Abstract
Glycosaminoglycans (GAG) are a group of negatively charged molecules that have been shown to bind and directly regulate the bioactivity of growth factors and cytokines such as basic fibroblast growth factor, transforming growth factor-β, IL-7, and interferon-γ. The ability of GAG to interact with human IL-10 (hIL-10) and the effect of these interactions on its biologic activity were analyzed. It was demonstrated by affinity chromatography that hIL-10 binds strongly to heparin–agarose at physiological pH. Biosensor-based binding kinetic analysis indicated an equilibrium dissociation constant, Kd, of 54 nmol/L for this interaction. Human IL-10 stimulated CD16 and CD64 expression on the monocyte/macrophage population within peripheral blood mononuclear cells, with optimal concentrations between 1 and 10 ng/mL. Soluble heparin, heparan sulfate, chondroitin sulfate, and dermatan sulfate were shown to inhibit the hIL-10–induced expression of CD16 and CD64 in a concentration-dependent manner. Heparin and heparan sulfate were most effective with IC50 values of 100 to 500 μg/mL. Considerably higher concentrations of dermatan sulfate and chondroitin 4-sulfate were required with an IC50 of 2000 to 5000 μg/mL, whereas chondroitin 6-sulfate was essentially inactive. The antagonistic effect of heparin on hIL-10 activity was shown to be dependent on N-sulfation, inasmuch as de-N-sulfated heparin had little or no inhibitory effect on the IL-10– induced expression of CD16, whereas the effect of de-O-sulfated heparin was comparable to that of unmodified heparin. Furthermore, the inhibition of cell-bound proteoglycan sulfation reduced the hIL-10–mediated expression of CD16 molecules on monocytes/macrophages. Taken together, these findings support the hypothesis that soluble and cell-surface GAG and, in particular, their sulfate groups are important in binding and modulation of hIL-10 activity.
Introduction
Interleukin-10 (IL-10) is an 18-kd glycoprotein produced by various cell types, including macrophages, activated T and B lymphocytes, B-cell lymphomas, keratinocytes, and mast cells.1-4 IL-10 was initially described as a novel cytokine produced by murine Th-2 cellular clones that inhibited the secretion of cytokines by Th-1 clones, notably interferon (IFN)-γ, and was, therefore, named cytokine synthesis inhibitory factor.5 Subsequently, it has been shown by in vitro and in vivo studies that IL-10 is a pleiotropic cytokine that exhibits suppressive and stimulatory effects on the immune system. For example, IL-10 potently inhibits the synthesis of pro-inflammatory cytokines by macrophages and dendritic cells and reduces their ability to serve as antigen-presenting cells for the stimulation of T cells.1-3,6 In contrast, IL-10 stimulates the proliferation and differentiation of B cells,7 murine thymocytes,8 mast cells, and megakaryocyte progenitor cells,9-11 and it enhances antibody-dependent cellular cytotoxicity of monocytes.12 Sequence studies have revealed that the C-terminus (Figure 1A) of human IL-10 (hIL-10)13,14 is rich in the basic amino acids arginine and lysine, and similar regions have been shown in other growth factors and cytokines—such as fibroblast growth factor (FGF), transforming growth factor (TGF)-β, and IL-8—to promote interaction with glycosaminoglycans (GAG).15-21
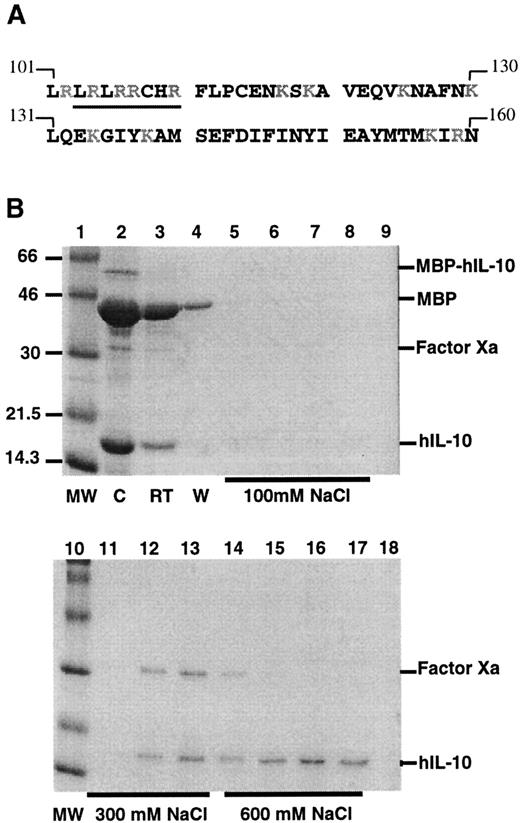
Binding of hIL-10 to heparin agarose.
(A) Amino acid sequence of the C-terminus of human IL-10. Basic residues appear in gray. The sequence −LRRCHR-(105-110), which is similar to one consensus site, XBBXBX (where B represents a cationic lysine, arginine, or histidine residue and X represents a hydropathic residue) and is described by several investigators16,18-20in GAG-binding proteins, is underlined. The sequence is taken from Moore et al13 and Vieira et al.14 (B) High-affinity binding of hIL-10 to heparin. Factor Xa-cleaved products were loaded onto a heparin–agarose column, and the binding of hIL-10 was analyzed on SDS-PAGE (14%) and visualized with Coomassie blue staining. Lanes 1 and 10, molecular weight markers in kd; lane 2, factor Xa-cleaved products before loading onto the heparin column (C); lane 3, run through from the column (RT); lane 4, wash from the column (W); lanes 5 to 8, eluate after 100 mmol/L NaCl; lanes 9 and 11 to 13, eluate after 300 mmol/L NaCl; lanes 14 to 17, elute after 600 mmol/L NaCl; lane 18, elute after 1 mol/L NaCl. MBP–hIL-10 fusion protein, MBP, factor Xa, and hIL-10 are indicated.
Binding of hIL-10 to heparin agarose.
(A) Amino acid sequence of the C-terminus of human IL-10. Basic residues appear in gray. The sequence −LRRCHR-(105-110), which is similar to one consensus site, XBBXBX (where B represents a cationic lysine, arginine, or histidine residue and X represents a hydropathic residue) and is described by several investigators16,18-20in GAG-binding proteins, is underlined. The sequence is taken from Moore et al13 and Vieira et al.14 (B) High-affinity binding of hIL-10 to heparin. Factor Xa-cleaved products were loaded onto a heparin–agarose column, and the binding of hIL-10 was analyzed on SDS-PAGE (14%) and visualized with Coomassie blue staining. Lanes 1 and 10, molecular weight markers in kd; lane 2, factor Xa-cleaved products before loading onto the heparin column (C); lane 3, run through from the column (RT); lane 4, wash from the column (W); lanes 5 to 8, eluate after 100 mmol/L NaCl; lanes 9 and 11 to 13, eluate after 300 mmol/L NaCl; lanes 14 to 17, elute after 600 mmol/L NaCl; lane 18, elute after 1 mol/L NaCl. MBP–hIL-10 fusion protein, MBP, factor Xa, and hIL-10 are indicated.
