Abstract
Low concentrations of As2O3 (≤1 μmol/L) induce long-lasting remission in patients with acute promyelocytic leukemia (APL) without significant myelosuppressive side effects. Several groups, including ours, have shown that 0.5 to 1 μmol/L As2O3 induces apoptosis in APL-derived NB4 cells, whereas other leukemic cells are resistant to As2O3 or undergo apoptosis only in response to greater than 2 μmol/L As2O3. In this report, we show that the ability of As2O3 to induce apoptosis in leukemic cells is dependent on the activity of the enzymes that regulate cellular H2O2 content. Thus, NB4 cells have relatively low levels of glutathione peroxidase (GPx) and catalase and have a constitutively higher H2O2content than U937 monocytic leukemia cells. Glutathione-S-transferase π (GSTπ), which is important for cellular efflux of As2O3, is also low in NB4 cells. Moreover, As2O3 further inhibits GPX activity and increases cellular H2O2 content in NB4 but not in U937 cells. Selenite pretreatment of NB4 cells increases the activity of GPX, lowers cellular H2O2 levels, and renders NB4 cells resistant to 1 μmol/L As2O3. In contrast, concentrations of As2O3 that alone are not capable of inducing apoptosis in NB4 cells induce apoptosis in the presence of the GPx inhibitor mercaptosuccinic acid. Similar effects are observed by modulating the activity of catalase with its inhibitor, aminotriazol. More important from a therapeutic point of view, U937 and HL-60 cells, which require high concentrations of As2O3 to undergo apoptosis, become sensitive to low, clinically acceptable concentrations of As2O3 when cotreated with these GPx and catalase inhibitors. The induction of apoptosis by As2O3 involves an early decrease in cellular mitochondrial membrane potential and increase in H2O2 content, followed by cytochrome c release, caspase 3 activation, DNA fragmentation, and the classic morphologic changes of apoptosis.
LOW CONCENTRATIONS (less than or equal to micromolar concentrations) of As2O3 have been shown to induce a high rate of clinical remission in patients with acute promyelocytic leukemia (APL) without severe toxicity.1-5 Thus, in stark contrast to the carcinogenic effect of chronic exposure to high doses of arsenic compound,6,7 low concentrations of As2O3 are of therapeutic value in APL and perhaps other leukemias.8-11 In vitro studies have shown that low concentrations of As2O3 induce apoptosis in APL derived-NB4 cells and primary cultures of APL. In other leukemic cells, As2O3 can induce apoptosis but only at higher concentrations that may be unacceptable in the clinic because of toxicity.8-11
The major feature that distinguishes APL cells from other malignant hematopoietic cells is the expression of PML-RARα, the product of the t(15;17) translocation.12-14 PML-RARα is a transcriptional repressor with dominant negative activity over RARα.15-17 Studies aimed at understanding the factors underlying the unique sensitivity of NB4 cells to As2O3 and, ultimately, how these relate to the expression of PML-RARα form the basis of this report. Our previous work indicated that As2O3-induced apoptosis was modulated by the cellular glutathione redox system, with increased intracellular levels of reduced glutathione (GSH) having an inhibitory effect.11 Because of the antioxidant function of GSH, we wondered whether 1 μmol/L As2O3 could trigger NB4 cell apoptosis through the generation of reactive oxygen species (ROS). We show here that, indeed, the induction of NB4 cell apoptosis and the refractoriness of other leukemic cells to 1 μmol/L As2O3 are explained, at least in part, by the accumulation of higher H2O2 levels in As2O3-treated NB4 as compared with other leukemic cells. This difference in intracellular H2O2 concentration is shown to be derived from a differential pattern of expression of H2O2-catabolizing enzymes. A causal link between H2O2 levels and apoptosis is supported by the coordinate regulation of these events in response to both positive and negative regulation of H2O2-catabolizing enzymes. Moreover, we show that, in the presence of glutathione peroxidase (GPx) and catalase inhibitors, it is possible to achieve high apoptotic rates in leukemic cells otherwise refractory to a therapeutic concentration of As2O3 (1 μmol/L). Finally, we demonstrate that the generation of H2O2 in As2O3-treated NB4 cells triggers apoptosis via a reduction in mitochondrial membrane potential, cytochrome c release, and caspase activation.
MATERIALS AND METHODS
Reagents.
As2O3 solution (0.1%) was kindly supplied by Dr Ting-Dong Zhang (Harbin Medical University, Harbin, China). N-acetylcysteine (NAC), sodium selenite, ethidium bromide, hydrogen peroxide, and acridine orange were purchased from Sigma Chemical Co (St Louis, MO) and dissolved in phosphate-buffered saline (PBS). Z-VAD-FMR and GPx assay kit were obtained from Calbiochem (San Diego, CA). Choromethyltetramethylrosamine methyl ester (CMTMR) and 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; C2938) were obtained from Molecular Probes (Eugene, OR).
