Abstract
So far, reproducible histomorphologic and immunological criteria to distinguish clinicopathologic subtypes of blastic peripheral B-cell non-Hodgkin’s lymphoma (BBCL), especially centroblastic (cb) and immunoblastic (ib) lymphomas, for daily diagnostic use are still lacking. Therefore, we correlated the cytogenetic findings in 126 patients with BBCL with histopathologic diagnoses. Subclassification of cb and ib lymphomas relied on the criteria defined in the updated Kiel classification; these subtypes are also listed in the Revised European-American Lymphoma (REAL) classification and in a preliminary report on the newly established World Health Organization classification, to investigate their clinical significance. Moreover, we performed a multivariate analysis to compare the prognostic significance of cytogenetic findings with the International Index. There were significant differences in the frequency of chromosome aberrations between different BBCL subtypes: t(8;14) was predominantly present in Burkitt’s lymphomas, t(14;18) in centroblastic lymphomas, deletions in 8q and 14q, changes of 4q and losses of chromosome 10 in immunoblastic lymphomas; t(11;14) was restricted to blastoid mantle cell lymphomas and associated with a poor prognosis. In cb lymphomas, deletions in 1q42-qter, duplications in 1q23-32, trisomy 5, and changes of 15q were identified as independent prognostic factors. In ib lymphomas, changes of 7q and 8q had stronger impact on survival than the International Index. These findings underline that Burkitt’s, cb, ib, and blastoid mantle-cell lymphoma are biologically distinct and clinically relevant entities and that cytogenetic findings can be helpful to subtype BBCL.
MOST CLASSIFICATION systems of non-Hodgkin’s lymphomas (NHL) are based on histological, cytomorphological, and immunological criteria. It has been a long debate whether these criteria alone may be sufficient to reproducibly define clinicopathologic entities. In the Kiel classification, different subtypes of blastic B-cell lymphomas (BBCL), mainly centroblastic (cb) and immunoblastic (ib), are distinguished according to their cytomorphologic and immunohistochemic appearance.1As indicated by the results of a prospective, randomized, multicenter trial, the survival of patients with these morphologically defined entities differs significantly.2 Many hematopathologists are not accustomed to using the Kiel classification, because, the Working Formulation predominating the Americal pathology practice in the past, the Kiel classification was only widely applied by European hematopathologists, whereas nowadays, the proposal of the International Lymphoma Study Group3 is generally accepted as the standard lymphoma classification system. This proposal questions the possibility to distinguish certain subtypes of large B-cell lymphomas, especially cb and ib lymphomas, based on morphological or cytomorphological criteria resulting in lumping a number of different subtypes as “diffuse large B-cell lymphoma” in the REAL classification. A preliminary report on the newly established WHO classification of NHL,4 for which a final publication is pending, suggested to list these morphologic variants of large B-cell lymphomas to test their clinical significance. Because typical cytogenetic changes of NHL, eg, the translocations t(8;14)(q24;q32) in Burkitt’s lymphomas (BL),5 t(14;18)(q32;q21) in follicular lymphomas,6 t(11;14)(q13;q32) in mantle cell lymphomas,7 or t(3;14)(q27;q32) in diffuse large cell lymphomas8 are associated with distinct clinicopathologic entities of NHL, it may be useful to integrate cytogenetic criteria in the definition of biologically relevant subtypes of NHL, which may differ in their biological behavior and, thus, may require different treatment strategies. All these translocations affecting the immunoglobulin heavy chain (IgH) locus in 14q32 lead to upregulation of oncogenes located at the chromosomal breakpoints of the translocation partners like c-myc in case of t(8;14),9 bcl-2 in case of t(14;18),10 CCND1 (bcl-1) in case of t(11;14),11 or bcl-6 in case of t(3;14).12Albeit all these translocations can be detected by polymerase chain reaction (PCR) or Southern blot analysis, chromosome analysis has been shown to be still more reliable at the time of diagnosis than these molecular methods.13 Certainly, molecular methods are reliable tools for the analysis of genetic aberrations with a sensitivity comparable with karyotyping for most applications, and even more reliable for selected investigations. In the present study, we investigated whether histomorphologic subtypes of BBCL can be distinguished according to the presence of distinct chromosomal abnormalities and whether cytogenetic findings are independent prognostic factors.
MATERIALS AND METHODS
One hundred twenty-six patients with blastic B-cell lymphomas consecutively referred to the Department of Human Genetics, University of Kiel, Germany, and to the Ludwig-Boltzmann Institute for Leukemia Research, Vienna, Austria, for cytogenetic analyses were included in this study, given that analyzable metaphases were found. The clinical characteristics of the patients are shown in Table1. Median observation time from primary diagnosis of a BBCL was 21 months (range, 1 to 112 months). Chromosome analyses were part of the diagnostic process at the time of the first diagnosis.