Glycosaminoglycans are a group of linear polysaccharides consisting of repeating disaccharide subunits in which one residue is an amino sugar, either d-glucosamine (GlcN) or d-galactosamine (GalN), and the other either a hexuronic acid (d-glucuronic acid [GlcA] or l-iduronic acid [IdoA]) or galactose. They can be divided into 4 subgroups: heparan sulfate (HS) and heparin (H), keratan sulfate (KS), chondroitin sulfates (CS; chondroitin 4-sulfate or chondroitin 6-sulfate) and dermatan sulfate (DS), and hyaluronic acid (HA). With the exception of HA, which is made as a free GAG and lacks sulfate, they are all synthesized as covalent complexes with core proteins, forming proteoglycans (PG), and are highly charged because of the addition of sulfates at various positions.22-26
GAG and PG are found in all tissue types as components of the extracellular matrix and the basement and cellular membranes and in the secretory granules of many cell types.22,24 At inflammatory sites, soluble PG are secreted by activated leukocytes, such as monocytes/macrophages, natural killer cells, T cells, mast cells, and basophils, and they are released as a consequence of extracellular matrix degradation.27-34 Besides their prominent role as structural components, GAG, PG, or both have been shown to modulate the activity of a number of cytokines by several different mechanisms.35,36 For example, binding to cell-surface PG, such as syndecans, or to the extracellular matrix PG may provide tissue-bound reservoirs of cytokines that can be presented to target cells, as has been previously described for basic FGF (bFGF) and hematopoietic growth factors such as IL-3 and granulocyte macrophage–colony-stimulating factor.37-39 Binding of IL-7, IFN-γ, bFGF, and acidic FGF (aFGF) to heparin and heparan sulfate has been shown to protect them from proteolytic and chemical inactivation.40-44 Interaction with cell-surface PG may also promote the binding of growth factors to their high-affinity receptors, as has been demonstrated for bFGF45 and IL-7.46 Interestingly, not all cytokines are positively regulated by interaction with GAG. Binding of IFN-γ to heparin competitively inhibits interaction with its receptor47 and also inhibits several of its activities, including the induction of major histocompatibility complex class II molecules and ICAM-1 on endothelial cells.48-53In addition, heparin has been shown to inhibit the antiviral54 and antiparasitic effects of IFN-γ.48 Collectively, the above studies highlight the potential for selective regulation of cytokine bioactivity by GAG.
In this study, we have identified hIL-10 as a heparin-binding cytokine. The physiological relevance of hIL-10 binding to heparin or other GAG can be expected to depend on the affinity of the cytokine for the GAG. Accordingly, we have measured the affinity of hIL-10 for heparin and investigated the biologic consequences of the interaction of hIL-10 with different types of GAG.
Materials and methods
Cytokines and reagents
Recombinant human IL-10, IFN-γ, and tumor necrosis factor (TNF)-α—all expressed in Escherichia coli—were obtained from PeproTech (London, UK), R&D Systems (Abingdon, UK), and Cambridge Bioscience (Cambridge, UK), respectively. Fluorescein isothiocyanate-conjugated CD16 (DJ130c), CD54 (ICAM-1; 6.5B5), and CD64 (clone 10.1) monoclonal antibodies were obtained from DAKO (Ely, UK) and PharMingen (Runcorn, UK), respectively. Avidin (from egg white), biotinylated albumin–heparin, heparin (grade I-A from porcine intestinal mucosa),N-acetyl-de-O-sulfated heparin, de-N-sulfated heparin (porcine mucosal heparin; completely de-N-sulfated), heparan sulfate (bovine kidney), chondroitin 4-sulfate (chondroitin sulfate A from bovine trachea), DS (from bovine mucosa), chondroitin 6-sulfate (chondroitin sulfate C from shark cartilage), and sodium chlorate were all purchased from Sigma (Poole, UK). De-O-sulfated heparin (completely de-O-sulfated) was obtained from Siekagaku Kogyo (Tokyo, Japan).
Binding of hIL-10 to heparin–agarose column
The hIL-10 gene was cloned into the pMAL-c2 (New England Biolabs) prokaryotic expression vector. This created a fusion protein with maltose binding protein (MBP) that could be purified by affinity chromatography on amylose resin, as described by the manufacturer. The pMAL-c2 vector used in these experiments contained the sequence coding for the specific recognition site (Ile Glu Gly Arg) of the protease factor Xa, adjacent to the polylinker insertion site, allowing MBP to be cleaved from hIL-10 without the addition of any vector-derived residues. The cleaved products, consisting of MBP, factor Xa, and hIL-10 (5 mL) in 20 mmol/L Tris-HCl, pH 7.4 (loading buffer), were loaded onto a 5-mL Hi-Trap heparin-agarose column (Amersham Pharmacia, Little Chalfont, UK) equilibrated with 10 column volumes of the same buffer. The sample was clarified by using a 0.45-μm filter (Gelman Sciences), and its pH and ionic strength were adjusted to that of the loading buffer. The sample was loaded onto the column and washed with 10 column volumes of loading buffer. Bound proteins were eluted using a stepwise salt gradient of 0.1 mol/L, 0.3 mol/L, 0.6 mol/L, and 1.0 mol/L NaCl in loading buffer. Fractions of 1 mL were collected and analyzed by Coomassie blue-stained (0.25% Coomassie brilliant blue G [Sigma], 10% glacial acetic acid, 45% methanol] sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).55
Solid-phase assay for binding hIL-10 to biotinylated albumin–heparin
The binding of hIL-10 to immobilized heparin–albumin (biotinylated) was monitored using an IAsys auto plus device (Affinity Sensors, Cambridge, UK), as described by Lyon et al56 with some modifications. A planar biotinylated surface was derivatized with avidin and then loaded with the biotinylated albumin–heparin according to the manufacturer's instructions. All hIL-10 binding reactions were performed in 20 mmol/L Tris-HCl, pH 7.4, at 25°C, and data were collected at 0.3-second intervals. The association of hIL-10 with the immobilized heparin was monitored until a plateau was reached. The cuvette was then rapidly washed 4 times with 20 mmol/L Tris-HCl, pH 7.4, and the dissociation of hIL-10 from immobilized heparin–albumin was followed. The heparin–albumin surface was regenerated with 1 mol/L NaCl in 20 mmol/L Tris-HCl, pH 7.4, for 2 minutes and re-equilibrated with 20 mmol/L Tris-HCl, pH 7.4. Three independent sets of binding reactions were obtained in duplicate for 4 different hIL-10 concentrations (13.5 nmol/L-54 nmol/L). The association rate (ka) and the dissociation rate (kd) constants were calculated from a plot of the observed on rates (kon) and the hIL-10 concentrations at which they were carried out. The dissociation equilibrium constant (Kd) for hIL-10 binding to heparin–albumin was calculated using the ka andkd values as described above, whereKd = kd (y-axis intercept)/ka (gradient).
