Abstract
Cellular methotrexate (MTX) resistance may cause treatment failure in childhood common/preB-acute lymphoblastic leukemia (c/preB-ALL), T-lineage ALL (T-ALL), and acute myeloid leukemia (AML). The ex vivo potency of several antifolates (MTX, trimetrexate [TMQ], GW1843U89, multitargeted antifolate [MTA], Raltitrexed, and ZD9331) was studied via in situ inhibition of thymidylate synthase (TS). After short-term exposure, relapsed c/preB-ALL (rALL, n = 21), T-ALL (n = 22), and AML (n = 22) were 3-fold, 10-fold, and 6-fold less sensitive to MTX (P ≤ .01) compared with initial c/preB-ALL (n = 43). This difference in resistance was not observed for TMQ. Also for GW1843U89 and MTA, no resistance was observed in rALL and AML compared with c/preB-ALL. T-ALL compared with c/preB-ALL tended to be less resistant to GW1843U89 (3-fold) and MTA (6-fold) than to MTX (10-fold) (P= .06). Raltitrexed was more active against c/preB-ALL compared with the other subtypes. After 21 hours continuous incubation, T-ALL and AML samples were equally sensitive as c/preB-ALL to MTX, but rALL was 3-fold resistant to MTX compared with initial c/preB-ALL (P = .003). The resistance of rALL was circumvented by TMQ (1-fold; P = .03) and GW1843U89 (1.4-fold; P= .004). Novel antifolates, except MTA, displayed a more potent TS inhibition than MTX during continuous exposure. These results suggest that MTX resistance in AML and T-ALL can be circumvented by continuous exposure, and that novel antifolates should be explored further for MTX-resistant T-ALL, rALL, and AML cells.
RESISTANCE TO methotrexate (MTX) may contribute to treatment failure as observed in one fifth of children diagnosed with common/preB-acute lymphoblastic leukemia (c/preB-ALL).1 The risk for relapse is partly associated with pharmacokinetic parameters, as individualized treatment to sustain MTX plasma levels at a specific level improved survival of B-lineage ALL patients.2 The correlation of accumulation of MTX and MTX polyglutamates in leukemic blasts with short-term antileukemic effect3 and with event-free survival4 suggested that also cellular MTX resistance is involved in treatment failure.
At relapse, ALL patients have a relatively poor prognosis with an event-free survival of 20% to 40%.5,6 Knowledge of the frequency of occurrence and mechanisms of MTX resistance in relapsed ALL is limited. Diminished MTX membrane transport via the reduced folate carrier (RFC)7 and elevated levels of dihydrofolate reductase (DHFR),8 the main target enzyme of MTX, have been detected in the majority of relapsed ALL samples. The latter might be associated with DHFR gene amplification as observed after MTX treatment in one third of relapsed ALL samples.9 Mutations in DHFR resulting in a lower affinity for MTX do not seem to play an important role in acquired MTX resistance in the clinical situation.10 11
Compared with B-lineage ALL, T-lineage ALL (T-ALL) requires more intensive therapy to be cured.1 Cellular MTX resistance, as was detected in T-ALL by an in situ enzyme inhibition assay,12 may be involved in this relative chemoresistance. T-ALL cells were reported to accumulate lower levels of MTX compared with B-lineage ALL cells, accompanied by a less efficient polyglutamylation, in vitro13,14 and in vivo.15,16 Additional studies demonstrated that the inefficient polyglutamylation was associated with a lower folylpolyglutamate synthetase (FPGS) activity,14,17 but not with a higher folylpolyglutamate hydrolase (FPGH) activity.14 In addition, DHFR levels were higher in blasts obtained from patients with T-ALL compared with B-lineage ALL,8 resulting in lower levels of unbound MTX available for polyglutamylation.18 No difference in MTX uptake was observed, which could have explained the lower accumulation in T-ALL compared with c/preB-ALL.8