Clinical Characteristics of 126 Cytogenetically Studied Patients With Different Subtypes of BBCL
| Histopathological Lymphoma Subtype According to: . | ||||||
|---|---|---|---|---|---|---|
| Kiel Classification . | cb Lymphoma . | ib Lymphoma . | BL . | Centrocytoid-cb Lymphoma . | Other BBCL‡ Lymphoma . | All . |
| REAL Classification . | Diffuse Large Cell Lymphoma . | BL . | bMCL Variant† . | Other High-Grade BCL‡ . | ||
| Clinical Data | ||||||
| Total | 68 (54.0%) | 28 (22.2%) | 11 (8.7%) | 11 (8.7%) | 8 (6.4%) | 126 (100%) |
| Gender (male:female) | 28:40 | 11:17 | 7:4 | 7:4 | 5:3 | 58:68 |
| Age (yr), median (range) | 61 (17-88) | 67 (21-94) | 44 (16-72) | 63 (60-71) | 48 (28-68) | 60 (19-94) |
| International Prognostic Index | ||||||
| Age >60 yr | 36 (53%) | 19 (68%) | 2 (18%) | 8 (73%) | 2 (25%) | 67 (53%) |
| Karnofsky Index ≤70% | 7 (10%) | 2 (7%) | 1 (9%) | 2 (18%) | 1 (12.5%) | 13 (10%) |
| Serum-LDH >240 U/I | 36 (53%) | 18 (64%) | 4 (36%) | 7 (63%) | 5 (63%) | 70 (56%) |
| Ann Arbor Stage III/IV | 39 (57%) | 19 (68%) | 5 (46%) | 10 (91%) | 7 (88%) | 80 (64%) |
| No. of E-sites* ≥2 | 6 (9%) | 5 (18%) | 2 (18%) | 3 (27%) | 1 (13%) | 17 (14%) |
| Median survival (mo) (95% CI) | 68.9 (27-112) | 16.3 (10-22) | 38.7 (44-112) | 14.4 (8-24) | 15.2 (12-33) | 24.2 (16-38) |
| Histopathological Lymphoma Subtype According to: . | ||||||
|---|---|---|---|---|---|---|
| Kiel Classification . | cb Lymphoma . | ib Lymphoma . | BL . | Centrocytoid-cb Lymphoma . | Other BBCL‡ Lymphoma . | All . |
| REAL Classification . | Diffuse Large Cell Lymphoma . | BL . | bMCL Variant† . | Other High-Grade BCL‡ . | ||
| Clinical Data | ||||||
| Total | 68 (54.0%) | 28 (22.2%) | 11 (8.7%) | 11 (8.7%) | 8 (6.4%) | 126 (100%) |
| Gender (male:female) | 28:40 | 11:17 | 7:4 | 7:4 | 5:3 | 58:68 |
| Age (yr), median (range) | 61 (17-88) | 67 (21-94) | 44 (16-72) | 63 (60-71) | 48 (28-68) | 60 (19-94) |
| International Prognostic Index | ||||||
| Age >60 yr | 36 (53%) | 19 (68%) | 2 (18%) | 8 (73%) | 2 (25%) | 67 (53%) |
| Karnofsky Index ≤70% | 7 (10%) | 2 (7%) | 1 (9%) | 2 (18%) | 1 (12.5%) | 13 (10%) |
| Serum-LDH >240 U/I | 36 (53%) | 18 (64%) | 4 (36%) | 7 (63%) | 5 (63%) | 70 (56%) |
| Ann Arbor Stage III/IV | 39 (57%) | 19 (68%) | 5 (46%) | 10 (91%) | 7 (88%) | 80 (64%) |
| No. of E-sites* ≥2 | 6 (9%) | 5 (18%) | 2 (18%) | 3 (27%) | 1 (13%) | 17 (14%) |
| Median survival (mo) (95% CI) | 68.9 (27-112) | 16.3 (10-22) | 38.7 (44-112) | 14.4 (8-24) | 15.2 (12-33) | 24.2 (16-38) |
E-sites, extranodal manifestation.
Defined according to Ott et al.14
Including unclassifiable BBCL and rare subtypes.
Parts of the same lymph node biopsies (n = 114), bone marrow (n = 8), spleen (n = 2), pleural effusion (n = 1), and tumor tissue from prostate (n = 1) were used both for chromosome analyses and for histopathological diagnoses. Giemsa- and hematoxylin-eosin–stained sections were carefully evaluated, and immunhistochemical staining against CD19, CD20, CD22, κ-, λ-light chain restriction, CD30, and CD3 was additionally applied if necessary. In a few selected cases, monoclonal proliferation of B cells was confirmed by clonal rearrangements of the CDR III IgH locus. Subclassification of BBCL relied on the criteria defined in the updated Kiel classification1 and in the REAL classification.3 In particular, within diffuse large cell lymphomas, cb lymphomas were diagnosed if more than 10% of the cells resembled centroblasts and if the tumor cells were multilobulated or admixed with up to 90% immunoblasts or other cells; ib lymphomas were diagnosed if more than 90% of the cells resembled immunoblasts. A centroblast was regarded as a medium-sized to large cell, with a rim of slightly to moderately basophilic cytoplasm, and round to oval nuclei with vesicular chromatin and peripheral nucleoli, or as a like cell with a multilobulated nucleus. An immunoblast was defined as a large cell with broad basophilic cytoplasm and a round vesicular nucleus with a solitary central nucleolus. For a detailed description of the different subtypes of BBCL, refer to Lennert and Feller.1One hundred twenty-six cases of this study comprised 96 diffuse large cell lymphomas according to the REAL classification, which were subtyped into 68 cb lymphomas, 28 B-ib lymphomas according to the Kiel classification, 11 BL, 11 blastoid mantle cell lymphoma (bMCL, which is a subtype previously termed centrocytoid centroblastic lymphoma and recently recognized as blastoid MCL14) and 8 unclassified BBCL. The selection and classification criteria were entirely based on the Kiel classification.