Control of endotoxin levels
All cell culture reagents used were either certified as low in endotoxin when purchased or were ensured to be low in endotoxin by the Pyrogenet-5000 turbidimetric LAL assay57 (BioWhittaker) using the Kinetic-QCL reader and WinKQCL software (BioWhittaker).
Isolation of mononuclear cells from peripheral blood
Peripheral blood from healthy donors was collected into tubes containing preservative-free heparin (CP Pharmaceuticals, Wrexham, UK) and processed within 1 hour of withdrawal. Mononuclear cells were isolated using a modification of the method described by Boyum.58 Peripheral blood was diluted 1:2.5 in phosphate-buffered saline (PBS), and 30 mL was then layered onto 15 mL Ficoll-Paque Plus (Pharmacia) and centrifuged at 400g for 35 minutes at 18°C. Mononuclear cells accumulating at the interface between the separating medium and the plasma were carefully transferred with a Pasteur pipette and washed twice with HEPES (25 mmol/L)-buffered RPMI 1640 medium (Gibco/BRL) supplemented with 10% fetal calf serum. Cell viability was determined by Trypan blue exclusion and found to be 95% to 98%.
Cytokine and GAG treatment of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were suspended at a density of 2 × 106 cells/mL in HEPES (25 mmol/L)-buffered RPMI 1640 supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin, 2 mmol/L l-glutamine, and 10% FCS (all from Gibco). PBMC suspension (0.1 mL) was transferred to each well of a 96-well U-bottomed tissue culture plate (Nunc). Human IL-10 (10 ng/mL) or IFN-γ (10 ng/mL) were preincubated with various concentrations of GAG for 20 minutes at room temperature in medium without FCS. Subsequently, 0.1 mL was added to the PBMC-containing 96-well plates in duplicate and incubated for 24 to 72 hours at 37°C in a humidified atmosphere containing 5% CO2. After the incubation period, cells were pelleted by centrifugation at 200g for 3 minutes followed by resuspension in 200 μL PBS. Cell-surface levels of CD16 or CD64 on monocytes/macrophages were determined by direct immunofluorescence and analyzed by flow cytometry.
Immunofluorescence staining of cells for FACS analysis
Harvested PBMC were washed twice with PBS, 2% bovine serum albumin, and 0.02% sodium azide (FACS buffer) by centrifugation and resuspended in 50 μL ice-cold FACS buffer containing mouse–antihuman CD16, or CD64 followed by incubation in the dark at 4°C for 30 minutes. Subsequently, the cells were washed 3 times with FACS buffer and resuspended in 200 μL PBS containing 1% formaldehyde. After a further incubation period of 1 hour on ice, the cells were washed and resuspended in 200 μL FACS buffer before analysis. Immunostained cells were analyzed on a FACScan flow cytometer (Becton Dickinson) using the CELLQuest software. Binding of antibodies was evaluated on monocyte/macrophage populations, which had been gated within PBMC by forward- and side-scatter patterns; the cells excluded from analysis were smaller and comprised less than 5% of the cell population. Monocyte/macrophage enrichment to more than 95% by the chosen flow cytometry gating was routinely confirmed by the expression of the monocyte-specific marker, CD14, of an identically gated control cell suspension. At least 5000 events gated for the forward- and side-scatter parameters characteristic of monocytes/macrophages were acquired. Results are expressed either as mean fluorescence intensity or percentage of maximum response within the gated region.
Inhibition of proteoglycan sulfation by sodium chlorate
Chlorate ions are potent inhibitors of GAG sulfation.59 60 Sodium chlorate (NaClO3) was used to test whether abrogation of GAG sulfation modified the mitogenic activity of hIL-10. PBMC, 2 × 105 cells, in 96-well U-bottomed tissue culture plates were incubated for 24 hours in the presence or absence of NaClO3 (5 or 20 mmol/L) in sulfate-free Dulbecco minimal essential medium (DMEM) containing 2 mmol/L l-glutamine, 2% FCS, 0.8 mmol/L magnesium chloride, and 1 mmol/L sodium pyruvate. Penicillin and streptomycin were omitted because they contain sulfur, and cysteine and methionine were reduced to 20% of normal concentration because they could also provide exogenous sulfate, which could negate the effect of chlorate. Ionic concentration was maintained at 0.15 mol/L by adjustment of NaCl concentrations. After 24 hours, cells were washed once and resuspended in culture medium containing hIL-10 (10 ng/mL) and chlorate. NaClO3 remained present at all times during the stimulation period. An equivalent concentration of NaCl + 1 mmol/L magnesium sulfate (MgSO4; sulfate-reconstituted medium) was used as the control. In some experiments, 15 mmol/L MgSO4was added with hIL-10 to reverse the effect of chlorate. Cultured cells were recovered 48 hours after stimulation with hIL-10, and the expression of CD16 was determined by immunofluorescence staining and analyzed by flow cytometry.
Results
Binding of hIL-10 to heparin agarose
The ability of hIL-10 to bind to heparin agarose was investigated by chromatography of a semipurified preparation of hIL-10, also containing MBP, and factor Xa. The heparin-bound proteins were eluted using a stepwise salt gradient. From the stained gels it can be seen that MBP (42 kd) has little or no interaction with heparin-agarose, whereas both hIL-10 (18 kd) and factor Xa (30 kd) bind (Figure 1B). Factor Xa typically eluted from the column at 300 mmol/L NaCl, hIL-10 had stronger affinity, with peak levels of protein eluting at 600 mmol/L NaCl.
Kinetics of hIL-10 binding to sensor chip-immobilized heparin
The IAsys technology, which uses an evanescent field to measure changes in refractive index when a soluble analyte (here, hIL-10) binds to an immobilized ligand (heparin–albumin), has been previously used to investigate the binding kinetics of hepatocyte growth factor/scatter factor to DS.56 Using this technology we investigated the kinetics of hIL-10 binding to immobilized heparin–albumin. Biotinylated albumin–heparin was immobilized on an avidin-activated sensor chip, at a density of 293 arc seconds (1 arc second = 1/3600 of 1°). When hIL-10 was injected over the heparin–albumin surface, a typical sensogram was obtained (Figure2), with an association phase (A), an equilibrium phase (E), and, when hIL-10 was replaced by running buffer alone, a dissociation phase (D). The association phase of the binding reaction between hIL-10 and heparin–albumin was fast, reaching saturation within 120 seconds (Figure 2A). Observed on rates (kon) were plotted against the hIL-10 concentration to calculate the association rate (ka, gradient) and dissociation rate (kd, y-axis intercept) constants (Figure 2B). The mean association rate (ka) was 3.5 × 105 mol/L−1 S−1 (Figure2B). The dissociation phase was considerably slower, with less than 25% of the bound hIL-10 dissociating from heparin after 5 minutes of washing with buffer (Figure 2A). The mean dissociation constant (kd) was 0.019 S−1 (Figure 2b). Association kinetics pointed to the existence of a single heparin-binding site on hIL-10. Combined data from 3 sets of interactions gave a calculated equilibrium dissociation constant,Kd of 54 (± 7) nmol/L.
Kinetics of hIL-10 binding to immobilized heparin.
(A) Association and dissociation curves of hIL-10 binding to immobilized biotinylated albumin–heparin. The binding of hIL-10 (13.5-54 nmol/L) was followed in the biosensor until a plateau was reached; after washing, the resultant dissociation was followed (arrow indicates initiation of dissociation). The binding response in response units (arc seconds) was recorded as a function of time and showed the association phase (A), the equilibrium (E), and the dissociation phase (D). Three separate sets of binding reactions were performed in duplicate for each hIL-10 concentration, of which one is illustrated (top panel). Association profiles are shown for all 4 concentrations, whereas only the dissociation profile for the highest concentration is shown for clarity. (B) Plot of the observed on rate (kon) values against hIL-10 concentration yields a straight line (r = 0.991). The slope of the line corresponds to the association rate constant, ka(3.44 ± 0.19 × 105 mol/L−1S−1). The dissociation rate constant,kd (0.019 ± 0.001 S−1) is given by the intercept. In this specific example, the resultantKd is 54 ± 7 nmol/L.
Kinetics of hIL-10 binding to immobilized heparin.
(A) Association and dissociation curves of hIL-10 binding to immobilized biotinylated albumin–heparin. The binding of hIL-10 (13.5-54 nmol/L) was followed in the biosensor until a plateau was reached; after washing, the resultant dissociation was followed (arrow indicates initiation of dissociation). The binding response in response units (arc seconds) was recorded as a function of time and showed the association phase (A), the equilibrium (E), and the dissociation phase (D). Three separate sets of binding reactions were performed in duplicate for each hIL-10 concentration, of which one is illustrated (top panel). Association profiles are shown for all 4 concentrations, whereas only the dissociation profile for the highest concentration is shown for clarity. (B) Plot of the observed on rate (kon) values against hIL-10 concentration yields a straight line (r = 0.991). The slope of the line corresponds to the association rate constant, ka(3.44 ± 0.19 × 105 mol/L−1S−1). The dissociation rate constant,kd (0.019 ± 0.001 S−1) is given by the intercept. In this specific example, the resultantKd is 54 ± 7 nmol/L.
Soluble heparin and heparan sulfate inhibit hIL-10 activity
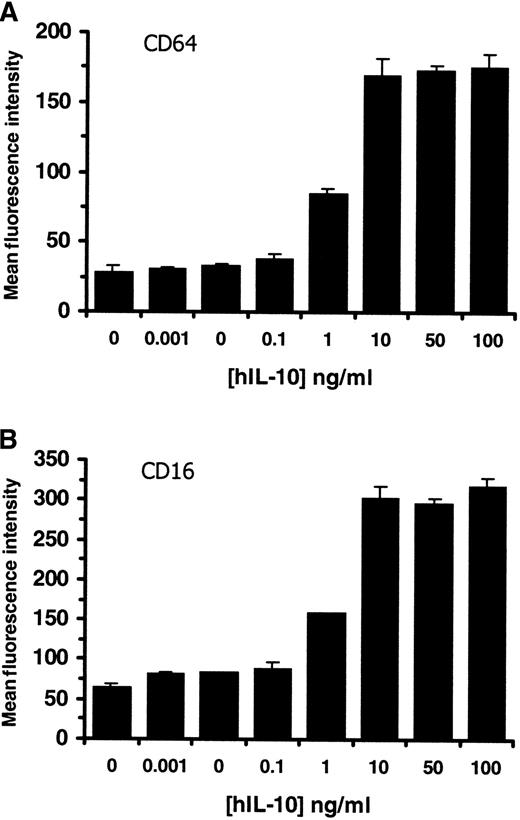
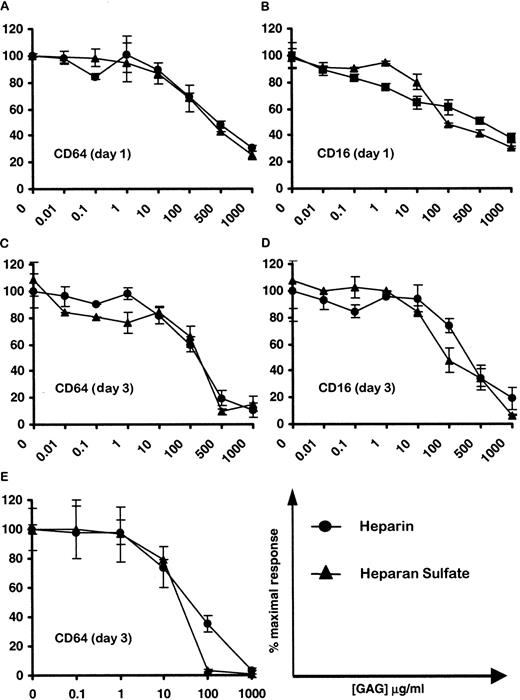
Based on our results showing that hIL-10 binds to heparin agarose at physiological pH, we have postulated that heparin or other GAG may participate in regulating the cytokine activity. Human IL-10 has been previously shown to up-regulate the expression of CD64 on purified monocytes12 and CD16 on macrophages within PBMCs.61 Initial experiments were carried out to determine the optimal concentration of hIL-10 required for the induction of CD16 and CD64 on monocytes/macrophages within PBMC. Figure3 shows that hIL-10 up-regulates the expression of both CD64 (Figure 3A) and CD16 (Figure 3B) on monocytes/macrophages in a concentration-dependent manner at 72 hours. Optimal concentrations of hIL-10 for CD16 and CD64 induction were found to be between 1 and 10 ng/mL, which corresponds to 2.47- to 4.68- and 2.95 to 6.25-fold increases in the levels of CD16 and CD64 expression, respectively. Similar percentage increases were seen at days 1 and 2; however, the background levels, which increased with time, were lower at day 1 (data not shown). Subsequently, similar experiments were carried out to investigate the effects of soluble GAG on hIL-10 activity using the optimal concentration of hIL-10 (10 ng/mL). Figure4 shows that heparin and heparan sulfate markedly reduce (by up to 95%) the hIL-10–induced expression of CD64 (Figure 4A,C) and CD16 (Figure 4B,D) on monocytes/macrophages in a concentration-dependent manner at 24 and 72 hours. The 50% inhibitory concentrations (IC50) for heparin and heparan sulfate were found to be between 100 and 500 μg/mL on all days tested.
Effect of hIL-10 on the expression of CD16 and CD64 on monocytes/macrophages within PBMC.
PBMC were cultured in growth medium alone or in the presence of increasing concentrations of hIL-10 for 72 hours. Cells were stained with (A) CD64 or (B) CD16 mouse antihuman monoclonal antibodies, and the monocyte/macrophage population within PBMC was gated out on the basis of forward- and side-scatter. CD16 and CD64 expression on monocytes were determined by FACS and are presented as mean fluorescence intensity ± 2 SD. Data are representative of 3 separate experiments.
Effect of hIL-10 on the expression of CD16 and CD64 on monocytes/macrophages within PBMC.
PBMC were cultured in growth medium alone or in the presence of increasing concentrations of hIL-10 for 72 hours. Cells were stained with (A) CD64 or (B) CD16 mouse antihuman monoclonal antibodies, and the monocyte/macrophage population within PBMC was gated out on the basis of forward- and side-scatter. CD16 and CD64 expression on monocytes were determined by FACS and are presented as mean fluorescence intensity ± 2 SD. Data are representative of 3 separate experiments.
Effect of soluble heparin and heparan sulfate on the capacity of hIL-10 to up-regulate the expression of CD16 and CD64 on monocytes/macrophages.
PBMC were stimulated with either hIL-10 (10 ng/mL; A-D) or IFN-γ (10 ng/mL; E) in the presence or absence of increasing concentrations of heparin (●) or heparan sulfate (▴) for 24 hours (A,B) or 72 hours (C,D). CD64 and CD16 expression on monocytes/macrophages were determined as described in Figure 3. Results are expressed as percentage inhibition of CD16 and CD64 induced by hIL-10 ± 2 SD. For the mean fluorescence intensity induced by IL-10 compared to controls, see Figure 3. Mean fluorescence intensity induced by IFN-γ was 208.5 ± 20. Data are representative of at least 4 separate experiments.
Effect of soluble heparin and heparan sulfate on the capacity of hIL-10 to up-regulate the expression of CD16 and CD64 on monocytes/macrophages.
PBMC were stimulated with either hIL-10 (10 ng/mL; A-D) or IFN-γ (10 ng/mL; E) in the presence or absence of increasing concentrations of heparin (●) or heparan sulfate (▴) for 24 hours (A,B) or 72 hours (C,D). CD64 and CD16 expression on monocytes/macrophages were determined as described in Figure 3. Results are expressed as percentage inhibition of CD16 and CD64 induced by hIL-10 ± 2 SD. For the mean fluorescence intensity induced by IL-10 compared to controls, see Figure 3. Mean fluorescence intensity induced by IFN-γ was 208.5 ± 20. Data are representative of at least 4 separate experiments.
Like hIL-10, IFN-γ has been shown to up-regulate the expression of CD64 on monocytes.12 Thus, the effects of increasing concentrations of heparin and heparan sulfate on the expression of CD64 by IFN-γ–stimulated PBMC were investigated. This provided us with a positive control for our experimental design because exogenously added heparin and heparan sulfate have been previously shown to inhibit the activity of IFN-γ.49,51,53 62 Figure 4, panel E demonstrates that heparin and heparan sulfate exhibit similar antagonistic effects on the IFN-γ–induced expression of CD64 at 72 hours. Consistent with other published reports, the inhibitory effects of heparin and heparan sulfate were shown to be concentration dependent, with IC50 values between 10 and 100 μg/mL.
Soluble C-4S and DS, but not C-6S, inhibit hIL 10–activity
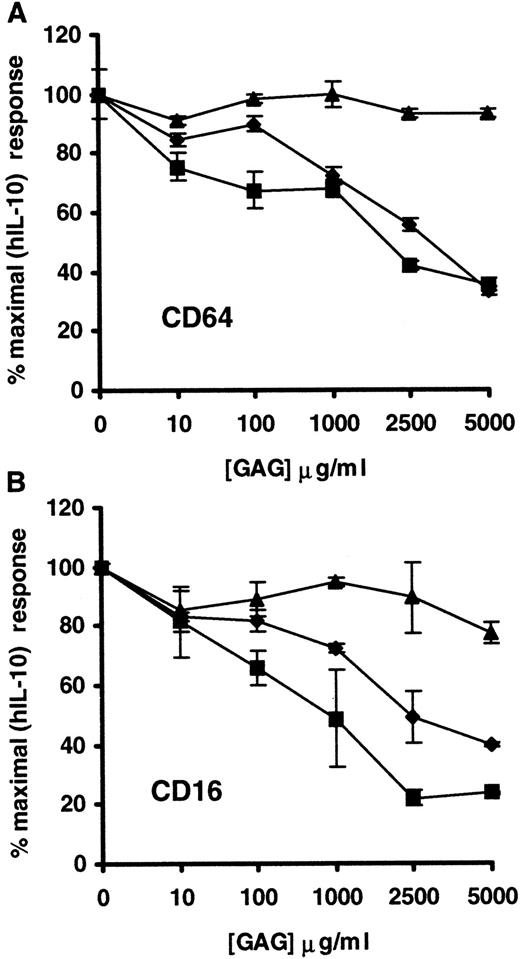
The ability of other structurally distinct GAG, such as C-4S, DS, and C-6S, to regulate the activity of hIL-10 was also investigated. Preliminary experiments indicated that C-4S, DS, and C-6S had a much lower ability than heparin or heparan sulfate to modulate hIL-10 activity (data not shown). As a result, subsequent experiments were carried out using GAG concentrations ranging from 10 to 5000 μg/mL. Figure 5 shows that the biologic activity of hIL-10 on monocytes/macrophages, as determined by the induction of CD16 and CD64, were inhibited by soluble C-4S and DS. The addition of these GAGs at 5000 μg/mL resulted in a 60% to 75% reduction in the levels of CD64 and CD16 expression on hIL-10–treated monocytes/macrophages. C-6S, on the other hand, was shown to have a considerably lower capacity to inhibit hIL-10 function because it reduced the expression of CD16 and CD64 only by approximately 25% and 10%, respectively. The IC50 values for C-4S and DS (2000-2500 μg/mL) were considerably higher than those of heparin and heparan sulfate (100-500 μg/mL).
Effect of soluble C-4S, DS, and C-6S on the capacity of hIL-10 to up-regulate the expression of CD64 and CD16 on monocytes/macrophages.
PBMC were stimulated with hIL-10 in the presence or absence of increasing concentrations of C-4S (▪), DS (♦), or C-6S (▴) for 24 hours. After the incubation period, CD64 (A) and CD16 (B) expression on monocytes/macrophages were determined as described in Figure 3. Data are presented as percentage inhibition of CD16 and CD64 induced by optimal concentration of hIL-10 (10 ng/mL) ± 2 SD. Data are representative of 2 separate experiments.
Effect of soluble C-4S, DS, and C-6S on the capacity of hIL-10 to up-regulate the expression of CD64 and CD16 on monocytes/macrophages.
PBMC were stimulated with hIL-10 in the presence or absence of increasing concentrations of C-4S (▪), DS (♦), or C-6S (▴) for 24 hours. After the incubation period, CD64 (A) and CD16 (B) expression on monocytes/macrophages were determined as described in Figure 3. Data are presented as percentage inhibition of CD16 and CD64 induced by optimal concentration of hIL-10 (10 ng/mL) ± 2 SD. Data are representative of 2 separate experiments.
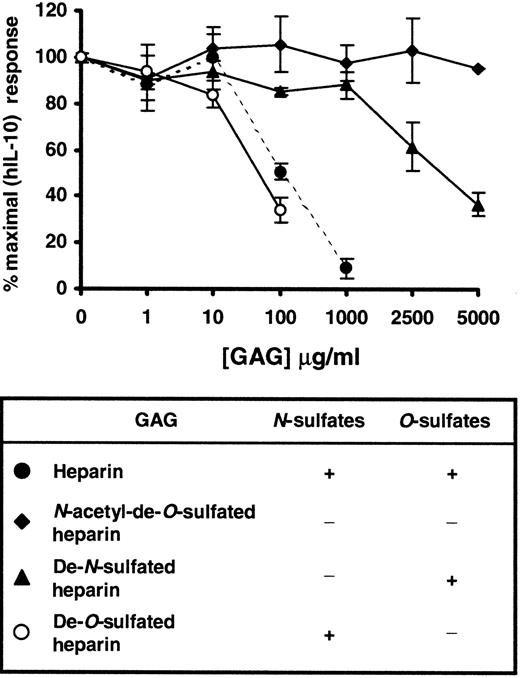
N-sulfate dependence of heparin binding to hIL-10
To examine the relative importance of different sulfate groups in heparin for the binding of hIL-10, specifically desulfated heparins were tested for their ability to inhibit the hIL-10–induced expression of CD16 on monocytes/macrophages. Figure6 shows thatN-acetyl-de-O-sulfated heparin, which lacks bothN- and O-sulfate groups, had little or no effect compared to unmodified heparin (IC50 value of approximately 100 μg/mL) on the hIL-10–induced expression of CD16. Similarly, the replacement of N-sulfate groups with N-acetyl groups (de-N-sulfated heparin) resulted in a modest (approximately 20%) reduction in hIL-10 activity when used at 1000 μg/mL. When high concentrations were used, O-sulfate groups appeared partially to compensate for the absence ofN-sulfates; a 60% reduction in hIL-10 activity was seen at 5000 μg/mL. Removal of O-sulfate groups had little or no effect on the inhibitory activity of heparin. Similar results were also obtained for CD64 (data not shown). Taken together, our results indicate that sulfate groups, specifically N-sulfates, are essential in the binding of heparin to hIL-10.
Effect of variably desulfated heparins on the hIL-10–induced expression of CD16 on monocytes/macrophages.
PBMC were stimulated with hIL-10 in the presence or absence of increasing concentrations of heparin (●),N-acetyl-de-O-sulfated heparin (♦), de-N-sulfated heparin (▴), de-O-sulfated heparin (○) for 72 hours. After the incubation period, CD16 expression on monocytes/macrophages was determined as described in Figure 3. Results are expressed as percentage inhibition of CD16 induced by hIL-10 ± 2 SD. Data are representative of 2 separate experiments.
Effect of variably desulfated heparins on the hIL-10–induced expression of CD16 on monocytes/macrophages.
PBMC were stimulated with hIL-10 in the presence or absence of increasing concentrations of heparin (●),N-acetyl-de-O-sulfated heparin (♦), de-N-sulfated heparin (▴), de-O-sulfated heparin (○) for 72 hours. After the incubation period, CD16 expression on monocytes/macrophages was determined as described in Figure 3. Results are expressed as percentage inhibition of CD16 induced by hIL-10 ± 2 SD. Data are representative of 2 separate experiments.
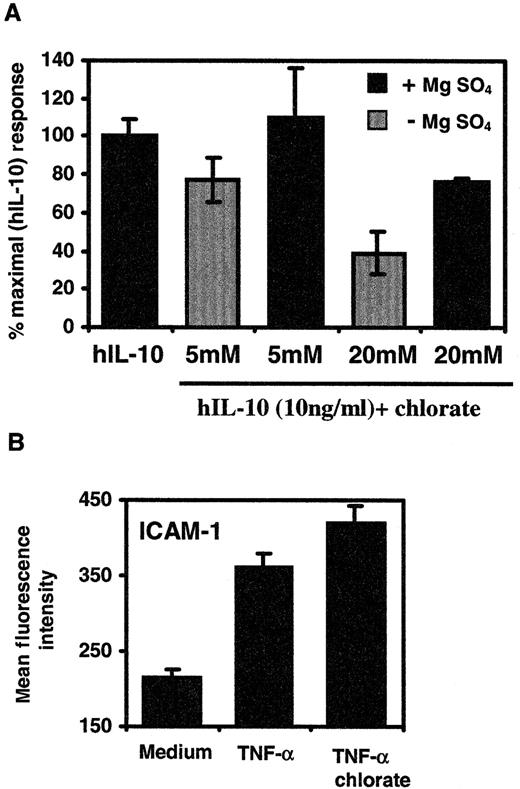
Sodium chlorate treatment of PBMC reduces activity of hIL-10 on monocytes/macrophages
Our results suggest that the sulfation of exogenously added heparin is important in enabling it to inhibit the activity of hIL-10. Thus, we addressed the question of whether sulfation of cell-bound HS or CS/DS PG is required for hIL-10 to modulate CD16 expression. Sodium chlorate has been shown by several investigators to inhibit the sulfation of proteoglycans in intact cells.59,60 Figure7A shows that treatment of PBMC with 20 mmol/L NaClO3 reduced the ability of hIL-10 to up-regulate the expression of CD16 by approximately 60%. However, this inhibition could be reversed by the simultaneous addition of 15 mmol/L MgSO4 to the culture medium. No significant effect on hIL-10 activity was seen when 5 mmol/L NaClO3 was used. In the absence of exogenous hIL-10, chlorate or MgSO4treatment had no effect on the constitutive levels of CD16 (data not shown). In addition, we investigated the influence of chlorate on the capacity of TNF-α to up-regulate the expression of ICAM-1 molecules on monocytes/macrophages.63 Soluble heparin does not affect the ability of TNF-α to up-regulate the expression of ICAM-1 on human umbilical vein endothelial cells,49 an observation that is consistent with its inability to bind to immobilized heparin under physiological conditions.62Therefore, chlorate should not affect its mitogenic activity. Figure 7B shows that the stimulation of PBMC with TNF-α (20 ng/mL) alone resulted in a 1.7-fold increase in the levels of ICAM-1 on monocytes/macrophages compared to untreated cells. In contrast to hIL-10, treatment of PBMC with chlorate did not inhibit the mitogenic activity of TNF-α. These data indicate that sulfate groups of PG are required for the full mitogenic activity of hIL-10 on monocytes/macrophages within PBMC.
Effect of sodium chlorate on the ability of hIL-10 or TNF-α to up-regulate the expression of CD16 or ICAM-1 on monocytes/macrophages.
