical, St Louis, MO) or opsonized zymosan (0.18 mg/mL) prepared as previously described.43 Absorbance at 550 nM was measured in a microplate reader (THERMOmax, Molecular Devices, Menlo Park, CA).
Intracellular calcium.
Cells were loaded with the calcium-sensitive fluorescent dyes Indo-1 (neutrophils) or Fura-2 am (EBV-transformed lymphoblasts) by incubation with 10−6 M of the methyl ester from a 1 mM DMSO stock (Molecular Probes, Eugene, OR) for 1 hour at room temperature in HBSS/0.5% HSA. They were then centrifuged (at 250g for 5 minutes at 4°C), washed twice, and resuspended in HBSS/HSA. Intracellular calcium transients in response to various agonists was measured as described.33 44
Actin polymerization.
Neutrophils at 5 × 106 cells/mL were combined with 8% formaldehyde, 150 μg/mL lysophosphatidylcholine, and 50 μg/mL FITC-conjugated phalloidin (2.5 μg/mL, Sigma Chemical) for 4 hours on ice and then washed with phosphate-buffered saline (PBS). Filamentous actin was detected by flow cytometry as previously described.45
Preparation of transformed lymphoblasts
Lymphocytes from the patient or from control subjects were suspended at 2 × 106 cells/m in RPMI 1640 (Biowhittaker, Walkersville, MD) with 2 mM L-glutamine, 20% fetal calf serum (Hyclone, Logan, UT), and 0.4 μg/mL cyclosporin A,46 and plated at 2 × 105 cells per well into a 96-well flat-bottomed plate. The 100-μL filtered (0.45 μm) supernatant from a culture of the EBV-shedding line B958 (American Tissue Culture Collection, Rockville, MD) was added to each well.47 The cells were fed as needed with media containing 10% fetal calf serum and 0.2 μg/mL cyclosporin A. Wells were examined for growth, and cells were transferred first to 24-well plates and later to flasks. Cyclosporin A was discontinued after 3 weeks. The transformed lymphoblasts were then maintained in RPMI-1640 with 5% fetal calf serum, 5% fetal clone 1 (Hyclone), and 2 mM glutamine with antibiotic supplementation (penicillin-streptomycin and amphotericin B). The patient-derived EBV-transformed lymphoblasts (LADV cells) and age-matched control EBV-transformed lymphoblasts (control lymphoblasts) were used in subsequent experiments.
Transformed lymphoblast adhesion assay
EBV-transformed lymphoblasts were labeled with 0.185 MBq (5 μCi) 111Indium per 106 cells using the same protocol as previously described for neutrophils.36 The labeled lymphoblasts were then added to wells coated with immobilized ligands (details in figure legends) and allowed to settle for 60 minutes at 4°C, and the adhesion assay was performed as described for neutrophils (above). In other experiments, lymphoblast adhesion was measured by using fluorescent labeling as previously described.48 49 Briefly, 96-well microtiter plates (Costar, Cambridge, MA) were incubated with the indicated concentrations of fibronectin or a recombinant ICAM-1 IgG chimera (a gift from Joel Hayflick and Pat Hoffman, Icos, Bothell, WA) overnight at 4°C and then blocked with HBSS/2.5% bovine serum albumin (BSA). Transformed lymphoblasts were labeled with 2 μg/mL Calcein-am (Molecular Probes), washed according to the manufacturer's protocol, added to wells (5 × 104 cells per well with appropriate stimuli in a final volume of 100 μL), and stimulated with agonists as indicated. The lymphoblasts were then allowed to settle for 60 minutes at 4°C, warmed to 37°C for the indicated times, and nonadherent cells were removed by washing. Adherent cells were quantified by using a fluorescence plate reader (Cytofluor 2300, Millipore, Bedford, MA). The fraction of adherent cells was assessed as the level of fluorescence in the well after washing, divided by total input fluorescence, and multiplied by 100. In experiments examining divalent cation substitution, the incubations were performed as described previously for primary neutrophils, except that EDTA was not added.
Lymphocyte aggregation assay
EBV-transformed lymphoblasts were resuspended at a concentration of 0.85 × 106 cells/mL with PMA at final 10−7 M concentration in the aggregometer, using the same protocol used for PMNs (previously described). PMN aggregation was assayed in parallel as a positive control.
Western immunoblotting
EBV-transformed lymphoblasts at 20 × 106 cells/mL were lysed in RIPA buffer with 1% Nonidet P-40 (Sigma Chemical) according to the manufacturer's instructions (Santa Cruz Biotechnology, Santa Cruz, CA) and centrifuged at 15 000gfor 20 minutes at 4°C. For β2 integrin detection, the lysates (1 mL) were collected and 10 μg anti-β2 mAb, 60.3, was added for 1 hour at 4°C. Protein A–Agarose (20 μL, Santa Cruz Biotechnology) was added, and the lysates were kept at 4°C, with mixing overnight. The agarose pellet was washed 4 times with RIPA buffer and then boiled with 40 μL electrophoresis sample buffer for 90 seconds. Western blot analysis was performed as previously described50 using goat antimouse IgG conjugated to horseradish peroxidase as the secondary antibody (BioSource International, Camarillo, CA) and ECL Western blot detection reagents (Amersham Life Science, Arlington Heights, IL). For β1subunit detection, 6 × 106 of the patient-derived EBV-transformed lymphoblasts (LADV cells), control EBV-transformed lymphoblasts, and Jurkat T cells were lysed in 100 μL RIPA buffer, passed through an 18-gauge needle 3 times, placed in boiling water for 2 minutes, and stored at −20°C. Lysates in nonreducing buffer were then run on a 7.5% SDS-PAGE gel, and Western blot analysis was performed as described previously using the anti-β1 mAb MAB 13 as the primary antibody.