Cell lines.
NB4 t(15;17) (obtained from Dr M. Lanotte, Hospital Saint Louis, Paris, France18), HL-60, U937, K562, and KG1 cells (from American Type Culture Collection, Rockville, MD) were cultured in RPMI-1640 medium supplemented with 100 U/mL pencillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine, and 10% heat-inactivated fetal bovine serum. Cells in logarithmic growth were seeded at 1 × 105 cells/mL for studies performed in duplicate and repeated at least 3 times.
Quantitation of apoptotic cells.
Apoptotic cells were determined by morphology and fluorescence-activated cell sorting (FACS) analysis with propidium iodide (PI) as well as TUNEL assay. For morphologic evaluation, cells were stained with acridine orange (AO) and ethidium bromide (EB) and assessed by fluorescence microscopy as described previously.19 Briefly, 1 μL of stock solution containing 100 μg/mL AO and 100 μg/mL EB was added to 25 μL of cell suspension. EB-negative cells with nuclear shrinkage, blebbing, and apoptotic bodies were counted as apoptotic cells. For FACS analysis with PI staining, cells were fixed with ice-cold 70% ethanol at a cell density of 1 × 106/mL and treated with 1 mg/mL RNase for 30 minutes at 37°C. PI was then added to the solution at a final concentration of 50 μg/mL and DNA content was quantitated by flow cytometry.20 TUNEL assay was performed according to the manufacturer’s instructions (PharMingen, San Diego, CA). Cells were analyzed by fluorescence microscopy and by flow cytometry using a FACScan. All data were collected, stored, and analyzed by LYSIS II software (Becton Dickinson, San Jose, CA). Cells were also analyzed for their DNA content and cell cycle distribution by flow cytometry analysis of PI-stained nuclei.
Mitochondria membrane potential assay.21
After As2O3 treatment, NB4 cells were washed, and the medium in each well was supplemented with 50 nmol/L CMTMR and incubated at 37°C for 15 minutes. The solution was replaced with dye-free media for a further 10 minutes before washing with PBS. The cells were cytospun onto slides and were then fixed on ice for 10 minutes with 4% paraformaldehyde. After fixation, the cells were rinsed with PBS, and the coverslips were mounted onto slides using Aquamount. Confocal microscopy was then used to resolve individual mitochondria labeled with CMTMR at the indicated time points. A Leica TCD confocal scanning microscope coupled to an argon-krypton laser (Omnichrome, USA) was used. A pinhole of 20 was used with an excitation filter wavelength of 568 nm and a emission filter of 615/30 nm. Images were scanned using an oil immersion (100×), 1.4 NA objective at 512 × 512 × 8 bits per pixel resolution, background offset of 0, and averaged 32 times in bidirectional scan mode. The images were saved in tagged image file format (TIFF) and transferred to a 100 MHz Pentium PC (Gateway, North Sioux, SD). Metamorph (Universal Imaging Corp, West Chester, PA) was used to threshold individual mitochondrial outlines and then to measure the mean intensity within each mitochondrion.
Enzyme activity assays.
Cells (5 × 107) were washed twice with PBS, resuspended in PBS, sonicated for 10 seconds, and centrifuged at 14,000 rpm for 10 minutes, and the supernatants were subjected to enzyme assays. GPx activity was determined using commercial kits (Calbiochem, San Diego, CA). One milliunit of enzyme activity was defined as 1 nmol NADPH oxidized to NADP per milligram of protein per minute. Catalase activity was determined by monitoring the rate of decomposition of H2O2, as assessed by the decrease in absorbance at 240 nm.22 One unit of activity represented the consumption of 1 mmol hydrogen peroxide/min/mg protein. The assay mixture (1 mL) contained 19 mmol/L H2O2 and defined amounts of cell extract in 50 mmol/L phosphate buffer (pH 7.0) at 25°C. Glutathione-S-transferase (GST) π activity was measured using 1-chloro-2,4-dinitrobenzene (CDNB) and GSH as substrates.23 The cell pellets, obtained as described above, were resuspended in 300 μL of 100 mmol/L potassium phosphate buffer, pH 6.8, sonicated for 10 seconds at 4°C, and centrifuged at 14,000 rpm for 30 minutes. The assay mixture contained 850 μL of 0.1 mol/L sodium phosphate-1 mmol/L EDTA (pH 6.5), 50 μL of 20 mmol/L GSH, 50 mL of 20 mmol/L CDNB, and 50 μL of clear cell lysate. The absorbance at 340 nm was continuously recorded for 2 minutes.
H2O2 production.