Chromosome analyses were performed according to standard methods on unstimulated short-term cultures and cultures stimulated by B- and T-cell growth supplement or calcium ionophore A 23187 and phorbol-12,13-dibutyrate as described.15,16 If possible, at least 20 metaphases were analyzed after fluorescence R/C banding using chromomycin A3, methyl green, or 4,6-Diamidino-2-phenylindole (DAPI) as fluorescence dyes. Description of the karyotypes followed the rules of the International System for Human Cytogenetic Nomenclature (ISCN).17 The cytogenetic findings of 43 cases have been published previously.16,18,19 Patients underwent routine diagnostic procedures and were classified according to the Ann Arbor system. Clinical data and risk profiles according to the International Prognostic Index20 for patients with different morphological subtypes of BBCL are shown in Table 1.
With the exception of 2 patients of advanced age, all patients were treated with anthracyclin-containing chemotherapy (CHOP, CHOEP, COP-BLAM, PROMACE, CytaBOM).21 Eleven patients received high-dose chemotherapy with stem cell support or autologous bone marrow transplantation. In a multivariate analysis, we found no differences in survival between different therapeutic modalities used.
To evaluate survival probabilities and to define the prognostic significance of various chromosome aberrations, patients with cb and ib subtypes were analyzed separately. Survival times were estimated by Kaplan-Meier and compared by Log-Rank test. Cox proportional hazard model was used to investigate the influence of multiple risk factors simultaneously. Frequencies of certain chromosome abnormalities in defined subtapes of BBCL were compared and analyzed by the exact Fisher test. Statistical significance was defined as P < .05. No adjustment of the error probabilities for multiple testing was performed because of the explorative nature of the study.
RESULTS
Cytogenetic findings.
Aberrant clones were detected in 116 of 126 BBCL studied; complex aberrant clones containing 4 or more abnormalities were found in 88 of the 126 BBCL studied (Table 2). BL were found to have complex aberrant clones significantly less frequent than the other BBCL (P = .001). Hypodiploid chromosome numbers were found in 6 cb, 7 ib, 2 BL, 6 bMCL, and 1 other BBCL; pseudodiploid chromosome numbers were found in 16 cb, 5 ib, 4 BL, 3 bMCL, and 2 other BBCL; hyperdiploid chromosome numbers were found in 31 cb, 10 ib, 3 BL, 1 bMCL, and 1 other BBCL; near-triploid chromosome numbers in 2 cb; near-tetraploid chromosome numbers in 8 cb, 4 ib, 1 BL, 1 bMCL, and in 2 other BBCL. Unidentifiable marker chromosomes were present in 27 cb, 9 ib, 3 BL, 1 bMCL, and in 4 BBCL.
Presence of Characteristic Translocations in Different Subtypes of BBCL
| Histopathological Lymphoma Subtype According to: . | ||||||
|---|---|---|---|---|---|---|
| Kiel Classification . | cb Lymphoma . | ib Lymphoma . | BL . | Centrocytoid-cb Lymphoma . | Other BBCL . | All . |
| REAL Classification . | Diffuse Large Cell Lymphoma . | BL . | bMCL Variant . | Other High-Grade BCL‡ . | ||
| Aberrant clones | 63 (92.6%) | 26 (92.9%) | 10 (90.9%) | 11 (100%) | 6 (75.0%) | 116 (92.1%) |
| Complex aberrant clones* | 50 (73.5%) | 22 (78.6%) | 2 (18.2%) | 9 (81.8%) | 5 (62.5%) | 88 (69.8%) |
| Chromosome translocations | ||||||
| t(8;14) | 2 (2.9%) | 0 | 6 (54.5%) | 0 | 0 | 8 (6.3%) |
| t(11;14) | 0 | 0 | 0 | 11 (100%) | 0 | 11 (8.7%) |
| t(14;18) | 20 (29.4%) | 1 (3.6%) | 2 (18.2%) | 0 | 0 | 23 (18.3%) |
| t(3;14)† | 9 (12.3%) | 5 (17.9%) | 1 (9.1%) | 0 | 1 (12.5%) | 16 (12.7%) |
| Histopathological Lymphoma Subtype According to: . | ||||||
|---|---|---|---|---|---|---|
| Kiel Classification . | cb Lymphoma . | ib Lymphoma . | BL . | Centrocytoid-cb Lymphoma . | Other BBCL . | All . |
| REAL Classification . | Diffuse Large Cell Lymphoma . | BL . | bMCL Variant . | Other High-Grade BCL‡ . | ||
| Aberrant clones | 63 (92.6%) | 26 (92.9%) | 10 (90.9%) | 11 (100%) | 6 (75.0%) | 116 (92.1%) |
| Complex aberrant clones* | 50 (73.5%) | 22 (78.6%) | 2 (18.2%) | 9 (81.8%) | 5 (62.5%) | 88 (69.8%) |
| Chromosome translocations | ||||||
| t(8;14) | 2 (2.9%) | 0 | 6 (54.5%) | 0 | 0 | 8 (6.3%) |
| t(11;14) | 0 | 0 | 0 | 11 (100%) | 0 | 11 (8.7%) |
| t(14;18) | 20 (29.4%) | 1 (3.6%) | 2 (18.2%) | 0 | 0 | 23 (18.3%) |
| t(3;14)† | 9 (12.3%) | 5 (17.9%) | 1 (9.1%) | 0 | 1 (12.5%) | 16 (12.7%) |
Complex aberrant clones were defined as containing 4 or more abnormalities. Balanced translocations were considered as 1 abnormality.