Sequence analysis of β1 and β2
The RNA from 1 × 107–transformed lymphoblasts was isolated using Micro-Fast Track Kit (Invitrogen, Carlsbad, CA). Adapter-ligated double-strand complementary DNA (cDNA) was synthesized with a Marathon cDNA Amplication Kit (Clontech, Palo Alto, CA). Three pairs of primers were designed to cover the entire coding sequence of human β1 integrin in 3 overlapping fragments. The 5′ end fragment was amplified by using the following primers: adapter primer 1 (supplied from Marathon cDNA Amplification Kit), 5′-CCA TCC TAA TAC GAC TCA CTA TAG GGC and 5′-GTA GCT AAA TGG GGT GGT GCA GTT CTG. The second fragment was amplified with the following primers: 5′-CAG CTA AGC TCA GGA ACC CTT GCA C and 5′-CTG CAC GCG CCA CAC TCA AAT GTC. The 3′ end fragment was amplified using the following primers: 5′-CCA AAG CGA AGG CAT CCC TGA AAG and 5′-CAG TGT TGT GGG ATT TGC ACG GGC AG. For β2 sequence, 3 pairs of primers were designed to cover the entire coding region in overlapping fragments. The 5′ end fragment was amplified from single strand cDNA using the primers: 5′-CCA GCA CAC CGA GGG ACA TGC TG and 5′-CGT GAC GTT GCG CCA GCC GAT TTC. The second fragment and the 3′ end fragment were amplified from double-strand cDNA by using the following 2 pairs of primers respectively: 5′-CTG GAC GCC ATG ATG CAG GTC GC and 5′-CTG GCC GTT GTA GCG CTC ACA GTT G, and 5′-CAA CTC CAT CAT CTG CTC AGG GCT G and 5′-CTG ACG GCC TTG TCT TCA CCA AGT G. A “touchdown” polymerase chain reaction (PCR) was carried out in PE GeneAmp Systems 2400, according to the instructions from the Marathon cDNA Amplification Kit. The PCR products were ligated into the PCR2.1 vector (Invitrogen) using the manufacturer's instructions. Individual white colonies were screened, and the plasmid DNAs were analyzed in the University of Utah core sequencing facility. Multiple clones were sequenced for each fragment.
Results
Clinical features of the affected infant indicated a unique syndrome of leukocyte adhesion deficiency
The patient, a male infant, first presented at 3 weeks of age with anemia, thrombocytopenia, leukocytosis (35-45 × 109cells/L), and a “blueberry muffin” rash. He was delivered after a full-term pregnancy, and his size was appropriate for gestational age. There was no family history of recurrent infection, immune deficiency, or consanguinity. Maternal screening was negative for infections, including human immunodeficiency virus and hepatitis B. At the time of presentation, assay of the infant's serum for IgM antibodies directed against cytomegalovirus, rubella, toxoplasmosis, EBV, parvovirus, human herpesvirus 6, and herpes simplex 1 and 2 were all negative. Viral cultures were also negative. In the fourth through seventh months of life, he had several bacterial infections: an infected urachal duct cyst (cultures grew Bacteroides vulgaris, Enterococcus faecalis, Enterococcus avium, and α hemolytic streptococcus), pneumonia (B vulgaris and Candida albicans were cultured from the bronchoalveolar lavage fluid), nonhemolytic streptococcal septicemia, and a perirectal ulceration and cellulitis without pus collection that grew Escherichia colion culture. He also had episodes of pneumonia caused by respiratory syncitial and parainfluenza viruses, oral candidiasis, and a C albicans urinary tract infection, and pneumonia caused byPneumocystis carinii. The leukocytosis found at the time of presentation persisted until bone marrow transplant, and leukocyte counts as high as 96 × 109 cells/L were recorded. Because the persistent leukocytosis and recurrent severe infections suggested the possibility of LAD-1, analysis of cell surface β2 integrins on the infant's circulating neutrophils was performed. Normal levels were detected (Table1 and data not shown). Studies of adhesive function of the child's circulating neutrophils were then performed as described in detail below. A biopsy of the “blueberry muffin” rash showed dermal erythropoiesis. At 6 weeks of age, the infant had normal quantitative IgG, IgA, and IgM levels. CD4 and CD8 T lymphocyte numbers were normal. At 6 months of age, the T-lymphocyte function was assessed, and there were positive responses in vitro to the mitogens phytohemagglutinin, concanavalin A, and pokeweed and to specific antigens (tetanus and candida) after immunization and infection, respectively.
In addition to the leukocyte abnormalities, the child was anemic and thrombocytopenic from the time of presentation. Later, the patient had hepatosplenomegaly and mucosal bleeding develop coincident with omphalitis caused by Bacteroides and an enterococcal species. In vitro analysis of the patient's platelet function demonstrated that platelet suspensions did not aggregate in response to collagen, adenosine diphosphate, arachidonic acid, or ristocetin, and there was absent clot retraction. The template bleeding time was more than 15 minutes. A bone marrow biopsy at 1 month of age revealed that the marrow was markedly hypocellular, but there were no dysplastic changes. A bone marrow biopsy at 7 months of age revealed trilineage dysplasia most pronounced in the granulocytic elements. Cytogenetic analysis was normal; specifically, monosomy 7 and deletion of 5q, 8q, or 17q alterations that are associated with myeloid malignancies,51 52 were not detected. At 8 months, the patient underwent successful bone marrow transplantation using an HLA-matched sibling donor. Three years after engraftment, he has no clinical or laboratory evidence for an immune deficit. A recent circulating leukocyte count was 10.7 × 109 cells/L with an absolute neutrophil count of 3.9 × 109 cells/L. There have been no further severe bacterial or viral infections.
Primary neutrophils isolated from the subject expressed β2 integrins on their surfaces but had impaired β2 integrin–dependent adhesive function
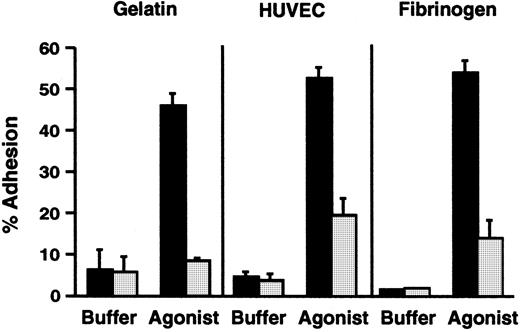
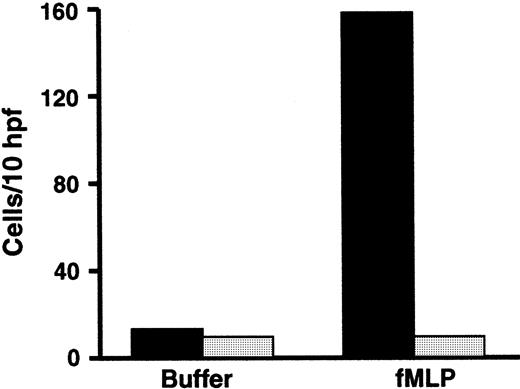
The subject had a clinical phenotype suggestive of the severe form of LAD-16,21 but his PMNs had normal surface expression of a β2 integrin marker, αMβ2(Table 1). In addition, the surface levels of αM and β2 increased 1.5-fold in response to stimulation of the PMNs with PMA. Therefore, we assessed the adhesive functions of the patient's neutrophils by using in vitro assays. There was dramatic impairment of adhesion to gelatin, which depends on inside-out signaling of β2 integrins,34,35,53 when freshly isolated PMNs from the subject were stimulated with N-formyl-met-leu-phe (fMLP) and compared with neutrophils from a control subject (Figure 1). The impairment in adhesion was similar to that seen previously in studies of neutrophils with absent surface expression of β2integrins from a patient with LAD-1, and when neutrophils from control subjects were treated with function-blocking anti-β2integrin antibodies.33-35 A similar impairment in adhesion was seen when the patient's PMNs were stimulated with platelet-activating factor (PAF), which is recognized by a G-protein–linked receptor on the leukocyte surface that is different from the receptor for fMLP,54 and when the neutrophils were treated with the receptor-independent agonists PMA and calcium ionophore A23187 (not shown). Stimulated adhesion of the patient's neutrophils to fibrinogen, a ligand for αMβ2 and αXβ2,6 and to monolayers of cultured human endothelial cells was also dramatically impaired compared with that of control PMNs (Figure 1 and data not shown), although there was a small increase in adhesion in some experiments.