Children diagnosed with acute myeloid leukemia (AML) are usually not treated with MTX because early clinical trials demonstrated low response rates to MTX in this type of leukemia.19Combination chemotherapy for AML, applying maximum tolerated doses that are sometimes even surpassed, results in an event-free survival of 40% to 50%.20 In addition to the toxicity of the treatment, the high rate of relapse in AML explains the poorer prognosis as compared with ALL. The high rate of relapse is likely to be associated with cellular drug resistance.21 To improve prognosis of these children, novel drugs with activity against AML are required.22,23 The presumed intrinsic MTX resistance in AML cells has been ascribed to an impaired MTX polyglutamylation14,24,25 associated with both a decrease in FPGS activity14,17,26 and an increase in activity of FPGH.14 In addition, MTX uptake may be defective as observed in one third of the AML samples,7 whereas also high levels of (altered) DHFR have been reported for AML blasts.27
We recently described that cellular resistance to MTX and the potency of new antifolates against blast cells from leukemia patients can be evaluated using an in situ thymidylate synthase (TS) inhibition assay.12,28 In the present study, we investigated the potency of novel antifolates designed to circumvent MTX resistance by (1) bypassing transport deficiency (trimetrexate29[TMQ]), (2) not being dependent on polyglutamylation (TMQ and ZD933130), (3) having an improved affinity towards FPGS and RFC (GW1843U89,31 Raltitrexed,32 multitargeted antifolate33 [MTA]), or by (4) targeting other enzymes than DHFR (GW1843U89,31 Raltitrexed,32ZD9331,30 and MTA33). We show that continuous long-term MTX exposure can circumvent MTX resistance in T-ALL and AML and that novel antifolates show potency to circumvent MTX resistance after short-term exposure in relapsed ALL, T-ALL, and AML.
MATERIALS AND METHODS
Patient Specimens
Bone marrow and peripheral blood samples were obtained after informed consent from 49 children with newly diagnosed common/preB-ALL, 22 with B-precursor ALL at relapse (rALL) previously treated with MTX, 24 with newly diagnosed T-lineage ALL, and from 26 AML patients (French-American-British [FAB] classifications: 1 M0; 5 M1; 6 M2; 2 M3; 6 M4; 2 M5; 1 M6; 1 M7; and 2 unknown). None of the patients have been treated with 1 of the novel antifolates. The number of antifolates screened per sample was dependent on the number of cells available. MTX sensitivity data for 75% of the untreated ALL samples, presented as reference values in the present report, have been reported previously.12 Immunophenotype of the ALL patients was determined by immunocytochemistry: c/preB-ALL was defined as HLA-DR+/CD19+ and terminal deoxynucleotidyl transferase (TdT) positive, excluding the proB-ALL phenotype (CD10−, cytoplasmic μ-chain negative). Samples were scored T-ALL when positive for TdT, CD3, and CD7. Newly diagnosed ALL patients were classified as high-risk if 1 or more of the following features were present: T-lineage phenotype, white blood cell counts ≥25/nL and younger than 1 or older than 10 years of age. Eighty percent of the newly diagnosed c/preB-ALL samples were derived from high-risk patients.
Mononuclear cells were isolated within 48 hours after sampling by centrifugation (500g, 25 minutes) with Ficoll Isopaque and washed twice in RPMI containing 1% fetal calf serum (FCS) with 10-minute periods of centrifugation at 300g and suspended at 1 × 106 cells/mL in culture medium (RPMI 1640 containing 20% heat inactivated FCS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.125 μg/mL fungizone, 200 μg/mL gentamycin, 2 mmol/L L-glutamine, 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite). If necessary, contaminating normal cells were eliminated using monoclonal antibodies linked to magnetic beads, as previously described.34 At the time of assay, the percentage of blasts was greater than 80% as morphologically determined by May-Grünwald-Giemsa staining of the cytospins. Samples, which had been cryopreserved in 10% dimethyl sulfoxide and 20% FCS, were assayed shortly after cells were thawed, washed, and resuspended in prewarmed culture medium.