Including other changes of 3q27, namely t(1;3)(q34;q27), t(2;3)(q35;q27), t(3;12)(q27;q13q24), t(3;22)(q27;q11), inv(3)(q13q27), dup(3)(q21q27), r(3)(p2?q2?7), add(3)(q27).
Including unclassifiable BBCL and rare subtypes.
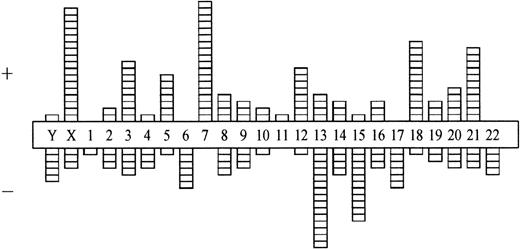
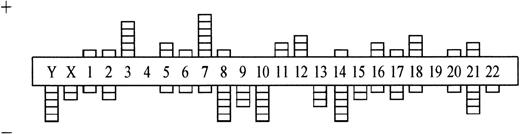
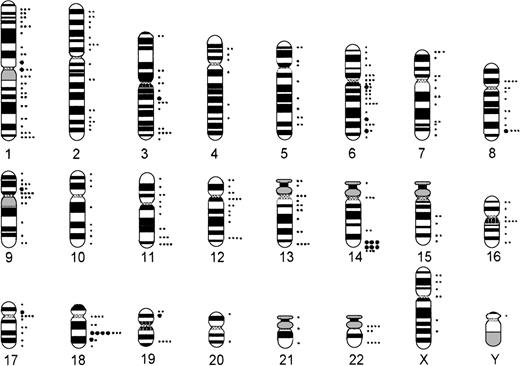
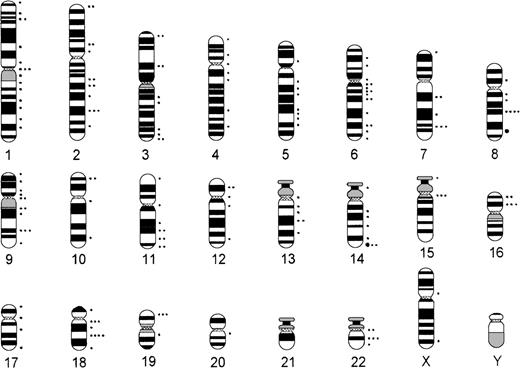
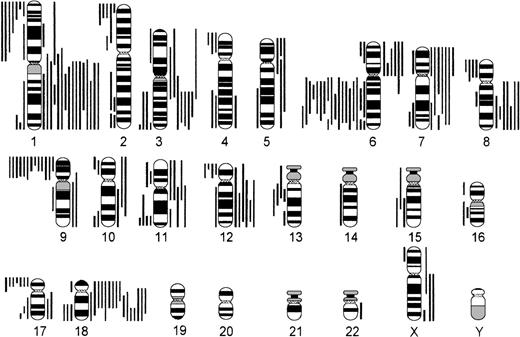
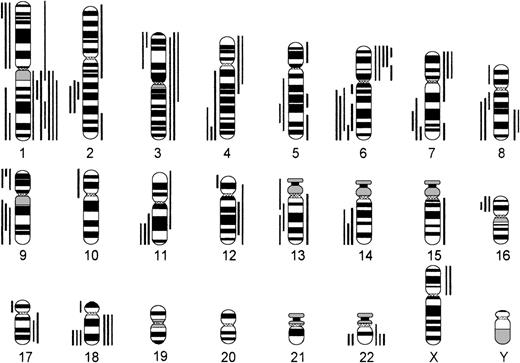
Comparing numerical and structural abnormalities in cb and ib lymphomas, which were the 2 largest groups of this series, gains of chromosomes X, 3, 5, 7, 12, and 18 and losses of chromosomes Y, 6, 13, 15, and 17 were the most frequent numerical aberrations in cb lymphomas (Fig 1); gains of chromosomes 3, 7, 12, and 18 and losses of chromosomes Y, 8, 10, 14, and 21 were most frequent in ib lymphomas (Fig 2). The series of BL and bMCL were too small for a detailed analysis. Recurrent breakpoints were localized in the following regions: 1cen-p13, 1p34-36, 1q42-43, 3q21-22, 3q27-29, 6q12-16, 6q23, 6q25, 8cen-p12, 8q24, 9cen-p21, 12cen-p12, 14q32, 17cen-p11, 18q21, 19p13, and 19q13 in cb lymphomas (Fig 3); and in 1cen, 2q32, 7q34, 8q21, 8q24, 9q32, 14q32, 15cen, 16p12, 18cen, 18q21, 19p13, and 22q12 in ib lymphomas (Fig 4). Commonly gained chromosomal regions were: 1q23-31, 3q21-22, 6p, 7p, 7q31-32, 8q22-24, 11q12-13, 12q14-24, and 18q11-21 in cb lymphomas (Fig5); 1q21-25, 3p24-q21, 6p21, 7p12-21, 18q, and 22q12-ter in ib lymphomas (Fig 6). Commonly deleted chromosomal regions were: 1p35-ter, 2p23-ter, 6q21-22, 6q25-ter, 8p12-ter, 9p21-ter, 11q23-ter, 12p12-13, and 17p12-13 in cb lymphomas (Fig 5); 1p35-36, 2q22-24, 4q32-ter, 6q21-25, 7q33, 8q21, 9p24, 9q21-32, 11q21-ter, 14q23-ter, 16p13, and 18q21-ter in ib lymphomas (Fig 6).