PMNs from the subject with variant LAD had deficient β2 integrin–dependent adhesive functions.
Adhesion of resting unstimulated PMNs or PMNs activated with fMLP (10−7 M) for 10 minutes at 37°C was measured using neutrophils from the affected subject (░) or from a control subject (▪) studied in parallel. This figure represents a single experiment (n = 2 for each condition) and is representative of 9 studies, using one or more of the indicated surfaces, each of which demonstrated decreased adhesion of the patient's PMNs compared with control neutrophils. The error bars in this figure represent the range.
PMNs from the subject with variant LAD had deficient β2 integrin–dependent adhesive functions.
Adhesion of resting unstimulated PMNs or PMNs activated with fMLP (10−7 M) for 10 minutes at 37°C was measured using neutrophils from the affected subject (░) or from a control subject (▪) studied in parallel. This figure represents a single experiment (n = 2 for each condition) and is representative of 9 studies, using one or more of the indicated surfaces, each of which demonstrated decreased adhesion of the patient's PMNs compared with control neutrophils. The error bars in this figure represent the range.
The patient's neutrophils were found to express PSGL-1, the fucosylated leukocyte counterligand that recognizes the endothelial tethering molecules P-selectin and E-selectin,55 with cell surface levels that were greater than those on neutrophils from a control subject studied in parallel (Table 1). We assessed the function of PSGL-1 on the patient's PMNs and found that they adhered normally to purified, immobilized P-selectin in adhesion assays conducted as previously described33 (not shown). The adhesion was inhibited by a specific monoclonal antibody against P-selectin. This indicated that the PSGL-1 detected on the surfaces of the PMNs was functional and that the infant did not have LAD-2.22 25 In a second experiment that confirmed this conclusion, the patient's neutrophils adhered to purified immobilized E-selectin, and this was inhibited by an anti–E-selectin antibody (not shown).
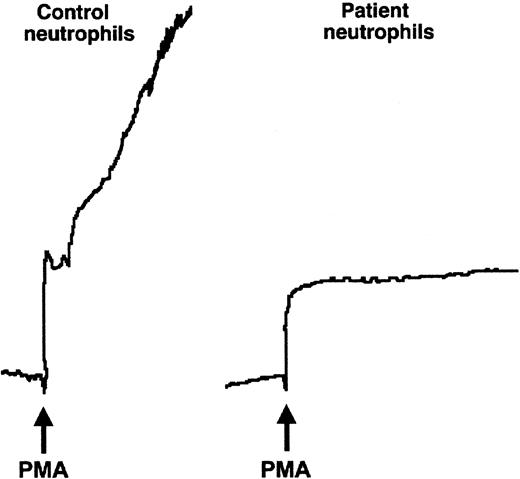
We then examined the performance of the patient's neutrophils in 2 additional assays that measure β2 integrin–dependent adhesive interactions, homotypic aggregation, and chemotaxis.6,56 Although control neutrophils aggregated briskly when stimulated with various agonists, there was no aggregation of PMNs from the affected infant, even when treated with the potent pharmacologic agent, PMA (Figure 2, and data not shown). There was also a dramatic defect in migration of the patient's neutrophils in response to fMLP in a Boyden chamber assay (Figure 3). Neutrophils from subjects with LAD-1 have similar abnormalities of stimulated aggregation and migration, as do control neutrophils treated with certain function-blocking antibodies against β2integrins.6 Thus, the analysis of adhesive interactions of the patient's neutrophils in 3 different assays indicated a defect in β2 integrin function.
The affected patient's neutrophils did not aggregate when stimulated.
Neutrophils (5.5 × 106 cells/mL) were stimulated in the cuvette of an aggregometer with fMLP (not shown) or PMA (10−7 M) at 37°C. Although the control neutrophils aggregated rapidly on addition of the agonist (arrow), the tracing from the cuvette containing the patient's neutrophils showed only the upward deflection caused by dilution.
The affected patient's neutrophils did not aggregate when stimulated.
Neutrophils (5.5 × 106 cells/mL) were stimulated in the cuvette of an aggregometer with fMLP (not shown) or PMA (10−7 M) at 37°C. Although the control neutrophils aggregated rapidly on addition of the agonist (arrow), the tracing from the cuvette containing the patient's neutrophils showed only the upward deflection caused by dilution.
Chemotaxis of the patient's neutrophils was impaired.
Leukocyte-rich plasma collected from the patient (░) and from an adult control subject (▪) in parallel was placed in modified Boyden chambers and chemotaxis in response to buffer or fMLP (10−8 M) was measured after a 3-hour incubation at 37°C. The number of cells migrating completely through the filter was counted microscopically in 10 randomly selected high-power fields. The samples from the patient and control subjects were assayed in triplicate.
Chemotaxis of the patient's neutrophils was impaired.
Leukocyte-rich plasma collected from the patient (░) and from an adult control subject (▪) in parallel was placed in modified Boyden chambers and chemotaxis in response to buffer or fMLP (10−8 M) was measured after a 3-hour incubation at 37°C. The number of cells migrating completely through the filter was counted microscopically in 10 randomly selected high-power fields. The samples from the patient and control subjects were assayed in triplicate.
In parallel studies, we found that intracellular calcium transients in response to fMLP or calcium ionophore A23187,33polymerization of actin in response to PMA as measured with FITC-conjugated phalloidin,45 superoxide generation in response to PMA,33 and shedding of L-selectin in response to PMA57 were each intact in the patient's neutrophils when compared with the PMNs from control subjects (data not shown). In addition, the patient's PMNs polarized in solution in response to fMLP (not shown). These experiments excluded a generalized defect in surface receptor function, intracellular signaling cascades, and signal-dependent effector functions of the PMNs from the subject.
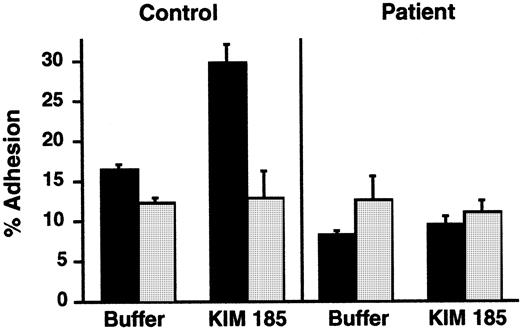
We then asked whether the extracellular domains of integrins on the infant's PMNs could recognize ligands in the presence of the exogenous cation Mn2+, a manipulation that eliminates the requirement for cellular activation and inside-out signaling via cytoplasmic pathways,37,38,58 or in response to an antibody that can induce ligand recognition. By using the protocols described previously,38 we found that, when Mn2+ was substituted for Ca++ and Mg2+ in a suspension of the patient's neutrophils, there was increased adhesiveness in 2 of 3 experiments. The increase in adhesion of the infant's PMNs to immobilized ligand was 2- to 6-fold compared with a 4- to 8-fold increase in adhesion of control neutrophils (data not shown). In a second experiment, we used KIM 185, a function-perturbing mAb against β2 that induces conformational alterations in αMβ2 and αLβ2and promotes adhesion via this mechanism.40 KIM 185 induced increased adhesion of control neutrophils in an assay similar to that shown in Figure 1, but there was no increased adhesion of neutrophils from the patient in parallel incubations (Figure4). There was equivalent binding of the KIM 185 antibody to PMNs from the patient and a control subject when measured by flow cytometry.
A β2-stimulating mAb did not increase adhesion of PMNs from the patient.
PMNs were pretreated with buffer (▪) or with the β2-stimulating mAb KIM 185 (10 μg/mL) for 20 minutes at 37°C, and adhesion to immobilized gelatin was measured after a 40-minute incubation. In some replicates, a blocking mAb directed against β2, 60.3 (10 μg/mL), was included to document that adhesion was dependent on β2 integrins (“anti-β2,” ░). The bars indicate the means and the error bars the ranges of the determinations (n = 2).
A β2-stimulating mAb did not increase adhesion of PMNs from the patient.
PMNs were pretreated with buffer (▪) or with the β2-stimulating mAb KIM 185 (10 μg/mL) for 20 minutes at 37°C, and adhesion to immobilized gelatin was measured after a 40-minute incubation. In some replicates, a blocking mAb directed against β2, 60.3 (10 μg/mL), was included to document that adhesion was dependent on β2 integrins (“anti-β2,” ░). The bars indicate the means and the error bars the ranges of the determinations (n = 2).
Transformed lymphoblasts from the affected subject have defective adhesive functions mediated by β1 and β2 integrins
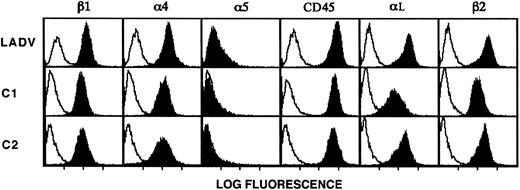
To further characterize the molecular basis for the adhesive defects exhibited by leukocytes from the patient, we developed a line of EBV-transformed lymphoblastoid cells from B lymphoblasts isolated from the infant before bone marrow transplantation (described in “Patient, materials, and methods”). This approach has been useful in characterizing cellular alterations in LAD-159 and in other syndromes. We termed the transformed lymphoblasts from the patient the LADV cell line. Flow cytometric analysis of these cells demonstrated that β2 and αL, the dominant α subunit paired with β2 on lymphocytes and lymphoblasts,60 were expressed at levels equal to or greater than those found on the surfaces of control lymphoblastoid cells from age-matched subjects (Figure5). In addition, the integrin β1 chain and the α4 and α5subunits were expressed on LADV lymphoblasts at levels equivalent to those on control lymphoblastoid cells (Figure 5). LADV cells and age-matched control lymphoblastoid cells each shed 50% or more of their surface L-selectin when treated with 10−7 M PMA,61 and the intracellular calcium transients triggered by treatment with calcium ionophore were similar in both LADV cells and control lymphoblasts (not shown). In addition, LADV cells demonstrated a calcium transient in response to concanavalin A (not shown). These features indicated that the LADV cells display surface integrins at the expected density and perform signal-dependent functions and therefore could be used as surrogate cells in additional studies of the adhesive defects in the patient's leukocytes.
LADV cells express surface β1 and β2 integrins at levels similar to those on control lymphoblastoid cells.
LADV cells derived from the patient and age-matched control lymphoblastoid cell lines C1 and C2 were analyzed by flow cytometry as described in “Patient, materials, and methods.” Solid profiles indicate staining with the following mAbs: the β1-specific mAb UN29, the α4-specific mAb P4C2, the α5-specific mAb P1D6, the αL-specific mAb BCA1, the β2-specific mAb 60.3, and the CD45-specific mAb H130. Binding of the primary antibodies were detected with FITC-conjugated goat antimouse IgG secondary antibodies (heavy and light chain specific). Open profiles indicate fluorescence of cells incubated with the secondary antibody alone (anti-IgG2a,κ in panels showing staining with anti-β2or anti-αL and anti-IgG1a,κ in all others).
LADV cells express surface β1 and β2 integrins at levels similar to those on control lymphoblastoid cells.
LADV cells derived from the patient and age-matched control lymphoblastoid cell lines C1 and C2 were analyzed by flow cytometry as described in “Patient, materials, and methods.” Solid profiles indicate staining with the following mAbs: the β1-specific mAb UN29, the α4-specific mAb P4C2, the α5-specific mAb P1D6, the αL-specific mAb BCA1, the β2-specific mAb 60.3, and the CD45-specific mAb H130. Binding of the primary antibodies were detected with FITC-conjugated goat antimouse IgG secondary antibodies (heavy and light chain specific). Open profiles indicate fluorescence of cells incubated with the secondary antibody alone (anti-IgG2a,κ in panels showing staining with anti-β2or anti-αL and anti-IgG1a,κ in all others).
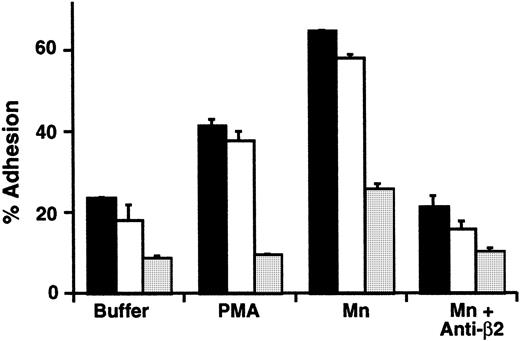
LADV cells did not aggregate when treated with PMA, whereas control lymphoblastoid cells aggregated as expected (not shown). In a second assay of their adhesive function, we examined the binding of LADV cells to an immobilized ICAM-1/IgG chimera. ICAM-1 is a ligand for αLβ2 and other β2integrins.6 Basal and stimulated adhesion of LADV cells, which used PMA as the agonist, were dramatically reduced compared with the control cell lines studied in parallel (Figure6). Thus, inside-out signaling of β2 integrin–dependent adhesive function of LADV cells was defective in a fashion that reproduced the impaired adhesive function of the patient's primary neutrophils (described above and in Figures 1,3). Treatment of LADV cells with Mn2+ caused increased adhesion to immobilized ICAM-1, but the level was substantially below adhesion of control cell lines (Figure 6). This pattern is again similar to that seen with primary leukocytes (described above). The enhanced adhesion induced by treatment of control and LADV cells with Mn2+ was inhibited by a blocking anti-β2 antibody, indicating that the adhesive interaction was specific (Figure 6).
The LADV EBV-transformed lymphoblastoid cell line demonstrates impaired β2 integrin–dependent adhesion.
LADV cells (░) or control lymphoblasts (2 different control lines, ▪ and ■) were incubated in wells coated with immobilized ICAM-1 for 15 minutes at 37°C. In parallel, the cells were treated with buffer, PMA (10−7 M), or Mn2+ using a cation substitution protocol as outlined in “Patient, materials, and methods.” In some wells, the blocking anti-β2 mAb, 60.3 (10 μg/mL), was added to the incubation mixture. The bars indicate the means and the error bars the ranges of duplicate determinations.
The LADV EBV-transformed lymphoblastoid cell line demonstrates impaired β2 integrin–dependent adhesion.
LADV cells (░) or control lymphoblasts (2 different control lines, ▪ and ■) were incubated in wells coated with immobilized ICAM-1 for 15 minutes at 37°C. In parallel, the cells were treated with buffer, PMA (10−7 M), or Mn2+ using a cation substitution protocol as outlined in “Patient, materials, and methods.” In some wells, the blocking anti-β2 mAb, 60.3 (10 μg/mL), was added to the incubation mixture. The bars indicate the means and the error bars the ranges of duplicate determinations.
We then asked whether the defect was specific for β2integrin function, or involved inside-out signaling of other integrin heterodimers. To examine this issue, we measured adhesion of LADV cells to immobilized fibronectin, a ligand for integrins of the β1 class. We found that there was reduced basal adhesion of LADV cells compared with that of the control transformed lymphoblasts, and no increase in response to PMA (Figure7). The defect in adhesion to fibronectin exhibited by the LADV cells was not corrected by varying the time of incubation (15 to 60 minutes) or by altering the concentration of fibronectin used to prepare the wells (2 to 60 μg/mL; 0.1 to 3 μg per well of a 96-well plate) (not shown). Because αLβ2, the dominant β2integrin on lymphoblastoid cells,60,62 does not recognize fibronectin,6 this result implied that LADV cells also have a defect in stimulated adhesion mediated by β1integrins. To further confirm this, we treated LADV cells with a monoclonal antibody that simulates β1 function, TS2/16,48 63 and found that this increased the adhesion of LADV cells to fibronectin to a level similar to that of control cells (Figure 7). Treatment with Mn2+ also caused increased adhesion of LADV cells to fibronectin (Figure 7). The increased adhesion of control lymphoblasts was inhibited by monoclonal antibody against β1 and α4, indicating that α4β1 integrin mediates recognition of fibronectin in this assay (Figure 7 and data not shown). This pattern was seen with the original LADV cell line, and with 3 additional cell lines made by independent EBV-mediated transformations of the infant's lymphoblasts (Figure 7 and data not shown).
β1 integrin–dependent adhesion is defective in LADV cells and is partially corrected by cation substitution and β1 integrin–activating antibody.
Wells were coated overnight with fibronectin (3 μg/well; 60 μg/mL), and adhesion of LADV cells (2 different populations from separate transformations, LADV-1 [■] and LADV-2 [░]) or control lymphoblastoid cells (▪) was measured after a 20-minute incubation at 37°C in the presence of control buffer, PMA (10−7 M), the β1-activating mAb TS2/16 (final 1:14 dilution of hybridoma supernatant), Mn++ (1 mM) or Mn++ and the β1 blocking mAb p4C10 (10 μg/mL). The error bars represent the standard deviation of triplicate determinations. In the experiment shown here, cation substitution did not result in increased adhesion of the control cell line to FN because of high basal binding. However, in additional experiments using the same control lymphoblastoid cells washed in HBSS containing the chelator EDTA (1 mM) there was a 2- to 4-fold increase in adhesion to FN in response to Mn++ substitution compared with buffer alone (not shown). The results with the LADV-1 and LADV-2 cells shown are representative of 3 separate experiments.
β1 integrin–dependent adhesion is defective in LADV cells and is partially corrected by cation substitution and β1 integrin–activating antibody.
Wells were coated overnight with fibronectin (3 μg/well; 60 μg/mL), and adhesion of LADV cells (2 different populations from separate transformations, LADV-1 [■] and LADV-2 [░]) or control lymphoblastoid cells (▪) was measured after a 20-minute incubation at 37°C in the presence of control buffer, PMA (10−7 M), the β1-activating mAb TS2/16 (final 1:14 dilution of hybridoma supernatant), Mn++ (1 mM) or Mn++ and the β1 blocking mAb p4C10 (10 μg/mL). The error bars represent the standard deviation of triplicate determinations. In the experiment shown here, cation substitution did not result in increased adhesion of the control cell line to FN because of high basal binding. However, in additional experiments using the same control lymphoblastoid cells washed in HBSS containing the chelator EDTA (1 mM) there was a 2- to 4-fold increase in adhesion to FN in response to Mn++ substitution compared with buffer alone (not shown). The results with the LADV-1 and LADV-2 cells shown are representative of 3 separate experiments.
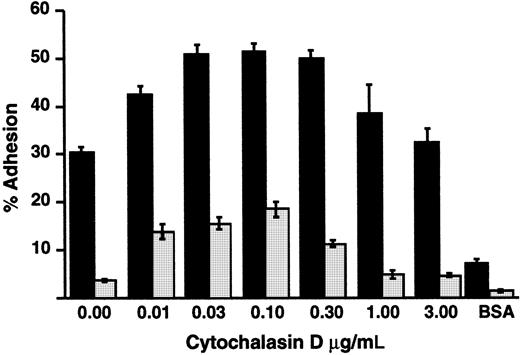
Inside-out signaling triggers clustering of integrins and consequent increased avidity for ligands, in addition to triggering increased affinity as a result of altered conformation.15,64-66Release of integrins from cytoskeletal constraints by treating cells with cytochalasins also induces increased avidity and consequent adhesion under some conditions.65 66 We found that treatment of LADV cells with low concentrations of cytochalasin D enhanced their adhesion to fibronectin in response to PMA, but did not correct the defect in adhesion in comparison to control lymphoblastoid cells (Figure 8). This result indicates that cytoskeletal interactions of β1 integrins can be modified by cytochalasins in LADV cells, as in control lymphoblasts, and suggests that dysregulated cytoskeletal linkage and/or impaired clustering of the integrins alone do not account for the adhesion defect.
Treatment with cytochalasin D does not correct defective adhesion of LADV cells to fibronectin.
Wells were coated overnight with 1.5 μg/well FN. LADV (░) or control (▪) lymphoblastoid cells were treated with increasing concentrations of cytochalasin D at 37°C for 15 minutes and their adhesion was measured after a subsequent 30-minute incubation on fibronectin with PMA (10−7 M). Adhesion without PMA was 2.4% for LADV cells and 17.4% for control lymphoblast cells in the absence of cytochalasin D and increased to 2.8% and 24%, respectively, with 0.1 mg/mL cytochalasin D. Adhesion to BSA in the presence of PMA was measured in parallel. Results shown are representative of at least 3 experiments.
Treatment with cytochalasin D does not correct defective adhesion of LADV cells to fibronectin.
Wells were coated overnight with 1.5 μg/well FN. LADV (░) or control (▪) lymphoblastoid cells were treated with increasing concentrations of cytochalasin D at 37°C for 15 minutes and their adhesion was measured after a subsequent 30-minute incubation on fibronectin with PMA (10−7 M). Adhesion without PMA was 2.4% for LADV cells and 17.4% for control lymphoblast cells in the absence of cytochalasin D and increased to 2.8% and 24%, respectively, with 0.1 mg/mL cytochalasin D. Adhesion to BSA in the presence of PMA was measured in parallel. Results shown are representative of at least 3 experiments.
The adhesion defects in LADV cells are not a result of altered structures of β1 or β2
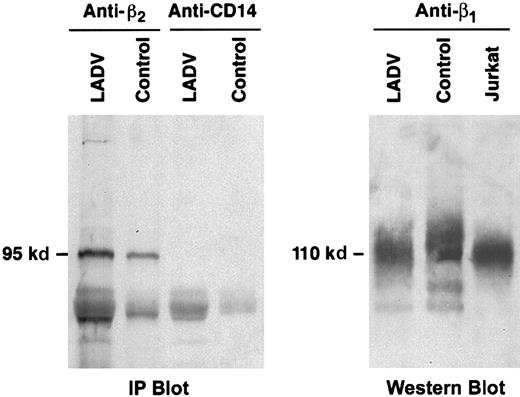
Immunoprecipitation and immunoblot analysis of the β2 protein from LADV cells demonstrated a band of the expected molecular size, 95 kd (Figure9A). Similarly, analysis of the β1 protein from LADV cells revealed a band at the expected size, 110 kd, on a nonreduced gel (Figure 9B). Further analysis of β2 cloned from LADV cells revealed that the cDNA sequence was normal in all clones examined (2 each) for fragments 2 and 3, and 4 of 5 clones for the 5′ proximal fragment (fragment 1, details in “Patient, materials, and methods”). The sequences of the β1 cDNA from LADV and control lymphoblastoid cells (2 clones each) were identical, but in each case, there was a histidine at position 112 rather than threonine as in the original published sequence.67-69 This polymorphism has previously been reported in human cDNA clones (Hillier et al, GenBank accessionAA112739, 1995). Position 112 is outside of the known β1ligand-binding regions.70 In addition, the control EBV-transformed lymphoblasts exhibited adhesion to fibronectin (Figure7), indicating that this polymorphism generated a functional protein. Thus, structural alterations in β1 and β2do not account for the adhesion defects in the LADV cells and appear not to account for the abnormalities of integrin-dependent adhesion in primary leukocytes from the patient.
The β1 and β2 proteins from LADV cells are of expected size.
(A) LADV cells and control lymphoblastoid cells were lysed and proteins in the lysates were immunoprecipitated with the anti-β2, mAb 60.3, or with an irrelevant control antibody, anti-CD14. The proteins were then separated on a 7.5% SDS-PAGE gel, transferred to a nitrocellulose membrane, and Western blot analysis was performed using the anti-β2 mAb 60.3. Equal amounts of protein were applied to each lane. The β2 protein band immunoprecipitated from LADV cells is of identical size and is at least as intense as that from control cells. (B) Transformed LADV cells, control lymphoblastoid cells, or Jurkat T cells were lysed, the proteins were separated on a 7.5% SDS-PAGE gel under nonreducing conditions, and analyzed by Western blot, using the anti-β1 mAb 13. Although there are variations in loading, the proteins from each cell type are of similar size.
The β1 and β2 proteins from LADV cells are of expected size.
(A) LADV cells and control lymphoblastoid cells were lysed and proteins in the lysates were immunoprecipitated with the anti-β2, mAb 60.3, or with an irrelevant control antibody, anti-CD14. The proteins were then separated on a 7.5% SDS-PAGE gel, transferred to a nitrocellulose membrane, and Western blot analysis was performed using the anti-β2 mAb 60.3. Equal amounts of protein were applied to each lane. The β2 protein band immunoprecipitated from LADV cells is of identical size and is at least as intense as that from control cells. (B) Transformed LADV cells, control lymphoblastoid cells, or Jurkat T cells were lysed, the proteins were separated on a 7.5% SDS-PAGE gel under nonreducing conditions, and analyzed by Western blot, using the anti-β1 mAb 13. Although there are variations in loading, the proteins from each cell type are of similar size.
Discussion
The clinical presentation and laboratory studies of the child described in this report document a syndrome of variant leukocyte adhesion deficiency in which surface levels of integrins were normal or increased, but integrin-dependent adhesive functions of circulating leukocytes were impaired. Furthermore, analysis of the LADV cell line developed from lymphocytes collected from the affected infant before bone marrow transplantation indicates a defect in inside-out signaling of β1, as well as β2, integrins. This feature, which is consistent with the diversity and severity of bacterial, viral, and fungal infections that the child had before marrow transplantation, is different from the characteristics of cells from the 2 other subjects with variant LAD-1 that have been identified to date.29,30 Dysfunction of β1 or β2 integrins has not previously been reported in association with myelodysplasia, which the child had, although terminally differentiated leukocytes often have functional defects in myelodysplastic syndromes.52
Dramatically reduced expression of β2 integrins on the surfaces of neutrophils and other leukocytes is the sine qua non for the diagnosis of LAD-1, and impaired ligand recognition and adhesion mediated by β2 integrins are the central pathogenetic mechanisms.6,20,21,27,28 The patient had the clinical features of LAD-1, but the surface levels of β2integrins on circulating PMNs were similar to those of control subjects. Although present at sufficient levels, the β2integrins were defective in their ability to mediate basal or activation-induced adhesion in several in vitro assays. In contrast, neutrophils from the child responded normally to chemotactic peptides and a pharmacologic agonist, PMA, with signal-dependent functional responses that included oxidative metabolism, intracellular calcium fluxes, actin polymerization, and shape change. Thus, the defect in integrin function was not due to a generalized disorder of signal transduction. This is similar to the phenotypes of leukocytes from the 2 subjects with variant LAD-1 that were reported earlier.29 30
The first of the previously identified patients with variant LAD-1 had clinical features consistent with the mild form of LAD-1 but normal levels of β2 integrins were found by cytometric analysis, and there was impaired activation-dependent binding of leukocytes to ligands recognized by β2 heterodimers.29 In vitro assays also demonstrated defective transmigration and aggregation of neutrophils from this subject. Sequence analysis of the β2 subunit by PCR yielded no abnormalities, and αLβ2 on the patient's EBV-transformed lymphoblasts could be forced into an active conformation by a function-perturbing antibody. In contrast, neutrophils from the subject did not display an activation-dependent epitope on αMβ2 integrin when they were treated with PMA. These features indicate a defect in inside-out signaling of β2 integrins.29 The LAD-variant patient also had a thrombasthenic-like bleeding disorder develop, with impaired antibody recognition of an activation-dependent epitope on platelet integrin αIIbβ3. Together, these features suggested deficiency or dysfunction of one or more intracellular factors that mediate inside-out signaling of β2 and β3 integrins, although the molecular defect was not identified.29 The abnormalities of integrin function and adhesive responses of leukocytes from the patient are generally similar to those of this previously identified subject with variant LAD-1, in terms of impaired inside-out signaling of β2heterodimers, but differ in that a function-perturbing antibody was not able to induce adhesiveness of PMNs to the same degree as with control neutrophils (Figure 4). This may be due to altered molecular associations involving cytoplasmic domains of the integrin heterodimers, because in model systems, specific interactions in these regions can influence function of the extracellular domains71 (also described below), or to differences in properties of the function-altering antibodies used in the 2 studies.72
The second individual with a variant LAD-1 syndrome to be reported also had features that differ from the child we describe here, including a mild LAD-1–like phenotype, compound mutations yielding 2 abnormal β2 alleles, and leukocytes with 40% to 60% of the expected levels of β2 heterodimers on their surfaces.30 The mutations together caused impaired expression of surface heterodimers and altered function of the extracellular metal ion–dependent adhesion site (MIDAS) motif of the β2 subunit,30 which is involved in ligand recognition.73-75 Thus, the impaired leukocyte adhesion in this variant of LAD-1 was caused by altered function of the “extracellular face”76 of the β2integrins compounded by a reduction in the number of copies on the leukocytes, rather than by aberrant inside-out signaling as in the subject reported here.
Analysis of the LADV lines of EBV-transformed lymphoblasts from the affected patient demonstrated defective β1, as well as β2, integrin function. We found normal surface expression of β1 and β2 heterodimers and normal molecular size and sequence of the proteins and cDNAs, but impaired basal and activation-dependent binding of the β1 and β2 integrins to their respective ligands in whole cell assays. However, manganese substitution and an activating anti-β1 antibody substantially corrected the adhesion defect of LADV cells and, to a lesser extent, manganese substitution improved defective adhesion of the primary neutrophils, indicating that the integrin proteins were capable of being forced into a conformation that can recognize ligands. Thus, it is likely that the adhesion defect in LADV cells involves one or more factors that interact directly or indirectly with the cytoplasmic tails of β1 and β2 integrins13 and thereby influence their responses to inside-out signals. Cytoskeletal associations regulate leukocyte integrin affinity, avidity, and distribution; thus, altered cytoskeletal responses to activation could potentially explain dysfunctional adhesion of LADV cells.7,13,15 Furthermore, cytoskeletal reorganization with cytochalasin D failed to overcome the β1 integrin binding defect (“Results” and Figure 8), in contrast to the ability of low concentrations of cytochalasins to induce clustering and β1 integrin–dependent adhesion in control cells and in model systems.64 However, low concentrations of cytochalasins induced a component of β1integrin–dependent adhesion (Figure 8), as it does in model systems.64 This finding, and studies demonstrating intact actin polymerization in primary neutrophils from the patient (“Results”) indicate that the adhesion defect was not due to a generalized abnormality of cytoskeletal regulation.
In contrast to the result with LADV cells, leukocytes from both of the other patients with variant LAD-1 had normal β1 adhesive functions in assays that use clonal T cells and cultured T lymphoblasts.29,30 The relatively mild clinical manifestations of these 2 subjects may have been the result of preservation of β1 adhesive functions.28,29Also, patients with the usual form of LAD-1 often do not have severe viral or opportunistic infections, a feature attributed to intact lymphocytic immune functions that depend on β1heterodimers.28,29,77 Dysregulated functions of both β1 and β2 integrins leading to impaired mononuclear immune surveillance likely contributed to the frequency and severity of infections in the patient we described; these infections included bacterial, viral, fungal, and protozoan (Pneumocystis) episodes, although we were only able to assess β2 integrin functions on primary leukocytes before bone marrow transplantation. In addition, β1 integrins have recently been reported to influence the migration of human neutrophils in in vitro assay systems.64 78
Although this is the first human syndrome associated with impaired signaling of both β1 and β2integrins to be identified, combined defects in β1- and β2-mediated functions were found in mutant Jurkat T-cell lines.32 The general adhesive phenotypes of the mutant Jurkat cells and the LADV cells derived from the affected patient that we reported are similar.32 Despite extensive characterization of the Jurkat mutant cell lines, the intracellular factors that were deficient or defective in inside-out signaling of β1 and β2 integrins were not identified, although one mutant cell type had an abnormal form of the mitogen-activated protein kinase ERK-1. Fusion of the mutants to one another, or to a different cell line that did not express β1, restored adhesive function indicating that one or more cellular factors could complement the adhesion defects.32 The defects in both of the mutant Jurkat cell lines appeared to be distal to protein kinase C (PKC),32which, like phosphoinositide-3-kinase,11 may be a point of convergence for signals that activate integrins of different classes.17 Similarly, the defect in the LADV cell line appears to be distal to PKC because the cells shed L-selectin but did not aggregate or adhere to fibronectin when treated with PMA (described in “Results”). Complementation analysis,14,79 using β1- and β2-deficient cell lines and other fusion partners for LADV cells, and screens for deficient cellular factors that regulate leukocyte integrin activation and/or cellular responses mediated by integrins, have been unrevealing to date (E.S.H. et al, unpublished observations, October 1998). It is possible that, instead of a factor critical to inside-out signaling of β1 and β2 integrin activation being missing or defective, there is a dominant suppressor effect 79-85or dysregulation of a pathway that suppresses integrin activation86 in LADV cells as a result of the myelodysplastic process. It is also possible that the defect in adhesion of LADV cells is more global and involves abnormal function of β3 integrins because the patient had impaired platelet aggregation develop (described in “Results”). This issue is under investigation. Of interest, the LADV cells grow extremely slowly in culture compared with other EBV-transformed lymphoblastoid cell lines, suggesting that defective inside-out signaling of adhesive function and, potentially, altered outside-in signaling may have contributed to the myelodysplasia and altered blood cell proliferation87-89 that the patient experienced before marrow transplantation.
The mechanisms of inside-out signaling of integrins leading to increases in their affinity and avidity remain elusive.7,14,17 Naturally occurring alterations in integrins of specific classes and in their regulatory pathways in syndromes that now include Glanzmann thrombasthenia,90LAD-1,21 and variant LAD-1 provide important insights into the functions and control of these ubiquitous tethering molecules. The LAD-1 variant syndrome of the infant we describe here and the in vitro behavior of cells from the subject are unique, and further analysis of the LADV cell line may yield additional useful information regarding the mechanisms by which β1 and β2 integrins on leukocytes are regulated.
Acknowledgments
We thank Michaeline Bunting, Aaron Pontsler, and Diana Stafforini for assisting with sequence analysis, Jim Jenkins for the development of the Epstein-Barr virus–transformed cell lines and Donelle Benson for technical assistance. We also thank Patrick Beatty, Dan Simon, Rodger McEver, Thomas Tedder, Joel Hayflick, and Pat Hoffman for providing us with antibodies and reagents. Lastly, we thank John Bohnsack and members of our laboratories for useful discussions and other contributions, Diana Lim for graphics, and Michelle Bills and Leona Montoya for the preparation of this manuscript.
Supported by the National Institutes of Health (KO8 HL03799, HL02726, HL44525, P5O HL50153) and by an Established Investigator Award from the American Heart Association (9640261N).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guy A. Zimmerman, Program in Human Molecular, Biology and Genetics, Eccles Institute of Human Genetics, 15 North 2030 East, Suite 4220, University of Utah Health Sciences Center, Salt Lake City, UT 84112.

![Fig. 7. β1 integrin–dependent adhesion is defective in LADV cells and is partially corrected by cation substitution and β1 integrin–activating antibody. / Wells were coated overnight with fibronectin (3 μg/well; 60 μg/mL), and adhesion of LADV cells (2 different populations from separate transformations, LADV-1 [■] and LADV-2 [░]) or control lymphoblastoid cells (▪) was measured after a 20-minute incubation at 37°C in the presence of control buffer, PMA (10−7 M), the β1-activating mAb TS2/16 (final 1:14 dilution of hybridoma supernatant), Mn++ (1 mM) or Mn++ and the β1 blocking mAb p4C10 (10 μg/mL). The error bars represent the standard deviation of triplicate determinations. In the experiment shown here, cation substitution did not result in increased adhesion of the control cell line to FN because of high basal binding. However, in additional experiments using the same control lymphoblastoid cells washed in HBSS containing the chelator EDTA (1 mM) there was a 2- to 4-fold increase in adhesion to FN in response to Mn++ substitution compared with buffer alone (not shown). The results with the LADV-1 and LADV-2 cells shown are representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/3/10.1182_blood.v97.3.767/6/m_h80310649007.jpeg?Expires=1769102806&Signature=IOWuj0qcqjQDOx-KeXh~0M5ubkCdzm7jzwz7I3KMeEi5NChUJ8qXO-fU2f0jCzUGOma0YIrvXRJN2nNNUkEsIPd0ura3SGR~NuwWswtVRuEZhjlfHx9JILamDwUSk-bxcsKy07szPWcs0OD8gp5VUw3VvRAHHEYYoAMGH9KWgTAErce4mCQrlopEfY~OHiGLhG9dqU~PU7GNcjfNiIRVTKdufGgq5FwbRmFCl89w93rz5q9b92QumrhF1SdI71Ey0JjuyDkHIydQX~sFTitAg8nZOVPjZN~QhWY2n3kHCyZ4yhTNmKnN11R5LQc60scHJICc8msyuMjNxD-PQ~TrKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)