Production of H2O2 was detected using DCFH-DA, an uncharged, cell permeant fluorescent probe. Inside the cells, DCFH-DA is cleaved by nonspecific esterases forming DCFH, which is the nonfluorescent form and is oxidized to the fluorescent compound 2′,7′-dichlorofluorescein (DCF) in the presence of H2O2.24 Exponentially growing cells (1 × 105 cells/mL) were labeled with 0.5 μmol/L DCFH-DA for 1 hour and then incubated in the absence or presence of As2O3 at 37°C for various periods of time. After washing with PBS, cells (10,000 per point) were analyzed by FACScan (Becton Dickinson) with excitation and emission settings of 495 and 525 nm, respectively.25 Arithemic histogram statistics analysis was used to determine the mean of the oxidized DCF peak in each group.
Quantification of DNA fragmentation.
DNA fragmentation was quantified as described previously.20Cells were harvested by centrifugation, and the pellets were suspended in lysis buffer containing 15 mmol/L Tris·HCl, 20 mmol/L EDTA, 0.5% Triton X-100, pH 8.0. After 30 minutes on ice, samples were centrifuged at 14,000g for 30 minutes, and cellular DNA was extracted. Electrophoresis was performed in 1% agarose gel in 40 mmol/L Tris-acetate buffer (pH 7.4) at 50 V. After electrophoresis, DNA was visualized by ethidium bromide staining.
Western blot analysis.
Protein extracts (50 μg) prepared with RIPA lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 100 μmol/L leupeptin, and 2 μg/mL aprotinin, pH 8.0) were separated on an 8% or 12% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were stained with 0.2% Ponceau S red to assure equal protein loading and transfer. After blocking with 5% nonfat milk, the membranes were incubated with polyclonal antibody to PARP (Boehringer Mannheim, Indianapolis, IN) and monoclonal antibodies to Cpp32 and Bcl-2 (Oncogene Research Products, Cambridge, MA). Immunocomplexes were visualized by chemiluminescence (ECL kit; Amersham, Arlington Heights, IL).11
RESULTS
As2O3-induced apoptosis is dependent on cellular H2O2 levels.
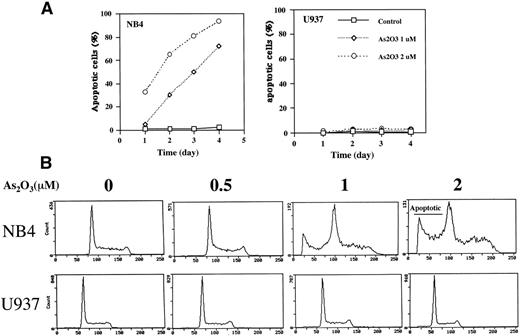
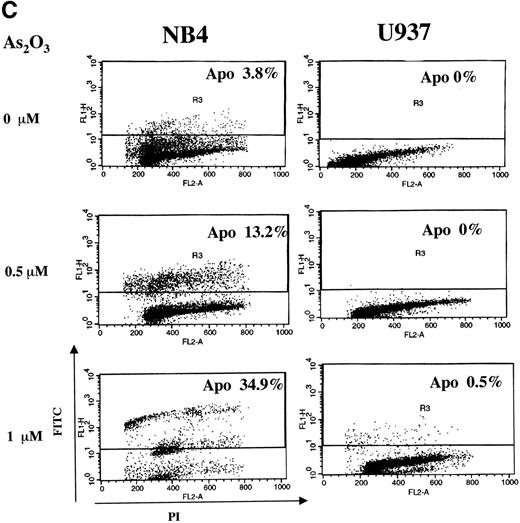
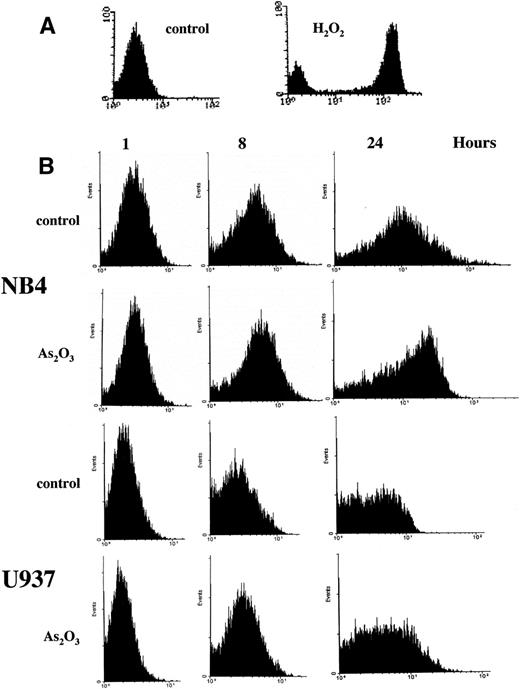
Treatment of NB4 cells with 1 μmol/L As2O3induced apoptosis, as indicated by morphologic analysis (Fig 1A), DNA distribution by FACS analysis demonstrating hypodiploid DNA (Fig 1B), and TUNEL assay (Fig 1C). In contrast, treatment of U937 cells with even 2 μmol/L As2O3 did not induce apoptosis (Fig 1A, B, and C). Although apoptosis was not detected in As2O3-treated U937 cells, cell growth was inhibited by As2O3 both in NB4 and U937 cells, with IC50 of about 0.7 and 1.2 mmol/L at 3 days of treatment, respectively. The growth inhibition was not correlated with arrest in a specific cell cycle phase (Fig 1B). These data were consistent with our previous report that As2O3inhibited cell growth in several lymphoma cells by prolongation of the cell cycle without blocking cells in a specific phase.26Thus, the selective effect of As2O3 in NB4 cells was due to apoptosis induction and not to growth inhibition. This differential sensitivity to As2O3-induced apoptosis in NB4 cells was associated with differences in cellular H2O2 levels that were determined by FACS analysis of cells labeled with DCFH-DA. The dramatic oxidation of DCFH-DA to DCF in NB4 cells treated with 50 μmol/L H2O2 for 1 hour served as a positive control (Fig 2A). NB4 cells in a standard culture had a higher oxidized DCF content as compared with U937 cells after adding DCFH-DA for 1 hour (Fig 2B). The oxidized DCF mean peak increased from 1.9 to 15.2 within 24 hours of incubation after initial loading of DCFH-DA in NB4 cells, but only increased from 0.9 to 4.3 in U937 cells. This is a reflection of a higher H2O2 content in NB4 cells due to either increased production or lower catabolism of H2O2. After treatment with 1 μmol/L As2O3, the mean of oxidized DCF peak increased compared with the untreated control from 1.9 to 2.4, 4.1 to 6.7, and 15.2 to 21.4 in 1, 8, and 24 hours after initial loading of DCFH-DA, respectively. In contrast, the mean of oxidized DCF peak was not increased by 1 μmol/L As2O3 in U937 cells even after 24 hours of treatment (Fig 2B). Thus, both the constitutive level of H2O2 and the ability of As2O3 to increase the level of H2O2 were correlated with the apoptotic activity of As2O3.
Comparison of apoptosis induction by As2O3 in NB4 versus U937 cells. (A) Fluorescence microscopy determination of apoptotic cells. Cells were treated with either 0 (control), 1, or 2 μmol/L As2O3 for up to 4 days, and the number of apoptotic cells was determined by fluorescence microscopy according to the morphology. The results are expressed as the percentage of apoptotic cells in the culture. Values shown are the mean of triplicate determinations with a standard deviation of less than 10%. (B) FACS analysis of apoptotic cells. Cells were treated for 3 days with the indicated concentrations of As2O3 and then evaluated for DNA content after propidium iodide staining. (C) TUNEL assay to determine apoptotic cells. Cells were treated with As2O3 at the indicated time for 3 days.
Comparison of apoptosis induction by As2O3 in NB4 versus U937 cells. (A) Fluorescence microscopy determination of apoptotic cells. Cells were treated with either 0 (control), 1, or 2 μmol/L As2O3 for up to 4 days, and the number of apoptotic cells was determined by fluorescence microscopy according to the morphology. The results are expressed as the percentage of apoptotic cells in the culture. Values shown are the mean of triplicate determinations with a standard deviation of less than 10%. (B) FACS analysis of apoptotic cells. Cells were treated for 3 days with the indicated concentrations of As2O3 and then evaluated for DNA content after propidium iodide staining. (C) TUNEL assay to determine apoptotic cells. Cells were treated with As2O3 at the indicated time for 3 days.
FACS scan analysis of H2O2accumulation. (A) NB4 cells were labled with DCFH-DA fluorescent probe for 1 hour and then treated with or without 50 μmol/L H2O2 for 1 hour as a positive control. (B) NB4 and U937 cells were treated with 1 μmol/L As2O3 for the indicated times, with DCFH-DA fluorescent probe being added 1 hour before the addition of As2O3. The oxidized DCF was analyzed by FACS.
FACS scan analysis of H2O2accumulation. (A) NB4 cells were labled with DCFH-DA fluorescent probe for 1 hour and then treated with or without 50 μmol/L H2O2 for 1 hour as a positive control. (B) NB4 and U937 cells were treated with 1 μmol/L As2O3 for the indicated times, with DCFH-DA fluorescent probe being added 1 hour before the addition of As2O3. The oxidized DCF was analyzed by FACS.
The role of H2O2 scavenging enzymes in As2O3-induced apoptosis.
To determine whether the higher basal levels of H2O2 in NB4 cells depends on lower H2O2 catabolism, the major cellular scavenging enzymes GPx and catalase were measured in several leukemia cell lines with different sensitivities to As2O3-induced apoptosis. The data in Table 1 show that the activity of H2O2 scavenging enzymes, GPx and catalase, was much higher in 4 cell lines that were insensitive to As2O3-induced apoptosis than in NB4 cells. The only exception is represented by K562 cells, in which the GPx activity was lower than that in NB4 cells. However, the very high activity in these cells of GSTπ, as compared with the low activity in NB4 cells may compensate for the low activity of GPx and catalase by greater efflux of cellular As2O3.23,27 28
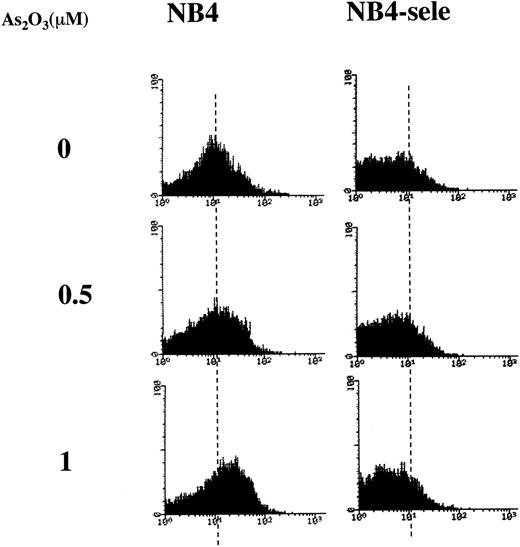
Based on the finding that NB4 cells have relatively low activities of GSTπ, catalase, and GPx (Table 1), we hypothesized that the higher sensitivity of NB4 cells towards As2O3-induced apoptosis is related to their low ability to metabolize the H2O2 produced during As2O3 treatment. To test this hypothesis, we tested the effect of selenite, an activator of GPx.29 To test the effect of a physiologic concentration of selenite, NB4 cells were grown for several generations in the presence of 100 nmol/L selenite. This treatment increased GPx activity by a factor of 7 while having no effect on cell growth. Under these conditions, As2O3 did not increase H2O2 levels (Fig3), and it induced apoptosis in only 7% of the cells, as compared with 58% in cultures not pretreated with selenite (Table 2). The data also demonstrated that As2O3 inhibited the activity of GPx and that this effect was less pronounced in cells pretreated with selenite (Table 2).
FACS analysis of H2O2accumulation in NB4 and selenite-pretreated NB4 cells. NB4 cells were pretreated without (NB4) or with 100 nmol/L sodium selenite for 9 days (NB4-sele) and then treated with the indicated concentrations of As2O3 for 24 hours. DCFH-DA was added 1 hour before the addition of As2O3, and the oxidized DCF was analyzed by FACS.
FACS analysis of H2O2accumulation in NB4 and selenite-pretreated NB4 cells. NB4 cells were pretreated without (NB4) or with 100 nmol/L sodium selenite for 9 days (NB4-sele) and then treated with the indicated concentrations of As2O3 for 24 hours. DCFH-DA was added 1 hour before the addition of As2O3, and the oxidized DCF was analyzed by FACS.
These results strongly suggested that the low H2O2-metabolizing activity of NB4 cells predispose them to As2O3-induced apoptosis. As a further test of this hypothesis, we determined whether the effect of As2O3 could be enhanced by treatment with mercaptosuccinic acid (MS),30 an inhibitor of GPx, and aminotriazol (AT), an inhibitor of catalase. As shown in Table 3, 100 μmol/L MS had no effect on cell apoptosis by itself, whereas 20 mmol/L AT moderately increased the percentage of apoptotic cells in NB4 and HL-60 cells. However, when combined with 0.5 μmol/L As2O3, which by itself was ineffective, both inhibitors raised the percentage of apoptotic NB4 cells to nearly 50%. Remarkably, in HL-60 and U937 cells, which normally respond to only greater than 5 μmol/L As2O3, cotreatment with 25 or 100 μmol/L MS and 1 μmol/L As2O3 raised the percentage of apoptotic cells to 39% and 43%, respectively (Table 3). The combination of 20 mmol/L AT and 1 μmol/L As2O3 was also more effective than either agent alone in HL-60 and U937 cells.
The mechanism of As2O3-induced apoptosis.
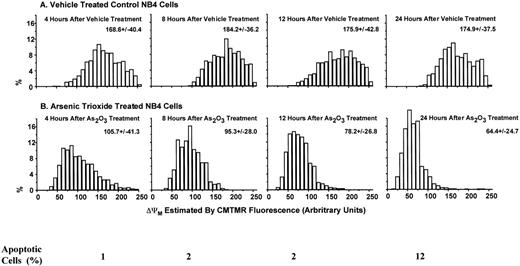
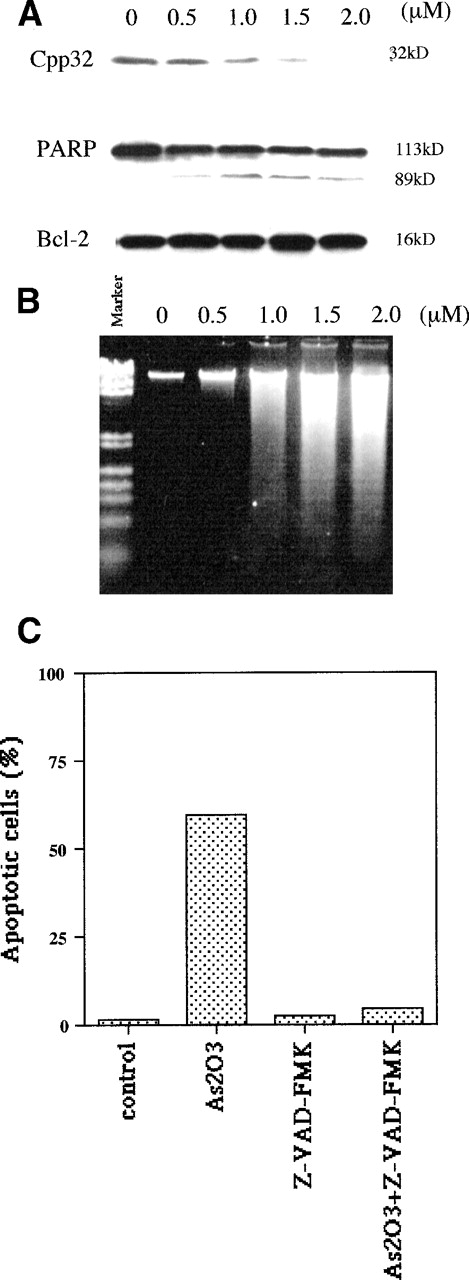
Apoptotic mechanisms are drug and cell-type–specific and are associated with the perturbation of mitochondrial functions.31 This process results in the activation of caspase and the fragmentation of DNA, coupled with characteristic morphologic changes. We tested whether As2O3-induced apoptosis is preceded by a decrease of the mitochondrial membrane potential (ΔΨM). We found that, in NB4 cells, ΔΨM decreased within 4 hours of treatment with 1 μmol/L As2O3 and decreased further with longer treatment times (Fig 4). As predicted from the decrease in ΔΨM, cytochrome c release into the cytoplasm was observed; the extent of release was slight 1 day after 1 μmol/L As2O3 treatment and complete 2 days later (Fig 5). Concomitant with the As2O3 induction of maximal cytochrome c release, Cpp32 was activated, as shown by the degradation of its precursor (32 kD) and the cleavage of PARP, a Cpp32 substrate. These events were independent of Bcl-2 degradation (Fig 6A) and were followed by DNA fragmentation (Fig 6B). As expected, high concentrations (200 μmol/L) of the general caspase inhibitor, Z-VAD-FMK, inhibited As2O3-induced apoptosis.
Decrease in mitochondrial membrane potential (▵ΨM) in NB4 cells treated with As2O3. The ▵ΨM of individual mitochondria was detected using confocal microscopy imaging of CMTMR fluorescence. NB4 cells were treated with 1 μmol/L As2O3 for the indicated times.
Decrease in mitochondrial membrane potential (▵ΨM) in NB4 cells treated with As2O3. The ▵ΨM of individual mitochondria was detected using confocal microscopy imaging of CMTMR fluorescence. NB4 cells were treated with 1 μmol/L As2O3 for the indicated times.
As2O3 treatment induces cytochrome c release from mitochondria. NB4 cells were treated with 1 μmol/L As2O3 for the indicated times and the cytochrome c content of mitochondrial and cytosolic fractions was detected by Western blot analysis as described.49
As2O3 treatment induces cytochrome c release from mitochondria. NB4 cells were treated with 1 μmol/L As2O3 for the indicated times and the cytochrome c content of mitochondrial and cytosolic fractions was detected by Western blot analysis as described.49
As2O3 treatment induces caspase-3 activation but not Bcl-2 degradation. (A) Activation of CPP32 and lack of Bcl-2 modulation after 3 days of treatment with the indicated doses of As2O3. (B) Effect of the same As2O3 treatment on DNA fragmentation. (C) Caspase inhibitor Z-VAD-FMK blocked As2O3-induced apoptosis. NB4 cells were treated for 3 days with 1 μmol/L As2O3 or 200 μmol/L Z-VAD-FMK alone or in combination; Z-VAD-FMK was added 4 hours before the addition of As2O3.
As2O3 treatment induces caspase-3 activation but not Bcl-2 degradation. (A) Activation of CPP32 and lack of Bcl-2 modulation after 3 days of treatment with the indicated doses of As2O3. (B) Effect of the same As2O3 treatment on DNA fragmentation. (C) Caspase inhibitor Z-VAD-FMK blocked As2O3-induced apoptosis. NB4 cells were treated for 3 days with 1 μmol/L As2O3 or 200 μmol/L Z-VAD-FMK alone or in combination; Z-VAD-FMK was added 4 hours before the addition of As2O3.
DISCUSSION
Recent developments suggest that a number of diverse apoptotic stimuli share a mechanistic pathway characterized by the generation of ROS and the loss of mitochondrial membrane potential, with subsequent outer mitochondrial membrane permeability changes, release of cytochrome c, and caspase activation.31-34 Thus, agents as diverse as ceramide, tumor necrosis factor-α, UV irradiation, and anthracyclines have been shown to trigger apoptosis via ROS production.24 35-38 Our findings indicate that As2O3 represents a novel apoptotic stimulus functioning via ROS, specifically H2O2, generation. This conclusion is supported by the following key observations. (1) Treatment of NB4 cells with an apoptotic concentration of As2O3 (1 μmol/L) inhibits the activity of GPx, an H2O2-catabolizing enzyme (Table 2). (2) The same treatment results in elevated intracellular H2O2 levels (Fig 2). (3) Pretreatment with selenite, a GPx activator, results in high GPx activity, lack of H2O2 accumulation, and inhibition of apoptosis (Table 2 and Fig 3). (4) Cotreatment with MS, a GPx inhibitor, and, to a lesser extent, with AT, a catalase inhibitor, potentiates the induction of apoptosis by As2O3(Table 3).
The high sensitivity of NB4 APL cells to As2O3appears to reside in a leukemic cell-specific pattern of expression of GPx and catalase,39 as well as GSTπ, which represents the major As2O3 detoxifying enzyme.40Thus, NB4 cells have low GSTπ, GPx, and catalase activities relative to 4 other non-APL leukemic cell lines (Table 1), suggesting that NB4 cells detoxify As2O3 and catabolize H2O2 less efficiently.
These findings are consistent with our previous work showing that As2O3-induced apoptosis is blocked by NAC and lipoic acid, agents that increase GSH, and is enhanced by BSO, a GSH-depleting agent.11 Thus, high levels of GSH, which is a proton donor for the GPx-catalyzed breakdown of H2O2 and for the GSTπ-catalyzed detoxification of As2O3, ensure rapid rates for these reactions, thus conferring a protective effect. Our findings are also consistent with the observations that 40 μmol/L arsenite induces apoptosis in hamster CHO41 and NIH3T3 cells42with generation of ROS, that H2O2-resistant CHO cells are less responsive,43 and that catalase-deficient CHO cells are hypersensitive to arsenic.44 The ascorbic acid synergism of As2O3-induced apoptosis in NB4 is mediated by H2O2 production, because it is inhibited by catalase.11
We suspect that As2O3 acts to increase H2O2 levels in NB4 cells mainly by inhibiting the activity of GPx, which is a major H2O2scavenging thiol-enzyme. We speculate that As2O3 inhibition of GPx is mediated by the binding of arsenic to vicinal thiol group in GPx,30 but this remains to be tested. The low levels of GSTπ limit detoxification of cellular As2O3, providing higher As2O3 binding ability that contributes to direct inactivation of GPx. On the other hand, it is possible that As2O3 also increases the mitochondrial production of H2O2. For instance, 20 μmol/L arsenite leads to H2O2 accumulation through a mechanism thought to depend on the activation of NADPH oxidase.42 It should also be pointed out that peroxidases other than GPx may also be targeted by As2O3. An example is thioredoxin peroxidase, another thiol peroxidase that protects against mitochondrial permeability transition by removing H2O2.45
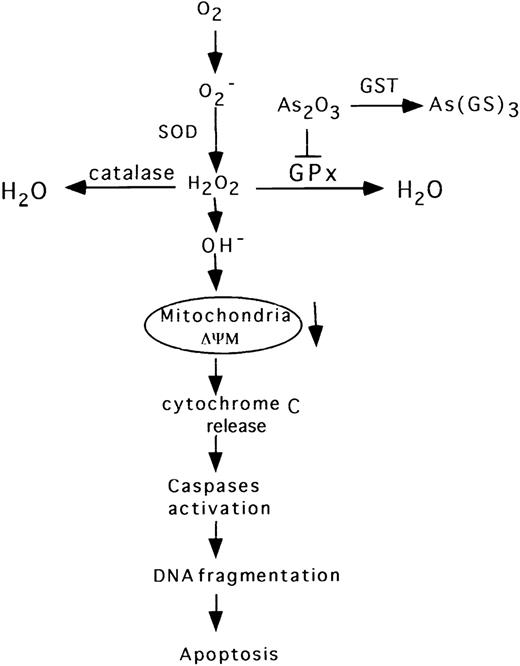
We have demonstrated that apoptosis in As2O3-treated NB4 cells proceeds via the classical pathway described for other ROS inductive signals.46-49 Thus, As2O3 elicited a rapid decrease in mitochondrial membrane potential (Fig 4), which preceded the release of cytochrome c, Cpp32 activation, DNA fragmentation, and morphologic evidence of apoptosis (Fig 5 and 6). However, in contrast to previous reports,8 we did not observe Bcl-2 degradation in NB4 cells treated with 1 to 2 μmol/L As2O3. This is consistent with a recent report showing that Bcl-2 is not degraded in NB4 cells, even when treated with 8 μmol/L As2O3.50 Moreover, human t(14;18) B-cell lymphoma su-DHL-4 cells overexpressing Bcl-2 are sensitive to 2 μmol/L As2O3 without degradation of Bcl-2.11 On the other hand, NIH3T342 and HL-60 cells with forced expression of Bcl-2 are more resistant to As2O3-induced apoptosis (Jing et al, unpublished data), consistent with the finding that cells overexpressing Bcl-2 are resistant to H2O2-induced cell death.25As2O3-induced apoptosis in NB4 cells is blocked by the caspase inhibitor Z-VAD-FMK (Fig 6). This result is consistent with the previous observation that Z-VAD-FMK blocked As2O3-induced apoptosis in lymphoma cells.51 Our current view of the apoptotic pathway induced by As2O3, as supported by the above-noted data, is depicted diagrammatically in Fig 7.
Diagrammatic representation of the proposed apoptotic pathway induced by As2O3 in NB4 cells.
Diagrammatic representation of the proposed apoptotic pathway induced by As2O3 in NB4 cells.
A fundamental question is why are NB4 APL cells uniquely sensitive to As2O3? In other words, how does the PML-RARα fusion protein that represents the molecular signature of APL confer sensitivity to As2O3? As already discussed, our data point to the low GSTπ, GPx, and catalase activities of NB4 cells as a logical explanation for their sensitivity to As2O3. What then is the relationship between PML-RARα and the activity of these enzymes? We suspect that the answer lies partly in the facts that GST is an RA-inducible gene52 and that PML-RARα is a dominant repressor of RARα function.15-17 Thus, it seems likely that PML-RARα inhibits the RARα activation of GSTπ in NB4 cells, leading to reduced enzyme expression and activity. RA has also been shown to upregulate GPx and catalase expression in some cell types, but whether this is a transcriptional effect remains to be demonstrated.53 Thus, it is conceivable that PML-RARα also interferes with GPx and catalase expression. These are testable hypotheses that we are currently pursuing. For instance, one prediction is that, if PML-RARα protein expression is decreased by pretreatment with RA,54 then NB4 cells may be less sensitive to As2O3-induced apoptosis. Our preliminary experiments suggest that this is in fact the case (Jing et al, unpublished data). In addition, cotreatment of NB4 cells with tRA and As2O3 has been shown to result in decreased apoptosis.10 Whatever the mechanism through which PML-RARα confers sensitivity to As2O3, it is clear that PML-RARα is indeed required for the therapeutic effect of As2O3; this is true both in vitro, as shown by the sensitization of U937 cells to As2O3 by ectopic PML-RARα expression,55 and in vivo, as shown by the finding that only 2 of 53 APL patients that did not experience clinical remission during As2O3 therapy had PML-RARα–negative disease.56
Interestingly, we have found that P388 lymphoma cells are growth inhibited by As2O3 with an IC50 of 1.7 μmol/L, and that P388/adr cells with multidrug resistance (MDR1) to adriamycin (482-fold) and taxol (111-fold)57 remain responsive to As2O3 (IC50 of 2.0 μmol/L). This suggests that As2O3 is not a substrate for the MDR1 drug efflux pathway, which is consistent with other reports40.58 and our proposal that GSTπ-mediated efflux represents the major As2O3 efflux pathway in NB4 cells. Furthermore, the lack of cross-resistance between adriamycin and As2O3 is consistent with the clinical observation that anthracycline-resistant APL is responsive to As2O3 therapy.2,4 5
It has been recently reported that the clinical remission induced by As2O3 in APL patients and in transgenic mice is accompanied in part by the induction of cell differentiation.5,59 It remains to be seen to what extent the H2O2-dependent pathway we have unmasked here contributes to the induction and/or maintenance of clinical remission. It will also be of interest to pursue the feasibility of extending As2O3 therapy to other leukemias or lymphomas that have shown variable sensitivity to As2O351 60 by appropriate cotreatment with drugs capable of altering the ROS balance in favor of cell death activation, as exemplified by our in vitro findings of apoptosis induction in HL-60 and U937 cells by As2O3 and MS.
ACKNOWLEDGMENT
The authors appreciate the advice of Dr George Acs throughout these studies and critical reading by Dr Rafael Mira-y-Lopez.
Supported by National Institutes of Health Grant No. 5RO1CA59936-03-05, the Gloria and Sidney Danziger Foundation, and the Samuel Waxman Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yongkui Jing, PhD, Division of Neoplastic Diseases, Department of Medicine, Box 1178, Mount Sinai Medical Center, One Gustave L. Levy Place, New York, NY 10029-6547.