Numerical chromosome aberrations in cb lymphomas (n = 68). +, gain; −, loss of the respective chromosome.
Numerical chromosome aberrations in cb lymphomas (n = 68). +, gain; −, loss of the respective chromosome.
Numerical chromosome aberrations in B-ib lymphomas (n = 28). +, gain; −, loss of the respective chromosome.
Numerical chromosome aberrations in B-ib lymphomas (n = 28). +, gain; −, loss of the respective chromosome.
Chromosomal breakpoints in cb lymphomas (n = 68). •, 5 breakpoints; •, 1 breakpoint.
Chromosomal breakpoints in cb lymphomas (n = 68). •, 5 breakpoints; •, 1 breakpoint.
Chromosomal breakpoints in B-ib lymphomas (n = 28). •, 5 breakpoints; •, 1 breakpoint.
Chromosomal breakpoints in B-ib lymphomas (n = 28). •, 5 breakpoints; •, 1 breakpoint.
Chromosomal imbalances in cb lymphomas (n = 68). Each bar indicates the gain (right side) or loss (left side) of the respective chromosomal region in 1 lymphoma case.
Chromosomal imbalances in cb lymphomas (n = 68). Each bar indicates the gain (right side) or loss (left side) of the respective chromosomal region in 1 lymphoma case.
Chromosomal imbalances in B-ib lymphomas (n = 28). Each bar indicates the gain (right side) or loss (left side) of the respective chromosomal region in 1 lymphoma case.
Chromosomal imbalances in B-ib lymphomas (n = 28). Each bar indicates the gain (right side) or loss (left side) of the respective chromosomal region in 1 lymphoma case.
Presence of chromosomal abnormalities in different subtypes of BBCL.
The groups of patients with morphologically defined subgroups of BBCL, ie, BL, cb, ib, bMCL subtypes were compared with regard to the frequency of chromosomal abnormalities. It turned out that t(8;14) was present most frequently in BL, t(14;18) in cb lymphomas, and t(11;14) in bMCL, whereas translocations involving 3q27 were seen in different subtypes of BBCL in similar frequencies (Table 2).
The typical Burkitt’s translocation t(8;14) was present in 6 of 11 BL and in 2 of 68 cb lymphomas, but not in any case of ib lymphoma. No variant translocations, t(2;8)(p13;q24) or t(8;22)(q24;q11), were seen. The differences between BL and cb lymphoma as well as between BL and ib lymphoma regarding the frequency of t(8;14) are highly significant (P < .0001 for both tests). Structural aberrations involving 8q24 other than t(8;14), eg, dup(8)(q22q24) or t(4;8)(q33;q24), were found in 1 case of BL, in 5 cases of cb, in 5 cases of ib, and in 1 case of bMCL subtypes. In 1 case of BL, in 4 cases of cb, and in 1 case of ib subtype, these changes of 8q24 occurred in addition to t(14;18) and in 1 case of bMCL in addition to t(11;14)(q13;q32). Thus, changes of 8q24 in these cases seem to represent secondary changes. t(14;18) was present in 2 of 11 BL, in 20 of 68 cb, and in 1 of 28 ib lymphomas. The difference between cb and ib subtypes with respect to the frequency of t(14;18) is highly significant (P = .006). t(11;14) was detected only in all 11 bMCL. There was no significant difference between these subtypes of BBCL regarding the frequency of t(3;14) or changes of 3q27.
When other chromosomal abnormalities were compared for their frequency in the 2 largest groups, ie, cb and ib subtypes, losses of the whole chromosome 10, deletions in 8q and 14q, as well as structural abnormalities of 4q, were significantly more frequent in ib than in cb lymphomas (P = .01 for −10; P = .017 for del(8)(q); P = .009 for del(14)(q); P = .049 for der(4)(q).
Survival.