Chemicals
MTX was a gift from Pharmachemie (Haarlem, The Netherlands). Trimetrexate35 (5-methyl-6-[(3,4,5-trimethoxyphenyl amino) methyl] 2,4-quinazolinediamide, TMQ), GW1843U8931((S)-2-(5-(((1,2-dihydro-3-methyl-1-oxo-benzo(F)quinazolin-9-yl)methyl)-amino)-1-oxo-2-isoindolinyl)glutaric acid) and MTA33(N-{4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]-benzoyl]-L-glutamic acid, LY231514) were provided by Warner-Lambert/ Parke Davis (Ann Arbor, MI), Glaxo Wellcome (Research Triangle Park, NC), and Eli Lilly Research Laboratories (Indianapolis, IN), respectively. Raltitrexed32{N-[5-[N-(3,4-dihydro-2-methyl-4-oxo-quinazolin-6-ylmethyl)-N-methylamino]-2-thenoyl]-L-glutamic acid} (Tomudex, ZD1694) and ZD933130 (gamma tetrazole analogue of 2-desamino-2,7-dimethyl-N10-propargyl-2’fluoro-5,8-dideazafolic acid) were obtained from Zeneca Pharmaceuticals (Macclesfield, UK).
FCS, penicillin, streptomycin, fungizone, gentamycin, and L-glutamine were obtained from Flow Laboratories (Irvine, UK), bovine serum albumin (BSA) from Organon Technika (Oss, The Netherlands), and Ficoll Isopaque (Lymphoprep 1.077 g/mL) was purchased from Nyegaard (Oslo, Norway). Insulin, transferrin, and sodium selenite were purchased from Sigma (Zwijndrecht, The Netherlands) and [5-3H]-2′-deoxycytidine (25 Ci/mmol) from Moravek Biochemicals (Brea, CA). RNAzol was purchased from Campro Scientific (Veenendaal, The Netherlands); Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV-RT) from Promega (Madison, WI), and Taq Polymerase from Pharmacia Biotech (Roosendaal, The Netherlands).
In Situ TS Inhibition Assay
Because antifolate sensitivity of patient-derived leukemia samples cannot be determined using conventional cytotoxicity assays,12 we determined TS inhibition in intact cells based on a previously described assay,12,28,36 measuring the TS-catalyzed conversion of 3H-deoxyuridylate (3H-dUMP) to deoxythymidylate (dTMP) and3H2O. In cell lines, we previously demonstrated a good correlation between antifolate sensitivity determined by conventional cytotoxicity assays and the in situ TS inhibition assay for MTX and for novel rationally designed antifolates.12 28 In short, 135-μL cell suspensions (1 × 106 cells/mL) were incubated at 37°C with either 15 μL RPMI as controls or with 15 μL drug solution. Blanks in triplicate were included containing 135 μL culture medium and 15 μL RPMI. Two conditions were tested: (1) long-term continuous incubation in which cells were incubated with drugs for 21 hours and (2) short-term exposure in which the drug was washed away after 3 hours followed by an 18-hour drug-free period. Five concentrations of each antifolate were tested in duplicate for each condition as demonstrated in Table 1. Controls without drug were included in triplicate for both conditions. [5-3H]-2′-deoxycytidine (final concentration 1 μmol/L, 2.5 Ci/mmol) was added 4 hours after the start of the experiment as precursor for [5-3H]-dUMP, the substrate for TS.
After a total incubation time of 21 hours, cells were put on ice and 150 μL 35% ice-cold trichloroacetic acid (TCA) was added together with 750 μL 10% activated charcoal solution (10 g washed charcoal, 0.5 g Dextran, and 2.5 g BSA in 100 mL demineralized water).37 After vortexing, samples were left on ice for 30 minutes and centrifuged (800g, 30 minutes, 4°C); 450 μL of the aqueous phase, containing 3H2O, was transferred to a scintillation vial and counted for radioactivity. After subtraction of the mean blank counts, the data were evaluated by calculation of the concentration of drug needed to inhibit 50% of the control TS activity, assuming a linear dose-response curve between the 2 flanking concentration points. Data were expressed as TSI50,cont referring to the continuous exposure condition and as TSI50,short for the short exposure condition. To evaluate the potency of novel antifolates to circumvent MTX resistance within individual samples, normalized TSI50 ratios were calculated by dividing the TSI50 values for each antifolate with the median TSI50 value obtained for that antifolate for the group of “MTX-sensitive” c/preB-ALL samples.
Competitive Template Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA was extracted from five million cells by the RNAzol method and reversed transcribed by Moloney Murine Leukemia Virus reverse transcriptase, as described by the manufacturer with minimal modifications. Competitive templates (CTs) were designed for RFC, DHFR, TS, FPGS, and FPGH and dissolved in standardized mixtures to avoid irreproducibilities as described elsewhere38 (manuscript submitted). PCRs were performed by adding different primer pairs to different aliquots of the same mastermix containing patients’ cDNA and CT mixture. PCR products (cDNA, CT, and heterodimers) were separated on agarose gel and measured by densitometry.
