Abstract
Paraffin blocks and clinical data from 521 patients with lymphocyte predominance Hodgkin disease (LPHD) diagnosed between 1970 and 1994 were collected from 16 European and United States oncological centers to establish the pathologic and clinical characteristics of a large patient cohort, to determine how frequent T-cell–rich large B-cell lymphoma (TCRLBCL) is among LPHD, and to find differential diagnostic criteria distinguishing between the 2 lymphoma categories. For this purpose, conventionally and immunohistologically stained sections were reviewed by a panel of hematopathologists. The diagnosis of LPHD was confirmed in only 219 of the 388 assessable cases (56.5%). This low confirmation rate was due mainly to the presence of a new variant of classical Hodgkin disease (CHD), which resembled, in terms of nodular growth and lymphocyte-richness, nodular LPHD and, in terms of the immunophenotype of the tumor cells, CHD and was designated nodular lymphocyte-rich CHD (NLRCHD). The nodules of LRCHD consisted—as in nodular LPHD—predominantly of B cells but differed from those present in LPHD in that they represented expanded mantle zones with atrophic germinal centers. Clinically, patients with LPHD and NLRCHD showed similar disease characteristics at presentation but differed in the frequency of multiple relapses and prognosis after relapse. Patients with LPHD and NLRCHD clearly differed from patients with CHD with nodular sclerosis or mixed cellularity, as they presented with an earlier disease stage and infrequent mediastinal involvement. As 97% of the LPHD cases showed a complete or partial nodular growth pattern, their differentiation from TCRLBCL was a rare problem in the present series.
Introduction
The Rye classification scheme of Hodgkin disease (HD)1 is based on the concept that the histologic subtypes represent morphologic variations of a neoplasm in which Hodgkin and Reed-Sternberg (HRS) cells are embedded in a reactive background, showing a characteristic cellular composition for each histotype. This concept remained unquestioned in the many years of its existence, except for the issue of the relationship of lymphocyte predominance HD (LPHD) to the other subtypes. LPHD, characterized by a lymphocyte-rich background with admixed histiocytes, was first described by Jackson in 1937 under the term “early HD.”2 The observation of a long, indolent disease course in most cases led Jackson and Parker3 to designate it in 1944 as “paragranuloma” to separate it from Hodgkin “granuloma.” Rappaport4distinguished in 1956 a nodular form of paragranuloma and separated it from follicular lymphoma. In 1966 Lukes and Butler5 renamed paragranuloma “lymphocytic and/or histiocytic predominance HD,” described a nodular and a diffuse type and established the term of lymphocytic and histiocytic (L&H) RS-cell variant for the predominant diagnostic cell.6 At the Rye symposium, it was decided, for practical reasons, to combine the nodular and diffuse types of the Lukes and Butler classification into LPHD.1 In the last decade, a considerable body of evidence has accumulated establishing that LPHD exhibits features of a B-cell lymphoma, with a characteristic antigen profile and clinical behavior.7-19 This was taken into account by the REAL classification proposal: LPHD was separated as a distinct clinicopathologic entity from the other subtypes of HD, which were subsumed under the term “classical HD” (CHD).20 21
The merging of nodular and diffuse LPHD in the Rye classification was not unanimously accepted by pathologists and oncologists. Unfortunately, the subsequently published studies did not clarify the issue, as they led to discordant results. This was caused by both the lack of precise immunophenotypical criteria and the fact that the clinical studies analyzed cases without consideration of their immunophenotypic features.22-25 The question of the existence of diffuse LPHD became more complex when several authors described a diffuse large B-cell lymphoma variant, the T-cell or histiocyte-rich large B-cell lymphoma (TCRLBCL), which frequently simulated the morphology of diffuse LPHD but exhibited a more aggressive disease course and frequent bone marrow involvement at presentation.26-35 Furthermore, more recently, 2 additional types of HD with an abundance of lymphocytes were recognized: (1) a “lymphocyte-rich form of classical HD” included in the REAL classification, which was thought to have a diffuse growth pattern in most instances, and (2) “follicular HD,” forming B-cell–rich nodules, which was identified at the lymphoma workshop of the European Association of Haematopathology in Toledo, Spain, in 1994 and subsequently published by Ashton-Key and colleagues.36
The need to establish widely accepted criteria for the diagnosis of LPHD and its differentiation from TCRLBCL led H.S. and V.D. to initiate a multinational study, under the auspices of the European Task Force on Lymphoma (ETFL). Sixteen oncology centers from Europe and the United States participated by submitting paraffin blocks and clinical data from 521 patients who had initially been diagnosed as LPHD according to the Rye criteria. All submitted samples were immunostained with a broad array of antibodies and reviewed by a panel of expert hematopathologists without prior knowledge of the initial diagnosis and clinical features, and a consensus diagnosis was reached. This study revealed that the diagnosis of LPHD can be correctly made by morphologic criteria in only two thirds of the cases. Difficult and borderline cases, however, require consideration of both the morphologic features and the immunophenotypical findings. The most unexpected finding of this combined approach was that the tumor cells of a large proportion (21%) of the cases submitted under the diagnosis LPHD had an antigen profile of classical HRS cells and thus represented cases of CHD rather than of LPHD. These cases exhibited a nodular growth pattern. The nodules consisting mainly of IgM+ and IgD+ B cells frequently contained—in contrast to those present in NLPHD—eccentrically located atrophic germinal centers. This variant of CHD was designated nodular lymphocyte-rich classical HD (NLRCHD). The second unexpected finding was that the problem of differentiating between TCRLBCL and diffuse LPHD was quite uncommon in this collection, as it occurred only in 7 (2%) of the cases.
In this paper we present the morphologic and immunohistologic criteria developed by the panel that proved the most useful for establishing the diagnosis of LPHD, LRCHD, and other types of CHD. An extensive report on clinical presentation, course, and prognostic factors has been published elsewhere.37
Material and methods
Submitted cases
A total of 563 paraffin-embedded tissue samples from 521 patients initially classified as LPHD according to the Rye classification in the period 1970–1994 were submitted from 16 oncological centers for this study (Table1).
Histology and immunohistology
Four micrometer sections were cut and stained with hematoxylin and eosin (H&E) and Giemsa. Table 2 shows the antibody panel used for immunohistologic analysis. With the exception of anti-CD21 antibody 1F8, which required a proteolytic pretreatment, all other primary antibodies were applied to dewaxed sections after an antigen-demasking procedure involving high-pressure cooking.38 Bound antibodies were made visible by the alkaline phosphatase antialkaline phosphatase (APAAP) method in association with new fuchsin (Merck, Darmstadt, Germany) or Fast Red development (DAKO, Carpinteria, CA). For the demonstration of EMA and membrane-bound immunoglobulin heavy chains, IgM and IgD, the streptavidin biotin complex method with conjugated peroxidase was used with the use of diaminobenzidine for development.
In-situ hybridization
In-situ hybridization for detection of Epstein-Barr virus (EBV)-encoded small nuclear RNAs (EBERs) was performed as described elsewhere,39 using in vitro transcribed digoxigenin-labeled sense (negative controls) and antisense RNA probes on paraffin sections. Detection of the bound labeled probes was achieved by incubation with a monoclonal digoxigenin-specific antibody conjugated with alkaline phosphatase that was developed afterward using naphtol-as-biphosphate and new fuchsin.
Evaluation of submitted cases
Each case was evaluated by a panel of pathologists (H.S, M.-L.H, I.A., and T.M.) without knowledge of the submitting diagnosis or the clinical data. As a first step, H&E- and Giemsa-stained slides were assessed for the presence of atypical cells with morphologic features of L&H and RS cells. The number and distribution of these cells, the architecture of the infiltrate as well as the number of epithelioid cells, of eosinophils and neutrophils, and the presence of fibrosis were noted. The panel then proposed a diagnosis based solely on histomorphologic features. The immunostained and in-situ hybridization slides were then reviewed. The immunophenotype of the atypical cells was evaluated as well as the pattern of the meshwork of follicular dendritic cells, the number and distribution of CD3- and CD57-expressing cells within nodular tumor areas, the presence of T-cell rosettes around L&H or RS cells, and the number of small B lymphocytes within nodular structures. All these parameters were considered for establishing a final diagnosis. A large number of cases (approximately 300), comprising all unusual or difficult cases, were reviewed in the same manner in several sessions by an extended panel, including K.F., M.H., N.L.H., E.S.J., J.H.J.M.vK., and S.P.
Results
Excluded cases
Of the 521 cases submitted, 86 were initially excluded because of missing clinical data, or the patient's age being less than 15 years. From the remaining 435 cases, 47 were excluded after histologic evaluation, as they were found either to contain too little tissue in the submitted paraffin blocks, making immunophenotypical analysis impossible, or because they represented errors of the submitting centers (eg, the paraffin blocks contained unrelated tissues such as ovaries, skin, lung, or were devoid of any lymphoid infiltrates). The final study was based on 388 cases with complete clinical data and adequate histologic material.
Morphology versus immunohistology
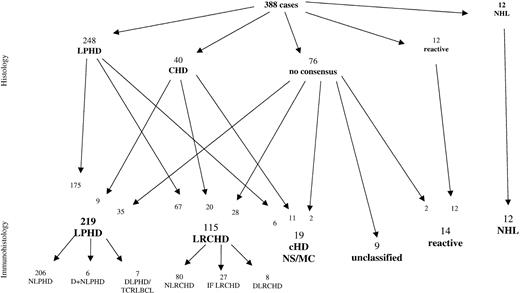
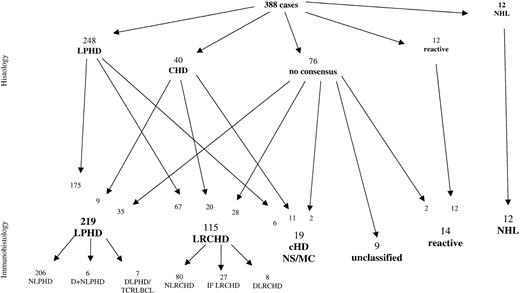
After screening of the H&E- and Giemsa-stained sections alone, the 388 cases were classified as follows (Figure1): 248 cases as LPHD, 40 cases as CHD of mixed cellularity (MC) or nodular sclerosis (NS) type, 12 cases as non-Hodgkin lymphomas (NHL), and 12 cases as reactive, usually corresponding to progressively transformed germinal centers. In the remaining 76 cases, no consensus diagnosis could be reached between the panel members. Within the LPHD group, approximately one third of the cases were regarded as not totally typical, as these cases frequently contained neoplastic cells resembling classic RS cells as well as L&H cells. In addition, occasional atrophic germinal centers were encountered within the lymphocyte-rich nodular background, whereas these proved to be absent in the “typical LPHD” cases.
Comparison of diagnoses based on conventional histology with diagnoses established after additional immunohistologic review of the submitted cases.
LPHD indicates lymphocyte predominance Hodgkin disease; CHD, classical Hodgkin disease; NS, nodular sclerosis, MC, mixed cellularity; NHL, non-Hodgkin lymphoma; LRCH, lymphocyte rich classical HD; N, nodular; D, diffuse; IF, interfollicular.
Comparison of diagnoses based on conventional histology with diagnoses established after additional immunohistologic review of the submitted cases.
LPHD indicates lymphocyte predominance Hodgkin disease; CHD, classical Hodgkin disease; NS, nodular sclerosis, MC, mixed cellularity; NHL, non-Hodgkin lymphoma; LRCH, lymphocyte rich classical HD; N, nodular; D, diffuse; IF, interfollicular.
Reevaluation of the cases after the review of immunohistologic slides confirmed the diagnosis of LPHD in 175 cases. The 12 NHL and the 12 reactive cases were also confirmed. Thirty-one cases classified as CHD remained in this category. However, immunophenotyping resulted in the reclassification of following cases:
1. Seventy-three of the cases initially classified as LPHD showed CD30+ and CD15+ neoplastic cells and were reclassified as CHD. Because of the presence of neutrophils and/or eosinophils, 6 of these cases were subtyped as CHD, either of MC type or because of the presence of nodular sclerosing collagen bundles as NS type. The remaining 67 cases exhibited a lymphocyte-rich background infiltrate with a nodular growth pattern. For the classification of these cases, a new term was created, which is “nodular lymphocyte-rich CHD” (NLRCHD).
2. Thirty-five of the 76 cases without morphologic consensus diagnosis were reclassified after immunophenotyping as LPHD (CD20+, CD30−, CD15−). Thirty additional cases were reclassified as CHD, 2 of MC, and 28 of the LRCHD subtype, whereas 2 additional cases were reevaluated as reactive lymph nodes with progressively transformed germinal centers.
3. Nine of 40 cases initially classified as CHD were reclassified as LPHD after immunostaining (CD20+, CD30−, CD15−). The remaining 31 cases represented CHD with CD30+ and CD15+ tumor cells. Twenty of the latter cases had a lymphocyte-rich background in which the neoplastic cells were frequently found within the interfollicular areas rich in T cells and venules. These cases were classified as LRCHD with interfollicular, or more rarely, nodular growth pattern. The remaining 11 cases corresponded to CHD of MC or NS type.
In conclusion, the diagnoses established after morphologic and immunohistologic evaluation showed that 219 cases fulfilled the criteria of LPHD, whereas an additional 134 cases belonged to the category of CHD. The largest group within the CHD cases (115 of 134) was characterized by a lymphocyte-rich background, termed LRCHD. Fourteen additional cases were classified as reactive, 12 cases represented NHL, whereas 9 cases remained unclassified.
Cases classified as LPHD
Morphology of neoplastic cells.
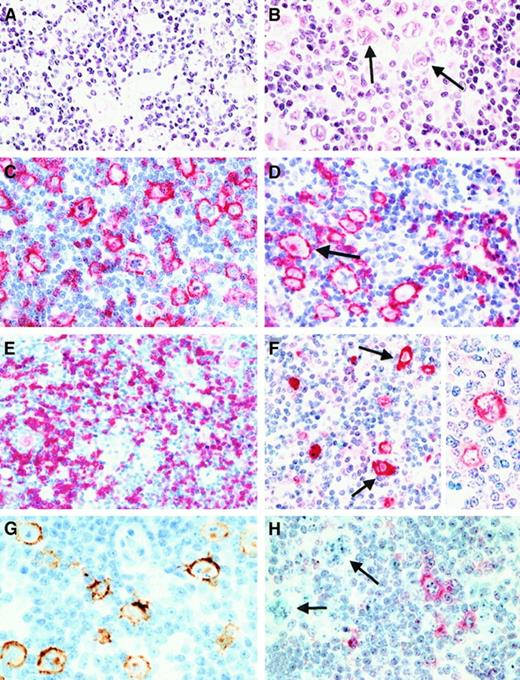
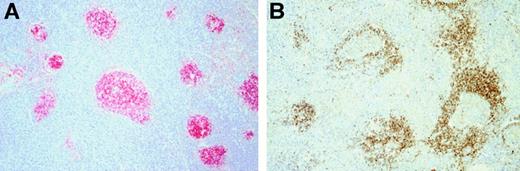
In all 219 LPHD cases, at least some of the neoplastic cells exhibited the characteristic morphology of L&H cells with folded and lobated (“popcorn”) nuclei and inconspicuous nucleoli (Figure2A). Mono- and binuleated cells with large acidophilic nucleoli resembling RS cells of CHD were observed in 120 cases (55%) (Figure 2B). These cells represented only a small proportion of the neoplastic cells in most (n = 108) cases. They were frequent in 11 cases, and in one case, they made up the majority of the neoplastic cell population.
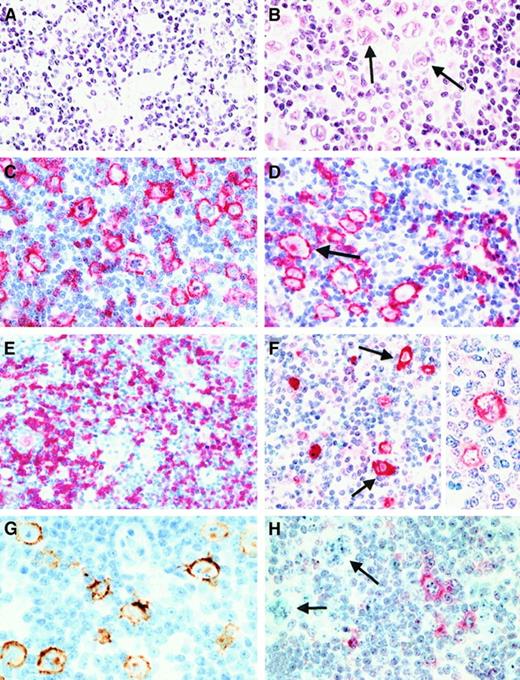
Morphology and immunophenotype of neoplastic cells in cases classified as lymphocyte predominance Hodgkin disease (LPHD).
(A) In all cases neoplastic cells with folded and lobated nuclei were observed (H&E-stained). (B) In more than half of the cases, there was a varying number of neoplastic cells with features of Reed-Sternberg (RS) cells of classical HD (arrows, H&E-stained). (C) and (D) Both types of tumor cells (an RS cell is highlighted by an arrow) showed a strong expression of the CD20 antigen (immunostain with the anti-CD20 monoclonal antibody L26, APAAP method with new fuchsin as chromogen). (E) A CD79a expression was also found in the neoplastic cells in most cases, its expression intensity being lower than that of CD20 (immunostain with the anti-CD79a monoclonal antibody JCB117, APAAP method). (F) Expression of J-chain was detectable in both tumor cell types (arrows indicate RS cells) of more than 90% of the cases. Insert highlights the cytoplasmic expression with labeling of the nuclear membrane (immunostain with the anti–J-chain polyclonal antibody, APAAP method). (G) EMA expression by the neoplastic cells was found in up to 50% of the cases (immunostain with the anti-EMA monoclonal antibody E29 using a peroxidase method with diaminobenzidine for development). (H) Immunostaining for CD30 (using the monoclonal anti-CD30 antibody Ber-H2 and the APAAP method) disclosed that the neoplastic cells (highlighted by arrows) did not express this antigen. The CD30+ cells corresponded to small mononuclear blasts.
Morphology and immunophenotype of neoplastic cells in cases classified as lymphocyte predominance Hodgkin disease (LPHD).
(A) In all cases neoplastic cells with folded and lobated nuclei were observed (H&E-stained). (B) In more than half of the cases, there was a varying number of neoplastic cells with features of Reed-Sternberg (RS) cells of classical HD (arrows, H&E-stained). (C) and (D) Both types of tumor cells (an RS cell is highlighted by an arrow) showed a strong expression of the CD20 antigen (immunostain with the anti-CD20 monoclonal antibody L26, APAAP method with new fuchsin as chromogen). (E) A CD79a expression was also found in the neoplastic cells in most cases, its expression intensity being lower than that of CD20 (immunostain with the anti-CD79a monoclonal antibody JCB117, APAAP method). (F) Expression of J-chain was detectable in both tumor cell types (arrows indicate RS cells) of more than 90% of the cases. Insert highlights the cytoplasmic expression with labeling of the nuclear membrane (immunostain with the anti–J-chain polyclonal antibody, APAAP method). (G) EMA expression by the neoplastic cells was found in up to 50% of the cases (immunostain with the anti-EMA monoclonal antibody E29 using a peroxidase method with diaminobenzidine for development). (H) Immunostaining for CD30 (using the monoclonal anti-CD30 antibody Ber-H2 and the APAAP method) disclosed that the neoplastic cells (highlighted by arrows) did not express this antigen. The CD30+ cells corresponded to small mononuclear blasts.
Immunophenotype of the neoplastic cells (Table3).
CD20 was expressed in 211 cases (Figure 2C, D). Tissue artifacts did not allow CD20 detection in an additional 3 cases, whereas 5 cases were negative. Only one of the CD20-negative cases showed a complete absence of B-cell–related antigens (CD79a, J-chain).
CD79a expression was detectable in 172 cases (Figure 2E); 43 cases were negative, and in 4 cases, the reactivity was not assessable because of technical problems.
The J-chain was found in 196 cases (Figure 2F). In 18 cases, neoplastic cells lacked the J-chain, and tissue artifacts led to unsatisfactory results in the remaining 5 cases.
During the initial review of the cases, 18 cases were identified with what appeared to be a “hybrid” phenotype between LPHD and CHD: many large cells seemed to express CD30 in addition to CD20 and J-chain. However, closer evaluation disclosed that the CD30+ cells were usually smaller than L&H cells, their nuclei were unfolded, their nucleoli were rodlike, and they lacked strong CD20 expression. Thus, these CD30+ cells corresponded to extrafollicular mononuclear blasts, which are regularly encountered in non-neoplastic reactive lymphoid tissues (Figure 2H). In general, no expression of CD30 by the neoplastic cell population could be identified in LPHD. Only occasionally a faint staining of single L&H cells was discernible.
The neoplastic cells were CD15 negative in all 219 cases. EMA was detectable in 118 cases (Figure 2G), whereas 100 cases were EMA negative and one case was not assessable.
Association with EBV infection.
In-situ hybridization for detection of EBER-transcripts was assessable only in 174 cases, as inappropriate tissue fixation did not permit analysis of 45 cases. EBER transcripts were detectable only in 43 of the 174 assessable cases. The labeled cells corresponded to small lymphocytes.
Composition of the reactive background.
The background infiltrate in all cases was rich in CD20+ B cells (Figure 3B). There were rare cases with a predominant T-cell population in some (Figure 3C) or in the majority of the nodules. CD3+ cells showed prominent rosetting around the neoplastic cells in 154 cases (70%). CD57+ cells were found in all cases, being most prominent within the nodular structures (Figure 3G). Rosettes composed of CD57+ cells occurred in 40 cases (18%) (Figure 3H). Epithelioid cells were present in almost all cases, and were very prominent in 22 (10%), occasionally forming large aggregates with a granuloma-like appearance. Neutrophils were completely absent from 165 cases (75%), being solely found within blood vessels. The remaining cases contained only scattered single neutrophils within the background infiltrate. Eosinophils were not observed in any case.
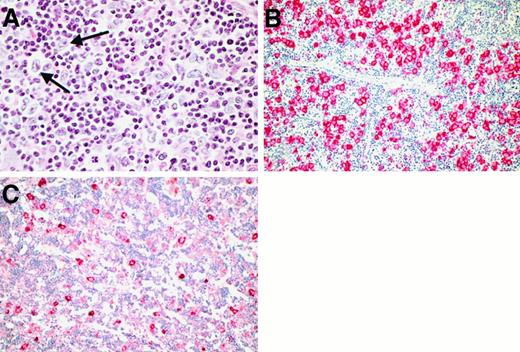
Growth patterns in the cases classified as LPHD.
(A) In most cases, a nodular growth pattern was conceivable at low power (H&E-stained). (B) and (C) Immunostains for CD20 disclosed that the nodules in nodular LPHD usually consisted of accumulations of small B cells (B). Occasionally, nodules rich in T cells were encountered admixed with B-cell rich ones (C). (D) Immunostains for CD20 proved useful also in detecting residual nodular structures in LPHD cases with predominantly diffuse growth pattern. (E) and (F) Labeling for CD21 (using the monoclonal anti-CD21 antibody 1F8 and the APAAP method) illustrated the presence of a follicular dendritic cell meshwork within the LPHD nodules, which engulfed the neoplastic cells with their rosettes (F). (G) and (H) Within the LPHD nodules, a varying number of CD57+ T cells was found (G). These cells often formed rosettes around the neoplastic cells as shown at higher magnification (H). (Immunostains using the anti-CD57 monoclonal antibody Leu7 and the APAAP technique).
Growth patterns in the cases classified as LPHD.
(A) In most cases, a nodular growth pattern was conceivable at low power (H&E-stained). (B) and (C) Immunostains for CD20 disclosed that the nodules in nodular LPHD usually consisted of accumulations of small B cells (B). Occasionally, nodules rich in T cells were encountered admixed with B-cell rich ones (C). (D) Immunostains for CD20 proved useful also in detecting residual nodular structures in LPHD cases with predominantly diffuse growth pattern. (E) and (F) Labeling for CD21 (using the monoclonal anti-CD21 antibody 1F8 and the APAAP method) illustrated the presence of a follicular dendritic cell meshwork within the LPHD nodules, which engulfed the neoplastic cells with their rosettes (F). (G) and (H) Within the LPHD nodules, a varying number of CD57+ T cells was found (G). These cells often formed rosettes around the neoplastic cells as shown at higher magnification (H). (Immunostains using the anti-CD57 monoclonal antibody Leu7 and the APAAP technique).
Growth patterns.
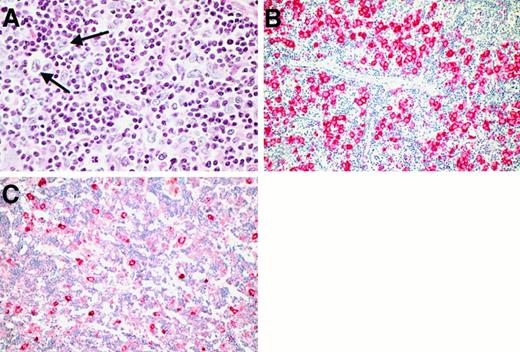
The most prominent architectural hallmark was the presence of nodular structures within the reactive cellular background. By conventional histology, the usually large nodules were composed of aggregates of small lymphocytes with slightly irregular hyperchromatic nuclei, thus simulating expanded primary follicles (Figure 3A). A variable number of epithelioid cells was admixed with this lymphoid population. Germinal center cells corresponding to centrocytes and centroblasts were usually absent from these nodules. Fine and rarely coarse bands of hyalinized connective tissue were observed in most cases, frequently surrounding the nodular structures. The nodularity became more evident on evaluation of the following immunostains (Table4): (1) labeling for CD20 and CD79a revealed aggregates of small B cells within the nodules (Figure3B,C,D), (2) CD21 detection disclosed an extended meshwork of follicular dendritic cells within the nodules (Figure 3E), which engulfed the neoplastic cells and the surrounding T-cell rosettes (Figure 3F), and (3) CD57 detection highlighted a T-cell population concentrated within the nodular structures (Figure 3G).
After assessment of the extent of the nodularity, LPHD cases were classified as follows:
Cases with nodular growth pattern occupying 30% to 100% of the infiltrated tissue were diagnosed as nodular LPHD (NLPHD) with or without diffuse areas. This corresponded to the largest group constituting 206 of the 219 LPHD cases (94%). In 61 of these cases, the neoplastic cells were found to be located solely within the nodular structures, whereas in the remaining cases, they also spilled out of the nodules and infiltrated the perinodular space. Cases in which the nodular growth pattern occupied less than 30% of the tumor area were classified as diffuse LPHD with nodular areas. This pattern was observed in 6 of the 219 LPHD cases (3%).
The remaining 7 cases exhibited loosely distributed neoplastic cells embedded in a background infiltrate without evidence of nodularity (Figure 4A). These cases closely resembled TCRLBCL by conventional histology (their findings are summarized in Table 5). In all of these cases, a majority of the neoplastic cells had the morphologic features of L&H cells. Immunophenotypic analysis disclosed an L&H cell-characteristic phenotype with expression of CD20 (7 of 7; Figure4B), CD79a (5 of 7), EMA (6 of 7) and of J-chain (4 of 6; Figure 4C), whereas in-situ hybridization for EBER-transcripts did not provide evidence of a latent EBV infection. In most cases, the neoplastic cells were arranged in a pattern vaguely simulating nodular structures (Figure 4B). In 3 cases, these nodular arrangements of the L&H cells were accompanied by small aggregates of small B cells and/or CD57+ T cells without any detectable follicular dendritic cells.
Morphology and immunophenotype in the LPHD cases with entirely diffuse growth pattern.
(A) By conventional histology, no evidence of nodularity was visible (H&E-stained; the neoplastic cells are highlighted with arrows). (B) Immunostains for CD20 showed that the positive neoplastic cells were arranged in a vaguely nodular pattern without evidence of admixed small B cells. (C) In several cases, the neoplastic cells showed an additional expression of J-chain.
Morphology and immunophenotype in the LPHD cases with entirely diffuse growth pattern.
(A) By conventional histology, no evidence of nodularity was visible (H&E-stained; the neoplastic cells are highlighted with arrows). (B) Immunostains for CD20 showed that the positive neoplastic cells were arranged in a vaguely nodular pattern without evidence of admixed small B cells. (C) In several cases, the neoplastic cells showed an additional expression of J-chain.
Cases classified as lymphocyte-rich classical Hodgkin disease
Morphology of the neoplastic cells.
At least some mono-, bi-, or multinucleated cells with large, slightly irregularly shaped nuclei and prominent broad, round, or clublike nucleoli, corresponding to classic RS cells were present in 103 of 115 cases (89.5%). In 57 cases (49.5%), these cells were rare, being more prominent in the remaining cases. Mononuclear neoplastic cells with folded nuclei and small nucleoli closely resembling the L&H cells were found in almost all cases (113 of 115; 98%). Such L&H-like cells were rare in 43 cases (37%), whereas they constituted the majority of the neoplastic cell population in 5 cases (4%).
Immunophenotype of the neoplastic cells.
CD30 expression was found in 107 cases; 2 cases showed complete absence of expression, whereaas the immunostaining in the remaining 6 cases was unsatisfactory, mostly for technical reasons (improper fixation of specimen, section artifacts). Expression of CD15 was detectable in 85 of 115 cases. There was not a single case with absent expression either of CD30 or of CD15 antigen.
Expression of CD20 was identified in 37 of 115 cases, CD79a was detected in 15 of 115 cases, and EMA in 7 of 115 cases. J-chain expression could not be found in any of the cases.
Association with EBV infection.
EBER-transcripts were identified in 55 of the 98 assessable cases. Inappropriate fixation of the specimens in the remaining 17 cases led to deterioration of the morphology during the procedure of in-situ hybridization, thus making any analysis impossible The labeled cells corresponded to neoplastic cells in 46 of 98 cases, and in an additional 9 cases, only to small non-neoplastic lymphocytes. In 15 cases, a mixture of labeled neoplastic and small bystander lymphocytes was identified. The immunophenotypical and in-situ hybridization data characteristic for LRCHD are summarized in Table 6.
Growth patterns and composition of the reactive background.
Three growth patterns were encountered: a nodular pattern without prominent interfollicular zones (80 cases), a pattern with small nodules and expanded interfollicular zones (27 cases), and a diffuse growth pattern (8 cases).
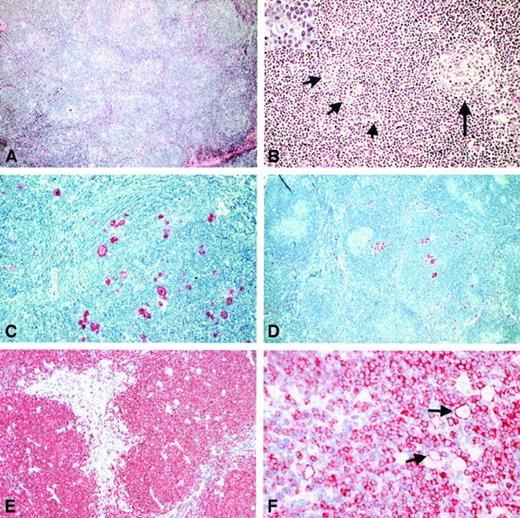
LRCHD, nodular. In 80 of 115 cases, a nodular growth pattern was discernible by conventional histology (Figure5A). The nodules consisted of small lymphoid cells exhibiting morphologic features of follicular mantle zone cells, showing round nuclei with dense chromatin. In occasional nodules, small, atrophic germinal centers could be identified lying eccentrically to the expanded mantle zone (Figure 5B). The neoplastic cells were usually embedded within the expanded mantle zones in a dispersed pattern, only rarely forming aggregates (Figure 5B). In some cases, the neoplastic cells were found in the outer rim of the mantle zones, expanding into the adjacent interfollicular area. Within the expanded mantle zones, epithelioid cells occasionally occurred. This arrangement of nodular aggregates of small lymphocytes admixed with epithelioid cells was highly reminiscent of the nodules found in LPHD. Fine bands of frequently hyalinized, connective tissue were identified in all cases, often engulfing nodular structures. Neutrophils and eosinophils were absent or rare in all of these cases.
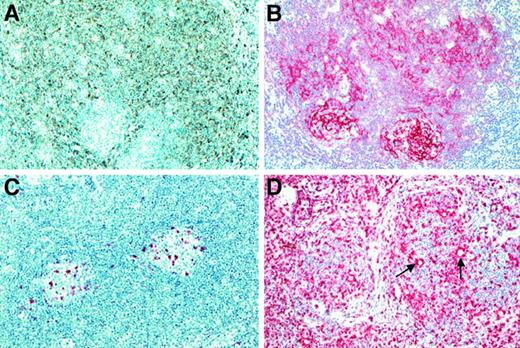
Histologic and immunohistologic features of the cases classified as NLRCHD.
(A) By conventional histology a nodular growth pattern reminiscent of NLPHD was visible at low power (H&E-stained). (B) In most of the cases, an intact or atrophic germinal center (highlighted by long arrow) could be found at the periphery of such a nodule (H&E-stained). The neoplastic cells were found to be distributed within the expanded mantle zone (short arrows). By morphology, they usually corresponded to RS cells. Also, cells without prominent eosinophilic nucleoli could be found (inset). (C) The neoplastic cells showed strong expression of CD30. (D) An expression of CD15 by the neoplastic cells was also noted (immunostain with the anti-CD15 monoclonal antibody C3D1, APAAP method). (E) The NLRCHD nodules consisted of small B cells as disclosed by the immunostains for CD20. (F) In 30% of the cases, the neoplastic cells (arrows) did show a CD20 expression.
Histologic and immunohistologic features of the cases classified as NLRCHD.
(A) By conventional histology a nodular growth pattern reminiscent of NLPHD was visible at low power (H&E-stained). (B) In most of the cases, an intact or atrophic germinal center (highlighted by long arrow) could be found at the periphery of such a nodule (H&E-stained). The neoplastic cells were found to be distributed within the expanded mantle zone (short arrows). By morphology, they usually corresponded to RS cells. Also, cells without prominent eosinophilic nucleoli could be found (inset). (C) The neoplastic cells showed strong expression of CD30. (D) An expression of CD15 by the neoplastic cells was also noted (immunostain with the anti-CD15 monoclonal antibody C3D1, APAAP method). (E) The NLRCHD nodules consisted of small B cells as disclosed by the immunostains for CD20. (F) In 30% of the cases, the neoplastic cells (arrows) did show a CD20 expression.
Immunohistologic analysis highlighted the nodular growth and revealed that the nodules corresponded to expanded mantle zones: the small cells expressed the B-cell antigens CD20 (Figure 5E) and CD79a and exhibited surface immunoglobulin heavy chains IgD (Figure6A) and IgM. Immunostaining for CD21 highlighted the small, atrophic germinal centers located eccentrically within the mantle zones (Figure 6B). In addition, an expanded meshwork of follicular dendritic reticulum cells was identified within the mantle zones. The neoplastic cells within the mantle zones were easily identified by the CD3 immunostaining, which highlighted the rosettes of CD3+ lymphocytes around these cells (Figure 6D). Only a small number of CD57+ T cells was observed. These cells were encountered within the atrophic follicle centres (Figure 6C), whereas rosettes composed of CD57+ cells were not identified. The neoplastic cells expressed CD30 (74 of 80; Figure 5C) and CD15 (65 of 80; Figure 5D), whereas B-cell antigens were identified in a minority of the cases: CD20 expression was found in 26 of 80 (Figure 5F) and CD79a in 7 of 80 cases, respectively (Table6). Expression of EMA was seen in 2 cases. The morphologic and immunophenotypical criteria useful for differentiating NLRCHD from NLPHD are summarized in Table7.
Immunohistologic features of cases classified as NLRCHD (continued).
(A) Immunostains for IgD revealed that the nodules corresponded to an expansion of IgD+ B cells of the mantle zone (polyclonal anti-IgD antibody, peroxidase method with diaminobenzidine development). (B) Labeling for CD21 highlighted the eccentrically placed atrophic germinal centers. (C) CD57+ T cells were encountered only within germinal centers. (D) Within the expanded mantle zones, the T cell rosettes surrounding the neoplastic cells were easily identified in CD3 immunostains (using polyclonal anti-CD3 antibody and the APAAP method).
Immunohistologic features of cases classified as NLRCHD (continued).
(A) Immunostains for IgD revealed that the nodules corresponded to an expansion of IgD+ B cells of the mantle zone (polyclonal anti-IgD antibody, peroxidase method with diaminobenzidine development). (B) Labeling for CD21 highlighted the eccentrically placed atrophic germinal centers. (C) CD57+ T cells were encountered only within germinal centers. (D) Within the expanded mantle zones, the T cell rosettes surrounding the neoplastic cells were easily identified in CD3 immunostains (using polyclonal anti-CD3 antibody and the APAAP method).
LRCHD, interfollicular, and diffuse. The remaining 35 cases showed a lymphocyte-rich background with 2 different growth patterns: twenty-seven cases presented with regular follicles and expanded interfollicular zones (Figure 7A,B). The neoplastic cells were located within the expanded interfollicular areas, sometimes infiltrating the adjacent mantle zones. The remaining 8 cases exhibited a diffuse growth pattern of the background infiltrate without discernible follicular structures. A variable number of epithelioid cells was present in almost all cases. Fourteen cases presented with a moderate number of neutrophils outside the blood vessels, whereas eosinophils were only rarely present.
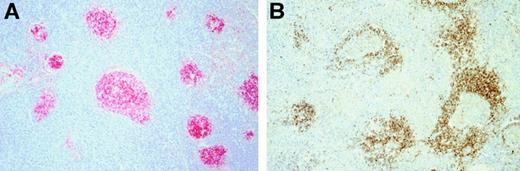
Immunohistologic features of cases classified as LRCHD with interfollicular growth pattern.
(A) Immunostains for CD20 illustrated the expanded interfollicular zones where the tumor infiltrate was located. (B) Labeling the mantle zones for IgD was of assistance in highlighting the interfollicular growth pattern.
Immunohistologic features of cases classified as LRCHD with interfollicular growth pattern.
(A) Immunostains for CD20 illustrated the expanded interfollicular zones where the tumor infiltrate was located. (B) Labeling the mantle zones for IgD was of assistance in highlighting the interfollicular growth pattern.
The phenotype of the neoplastic cells (Table 6) showed a predominance of CD30 (32 of 35), and CD15 (25 of 35) expression, whereas B-cell antigens were less frequently encountered (CD20: 11 of 35; CD79a: 2 of 35). Weak expression of EMA was rare (4 of 35). Most of the lymphocytes present in the interfollicular zones, as well as in the diffuse cellular background, expressed the CD3 antigen and formed rosettes around the neoplastic cells in all cases. Only rare CD57+ T cells were observed in these cases, mostly located within the reactive follicles. Rosettes composed of such cells surrounding neoplastic cells were not detectable in any case.
Clinical presentation of LPHD and LRCHD versus NS/MC CHD
The characteristics of clinical presentation of the 219 LPHD and 115 LRCHD cases were compared with those from 599 NS and 174 MC CHD patients recruited in the multicenter trials of the German Hodgkin Study Group from 1988 to 1992.40
LPHD and LRCHD showed great similarities in the presence of B symptoms and the stage of the disease as well as in extension of organ involvements (Table 8). The only differences were the patient's age (more than 50 years: 18% in LPHD vs 32% in LRCHD), presence of stage III disease (14% in LPHD vs 24% in LRCHD), and the presence of mediastinal involvement (7% in LPHD vs 15% in LRCHD).
The principal differences between LRCHD and the other types of CHD were the mainly early stage (stage I: 46% in LRCHD vs 10% in NS and 21% in MC), infrequent B symptoms (11% in LRCHD vs 42% in NS and 35% in MC), infrequent bulky disease (11% in LRCHD vs 54% in NS and 40% in MC), and infrequent mediastinal involvement (15% in LRCHD vs 80% in NS and 40% in MC).
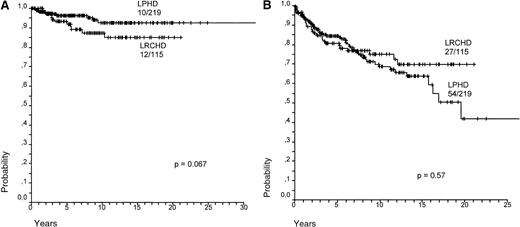
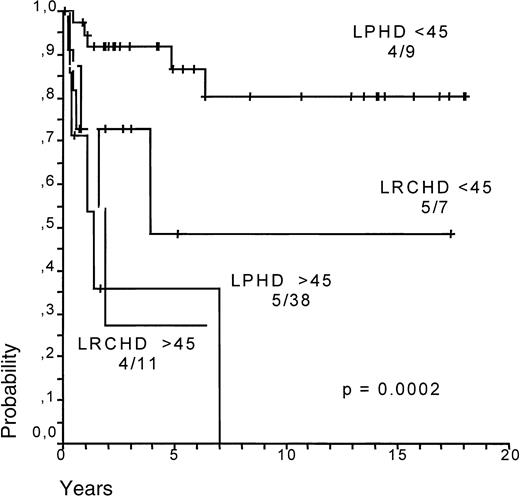
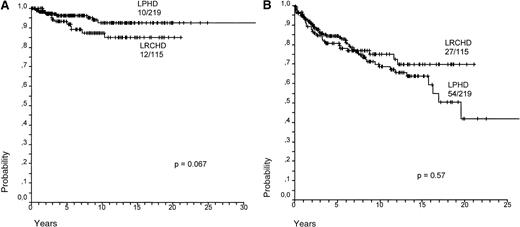
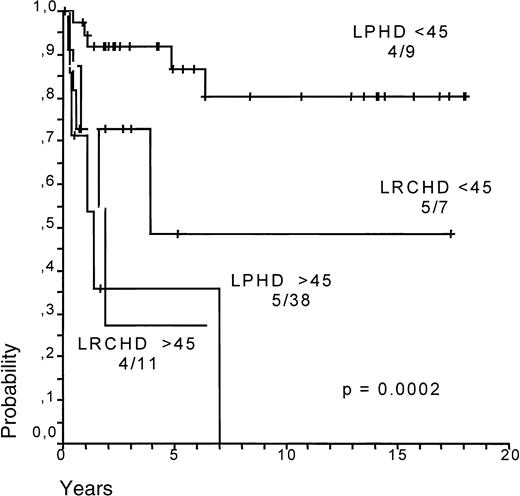
Survival (SV) and failure-free survival (FFS) for LPHD and LRCHD patients were analyzed (Figure8A,B).37 Although survival appears to be slightly worse for LRCHD patients, no significant difference was observed between the 2 groups (P = .067 for SV; P = .57 for FFS). Analysis of the data from disease relapses showed that 21% of the LPHD patients experienced a first relapse after achieving a complete remission, whereas the same was the case in 17% of LRCHD patients. The LPHD patient group was also characterized by the occurrence of multiple relapses in 12 of the relapsing patients, whereas this was observed only in one of the 19 relapsing patients with LRCHD (data not shown).37 LRCHD patients younger than 45 had a worse prognosis after relapse when compared with the LPHD patient group. Patients older than 45 had an equally bad prognosis in both disease groups (Figure9)..37
Survival for LPHD and LRCHD.
(A) Hodgkin disease-specific overall survival for LPHD and LRCHD patients (data from Diehl et al37). (B) Hodgkin disease-specific failure-free survival for LPHD and LRCHD patients (data from Diehl et al37).
Survival after relapse for LPHD and LRCHD patients related to age (younger or older than 45 years).
Data from Diehl et al.37
Survival after relapse for LPHD and LRCHD patients related to age (younger or older than 45 years).
Data from Diehl et al.37
Discussion
We report the largest series of cases submitted with the diagnosis of LPHD. The cases were reviewed by a large panel of hematopathologists who achieved a consensus diagnosis in all cases after the evaluation of immunohistologic stains. The initial attempt by the panel to classify the cases according to the H&E stains alone led to the diagnosis of LPHD in 248 of the 388 assessable cases, of CHD in 40 cases, of reactive lesions in 12 cases, and of NHL in 12 cases, whereas in 76 cases no consensus could be reached. Most of the diagnostic problems in the cases without consensus could be solved after combined evaluation of histologic and immunohistologic findings, leaving only 9 cases unclassified. This combined approach led also to the reclassification of 73 cases diagnosed as LPHD, according to the H&E morphology as CHD. The final classification of the 388 assessable cases revealed that the submitting pathologists' diagnosis of LPHD could be confirmed only in 219 cases (56.5%), whereas the remaining cases were found to represent CHD (134 cases; 34.5%), reactive lesions (14 cases; 4%), and NHL (12 cases; 3%). This unexpected result appears to be due to the fact that most of the CHD cases (115 of 134) exhibited a lymphocyte-rich background.
In particular, the majority of the lymphocyte-rich CHD cases (80 of 115) were characterized by a nodular background with admixed histiocytes and absent neutrophils and eosinophils closely resembling NLPHD, particularly at low power. Furthermore, a varying proportion of the neoplastic cells exhibited morphologic features of L&H cells. The finding that many of the neoplastic cells had the cytomorphologic features of classical RS cells was the first hint that these cases differed from typical NLPHD. In addition, the identification of small germinal centers at the periphery of the nodular structures distinguished these nodules from those of NLPHD, which do not contain germinal centers. Immunohistologic analysis revealed that the nodular structures corresponded to expanded mantle zones composed of B cells with surface IgD and IgM expression. CD21 immunostaining highlighted the presence of eccentrically placed small germinal centers with a dense, sharply defined meshwork of follicular dendritic cells (FDC) and of an expanded mantle zone with a loose, ill-defined FDC meshwork. The neoplastic cells were found to be engulfed by this meshwork and were often surrounded by CD3+ T-cell rosettes.
The phenotype of the neoplastic cells was characteristic of CHD with expression of CD30 in 92.5% and of CD15 in 81% of the cases. Expression of B-cell antigen CD20 by the neoplastic cells was found in only 32.5% and of CD79a in 8.7% of the cases, a much lower frequency than that observed in LPHD (see below). A further difference from LPHD was the complete absence of J-chain in all cases and a weak expression of EMA in only 2.5% of the cases.
Examples of this CHD variant had been first identified at the meeting of the European Society of Haematopathology in 1994 in Toledo, Spain, followed by a publication of 4 such cases under the term “follicular HD” by Ashton-Key et al.36 The finding that this CHD variant made up a significant proportion of the cases in the present series (80 of 388; 21%) indicates that it is not as rare as initially suspected. It seems also that this CHD-variant has remained unrecognized even in the recently published large study on LPHD from the German Hodgkin Study Group.41 In that study, 25 cases were described, which had been classified as LPHD according to morphology but became reclassified as CHD after immunophenotyping. Unfortunately, no details on the characteristics of these cases have been presented.
During the review of these cases, some of the members of the panel proposed that some of these might represent a variant of “cellular phase of nodular sclerosis.” However, the original description of cellular phase of NSHD was imprecise, and there appears to be no consensus as to what cases this term should cover.5,7,42 43 The additional observation of the low frequency of mediastinal involvement led to the conclusion that these cases are not related to NSHD. Therefore, the panel members decided unanimously to propose the term “nodular lymphocyte-rich classical HD (NLRCHD)” for these cases to emphasize (1) that they simulate NLPHD and might be mistaken for it and (2) that they are a variant of CHD. For these reasons, the term NLRCHD was also favored over “follicular HD.”
In addition to the 80 cases with a nodular pattern, 35 LRCHD cases with an interfollicular or a diffuse growth pattern were recognized. These cases corresponded to the provisional LRCHD entity established in the REAL classification.20 The background infiltrate was rich in CD3+ T cells with almost absent neutrophils and eosinophils, whereas the phenotype of the neoplastic cells was similar to that of the nodular variant.
Analysis of the patient characteristics at presentation disclosed marked differences between the LRCHD patient group and the NS and MC types of CHD: LRCHD patients usually presented with early stage disease, infrequent B symptoms, infrequent bulky disease, and infrequent mediastinal involvement. These data further justify the separation of LRCHD as a distinct type of CHD. LRCHD cases showed many similarities to LPHD; however, they differed in having an older age (more than 50 years: 32% in LRCHD vs 18% in LPHD), more frequent involvement of mediastinum (15% in LRCHD vs 7% in LPHD), and a higher incidence of stage III disease (24% in LRCHD vs 14% in LPHD). Interestingly, both LPHD and LRCHD patients were found to have a good-to-excellent prognosis. Relapses were frequent in both groups, and patients continued to relapse within the observation period. Multiple relapses were more common and survival after relapse was slightly better in LPHD patients, which may in part reflect a more benign character of relapse. However, LRCHD patients were older than LPHD patients and this may have influenced the outcome of a relapse.37
LPHD was correctly recognized using H&E sections in 175 cases. Immunohistologic analysis, however, revealed that 73 cases classified as LPHD, according to conventional histology, exhibited the phenotypical criteria of CHD. Also, 9 cases initially classified as CHD were reclassified as LPHD after review of the immunostains. A particular finding posing diagnostic problems in conventional histology was the fact that neoplastic cells with morphologic features of classic RS cells were not as infrequent in LPHD, as previously reported in the literature.17,44 Such cells were observed in low numbers in most cases and were a frequent and often prominent finding in 5% of the cases. The immunophenotype of the neoplastic cells in LPHD, including those with classic RS morphology, was found to be characteristic: constant expression of CD20 in almost all cases, expression of CD79a in 80%, and expression of J-chain in 91.5% of the cases. These findings are largely in line with published data with one exception: the slightly lower frequency of CD79a expression compared with the 100% figure given in one study.45 In contrast to the published data on CD15 expression in LPHD,46 CD15 was never found to be expressed by the neoplastic cells in the current series. It has also been repeatedly reported that CD30 is expressed on L&H cells in a proportion of cases.17,15,47 48 However, careful review of the CD30 immunostains of the current study revealed that this antigen was not expressed by the neoplastic cell population, but rather by blasts that corresponded in morphology to normally occurring CD30+ extrafollicular blast cells in reactive lymphoid tissues. Only in rare cases was an extremely faint staining of isolated L&H cells observed.
In-situ hybridization for detection of EBV-encoded small nuclear transcripts (EBER-1 and -2) was found to represent a valuable tool in differentiating LPHD from LRCHD. In all LPHD cases studied, the neoplastic cells were devoid of EBER-specific signals. The only cells found to be EBV-infected in LPHD were single, small non-neoplastic lymphocytes occurring in 25% of the cases. In contrast, 47% of the LRCHD cases harbored EBER-positive RS cells. Thus, the current data clarify the previously confusing picture of the association of LPHD with an EBV infection with some reports showing positive,39-52 and others negative53-55 cases. It seems plausible that errors in classification have been the reason for the discordant data published.
The growth patterns of LPHD were highlighted with immunohistology. In particular, immunostaining for CD20 was helpful in identifying nodular aggregates of small B cells with an accompanying CD21+ meshwork of FDC. A useful adjunct was the demonstration of CD57+ T cells, which were preferentially found within nodular structures occasionally forming rosettes around the neoplastic cells. As precise definitions on the classification of a given LPHD case according to its growth pattern do not exist in the literature, a pragmatic approach to classification was chosen: The presence of nodular structures occupying more than 30% of the involved lymph node area led to the diagnosis of NLPHD, with or without diffuse areas, whereas the absence of any nodularity in up to 70% of the tumor area was classified as diffuse LPHD (DLPHD) with nodular areas.
The evaluation of the LPHD cases according to this approach led to the finding that at least partial nodularity was present in 97% (212 of 219) of the cases. The problem of discrimination between DLPHD and TCRLBCL occurred only in 7 cases, a much lower number than initially expected. These cases contained neoplastic cells that mostly resembled L&H cells in morphology and immunophenotype. The neoplastic cells were frequently arranged in a vaguely nodular pattern, but the background consisted predominantly of T cells. A small number of accompanying small B lymphocytes and CD57+ cells was observed only in 3 of the cases. Although these findings would support the diagnosis of DLPHD, they have also been described in TCRLBCL. It has been reported that the morphology of the T cells in the background infiltrate can assist in differentiation between LPHD and TCRLBCL, as these cells can show nuclear atypia in latter entity.26,28The panel members did not find that this phenomenon was of particular value, as it is rather subjective. In addition, atypical T cells were also encountered within nodular and diffuse structures of LPHD in a number of cases of the present series. The most important finding supporting the classification of these cases as DLPHD was that they did not show any significant differences in clinical presentation and follow-up to the NLPHD group of cases.37 Although the differential diagnosis of predominantly diffuse LPHD from TCRLBCL remains difficult and precise criteria were not established in this study, this differential diagnostic problem was extremely rare in this series of cases.
In conclusion, this study has shown that HD with a nodular growth pattern and a lymphocyte-rich background encompasses 2 entities with distinct morphologic, phenotypical, and clinical features. Therefore, the precise classification of such cases requires a combination of conventional histology and immunohistology using a distinct panel of antibodies. LPHD may contain a broad morphologic spectrum of neoplastic cells, which nonetheless always exhibit a characteristic immunophenotype with expression of B-cell–specific antigens and/or J-chain and absent expression of CD30 and CD15. Furthermore, it could be shown by means of immunostains that the vast majority of LPHD cases contain areas with nodular growth pattern, whereas purely diffuse cases are extremely rare. LRCHD cases also exhibit, in the majority of cases, a nodular growth pattern as well as a broad morphologic spectrum of the neoplastic cells. The tumor cell phenotype, however, is always characteristic of CHD with expression of CD30 and CD15, infrequent expression of B-cell antigens, and absent expression of J-chain. In addition to immunophenotyping, in-situ hybridization for EBER detection can assist in the differential diagnosis between the 2 HD entities as the neoplastic cells in LPHD appear not to be permissive for an EBV infection.
Acknowledgments
We thank the following colleagues who contributed essentially to this study:
Clinicians: A. Anselmo, U. Axdorf, M. Björkholm, G. Bonadonna, V. Bonfante, B. Coiffier, D. Crowther, H. Eghbali, A. M. Gianni, B. Glimelius, A. Gustavsson, T. Habermann, F. B. Hagemeister, T. Lister, F. Mandelli, E. Noordjik, S. Proctor, J. Radford, L. Specht, P. Taylor, and L. Teerenhovi.
Pathologists: B. Angus, C. Baroni, F. Berger, M. Dictor, A. Georgii, K. Hou-Jensen, P. Kluin, P. Kurtin, A. MacDonald, J. A. McBride, J. Menarguez, A. Norton, A. Öst, S. Pilotti, W. Pugh, I. Soubeyran, and C. Sundström.
We are also particularly indebted to C. Cieluch, H. Karg, H. Protz, P. Wendler, I. Winter, and C. Kreschel for their excellent technical assistance, and to L. Udvarhelyi for help with the text.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Harald Stein, Professor of Pathology, Klinikum Benjamin Franklin, Free University Berlin, Hindenburgdamm 30, D-12200 Berlin, Germany; e-mail: stein@ukbf.fu-berlin.de.