Median survival was 68.9 months for the patients with cb lymphoma, 16.3 months for the patients with ib lymphoma, 38.7 months for patients with BL, 14.4 months for patients with bMCL, and 15.2 months for patients with unclassified BBCL, which is in agreement with the results of a prospective, randomized, multicenter therapy trial that showed that the distinction of cb and ib lymphomas is a significant prognostic risk factor.2 As in this study, the difference between our patients with cb lymphoma and patients with ib lymphoma was statistically significant (P = .004).
Prognostic significance of chromosomal abnormalities in different subtypes of BBCL.
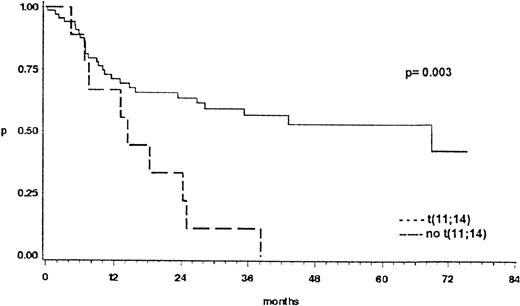
The prognostic significance of cytogenetic findings was separately analyzed for patients with cb and B-ib lymphoma. The presence or absence of t(8;14), or other changes of 8q24, of t(14;18), and of t(3;14), or other changes of 3q27 did not influence the survival probability. However, as shown in Fig 7, patients with t(11;14)-positive bMCL had a significant shorter survival than the patients with cb lymphoma, 2 subtypes that, based on morphologic appearance, can sometimes hardly be distinguished (P = .003). Other chromosome abnormalities associated with a significantly shorter survival are given in Table3. In multivariate testing performed to evaluate a possible relationship between cytogenetic findings and established prognostic risk factors of the International Index, deletions and duplications of 1q were independent adverse risk factors, whereas trisomy 5 and changes of 15q were independent favorable risk factors in patients with cb subtypes. In ib subtypes, changes of 7q and 8q had a stronger impact on survival than the International Prognostic Index (Table 4).
Overall survival of 68 patients with cb lymphomas compared to 11 patients with t(11;14)-positive lymphomas. This translocation was only present in the blastic variant of mantle cell lymphomas previously termed anaplastic mantle cell lymphoma or centrocytoid cb lymphoma.
Overall survival of 68 patients with cb lymphomas compared to 11 patients with t(11;14)-positive lymphomas. This translocation was only present in the blastic variant of mantle cell lymphomas previously termed anaplastic mantle cell lymphoma or centrocytoid cb lymphoma.
Chromosomal Abnormalities as Adverse Risk Factors for Survival in 68 Patients With cb and 28 Patients With B-ib Lymphoma (Univariate Analysis, Log-Rank test)
| Chromosomal Abnormality in: . | P Value . |
|---|---|
| cb Lymphomas | |
| del(1)(q) | .032 |
| dup(1)(q) | .012 |
| der(3)(p) | .030 |
| der(4)(p) | .027 |
| −6 | .000 |
| der(22p) | .000 |
| ib Lymphomas | |
| del(6q)(14) | .045 |
| del(6q)(22) | .043 |
| del(6q)(25) | .034 |
| der(7)(q) | .048 |
| der(8)(q) | .013 |
| del(9)(p) | .010 |
| der(11)(q23) | .003 |
| Chromosomal Abnormality in: . | P Value . |
|---|---|
| cb Lymphomas | |
| del(1)(q) | .032 |
| dup(1)(q) | .012 |
| der(3)(p) | .030 |
| der(4)(p) | .027 |
| −6 | .000 |
| der(22p) | .000 |
| ib Lymphomas | |
| del(6q)(14) | .045 |
| del(6q)(22) | .043 |
| del(6q)(25) | .034 |
| der(7)(q) | .048 |
| der(8)(q) | .013 |
| del(9)(p) | .010 |
| der(11)(q23) | .003 |
Clinical Parameters of the International Index and Chromosomal Abnormalities as Risk Factors for Survival in 68 Patients With cb and 28 Patients With B-ib Lymphoma (Multivariate Analyses)
| . | cb Lymphomas . | ib Lymphomas . | ||
|---|---|---|---|---|
| P Value . | Risk Ratio . | P Value . | Risk Ratio . | |
| Clinical Risk Factor | ||||
| Age >60 yr | .37 | 1.47 | 0.81 | 1.14 |
| Clinical Stage (Ann Arbor Stage III or IV) | .61 | 0.80 | 0.76 | 0.83 |
| Serum LDH >240 U/l | .19 | 1.79 | 0.36 | 1.88 |
| Karnofsky Index ≤70% | .01 | 0.25 | 0.03 | 0.12 |
| No. of E-sites4-150 ≥2 | .32 | 0.53 | 0.60 | 0.65 |
| Clinical Risk Factor or Chromosomal Abnormality | ||||
| Age >60 yr | .018 | 1.05 | — | — |
| Clinical Stage (Ann Arbor Stage III or IV) | .035 | 2.44 | — | — |
| dup(1)(q) | .065 | 2.27 | — | — |
| del(1)(q) | .084 | 2.39 | — | — |
| +5 | .089 | 0.17 | — | — |
| der(15)(q) | .125 | 0.19 | — | — |
| Karnofsky Index ≥70% | — | — | .001 | 0.01 |
| der(8)(q) | — | — | .003 | 13.20 |
| der(7)(q) | — | — | .009 | 6.91 |
| . | cb Lymphomas . | ib Lymphomas . | ||
|---|---|---|---|---|
| P Value . | Risk Ratio . | P Value . | Risk Ratio . | |
| Clinical Risk Factor | ||||
| Age >60 yr | .37 | 1.47 | 0.81 | 1.14 |
| Clinical Stage (Ann Arbor Stage III or IV) | .61 | 0.80 | 0.76 | 0.83 |
| Serum LDH >240 U/l | .19 | 1.79 | 0.36 | 1.88 |
| Karnofsky Index ≤70% | .01 | 0.25 | 0.03 | 0.12 |
| No. of E-sites4-150 ≥2 | .32 | 0.53 | 0.60 | 0.65 |
| Clinical Risk Factor or Chromosomal Abnormality | ||||
| Age >60 yr | .018 | 1.05 | — | — |
| Clinical Stage (Ann Arbor Stage III or IV) | .035 | 2.44 | — | — |
| dup(1)(q) | .065 | 2.27 | — | — |
| del(1)(q) | .084 | 2.39 | — | — |
| +5 | .089 | 0.17 | — | — |
| der(15)(q) | .125 | 0.19 | — | — |