Statistics
The Mann-Whitney U test was applied to evaluate differences in antifolate sensitivity between the group of c/preB-ALL versus the group of T-ALL, relapsed ALL, or AML cells. The Wilcoxon signed ranks test was conducted to analyze the potency of a novel antifolate to circumvent MTX resistance within individual samples by comparing the normalized TSI50 ratios for MTX with those for a novel antifolate. To analyze any correlation between antifolate sensitivity profiles and mRNA expression levels, Spearman rank correlation coefficients were calculated.
RESULTS
Potency of MTX to Inhibit In Situ TS Activity in Childhood Leukemia Subtypes
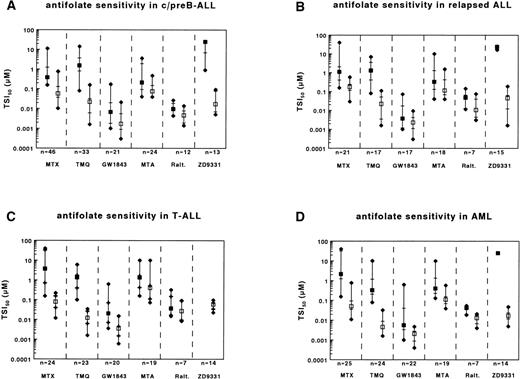
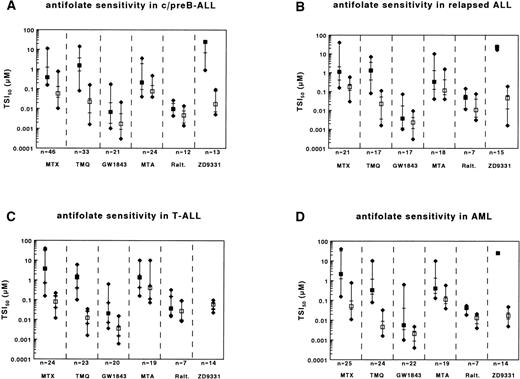
To determine whether relative clinical chemoresistance of relapsed c/preB-ALL, T-lineage ALL and AML compared with c/preB-ALL was reflected by ex vivo testing of MTX sensitivity, we used the in situ TS inhibition assay as described previously.12Ranges of TSI50 values are presented in Table 2. Compared with c/preB-ALL, the median TSI50,short values for MTX were higher for relapsed c/preB-ALL, T-lineage ALL and AML (Table 3and Fig 1A through D [closed squares]).
Ranges of sensitivities to MTX, TMQ, GW1843U89, MTA, Raltitrexed, and ZD9331 as determined by the in situ TS inhibition assay for (A) c/preB-ALL; (B) relapsed c/preB-ALL (rALL); (C) T-ALL; and (D) AML. Number of samples tested are depicted at the bottom. Median values are represented by squares, the 25th and 75th percentiles by horizontal lines. Closed squares represent the short-term exposure, open squares the continuous exposure. For ZD9331, which has not been assayed for T-ALL samples, the closed squares represent a median TSI50,short of >25 μmol/L.
Ranges of sensitivities to MTX, TMQ, GW1843U89, MTA, Raltitrexed, and ZD9331 as determined by the in situ TS inhibition assay for (A) c/preB-ALL; (B) relapsed c/preB-ALL (rALL); (C) T-ALL; and (D) AML. Number of samples tested are depicted at the bottom. Median values are represented by squares, the 25th and 75th percentiles by horizontal lines. Closed squares represent the short-term exposure, open squares the continuous exposure. For ZD9331, which has not been assayed for T-ALL samples, the closed squares represent a median TSI50,short of >25 μmol/L.
Because a continuous exposure to MTX can bypass some potential mechanisms of MTX resistance,12,39 40 cells were continuously exposed to MTX. TSI50,cont. values for MTX were higher for relapsed c/preB-ALL cells compared with initial c/preB-ALL cells. In contrast, TSI50,cont. values for MTX in T-ALL and AML samples were comparable to those in c/preB-ALL (Table 4 and Fig 1A through D [open squares]).
Potency of Novel Antifolates to Inhibit In Situ TS Activity in c/preB-ALL
Of the novel antifolates, only the polyglutamatable TS-inhibitors (GW1843U89 and Raltitrexed) displayed lower TSI50,shortvalues than MTX (Table 3 and Fig 1A). MTA, an inhibitor of both DHFR and TS displayed similar TSI50,short values. Both nonpolyglutamatable antifolates, TMQ, and ZD9331 showed a lower activity than MTX; the median TSI50,short for the DHFR inhibitor TMQ was 4-fold higher than for MTX, but for ZD9331, TSI50,short values were generally higher than the highest concentration used in the assay (25 μmol/L).
Potency of Novel Antifolates to Circumvent MTX Resistance in Relapsed c/preB-ALL (rALL)
Relapsed c/preB-ALL compared with initial c/preB-ALL.
Because several of the novel antifolates displayed a differential better activity than MTX in c/preB-ALL, we also tested these compounds in the MTX-resistant subtypes. No significant differences in TSI50,short values were observed between rALL and initial c/preB-ALL for TMQ, GW1843U89, MTA, or ZD9331 (Table 3 and Fig 1B). When exposed to Raltitrexed, the median TSI50,short was higher for rALL compared with initial c/preB-ALL.
Relapsed c/preB-ALL is the only subgroup in this study that remained more resistant to MTX during continuous incubation compared with initial c/preB-ALL (Table 4 and Fig 1B). In contrast, no significant differences in TSI50,cont. values between rALL and initial c/preB-ALL cells were observed for TMQ, GW1843U89, MTA, or ZD9331. For Raltitrexed, however, the median TSI50,cont. was higher for rALL compared with c/preB-ALL.
Novel antifolates compared with MTX (relapsed c/preB-ALL).
To evaluate the relative potency of each antifolate compared with MTX within individual samples, normalized TSI50 ratios were calculated by dividing the TSI50 values obtained for each individual rALL sample by the median TSI50 value obtained for the group of initial c/preB-ALL samples. The median normalized TSI50,short ratio for rALL was 3 for MTX (Table 3). The median normalized TSI50,short ratio was lower for TMQ and for GW1843U89 compared with MTX. For MTA and Raltitrexed, the normalized TSI50,short ratios were not different from the normalized TSI50,short ratios calculated for MTX. For ZD9331, again the TSI50,short values were higher than the highest concentration tested.
The normalized TSI50,cont. ratio for MTX was 3 for rALL. The median normalized TSI50,cont. ratios were lower for TMQ and GW1843U89 than for MTX (Table 4). No significant decrease in normalized TSI50,cont. ratios was observed for MTA, Raltitrexed, and for ZD9331 compared with that for MTX.
Potency of Novel Antifolates to Circumvent MTX Resistance in T-ALL
T-ALL compared with initial c/preB-ALL.
TSI50,short values for GW1843U89 and Raltitrexed were higher for T-ALL samples compared with c/preB-ALL samples, while no significant differences between T-ALL and c/preB-ALL were observed for TMQ and for MTA (Table 3 and Fig 1C). As for MTX, no differences in TSI50,cont. values were observed between the group of T-ALL samples and the group of c/preB-ALL samples for TMQ, GW1843U89, and ZD9331 (Table 4). T-ALL samples, however, were more resistant to MTA and to Raltitrexed compared with c/preB-ALL after continuous exposure.
Novel antifolates compared with MTX (T-ALL).
The median normalized TSI50,short was 10 for MTX in the group of T-ALL samples (Table 3). The median normalized TSI50,short ratio was significantly lower for TMQ and tended to be lower for GW1843U89 and MTA. For Raltitrexed, normalized TSI50,short ratios were not significantly different from those obtained for MTX. The median normalized TSI50,cont.was 1.4 for MTX in the group of T-ALL samples (Table 4). Normalized TSI50,cont. ratios were lower for TMQ, equal for GW1843U89, but higher for MTA, Raltitrexed, and ZD9331 compared with MTX.
Potency of Novel Antifolates to Circumvent MTX Resistance in AML
AML compared with c/preB-ALL.
The median TSI50,short value was lower for TMQ and higher for Raltitrexed in the group of AML compared with c/preB-ALL samples (Table 3 and Fig 1D). TSI50,cont. values were also lower for TMQ and higher for Raltitrexed for AML compared with c/preB-ALL (Table 4). No significant differences between AML and c/preB-ALL were observed for GW1843U89 and MTA after short exposure or during continuous exposure.
Novel antifolates compared with MTX (AML).
The median normalized TSI50,short ratio for MTX was 6 for AML samples (Table 3). Median normalized TSI50,short ratios were lower for TMQ, GW1843U89, and MTA than for MTX. The median normalized TSI50,cont. for MTX was 0.9 for AML samples (Table 4). Normalized TSI50,cont. ratios were lower for TMQ, equal for GW1843U89 and ZD9331, and higher for MTA and Raltitrexed compared with MTX.
Molecular Biological Correlates
To investigate whether antifolate sensitivity was correlated with mRNA levels of proteins involved in folate metabolism, we developed competitive templates to determine the mRNA levels of RFC, DHFR, TS, FPGS, and FPGH. No correlations were observed for antifolate sensitivity and mRNA levels of RFC, DHFR, or TS in a group of 16 samples for which these parameters were determined. However, for the enzymes involved in polyglutamylation, we observed a significant correlation between a high ratio of FPGH/FPGS mRNA levels and high TSI50,short values for MTX (r = .64, P = .007) and for MTA (r = .58, P = .030). For MTX, this could not be explained by a correlation with the mRNA levels of one of the enzymes independently. For MTA, however, high FPGH mRNA levels were correlated with high TSI50,short values (r = .58,P = .024), whereas low FPGS mRNA levels were correlated with high TSI50,cont. values (r = −.44, P= .051). Also for Raltitrexed and ZD9331, low FPGS mRNA levels were correlated with high TSI50,cont. values (r = −.77, P = .009 and r = −.49, P = .055, respectively). For GW1843U89, a trend was observed between low TSI50,short and high FPGS mRNA levels (r = −.45, P = .056).
DISCUSSION
In the present study, ex vivo experiments showed relative MTX resistance in relapsed c/preB-ALL, T-ALL, and AML samples compared with initial c/preB-ALL after short-term drug exposure. This MTX resistance could be circumvented by continuous drug exposure for T-ALL and AML. Thus, with respect to long-term continuous drug exposure, ALL at relapse was the only leukemia subgroup for which resistance to MTX was still observed. Five rationally designed folate antagonists (TMQ, GW1843U89, MTA, Raltitrexed, and ZD9331) displayed a marked potency to circumvent the observed MTX resistance in childhood leukemia cells.
We previously demonstrated that for cell lines, sensitivity to MTX and to novel antifolates, as measured via conventional cytotoxicity assays, correlated with sensitivity measured via the in situ TS inhibition assay, both for short and continuous exposure conditions.12,28 As antifolate sensitivity of primary leukemia samples cannot be determined using conventional assays, as demonstrated and discussed previously,12 we determined the potency of novel antifolates versus MTX on the basis of in situ TS inhibition for all leukemia subgroups. The specific TS inhibitors GW1843U89, Raltitrexed, and ZD9331 showed markedly lower TSI50,cont. values than observed for MTX, which reflects their enhanced RFC-mediated membrane transport and more potent TS inhibition.30-32 Moreover, GW1843U89 and Raltitrexed were more efficiently retained during the drug-free period, as illustrated by a diminished difference between median values of TSI50,short and TSI50,cont compared with this difference for MTX (Fig 1). This result is consistent with a superior polyglutamylation of these compounds by FPGS as compared with MTX.31,32 ZD9331 as a nonpolyglutamatable drug required continuous exposure to inhibit TS; after a drug-free period, inhibition of TS activity was not sustained.28
To show whether MTX resistance can be circumvented by novel antifolates, we analyzed the differential potency of antifolates between the leukemia subtypes. rALL cells were resistant to MTX both after short-term exposure and after continuous exposure as compared with initial c/preB-ALL samples. Resistance to MTX after continuous drug exposure was not observed for T-ALL and AML, suggesting a different mechanism of MTX resistance in rALL compared with T-ALL and AML. Both after short-term and after continuous exposure, MTX resistance could be circumvented by TMQ and GW1843U89, but not by MTA and Raltitrexed in rALL. RFC transport defects, which have been reported to contribute to MTX resistance in rALL cells,7can also reduce transport of Raltitrexed.32 41 On the other hand, residual RFC activity may still allow sufficient accumulation of GW1843U89 in rALL cells to confer drug sensitivity.
T-ALL was MTX resistant only after short-term exposure as compared with initial c/preB-ALL. This resistance could be circumvented by continuous MTX exposure, while the nonpolyglutamatable antifolate, TMQ, was equally potent in both subtypes. Because polyglutamylation defects could be overcome by continuous exposure in cell line experiments,40 the circumvention of MTX resistance in these primary leukemia samples is in agreement with the observation that T-ALL samples are less capable of polyglutamylation compared with initial c/preB-ALL samples.13,14,40 For TMQ, the circumvention of MTX resistance is of particular interest because increased DHFR levels have been reported as an additional mechanism of MTX resistance in T-ALL cells.8 Although DHFR is the target enzyme of TMQ, these small elevations of DHFR apparently do not result in cross-resistance to TMQ. The improved activity of TMQ over MTX might reflect its sufficient intracellular accumulation42 to levels that can sustain DHFR inhibition both in c/preB-ALL and T-ALL cells. T-ALL tended to be less resistant to GW1843U89 and MTA, but not to Raltitrexed, as compared with MTX. Despite the low number of samples tested for Raltitrexed sensitivity, this suggests that resistance due to a low FPGS activity, as reported for T-ALL cells previously,14,17 cannot be circumvented by the increased affinity of FPGS for Raltitrexed. This is consistent with studies on MTX-resistant human leukemia cell lines.43 AML samples, which displayed MTX resistance only after short-term drug exposure, were sensitive to TMQ, GW1843U89, and MTA after short-term exposure. In contrast, Raltitrexed was not able to circumvent MTX resistance ex vivo in these AML samples. Whether this is solely due to the lower activity of FPGS14,17 along with altered kinetic properties,26 as reported for AML cells, or a multifactorial process involving decreased transport7 and increased FPGH activity,14 is presently unknown.
We and others previously described a good correlation between TSI50,short values, MTX polyglutamylation, and the ratio of FPGH/FPGS activities12,14 44 or FPGH/FPGS mRNA levels (present report). The present study shows no correlations for TSI50 values and mRNA expression of the target enzymes DHFR and TS, or of RFC, for any of the antifolates. This suggests that mutations or posttranslational modifications are of more importance in determining the overall response to these drugs. For GW1843U89 and MTA, correlations were observed between TSI50,short and mRNA expression levels of FPGH and FPGS, respectively. This might suggest that MTX resistance due to high or low mRNA expression of FPGH or FPGS, respectively, could not be circumvented by these antifolates. Despite these correlations, however, GW1843U89 and MTA demonstrated good potency to circumvent MTX resistance in childhood T-ALL and AML cells.
To date, only TMQ45 and Raltitrexed46 have been administered in pediatric clinical trials. Peak plasma levels obtained during these trials, together with plasma levels of GW1843U89 and MTA obtained in adults, are provided in Table 2. Although these values suggest that the presented TSI50 values for the novel antifolates are clinically achievable, some considerations have to be kept in mind. Binding of drugs to serum proteins (in vivo) or to plastic (in vitro) and technical parameters, such as incubation time, make it difficult to extrapolate in vitro data to in vivo values.
Although the poor prognosis of AML and ALL at relapse warrants novel treatment modalities, low numbers of pediatric patients are eligible for entry into phase I/II trials. This is due to the low incidence of childhood cancer and the relatively good prognosis of the majority of the cases. Therefore, the agents to be chosen for further studies need to be prioritized.22 The availability of biochemical assays, such as the in situ TS inhibition assay, and molecular probes7,16,47 to analyze MTX-resistance parameters, may facilitate the initiation of phase I/II clinical trials in childhood leukemia with novel antifolates.22,23 48
In conclusion, these ex vivo results suggest that novel antifolates could be promising candidates for further evaluation in clinical trials with T-ALL, rALL, and AML.
Supported by Grant No. VU 94-679 from the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marianne G. Rots, Department of Pediatric Hematology/Oncology, University Hospital Vrije Universi- teit, PO Box 7057, 1007 MB Amsterdam, The Netherlands; e-mail: marianne.rots@azvu.nl.