PBMC were grown in the presence or absence of sodium chlorate for 24 hours. (A) hIL-10 (10 ng/mL) or (B) TNF-α (20 ng/mL) was added in chlorate-containing medium for an additional 48 hours. In some experiments, MgSO4 (15 mmol/L) was added together with NaClO3 and hIL-10. After the incubation period, the cells were stained with (A) CD16 or (B) ICAM-1 mouse antihuman monoclonal antibodies. The monocyte/macrophage population within PBMC was gated out on the basis of forward- and side-scatter. CD16 or ICAM-1 expression on monocytes from quadruplicate cultures was determined by FACS and is presented as percentage control or mean fluorescence intensity ± 4 SD. Data are representative of 2 separate experiments.
Effect of sodium chlorate on the ability of hIL-10 or TNF-α to up-regulate the expression of CD16 or ICAM-1 on monocytes/macrophages.
PBMC were grown in the presence or absence of sodium chlorate for 24 hours. (A) hIL-10 (10 ng/mL) or (B) TNF-α (20 ng/mL) was added in chlorate-containing medium for an additional 48 hours. In some experiments, MgSO4 (15 mmol/L) was added together with NaClO3 and hIL-10. After the incubation period, the cells were stained with (A) CD16 or (B) ICAM-1 mouse antihuman monoclonal antibodies. The monocyte/macrophage population within PBMC was gated out on the basis of forward- and side-scatter. CD16 or ICAM-1 expression on monocytes from quadruplicate cultures was determined by FACS and is presented as percentage control or mean fluorescence intensity ± 4 SD. Data are representative of 2 separate experiments.
Discussion
Numerous growth factors and cytokines bind to heparin or heparan sulfate molecules.64,65 These include the FGF family,66 hepatocyte growth factor (HGF),67chemokine platelet factor 4 (PF4),68 and cytokines such as IL-7,40 IL-8,69 IL-12,70 and IFN-γ.71,72 Considerable data have been reported during the past few years regarding the physiologic significance of the binding of heparin to growth factors and cytokines, the prototype of which is FGF. Here for the first time we report on the interaction of hIL-10 with heparin and heparan sulfate. The sequence −LRRCHR-(105-110), which is conserved between all species of IL-10 sequenced to date, is similar to one consensus site, XBBXBX (where B represents a cationic lysine, arginine, or histidine residue and X represents a hydropathic residue), described by several investigators in GAG-binding proteins.16,18-20 The 3-dimensional structure of hIL-10, as determined by Zdanov et al,73identifies this particular region (residues 99-110; Figure 1A) as an external feature of the protein, suggesting that it may be involved in receptor binding. Thus, the purpose of this study was to investigate whether hIL-10 can interact with GAG, in particular heparin, and to investigate the potential role these molecules may exert on the biologic activity of hIL-10.
We have demonstrated by affinity chromatography that hIL-10 displays a strong affinity for heparin at pH 7.4. Human IL-10 could be eluted from the column with 0.3 to 0.6 mol/L NaCl. This compares with 0.3 to 0.6 mol/L for IL-7,40 0.4 to 1.2 mol/L for TGF-β1,16 and 1 mol/L and 1.5 mol/L for aFGF and bFGF, respectively.74 To further characterize the binding of hIL-10 to heparin, we used a biosensor-based assay to analyze the kinetics of hIL-10 binding to immobilized heparin–albumin. The measured affinity, Kd, of hIL-10 for heparin was shown to be 54 nmol/L (Figure 2). This compares with 1.5 nmol/L, 3 to 9 nmol/L, 25 nmol/L, and 500 nmol/L for IFN-γ,71bFGF,40 hIL-7,40 and IL-2,75respectively. It is important to note that the ability of GAG to modulate cytokine activity is dependent on their relative affinities for each other. Consistent with this, the interaction of hIL-2 with heparin (Kd 500 nmol/L) does not appear to alter its biologic activity in vitro.75,76 More recently, Fernandez-Botran et al62 demonstrated that not all GAG-binding cytokines are capable of binding to immobilized heparin at physiological pH. For example, of the cytokines IL-1β, IL-2, IL-4, IFN-γ, and TNF-α, only IFN-γ bound to heparin-agarose at pH 7, 8, or 9, whereas IL-2, IL-4, and TNF-α showed significant binding only at pH 5. Therefore, based on our results showing the ability of hIL-10 to bind to heparin with high affinity at pH 7.4, we have postulated that interactions between this cytokine and heparin, or other GAG, might take place in a physiological environment and that this interaction may play an important factor in the biology of hIL-10.
To test our hypothesis, we used an assay based on that described by te Velde et al12 and Olikowsky et al,61 where hIL-10 was shown to up-regulate the expression of CD64 on purified monocytes and CD16 on macrophages within PBMC. In our experiments we looked at the monocyte/macrophage (CD14+ cells) population within PBMC rather than purified cells. The main advantage of the PBMC system is that it allows critical intercellular contacts to take place and, hence, is more like an in vivo situation. In this system hIL-10 was shown to up-regulate the expression of CD16 and CD64 on monocytes/macrophages within PBMC in a concentration-dependent manner; optimal concentrations of hIL-10 were between 1 and 10 ng/mL (Figure3). Preincubation of hIL-10 with heparin or heparan sulfate reduced its ability to up-regulate the expression of CD16 and CD64 on monocytes/macrophages by up to 95% (Figure 4). The inhibitory effects of heparin and heparan sulfate were shown to be concentration dependent, with IC50 values ranging from 100 to 500 μg/mL. Heparin is the most negatively charged GAG (the average disaccharide contains 2.7 sulfate groups) and usually binds more strongly to ligands than do other GAGs. Although heparan sulfate is biosynthetically related to heparin, it contains an average of only one sulfate or less (depending on the source) per disaccharide. In general, it contains more GlcNAc and GlcA, whereas heparin has a high IdoA/GlcA ratio and predominantly contains GlcNSO3.22-26Another feature of heparan sulfates that makes their distinction possible from heparin is a chain structure consisting of alternating low-sulfated domains (N-acetylated; NA domains) and domains with high sulfation levels (N- and O-sulfated; S-domains). The levels of sulfation within these S-domains can approach the levels seen in heparin.22-26 Thus, the similar IC50 values exhibited by these 2 GAG in our experimental system may be accounted for by the presence of S-domains in heparan sulfate, and they may reflect a requirement for a specific, highly sulfated sequence for hIL-10 binding and not the overall negative charge. Consistent with our findings, S-domains have been implicated in the binding of heparan sulfate to IL-8,21PF4,68 TGFβ-1,78 and bFGF.79Further studies are required to determine whether the optimum heparan sulfate-binding sites are contained within one extended sulfated domain, as seen for bFGF,79 or several S-domains separated by N-acetylated sequences, as demonstrated for IL-8,21 PF4,68 and TGFβ-1.78
As for the requirement for anionic groups on GAG for the binding of hIL-10, we have clearly demonstrated the absolute requirement for sulfation because N-acetyl-de-O-sulfated heparin failed to inhibit the activity of hIL-10 compared to unmodified heparin. Additionally, the relative lack of effect of the solelyO-sulfated GAGs, CS and DS, coupled with the marked reduction in the inhibitory activity of heparin after the removal ofN-sulfates but not O-sulfates, indicates a strong specific requirement for N-sulfation in heparin binding to hIL-10. It must be noted, however, that at very high heparin concentrations, O-sulfate groups were able partially to compensate for the lack of N-sulfates. Even among theO-sulfated GAG, there seems to be some degree of selectivity; the 4-O-sulfated DS and C-4S were able to inhibit partially the biologic activity of hIL-10 at very high concentrations, whereas the 6-O-sulfated C-6S had little or no effect. The selective requirement for N-sulfates in the binding of hIL-10 by heparin described here is comparable to that seen for TGFβ-178 but differs from that for PF468and HGF,80 81 for which there is a strong dependence forO-sulfates and little or no apparent requirement forN-sulfates. Collectively, our results demonstrate a degree of specificity in the interaction of heparin and heparan sulfate with hIL-10, not simply a nonspecific interaction between a cationic protein and an anionic GAG of any type.
The antagonistic effects of GAG on hIL-10 function described here are consistent with the findings of several other studies in which soluble GAG have been shown to abrogate the biologic activity of cytokines such as IL-740,46 and IFN-γ.48,53,62 In the current study, we did not examine the mechanisms by which soluble GAGs inhibit hIL-10 function. However, it is not inconceivable that this may be as a result of GAGs competitively inhibiting the hIL-10–receptor complex formation, as has been demonstrated for IFN-γ.47,62 Furthermore, in the above experiments, the effect of GAG on hIL-10 activity was investigated using optimal concentrations of hIL-10, which may have some reflection on the dose of GAG required to inhibit its function. In support of this notion, the concentration of GAG required to inhibit the specific binding of IFN-γ to its receptor on COLO-205 cells has been shown to be proportional to the concentration of IFN-γ.62
Interestingly, whereas soluble GAG were shown to inhibit hIL-10 function, we could demonstrate that the inhibition of endogenous PG sulfation with sodium chlorate59,60,82 reduced the hIL-10–mediated expression of CD16 on monocyte/macrophages by up to 60%. This effect was shown to result from the specific depletion of sulfation on PG because PBMC treated simultaneously with both chlorate and sufficient exogenous sulfate to counter the effect of the chlorate retained normal responsiveness to hIL-10. Furthermore, the mitogenic activity of TNF-α, which does not bind to heparin under physiological conditions,62 was shown to be unaffected by chlorate treatment. This not only demonstrates the ability of cells to function normally in chlorate, it supports the specific requirement of PG for the optimum activity of hIL-10. Taken together, the above data are supportive of the hypothesis that, for optimum activity on monocytes/macrophages, hIL-10 requires the presence of sulfated PG at the cell surface, which may in turn facilitate its interaction with high-affinity hIL-10 receptors on these cells. This result adds hIL-10 to a relatively small number of GAG-binding proteins, such as IL-7,46 FGF,45 HGF,83 and IL-8,84 85 that have been shown to require cell-surface PG to exert their full mitogenic activity. However, we note that our results do not necessarily imply that receptor binding and activation by hIL-10 is totally dependent on PG in all cases. The requirement for PG by hIL-10 may vary according to the biologic assay and the receptor involved. In addition, because we found that hIL-10 binds preferentially to N-sulfated GAG, any dependence on GAG for binding or activation may vary according to tissue, cell type, or both.
Several investigators have shown that low concentrations of soluble heparin can overcome the inhibitory effects of chlorate on cellular responses to bFGF.86,87 We have examined the effects of exogenously-added heparin or heparan sulfate at low concentrations (0.1, 1, and 10 μg/mL) on chlorate-treated cells and found them to be unable to restore the full activity of hIL-10 (data not shown). As proposed for HGF,83 88 our findings imply that though soluble GAG can modulate the activity of hIL-10, they cannot replace the function of cell-surface–associated PG.
Although heparin and heparan sulfate are structurally similar, their distribution in vivo is radically different. Heparin is synthesized in connective tissue-type mast cells and is released in a soluble form on degranulation.22 Heparan sulfate, on the other hand, is found ubiquitously in an immobilized form in extracellular matrices, basement membranes, and cell surfaces.89 These different distributions suggest different functions with respect to hIL-10 binding. Thus, through its interaction with hIL-10, heparin may antagonize its activity by competing with membrane hIL-10R, or it may act as a carrier molecule protecting it from protease degradation and increasing its half-life, as has been shown for IL-740 and IFN-γ.42 Cell-surface heparan sulfate proteoglycans, however, may function to retain the cytokine and provide a local tissue-bound reservoir of hIL-10, which could then be displaced either through the release of competitive heparin during inflammation, as shown for FGF,38 or by their proteolytic degradation to release a soluble PG fragment with hIL-10 still attached, as also described for bFGF.90 Binding of hIL-10 to cell surface PG may be required for the presentation of hIL-10 to its high-affinity receptor or the formation of hIL-10 dimers, thus leading to the hypothesis that the presentation of hIL-10 molecules by PG may facilitate receptor dimerization and trigger biologic responses. Similarly, binding of hIL-10 to PG on endothelial cells may provide a suitable means by which hIL-10 could be presented to interacting leukocytes, such as CD8+ T cells, for which it has been shown to be chemotactic.91
The ability of GAG to interact with hIL-10 adds an additional layer of control to its biologic activities and may have important ramifications for the development of therapeutic agents in clinical situations in which hIL-10 has been implicated. For example, hIL-10 has been shown to inhibit macrophage-mediated immunity against intracellular and extracellular parasites, such as fungal and mycobacterial infections (eg Leishmaniasis, leprosy, and tuberculosis). In addition, hIL-10 is thought to act as an autocrine growth factor or inhibitor of antitumor immune responses in certain cancers, such as Burkitt lymphoma, Hodgkin disease, nasopharyngeal carcinoma, and AIDS-associated B lymphomas. Hence, our findings showing that GAG can bind to and inhibit the activity of hIL-10, coupled with the apparent requirement for specificN-sulfates, patterns of O-sulfation residues, and length of the sulfated segments, suggest possible clinical applications of chemically modified heparinoids as hIL-10 antagonists with low anticoagulant activity, alone or in combination with other therapies. In view of this, further studies are required to identify the minimum structural motifs for GAG binding to hIL-10.
Acknowledgments
The authors thank Dr M. Lyon and Prof J. Gallagher for review of the manuscript and helpful discussion, Mike Hughes and Jeff Barry for their technical assistance with flow cytometry analysis, and Jane Bolton for performing the lipopolysaccharide assays.
Supported by the Cancer Research Campaign, London, UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shahram Salek-Ardakani, CRC Section of Molecular Biology, Paterson Institute for Cancer Research, Christie Hospital NHS Trust, Wilmslow Road, Withington, Manchester, M20 4BX, UK; e-mailssalek@picr.man.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal