Abstract
Malignant cell contamination in autologous transplants is a potential origin of tumor relapse. Ex vivo expansion of CD34+ blood progenitor cells (BPC) has been proposed as a tool to eliminate tumor cells from autografts. To characterize the influence of culture conditions on survival, growth, and clonogenicity of malignant cells, we isolated primary mammary carcinoma cells from pleural effusions and ascites of patients with metastatic breast cancer and cultured them in the presence of stem cell factor (SCF), interleukin-1β (IL-1β), IL-3, IL-6, and erythropoietin (EPO), ie, conditions previously shown to allow efficient ex vivo expansion of CD34+ BPC. In the presence of serum, tumor cells proliferated during a 7-day culture period and no significant growth-modulatory effect was attributable to the presence of hematopoietic growth factors. When transforming growth factor-β1 (TGF-β1) was added to these cultures, proliferation of breast cancer cells was reduced. Expansion of clonogenic tumor cells was seen in the presence of SCF + IL-1β + IL-3 + IL-6 + EPO, but was suppressed by TGF-β1. Cocultures of tumor cells in direct cellular contact with hematopoietic cells showed that tumor cell growth could be stimulated by ex vivo expanded hematopoietic cells at high cell densities (5 × 105/mL). In contrast, culture under serum-free conditions resulted in death of greater than 90% of breast cancer cells within 7 days and a further decrease in tumor cell numbers thereafter. In the serum-free cultures, hematopoietic cytokines and cellular contact with CD34+ BPC could not protect the tumor cells from death. Therefore, ex vivo expansion of CD34+ BPC in serum-free medium provides an environment for efficient purging of contaminating mammary carcinoma cells. These results have clinical significance for future protocols in autologous progenitor cell transplantation in cancer patients.
IN RECENT YEARS, high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation has been increasingly used for the treatment of breast cancer.1Initial results from clinical trials using high-dose chemotherapy protocols to treat breast cancer patients have suggested an increased therapeutic benefit compared with conventional chemotherapy regimens, including improved response rates and better survival.2,3Because contamination of the autologous grafts with tumor cells does occur,4,5 and may be of clinical significance,6-11 attempts have been made to deplete the tumor cells within autologous grafts by manipulation of the harvests ex vivo.
One possibility to purge autologous grafts is antibody-mediated selection of hematopoietic progenitor cells, eg, enrichment for CD34+ progenitor cells. Yet, the reported clinical trials using currently available CD34+ selection technology have shown that it is difficult to achieve CD34+ cell purities of greater than 90%, which are equivalent to an expected 2 log depletion of breast cancer cells within the graft.12Contaminating breast cancer cells have indeed been detected in positively selected CD34+ fractions from both bone marrow12 and mobilized peripheral blood13 (and own unpublished results). Ex vivo expansion of blood progenitor cells has been proposed as an alternative or additional tumor cell purging strategy, if it selectively favors hematopoietic cell survival and expansion in culture conditions adverse for malignant cells. A number of studies have defined optimal culture conditions to generate increased numbers of progenitor cells from CD34+enriched cells.14,15 Hematopoietic progenitor cells expanded ex vivo in liquid cultures in the presence of cytokines have so far been used to initiate long-term bone marrow cultures,15,16 to repopulate the bone marrow of lethally irradiated mice,17 and to accelerate hematopoietic reconstitution in humans after myelosuppressive chemotherapy.18
Ex vivo expansion of CD34+ hematopoietic cells could be used as a means of tumor cell purging only if contaminating tumor cells do not coexpand in the ex vivo expansion systems. Although some studies have suggested the absence of detectable tumor cells in the ex vivo expanded hematopoietic cell collections, the question of whether tumor cells survive or proliferate in the cultures could not be formally answered in some previous studies, because the CD34+starting cell populations were not screened19 or were negative20 for tumor cell contamination. In contrast, Purdy et al21 reported the possibility that malignant cells may persist after ex vivo culture. Also, breast cancer cells contained in harvests of bone marrow or mobilized peripheral blood have been found to be viable and to possess the capacity for clonogenic growth in vitro.4,22 23 We hypothesized that culture conditions may alter the fate of tumor cells in ex vivo expansion cultures and we therefore analyzed the influence of ex vivo expansion parameters (ie, cytokines, culture medium, and hematopoietic cells) on the survival, growth, and clonogenicity of primary breast cancer cells ex vivo. Breast cancer cells were found not to be susceptible to growth stimulation by hematopoietic cytokines used to ex vivo expand CD34+ blood progenitor cells, but a tumor cell-suppressive effect was obtained when using serum-free medium preparations. These data have implications for future ex vivo expansion protocols used in patients with mammary carcinoma.
MATERIALS AND METHODS
Primary breast cancer cells.
Tumor cells were derived from pleural effusions or ascites from patients with disseminated stage of metastatic breast cancer (≥3 sites involved). Primary tumors were histologically classified as infiltrating ductal carcinomas and nuclear grading was II-III in all patients. All patients had received previous chemotherapy, but no chemotherapy had been administered throughout the last 3 months before the specimens were obtained. Cells from the malignant effusions were sedimented by centrifugation at 400g for 6 minutes. An aliquot was processed for immunocytological analyses (specified below) to assess the malignant cell content of the patient samples. Cells were seeded into T175 flasks (Falcon; Becton Dickinson, Heidelberg, Germany) in α-Minimal Essential Medium (α-MEM; GIBCO BRL, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS; Pan Systems, Passau, Germany) at a density of 1 to 5 × 106/mL. In later experiments (shown in Fig 5), cells were seeded directly after sedimentation into the respective assays at a density of 0.1 to 1 × 106/mL. When reaching confluency, cells were detached from the culture surface with trypsin-ethylenediaminetetraacetic acid (EDTA) solution (Sigma, Munich, Germany), washed twice with phosphate-buffered saline (PBS; BioWhittaker, Brussels, Belgium), counted, and either reseeded in culture medium or resuspended in HEPES (hydroxyethyl-piperazine-ethane sulfonic acid)-buffered saline (HBS) for subsequent immunocytologic staining with anticytokeratin, antiepithelial-specific, and antimesothelial-specific monoclonal antibodies (MoAbs; see below). In some experiments, both adherent and nonadherent cells were passaged as indicated in the respective figure legends. In all other experiments, only the adherent cell populations were subcultured. The tumor cell content of the successfully established cultures (termed samples 1 through 3) was documented serially by immunocytochemistry. Initially, the content of CK+/Ber-Ep4+ cells was heterogeneous (content of CK+ cells for samples 1 through 6: 10.6%, 9.6%, 79.2%, 0.24%, 11.3%, and 10.2%, respectively; contents of BER-Ep4+ cells for samples 1 through 6: 9.4%, 8.5%, and 80.7%), respectively. Stock cultures of greater than 95% tumor cells (CK+/Ber-Ep4+cells) were used as a source of tumor cells for proliferation and clonogenic assays. Population doubling times were calculated from the cell numbers determined at a given time point and a second time point between 4 and 7 days thereafter before the cultures reached confluency.
Media and growth factors.
Serum-free culture medium (SFM) containing Iscove’s Modified Dulbecco’s Medium (IMDM; BioWhittaker), bovine serum albumin (Boehringer Mannheim, Mannheim, Germany; 2%), cholesterol (Sigma; 2 × 10−6 mol/L), β-mercaptoethanol (Serva Heidelberg, Germany; 5 × 10−5 mol/L), and insulin (Sigma; 0.01 mg/mL) was prepared using a protocol originally developed for clonal hematopoietic culture assays.24 25 Suppliers of recombinant growth factors and concentrations used were: stem cell factor (SCF; Genzyme, Cambridge, MA; 10 ng/mL), interleukin-1β (IL-1β; Genzyme; 3 ng/mL), IL-3 (Genzyme; 100 ng/mL), IL-6 (Genzyme; 100 ng/mL), erythropoietin (EPO; Erypo 4000; Cilag, Sulzbach, Germany; 1 U/mL); transforming growth factor-β1 (TGF-β1; R&D Systems, Wiesbaden, Germany; 30 ng/mL), epidermal growth factor (EGF; Genzyme; 1 μg/mL), and insulin-like growth factor (IGF; Genzyme; 0.1 μg/mL). Long-term bone marrow culture (LTBMC) medium consisted of IMDM, 10% horse serum (Sigma), 10% fetal calf serum (Pan Systems), and 5 × 10−7 mol/L hydrocortisone (Sigma).
Tumor cell proliferation assays.
Tumor cells from the stock cultures were trypsinized and seeded into 24-well plates (Falcon) in FCS-supplemented α-MEM medium at 10,000 cells/cm2 and various concentrations of added growth factors. After 7 days, cells were trypsinized for 10 minutes at 37°C and cell counts were determined in a hemocytometer. Cell viability was greater than 95% by trypan blue exclusion. Aliquots were also processed for immunocytological analysis to document the tumor cell origin of the cells (see below).
Tumor cell colony assay.
Tumor cells from stock cultures were trypsinized, washed twice, and plated in duplicates in 35-mm Petri dishes (1 × 104/dish; Nunc, Wiesbadem, Germany) in a semisolid medium consisting of 55% LTBMC medium, 45% of a 2.1% methylcellulose solution in IMDM (WAK-Chemie, Bad Homburg, Germany), 1 μg/mL human recombinant EGF, and 0.1 μg/mL human recombinant IGF. All cultures were evaluated light-microscopically and contained single-cell suspensions after plating. Initial seeding of tumor cell aggregates was never recorded. Dishes were incubated at 37°C in 5% CO2 and colonies consisting of more than 30 cells were scored under a light microscope on days 17 to 20 after plating. Cultures without supplemental growth factors (EGF and IGF) did not give rise to tumor cell colonies and were used as negative controls. Colonies from each dish were picked with a Pasteur pipette, diluted in 100 μL of PBS, and attached onto glass slides by cytocentrifugation (Cytospin 3; Shandon, Runcorn, UK). Slides were immunostained with fluorescein isothiocyanate (FITC)-anticytokeratin MoAbs (see below) to determine the epithelial cell origin of the colonies. Absolute numbers of clonogenic tumor cells per culture were calculated by multiplying the clonogenicity of tumor cells with the number of total cells in the cultures.
Immunocytological detection of tumor cells.
A mixture of two IgG1 anticytokeratin antibodies (AE1/AE3 [Boehringer Mannheim] and KL1 [Dianova, Hamburg, Germany]), an anticytokeratin antibody (MNF116; DAKO, Hamburg, Germany) that specifically recognizes members of the acidic as well of the basic subfamily of cytokeratins, the antiepithelial-specific Ber-Ep4 antibody (DAKO, Glastrup, Denmark) that recognizes two glycoproteins present on epithelial cells but not mesothelial cells,26 and a mesothelial-specific anticalretinin antibody (Zymed Laboratories, San Francisco, CA) were used to detect mammary carcinoma cells and distinguish them from mesothelial or hematopoietic cell populations. For staining with AE1/AE3 (1:50 dilution) + KL-1 (1:200 dilution) and with Ber-Ep4 (1:40 dilution) antibodies, cells were washed twice in HBS, attached in duplicate to poly-L-lysine–coated adhesion slides (Bio-Rad, Munich, Germany) consisting of 12 spots of 5-mm diameter, and dried on the spots according to the manufacturer’s recommendations. Slides were stored in sealed plastic bags at −20°C until use. Cells were fixed and permeabilized in serial dilutions of acetone in ethanol for 5 minutes at 4°C, incubated with the MoAbs, and immunostained in an alkaline phosphatase-antialkaline phosphatase assay using the APAAP assay (DAKO). MCF-7 mammary carcinoma cells obtained from the American Type Culture Collection (ATCC; Rockville, MD) were used as a positive control in each assay, and an IgG1 isotype antibody served as a negative control. Staining with these antibodies of peripheral blood mononuclear cells, of bone marrow cells, or of CD34+ blood progenitor cell populations from patients with hematologic malignancies never gave positive results. Regularly, a total of 2,000 cells per spot was examined microscopically (at least 1,700), and the incidence of cells staining bright red was determined. Absolute numbers of tumor cells per culture were calculated from the incidence of immunocytologically detected tumor cells and the total cell counts within a culture at the end of the culture period. For immunostaining with the MNF116 anticytokeratin (1:700 dilution) and the anticalretinin (1:1 dilution) antibodies, cells were formalin-fixed and paraffin-embedded. Cytokeratin staining was performed after digestion for 10 minutes with 0.5% proteinase K (Sigma-Aldrich GmbH, Munich, Germany) using the avidin-biotin-complex method (Vectorstain-Kit; Vector, Burlingame, CA). Calretinin staining was performed after heat-induced epitope retrieval according to the manufacturer’s instructions. For immunfluorescence staining of colonies in the tumor colony assay, several colonies per dish were randomly selected, aspirated through a 100-μL pipette tip, resuspended in 100 μL PBS, spun onto glass slides using a cytocentrifuge, and fixed in acetone for 5 minutes. Immunostaining was performed for 1 hour at 4°C in a moist chamber using an FITC-conjugate of an anticytokeratin IgG1 (Cytokeratin-FITC, clone CAM 5.2; Becton Dickinson). After washing in PBS for 5 minutes, the slides were covered with a mixture of propidium iodide (Oncor, Gaithersburg, MD) and mounting medium (Vectashild; Vector) and examined under a fluorescence microscope (Axiophot; Zeiss, Stuttgart, Germany). The nuclei of both cytokeratin-positive and -negative cells stained red, and only cells with a bright green cytoplasm were scored as cytokeratin-positive. No staining with anticytokeratin MoAbs was observed in hematopoietic colonies derived from CD34+ blood progenitor clonogenic assays.
Detection of ErbB2-receptor expression by immunoblotting.
Western blot analyses of erbB2-protein expression were performed as previously described.27 Briefly, extracts from 2.5 to 5 × 105 patient cells (except sample 3, 2.5 × 106 cells) and from 5 to 7 × 105SKBR3 or A431 cells (ATCC) were subjected to a 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were electroblotted onto nylon membranes. The p185ErbB2 proteins were detected using the 21N antisera and an enhanced chemiluminescence detection kit (Amersham, Braunschweig, Germany) as described.
Coculture of CD34+ blood progenitor cells and primary mammary carcinoma cells.
CD34+ hematopoietic cells were obtained from patients with solid tumors or hematologic malignancies who underwent blood progenitor cell (BPC) mobilization by leukapheresis after informed consent. For the experiments using previously expanded hematopoietic cells, ex vivo expansion of CD34+ cells was performed in 24-well plates and 1 mL culture medium at 37°C and 5% CO2 in a humidified atmosphere using IMDM (BioWhittaker) containing FCS or SFM and 10 ng/mL SCF, 3 ng/mL IL-1β, 100 ng/mL IL-3, 100 ng/mL IL-6, and 1 U/mL EPO as described.24 25 Cells proliferated exponentially and reached a 8.8- to 10.2-fold increase in cell numbers by day 7 and a 20- to 80-fold increase by day 14. After 14 days, differentiated granulocytic and monocytic/macrophage cells predominated in the cultures. Fresh or 14-day expanded CD34+hematopoietic cells were cocultured for 3 days with 10 fluorescence-labeled tumor cells (see below) in 96-well flat-bottom plates (Becton Dickinson) or for 7 days with unlabeled mammary carcinoma cells at a ratio 1:10 in 3-mL slide flasks (Nunc, Wiesbaden, Germany). Cocultures were preformed in the presence of SCF, IL-1β, IL-3, IL-6, and EPO. Cell viability under these conditions remained greater than 80%. During the first 72 hours, no significant increase of cell numbers was recorded. For tumor cell labeling, the fluorescent dye PKH-26 (Sigma) was used according to the manufacturer’s instructions. Briefly, 105 tumor cells were washed two times in IMDM (BioWhittaker), and the pellet was suspended in 600 μL diluent buffer. One microliter of PKH-26 solution (10−3 mol/L) prediluted into 100 μL diluent buffer was added, and the mixture was left at room temperature for 3 minutes with gentle agitation for several times. The labeling reaction was stopped by the addition of 1 mL heat-inactivated FCS (Pan Systems) for 1 minute. This mixture was then carefully underlayered with 1 mL heat-inactivated FCS. Cells were cytocentrifuged for 5 minutes at 400g, the supernatant was removed, and the pellet was washed three times in serum-containing medium (IMDM/10% FCS) in a fresh tube for every washing step. Finally, cells were taken up in culture medium, counted, and used for the assays. The whole cultures of CD34+ cells and tumor cells were examined under a fluorescence microscope, and cells displaying a red fluorescence were scored positive. In the experiments performed for 7 days, both adherent and nonadherent cells were collected, fixed on adhesion slides, and stained with anticytokeratin and Ber-Ep4 antibodies as described above to determine the numbers of tumor cells.
Statistical analyses.
For statistical analysis, data for the control groups were normalized to 1, and the groups were compared using a two-tailed Student’st-test. A P value less than .05 was considered significant.
RESULTS
Isolation and enrichment of primary breast cancer cells.
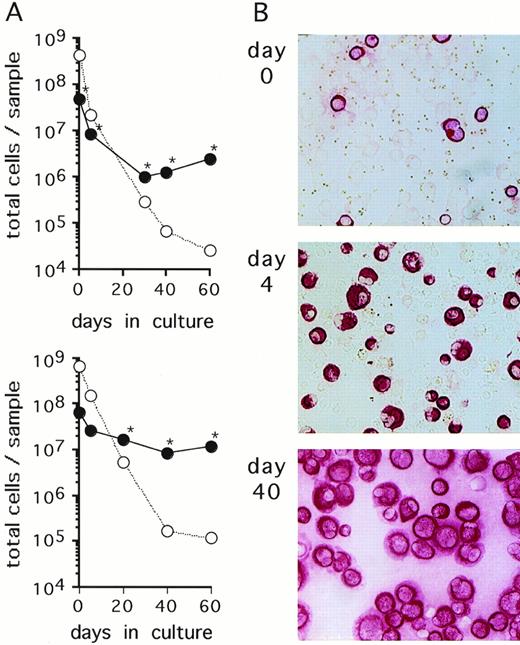
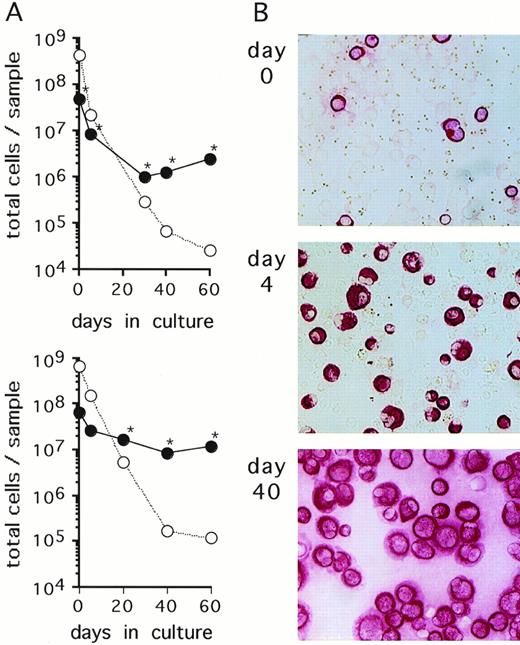
Cells isolated from serous fluids (ascites or pleural effusions) of patients with metastatic breast cancer were cultured in FCS-supplemented α-MEM. In 6 of 11 specimens obtained from different patients, an outgrowth of breast carcinoma cells as identified by positive immunostaining with anticytokeratin (CK) and Ber-Ep4 antibodies and negative immunostaining with anticalretinin antibodies was observed. The 6 samples that yielded growing mammary carcinoma cells initially presented with a very heterogenous content of CK-positive cells (0.2% to 79%). CK-negative cells consisted mostly of erythrocytes, macrophages, and lymphocytes, as evaluated through Wright-Giemsa–stained cytospin preparations. Growth kinetics of both CK-positive and CK-negative cells were monitored by staining aliquots with anti-CK antibodies. The absolute numbers of CK-positive cells decreased during the first few weeks of culture, indicating that a proportion of tumor cells did not survive in culture (Fig1). At later time points, numbers of CK-positive cells started to increase and, concomitantly, CK-negative cells completely disappeared from the cultures (Fig 1). Continuous subcultivation of the adherent cell populations for more than 10 to 30 days resulted in purities of CK-positive cells of greater than 95%. At these and later time points, cells in the cultures were also stained with the epithelial-specific Ber-Ep4 MoAb with all samples staining positive in greater than 95% of cells, indicating that the cells were of tumor origin and not derived from mesothelium which does not express this antigen.28,29 In addition, the cells stained negative with the mesothelial-specific anticalretinin antibody30(Table 1). Cells from 4 of the 5 analyzed cultured samples were found to express the erbB2-protein at levels comparable to SKBR3 or higher than A431 cell lines, as detected by Western blot analyses (Table 1). These levels of erbB2 expression reflect an overexpression of this protein according to the quantitative estimates for normal breast tissues, breast cancer tissues, and various cell lines.31-33 Enriched primary breast cancer cells proliferated ex vivo until the cultures terminated spontaneously within 60 to 130 days (Table 1). Cell aliquots taken during this growth period were used for subsequent experiments.
Primary breast cancer cell cultures. (A) Cells contained in an ascites (upper panel) and a pleural effusion sample (lower panel) from 2 representative breast cancer patients were cultured in -MEM/10% FCS. At the indicated time points, numbers of CK-positive cells (•) and CK-negative cells (○) were determined by immunocytochemical analysis as described in Materials and Methods. At the time points indicated with an asterisk (*), detection of Ber-Ep4 was also performed yielding nearly identical cell numbers as CK-positive cells. Cultures were maintained by subcultivating total cells, ie, adherent and nonadherent fractions. (B) Immunostaining with anti-CK MoAbs of the cultures shown in the upper panel of (A) at the indicated time points (original magnification, ×100).
Primary breast cancer cell cultures. (A) Cells contained in an ascites (upper panel) and a pleural effusion sample (lower panel) from 2 representative breast cancer patients were cultured in -MEM/10% FCS. At the indicated time points, numbers of CK-positive cells (•) and CK-negative cells (○) were determined by immunocytochemical analysis as described in Materials and Methods. At the time points indicated with an asterisk (*), detection of Ber-Ep4 was also performed yielding nearly identical cell numbers as CK-positive cells. Cultures were maintained by subcultivating total cells, ie, adherent and nonadherent fractions. (B) Immunostaining with anti-CK MoAbs of the cultures shown in the upper panel of (A) at the indicated time points (original magnification, ×100).
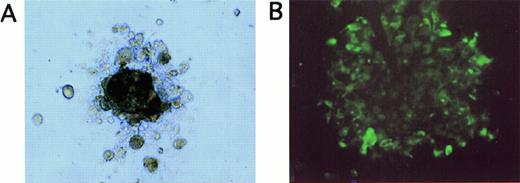
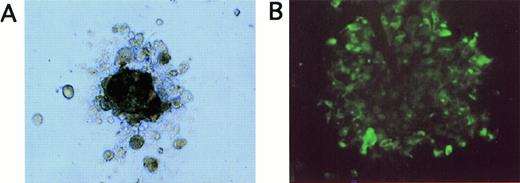
(A) Appearance of a tumor cell colony grown for 20 days in methylcellulose in LTBMC medium supplemented with EGF and IGF (original magnification, ×100). (B) Immunofluorescence staining of cells picked from tumor cell colonies shown in (A) after staining with FITC-labeled anticytokeratin antibodies (original magnification, ×40).
(A) Appearance of a tumor cell colony grown for 20 days in methylcellulose in LTBMC medium supplemented with EGF and IGF (original magnification, ×100). (B) Immunofluorescence staining of cells picked from tumor cell colonies shown in (A) after staining with FITC-labeled anticytokeratin antibodies (original magnification, ×40).
Effect of cytokines on breast cancer cells.
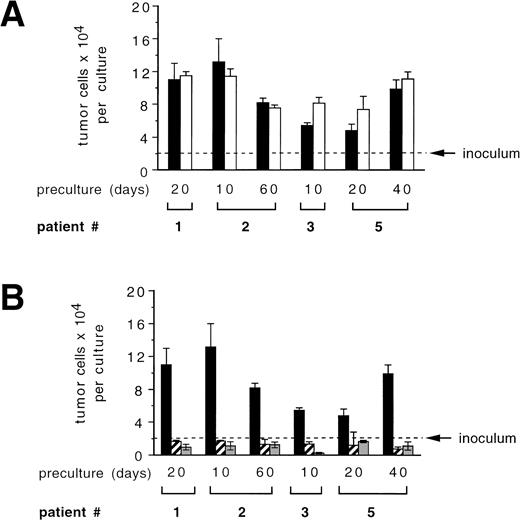
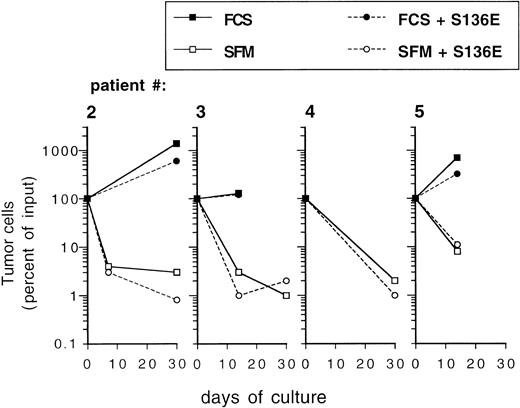
To address the question whether the cytokines used for ex vivo expansion of CD34+ blood progenitor cells may influence the growth of breast cancer cells in culture, we studied tumor cell proliferation in the presence and absence of SCF + IL-1β + IL-3 + IL-6 + EPO, a cytokine combination previously shown to efficiently mediate ex vivo expansion of CD34+ blood progenitor cells.34 Enriched primary mammary carcinoma cells used after various precultivation periods and derived from different patients showed a 2.4- to 6.6-fold increase in cell numbers over a 7-day period in control cultures (Fig 2). Overall, the addition of cytokines SCF + IL-1β + IL-3 + IL-6 + EPO did not result in a significant inhibitory or stimulatory effect on tumor cell growth compared with cultures without cytokines (Fig 2A). TGF-β1, a pleiotropic cytokine that has been shown to suppress the in vitro growth of epithelial-derived tumor cells,35 was recently found to allow reduced, but still significant expansion of colony-forming cells without loss of primitive LTBMC-initiating cells in cytokine-supported CD34+ BPC ex vivo expansion.36 To determine if TGF-β1 could be of use to purge CD34+ autografts from breast cancer cells during ex vivo expansion, we investigated its potential to influence the survival and proliferation of breast cancer cells. As outlined in Fig 2B, enriched primary tumor cells proliferated in control cultures, yet their growth was inhibited in the presence of TGF-β1. In all cases, cell numbers fell below the cell numbers initially seeded into the cultures, irrespective of the presence of cytokines, SCF + IL-1β + IL-3 + IL-6 + EPO.
Influence of hematopoietic stimulatory cytokines (A) or TGF-β1 (B) on proliferation of primary mammary carcinoma cells. Primary mammary carcinoma cells were precultured in -MEM/10% FCS for the indicated periods. Enriched tumor cells (purity >95% of CK-positive and Ber-Ep4–positive cells) were cultured for 7 days in -MEM/10% FCS alone or with 10 ng/mL SCF, 3 ng/mL IL-1β, 100 ng/mL IL-3, 100 ng/mL IL-6, and 1 U/mL EPO (S136E) and/or 30 ng/mL of TGF-β1. (▪) Controls; (□) S136E; (▨) TGF-β1; (▩) TGF-β1 + S136E. Results represent the mean values ± SD of duplicate determinations. A P value greater than .05 was obtained when comparing the mean values of both groups in a two-tailed Student’st-test in (A). Statistical significant differences (P < .05) were recorded when comparing the control group (no cytokines) with either the TGF-β1 or the S136E plus TGF-β1 group in (B) using a two-tailed Student’s t-test.
Influence of hematopoietic stimulatory cytokines (A) or TGF-β1 (B) on proliferation of primary mammary carcinoma cells. Primary mammary carcinoma cells were precultured in -MEM/10% FCS for the indicated periods. Enriched tumor cells (purity >95% of CK-positive and Ber-Ep4–positive cells) were cultured for 7 days in -MEM/10% FCS alone or with 10 ng/mL SCF, 3 ng/mL IL-1β, 100 ng/mL IL-3, 100 ng/mL IL-6, and 1 U/mL EPO (S136E) and/or 30 ng/mL of TGF-β1. (▪) Controls; (□) S136E; (▨) TGF-β1; (▩) TGF-β1 + S136E. Results represent the mean values ± SD of duplicate determinations. A P value greater than .05 was obtained when comparing the mean values of both groups in a two-tailed Student’st-test in (A). Statistical significant differences (P < .05) were recorded when comparing the control group (no cytokines) with either the TGF-β1 or the S136E plus TGF-β1 group in (B) using a two-tailed Student’s t-test.
Because induction of cell proliferation and differentiation by hematopoietic growth factors has been described in nonhematopoietic malignant cells,37 we investigated whether the treatment of breast cancer cells with cytokines influences subpopulations of tumor cells with in vitro clonogenic potential. Tumor cell colonies were grown in EGF- and IGF-supported semisolid assays and were of irregular shape, consisting of aggregates of closely attached cells (Fig3A). The epithelial origin of the clonogenic cells was confirmed by immunofluorescence staining of the cells picked from the colonies with anticytokeratin MoAbs (Fig 3B). As outlined in Table2, tumor cells from 3 of 5 patient samples scored positive in the tumor colony assay. When tumor cells had been pretreated with hematopoietic cytokines SCF + IL-1β + IL-3 + IL-6 + EPO for 7 days, tumor cells formed colonies with a comparable cloning efficiency as untreated controls (Table 2). Absolute numbers of clonogenic tumor cells per culture were also not significantly different in control and SCF + IL-1β + IL-3 + IL-6 + EPO-treated cultures. These results suggest that a cytokine combination used to ex vivo expand CD34+ hematopoietic cells, SCF + IL-1β + IL-3 + IL-6 + EPO, does not influence the growth of breast cancer cell subpopulations with in vitro clonogenic potential. In contrast, TGF-β1 suppressed clonogenic tumor cell populations as determined in tumor colony assays, resulting in a reduction of numbers of clonogenic progenitors compared to the respective control cultures (Table 2). The combined treatment of tumor cells with TGF-β1 and SCF + IL-1 + IL-3 + IL-6 + EPO resulted in a similar inhibitory effect as TGF-β1 alone, with reduced numbers of clonogenic cells compared with untreated control cultures (Table 2).
Influence of CD34+ cells on tumor cells during ex vivo expansion.
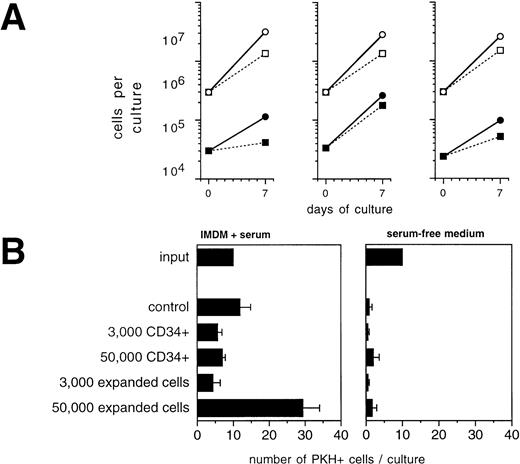
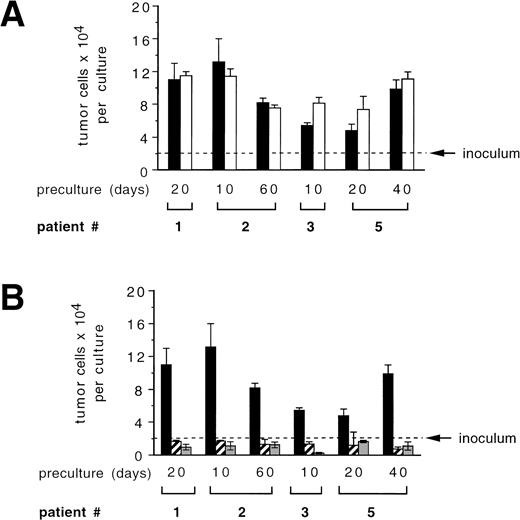
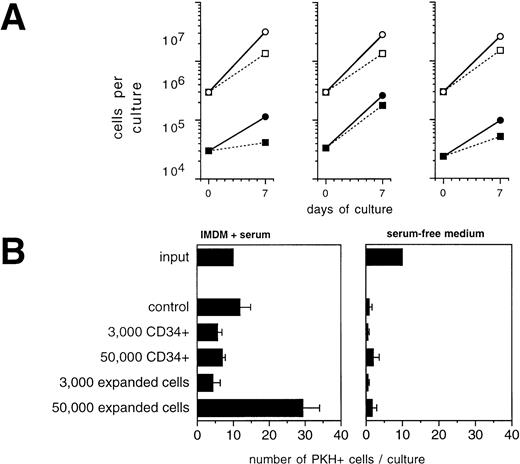
To analyze the potential of CD34+ cells or their progeny to influence the growth of tumor cells during ex vivo expansion, we performed mixing experiments of CD34+-enriched BPC with primary mammary carcinoma cells. During a 7-day culture period in the presence of SCF + IL-1 + IL-3 + IL-6 + EPO, which led to an overall 9.5- ± 0.7-fold expansion in total numbers of hematopoietic cells, CK+/Ber-Ep4+ cells coexpanded by a factor of 5.2 ± 1.4 (Fig 4A). Comparison with the results of the analogous experiments in the absence of CD34+ cells shown in Fig 2 demonstrates that CD34+ cells, under these conditions, did not detectably influence tumor cell growth. Interestingly, the addition of TGF-β1 still led to suppressed tumor cell growth, but in contrast to the previous experiments with TGF-β1 in the absence of CD34+cells, absolute numbers of tumor cells increased. Therefore, the presence of hematopoietic cells interferes with the potential of exogenously added TGF-β1 to inhibit tumor cell growth and survival. In another assay, highly enriched CD34+ hematopoietic cells or late-stage hematopoietic cell populations that are found on day 14 of ex vivo expansion were separately analyzed for their potential to influence growth of PKH-labeled tumor cells. In the presence of serum together with high numbers (5 × 105/mL) of 14-day ex vivo expanded CD34+ cells, numbers of PKH-labeled tumor cells increased up to 2.5-fold compared with control cultures (Fig 4B, left panel). In contrast, during this 72-hour period, no increases in tumor cell numbers were detected in the presence of CD34+BPC (Fig 4B, left panel). Therefore, mature hematopoietic cells at higher cell densities may harbor a risk to stimulate tumor cell growth.
Influence of hematopoietic cells on primary breast cancer cells during ex vivo expansion. (A) Culture-enriched mammary carcinoma cells (solid symbols) and CD34+ enriched BPCs (open symbols) were cocultured at a ratio of 1:10 in 3-mL flasks in the presence of SCF, IL-1β, IL-3, IL-6, and EPO with (squares) or without (circles) 30 ng/mL of TGF-β1. Tumor cells were enumerated by immunocytochemistry using anti-CK and Ber-Ep4 antibodies. Mean values using cells from three different patients are shown. (B) Ten PKH-26–labeled tumor cells were suspended in 100 μL of FCS-containing IMDM or serum-free medium together with the indicated numbers of enriched CD34+ BPCs that had either not been precultured (CD34+) or that had been ex vivo expanded for 14 days in the presence of SCF, IL-1β, IL-3, IL-6, and EPO (expanded). After 3 days, the entire cultures were examined under a fluorescence microscope and all cells displaying a red fluorescence were counted. Results are the mean values ± SD of triplicate cultures and show a representative experiment.
Influence of hematopoietic cells on primary breast cancer cells during ex vivo expansion. (A) Culture-enriched mammary carcinoma cells (solid symbols) and CD34+ enriched BPCs (open symbols) were cocultured at a ratio of 1:10 in 3-mL flasks in the presence of SCF, IL-1β, IL-3, IL-6, and EPO with (squares) or without (circles) 30 ng/mL of TGF-β1. Tumor cells were enumerated by immunocytochemistry using anti-CK and Ber-Ep4 antibodies. Mean values using cells from three different patients are shown. (B) Ten PKH-26–labeled tumor cells were suspended in 100 μL of FCS-containing IMDM or serum-free medium together with the indicated numbers of enriched CD34+ BPCs that had either not been precultured (CD34+) or that had been ex vivo expanded for 14 days in the presence of SCF, IL-1β, IL-3, IL-6, and EPO (expanded). After 3 days, the entire cultures were examined under a fluorescence microscope and all cells displaying a red fluorescence were counted. Results are the mean values ± SD of triplicate cultures and show a representative experiment.
Influence of serum on ex vivo proliferation and survival of breast cancer cells.
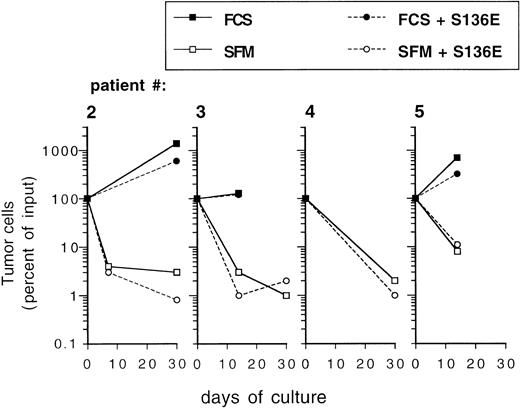
Ex vivo expansion of CD34+ blood progenitor cells has been efficiently performed in serum-free, chemically defined media.15,25 38 To investigate the influence of serum-free culture conditions on the behavior of epithelial-derived tumor cells during ex vivo expansion, primary breast cancer cells were inoculated into serum-free medium. To exclude that during the precultivation period tumor cells had adapted to serum and that they were influenced by serum withdrawal, primary breast cancer cells used in these experiments were not culture-enriched, but were directly inoculated immediately after isolation from patients. Whereas tumor cells survived during a culture period of 7 to 14 days or started to grow in serum-containing cultures, culture under serum-free conditions resulted in a 10- to 100-fold depletion of breast cancer cells (Fig5). Whereas prolonged culture until day 30 resulted in an increase in the absolute numbers of tumor cells in serum-supplemented cultures, a sharp decrease in tumor cell numbers was recorded in the serum-free cultures. Similar survival curves of tumor cells as in the serum-free medium preparation were observed in cultures containing α-MEM without FCS (data not shown). No difference was evident between cultures (serum-free or serum-supplemented) when performed in the presence or absence of SCF + IL-1 + IL-3 + IL-6 + EPO, indicating that the hematopoietic cytokines do not influence the survival (in serum-free cultures) or the proliferation (in serum-supplemented cultures) of mammary carcinoma cells (Fig 5). When low numbers of PKH-labeled breast cancer cells were cocultured with hematopoietic cells for 3 days in serum-free medium, very little or no influence of hematopoietic cells on the survival of tumor cells was seen, irrespective of the cell density used or if fresh CD34+ cells or more mature ex vivo expanded cell populations were analyzed (Fig 4C). These results demonstrate that breast cancer cells may be efficiently eliminated in serum-free culture medium ex vivo, irrespective of the presence of hematopoietic growth factors.
Influence of culture in SFM on survival of primary mammary carcinoma cells. Freshly isolated cells from malignant effusions were directly inoculated into 24-well plates at a cell density of 0.1 to 1 × 106/mL into the indicated culture media with or without cytokines (SCF, IL-1β, IL-3, IL-6, and EPO; S136E) as indicated. Independent cultures were set up in duplicates for the individual time points. After trypsinization both adherent and nonadherent cells were subjected to immunocytochemical analysis of tumor cells as described in Materials and Methods. Results represent mean values. FCS: -MEM + 10% fetal calf serum.
Influence of culture in SFM on survival of primary mammary carcinoma cells. Freshly isolated cells from malignant effusions were directly inoculated into 24-well plates at a cell density of 0.1 to 1 × 106/mL into the indicated culture media with or without cytokines (SCF, IL-1β, IL-3, IL-6, and EPO; S136E) as indicated. Independent cultures were set up in duplicates for the individual time points. After trypsinization both adherent and nonadherent cells were subjected to immunocytochemical analysis of tumor cells as described in Materials and Methods. Results represent mean values. FCS: -MEM + 10% fetal calf serum.
DISCUSSION
Tumor cells have been found in bone marrow or mobilized peripheral blood from patients with solid tumors, eg, breast cancer, in both unprocessed and in CD34+ selected harvests.4,12,13 Contaminating breast cancer cells have been found viable and capable of ex vivo growth4,22,23 and may therefore be of clinical concern.9-11 For ex vivo expansion of autologous hematopoietic progenitor cells, culture conditions have been optimized to generate large numbers of progenitor cells.14 39 Because results of a systematic analysis of the behavior of primary solid tumor cells during ex vivo expansion cultures have not been available so far, we investigated the influence of variables in culture conditions on mammary cancer cell growth, survival, and clonogenicity.
Because the incidence of contaminating tumor cells in autologous transplants is very low, we used mammary carcinoma cells from malignant effusions. We were able to grow breast cancer cells from 6 of 11 aspirates. Other or similar approaches performed from other investigators to isolate proliferating tumor cells from effusion fluids have also only been successful in a proportion of the samples used.40,41 Sharp et al23,42 and Ross et al4 were able to culture proliferating breast cancer cells isolated from bone marrow or mobilized blood specimens. Recently, Emerman et al43 could successfully grow primary breast cancer cells in liquid culture from 4 of 7 bone marrow specimens and 3 of 4 pleural effusions. Ethier et al44 also found growth of pleura-derived tumor cells in 3 of 7 samples from patients with metastatic breast cancer. The breast cancer origin of the cells from our 6 specimens that showed in vitro growth was documented and monitored by their positive immunostaining with anticytokeratin and Ber-Ep4 antibodies and negative immunostaining with anticalretinin antibodies. These antibodies in combination have been shown to distinguish epithelial tumor cells from mesothelial or mesenchymal cells in serous fluids.28-30 In addition, erbB2-protein overexpression, which has been described as a characteristic feature of a proportion of mammary carcinomas,31 was recorded in part of our cultured samples.
Initially, tumor cells grew very slowly, but in later passages their growth rate increased. Thus, our primary tumor cells displayed a similar growth behavior as tumor cells isolated from the site of the primary or metastatic tumor40,44,45 or to the micrometastatic mammary carcinoma cells isolated from bone marrow.22,46 Our primary cultures terminated spontaneously after 8 to 18 weeks of culture, and we did not obtain continuous cell lines. The incidence of clonogenic cells in semisolid medium (range, 0.025% to 0.75%) detected in our tumor cell population also represents previously described incidences in tumor biopsies47 or bone marrow samples.4
Our results indicate that under serum-containing conditions, primary tumor cells may remain viable and proliferate ex vivo. We did not find significant stimulatory or inhibitory effects of the combination of cytokines, SCF + IL-1β + IL-3 + IL-6 + EPO, on the growth rate of primary breast cancer cells. Previous studies using pre-established carcinoma cell lines found that IL-1 and IL-6 had an inhibitory influence,48-50 whereas IL-3, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been found to stimulate the growth of tumor cells.37,51-54 Using primary breast cancer cells, Emerman et al55 found no effect of hematopoietic stimulatory cytokines, which is in line with our results. We cannot exclude the possibility that each factor alone may positively or negatively influence the ex vivo growth of the primary breast cancer cells; however, the ultimate response to the cytokine combination that has been shown to mediate ex vivo hematopoietic expansion16was neither growth-stimulatory nor growth-inhibitory.
Tumor cell growth could be stimulated by mature hematopoietic cells at high cell densities in the presence of serum. These results are in analogy to the findings of Vogel et al,20 who suggested that direct cellular interactions between ex vivo expanded hematopoietic cells and solid tumor cells result in a better survival of tumor cells in the later phases of the ex vivo culture compared with earlier phases. This underscores a supportive effect of more mature hematopoietic cell types on in vitro tumor cell survival. Also, Emerman et al43 have shown that excess numbers of hematopoietic cells as present in LTBMC are able to stimulate the growth of mammary carcinoma cells. It is also known that, within LTBMC, high levels of TGF-β1 are prevalent.56 These findings can explain our observation that the suppressive effect of TGF-β1 on tumor cells that is recorded in our experiments in the absence of CD34+cells and that was also seen by other investigators with various epithelial tumor cell types57-59 is counteracted in the presence of excess numbers of hematopoietic cells.
We have observed that primary mammary carcinoma cells undergo rapid elimination during culture in serum-free medium. This effect persisted upon culture for extended time periods, indicating that the tumor cells could not adapt their in vitro growth to the serum-free conditions. A similar decrease of tumor cells was observed using both a basal culture medium (α-MEM) containing essentially only salts, glucose, amino acids, and vitamins or a serum-free medium preparation that, in addition, also contains supplemental bovine serum albumin, cholesterol, and transferrin. These results indicate that the medium supplements, which are of importance for the survival of hematopoietic cells,25 do not contribute to survival of breast cancer cells. We also found that the presence of hematopoietic cytokines in the serum-free cultures could not protect the primary mammary tumor cells from elimination. Thus, in addition to the results shown in Fig2A, where influences on the proliferation of tumor cells by cytokines were determined, the survival of primary breast cancer cells, assayed in the cultures in serum-free medium, is not affected by the hematopoietic stimulatory cytokines.
Taken together, we have shown that culture conditions used for ex vivo expansion influence tumor cell survival and growth. A strong suppression of tumor cells was achieved in serum-free medium preparations. In contrast, ex vivo expansion for prolonged time periods reaching relatively high cell densities of mature hematopoietic cells may harbor a risk of stimulating tumor cell growth. These results will be of importance for ex vivo expansion of hematopoietic transplants for maximal efficiency of tumor cell purging. Our findings should be essential when designing clinical trials that investigate the value of tumor cell purging in patients with breast cancer.
ACKNOWLEDGMENT
The authors thank Prof Dr R. Mertelsmann for his continuous encouragement and conceptional advice. We also thank Dr M. Schmidt for providing Western Blot analyses and E. de Lima-Hahn for excellent technical support.
A.S. and W.B. contributed equally to this work.
Supported by the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 364 (Project A1 to R.H.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R. Henschler, MD, Abt. Hämatologie, Medizinische Universitätsklinik, Hugstetter Strasse 55, D-79106 Freiburg, Germany.