| Karnofsky Index ≥70% | — | — | .001 | 0.01 |
| der(8)(q) | — | — | .003 | 13.20 |
| der(7)(q) | — | — | .009 | 6.91 |
E-sites, extranodal manifestation.
DISCUSSION
Cytogenetic findings may help to recognize biologically relevant entities that are sometimes hard to distinguish by morphologic, cytomorphologic, and immunologic criteria alone.22 This distinction is especially important if patients with defined entities run a distinct clinical course as has been shown by Engelhard et al2 for patients with cb and ib lymphomas, 2 BBCL subtypes of similar morphological appearance originally described in the Kiel classification.1 They were able to show in a prospective, randomized, multicenter trial that the distinction between cb and ib lymphomas is a significant prognostic risk factor. These results were confirmed in our study. The patients with cb and ib lymphomas had about the same survival times as in the study by Engelhard et al,2 although they were not homogenously treated. However, except for 2 patients, all of them had received anthracyclin-containing regimes. In line with a study comparing a standard anthracyclin-containing regime (CHOP) with intensified protocols,21 patients treated with different therapy modalities did not have any differences in survival. Thus, the diagnosis of an ib lymphoma seems to be an independent adverse risk factor. Nevertheless, there is an ongoing discussion as to whether diffuse large cell B-cell lymphomas as defined in the REAL classification should be subclassified into cb and ib lymphomas.
The presence of typical chromosome abnormalities in morphologically defined entities of BBCL seems, for the first time, to provide evidence that ib lymphomas are a distinct biological entity. As to the frequency of t(8;14), there was a significant difference between ib lymphomas and BL. This appears to be important, because based on morphology, it is often difficult to distinguish ib lymphomas with plasmocytic differentiation from Burkitt-like lymphomas. Moreover, we found significant differences in the frequency of translocations t(14;18) between ib and cb lymphomas, and in the frequency of t(11;14) between ib lymphomas and bMCL. Thus, translocations involving the IgH locus at 14q32 occur less frequently in ib lymphomas than in the other BBCL studied. These karyotypic findings are confirmed by fluorescence in situ hybridization (FISH) analyses using a Cα/VH assay to detect break events in the IgH locus (R.S., T. Springer, B.S., unpublished data, March 1999). Comparably, in small cell B-cell lymphomas, chronic lymphocytic leukemias (CLLs) (small lymphocytic lymphomas) only rarely have translocations involving the IgH locus in 14q32 (H. Doehner, personal communication, November 1998), in contrast to other low grade B-cell lymphomas, eg, centrocytic cb lymphomas (follicular lymphomas grade 1 or 2)23 with t(14;18), mantle-cell lymphomas with t(11;14) or plasmocytomas.24 Moreover, deletions in 14q, a recurrent abnormality of ib subtypes in this study, seem to be a characteristic finding in diffuse B-lymphocytic lymphomas25 and in CLL.26 In this cytogenetic study, characteristic chromosome aberrations of CLL, ie, del(11)(q21-q22), +12, del(13)(q14-21), were present more frequently in ib than in cb lymphomas. However, these differences were not statistically significant. Trisomy 12 may be only regarded, if it is present as primary change, meaning, if it does not occur as a secondary abnormality in addition to t(14;18) or t(8;14). Within this series, there was 1 ib lymphoma with trisomy 12 as single change. Because FISH has a higher sensitivity than karyotyping to detect trisomies and chromosomal deletions, FISH seems to be the method of choice to clarify whether chromosome aberrations characteristic of B-CLL indeed occur more frequently in ib than in cb lymphomas and whether some ib lymphomas may represent the blastic counterpart of CLL. This hypothesis would be in accordance with the fact that cases of CLL repeatedly transformed into ib lymphomas.1
One of 28 ib lymphomas in this study and 7 of 153 ib lymphomas reported in Mitelman’s catalogue of chromosome aberrations27 in cancer, 104 of them diagnosed according to the Working Formulation, had a t(14;18). Remarkably, the ib lymphoma of this study and 3 ib lymphomas reported by Mitelman27 additionally showed a Burkitt’s translocation or other structural changes of 8q24. In these cases, changes of 8q24 may appear as secondary changes during the karyotypic evolution.28 Recently, amplifications of the chromosomal region 8q24, resulting in an overexpression of the c-myc gene, were identified by comparative genomic hybridization in follicular lymphomas with a large cell component that often habor a t(14;18).29 Patients with both t(14;18) and t(8;14) or t(8;22) often present in a leukemic phase and run an extremely aggressive course.30 Thus, lymphomas with both t(14;18) and alterations of 8q24 may be a unique clinicopathologic entity.
So far no characteristic chromosome abnormalities of ib lymphomas have been identified.31 Schouten et al32 assumed that patients with an abnormal chromosome 6 had an increased frequency to suffer from ib lymphoma.29 In this study, there was no significant difference between cb and ib lymphomas with respect to deletions in 6q. However, deletions in 6q turned out to have prognostic significance for patients with ib lymphomas. Offit et al33and Bastard et al34 described bcl-6 or LAZ3 rearrangements, the molecular counterpart of t(3;14) or other changes of 3q27, in patients with different subtypes of BBCL, among them follicular and ib lymphomas. In line with these findings t(3;14)(q27;q32) or other changes of 3q27 were not associated with a certain subtype of BBCL in this study. In contrast, loss of chromosome 10, deletions in 8q and 14q, as well as structural abnormalities of 4q, were found significantly more frequent in ib than in cb lymphomas. In a study of 78 previously untreated patients with NHL, monosomy 14 was the only abnormality associated with a statistically significant difference in survival duration.35 When analyzing 104 patients with diffuse large cell lymphomas from a cytogenetic study of 434 consecutively ascertained specimens, breaks at 1q21-23 and the presence of more than 4 marker chromosomes were associated with a shortened median survival.8 Deletions of 1q and duplications of 1q were also identified in our survival analysis as independent cytogenetic risk factors for patients with cb subtypes. These findings appear contradictory; detailed analyses of the cytogenetic data, however, shows that the deletions involve the terminal region 1q42 → qter, the duplications involve the region 1q23-32. Moreover, we found a significant, but favorable, impact on survival of trisomy 5 and changes of 15q. In the multivariate analysis on patients with ib subtypes, changes of 7q and 8q even had more impact on survival than the parameter of the International Index.20 Remarkably, the recently cloned gene for Nijmegen breakage syndrome, a chromosomal instability syndrome with an increased predisposition for lymphomas, especially of ib subtype, has been localized to 8q21.36Possibly, mutations of this gene play a role for the development of B-ib lymphomas, as has been shown for mutations of the related ataxia-telangiectasia gene in patients with T-PLL.37
The t(11;14) was exclusively present in bMCL, an entity previously termed centrocytoid centroblastic lymphoma and recently recognized to represent the blastic counterpart of mantle-cell lymphomas.14 bMCL subtypes were characterized by elevated mitotic counts, proliferation indices, frequent bcl-1 rearrangements at the major translocation cluster locus, overexpression of p53, and tetraploid chromosome numbers. Weisenburger and Armitage38reported patients with diffuse MCL to have a significantly shorter survival of 30 to 33 months when compared to those with a nodular pattern who had a median survival of 77 to 88 months. In the present study, t(11;14) turned out to be an adverse prognostic factor. Thus, BBCL with t(11;14) seem to be a distinct histomorphologic entity associated with a remarkably poor outcome.
Recently, FISH assays for the detection of the typical translocations t(8;14), t(11;14), t(14;18), and t(3;14) have been developed. At the time of primary diagnosis, their sensitivity exceeded that of chromosome analysis and even that of molecular techniques like PCR or Southern blotting.13 19 Studies are underway using these highly sensitive FISH techniques to approve our findings that different clinicopathologic entities of BBCL can be distinguished based on the significantly different frequencies of typical chromosome translocations.
The cytogeneticists H. Grüner (Wien), A. Borowski, K. Rohde, the pathologists Dr Hanak (Wien), Dr Merz (Lübeck), Prof Dr Parwaresch (Kiel), and clinicians from different hospitals in Germany, in particular Dr Kuse (Hamburg), Prof Dr Löffler, Prof Dr Schmitz (Kiel), Dr K. Hoffmann, and B. Liedtke also contributed to this work.
Submitted December 21, 1998; accepted June 28, 1999.
Supported by the Deutsche Krebshilfe, Grant No. 10-0992-Schl3, the Interdisciplinary Center for Clinical Cancer Research, University Hospital Kiel, and the Wilhelm Sander-Stiftung Grant No. 95.003.2. B.S. holds a Hermann und Lilly Schilling professorship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Brigitte Schlegelberger, MD, Department of Human Genetics, University of Kiel, Schwanenweg 24, 24105 Kiel, Germany; e-mail: schlegelberger@medgen.uni-kiel.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal