To the Editor:
Wiskott-Aldrich syndrome (WAS) is an X-linked recessive disorder characterized by thrombocytopenia with small platelets, severe eczema, and recurrent infections due to defects in the immune system.1 Recent improvement of the prognosis of WAS by bone marrow transplantation (BMT) made early diagnosis more important. However, early diagnosis is sometimes difficult, because WAS patients often show atypical clinical phenotypes in infancy.2Although the cause of WAS has been defined as mutations in the WAS protein (WASP) gene, it is difficult to perform mutation analysis of the gene in all the possible cases. In this study, we performed flow cytometric analysis of the WASP expression in lymphocytes using an anti-WASP monoclonal antibody to assess its usefulness in the diagnosis of WAS. The results suggested that the method is simple, rapid, and applicable for screening of WAS.
Eight normal individuals, three WAS patients, and their mothers were included in this study. The diagnosis of WAS was made by their clinical and laboratory findings and was confirmed by mutation analysis (Table 1). Restriction enzyme digestion of polymerase chain reaction (PCR) fragments, allele-specific PCR, and/or direct sequence analysis of genomic DNA, which were based on mutation information, were performed for carrier diagnosis as reported by Ariga et al.3 All of the mothers of the WAS patients were proved to be carriers of WAS (data not shown).
Characterization of Three WAS Patients
| Patient No. . | Patient Age . | Exon . | Mutation . | Western Blot Analysis . |
|---|---|---|---|---|
| 1 | 1 yr | 7 | C-665 → T | — |
| Arg-211 → stop | ||||
| 2 | 10 mo | 1 | G-125 → A | — |
| Glu-31 → Lys | ||||
| 3 | 7 yr | 10 | C insertion between 980-984 | — |
| → premature termination |
| Patient No. . | Patient Age . | Exon . | Mutation . | Western Blot Analysis . |
|---|---|---|---|---|
| 1 | 1 yr | 7 | C-665 → T | — |
| Arg-211 → stop | ||||
| 2 | 10 mo | 1 | G-125 → A | — |
| Glu-31 → Lys | ||||
| 3 | 7 yr | 10 | C insertion between 980-984 | — |
| → premature termination |
Heparinized blood samples were collected and processed each in a set consisting of those from a WAS patient, his mother, and an appropriate normal individual under the same conditions. Samples necessary for more than 1-day transportation were kept at 4°C after collection. Peripheral blood mononuclear cells (PBMCs) were isolated by standard Ficoll-Hypaque gradient centrifugation methods. PBMCs were washed in phosphate-buffered saline (PBS) containing 1% fetal bovine serum. Cytofix/Cytoperm solution from CytoStain Kits (Pharmingen, San Diego, CA) was added to thoroughly suspended 2 × 106 PBMCs in each tube at 4°C for 20 minutes. After washing twice in Perm/Wash solution, they were reacted with 1:200 diluted mouse anti-WASP monoclonal antibody (3F3-A54) or 1:5 diluted mouse IgG1 control (Becton Dickinson, San Jose, CA) at 4°C for 30 minutes and washed twice. They were then reacted with 1:200 diluted fluorescein isothiocyanate (FITC)-conjugated goat antimouse Igs γ and light chains (Biosource, Camarillo, CA) at 4°C for 30 minutes. Samples, thus processed, were analyzed on a FACSCalibur (Becton Dickinson). A total of 20,000 events of lymphocytes, which were gated based on forward and side scatter, were counted. Fluorescence intensity was detected by FL1. M1 area (positive fluorescence) was set in each normal individual stained with mouse IgG1 control so that the proportion of positive cells in the area was 1%.
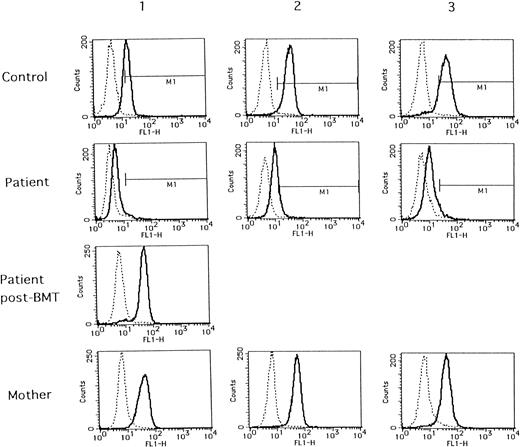
As shown in Fig 1 (solid lines), when compared with normal individuals, deficient WASP expressions were distinctly demonstrated in all of the WAS patients tested. WASP expressions in all of their mothers were the same as in those of normal individuals. The same normal WASP expression was also demonstrated in a patient with successful BMT (patient no. 1, post-BMT). WASP expressions in the remaining five normal individuals were the same as those of controls 1, 2, and 3, even when stored at 4°C for 2 days after collection (data not shown). Thus, so far, the method tested seems to be efficient in the diagnosis of WAS.
Flow cytometric analysis of WASP expression in normal individuals (control), WAS patients (patient), and their mothers (mother). Blood samples from controls 1, 2, and 3 and their respective mothers were collected with the same condition as those of patients no. 1, 2, and 3, respectively. A blood sample from patient no. 1 after BMT (patient post-BMT) was collected in the separate examination.
Flow cytometric analysis of WASP expression in normal individuals (control), WAS patients (patient), and their mothers (mother). Blood samples from controls 1, 2, and 3 and their respective mothers were collected with the same condition as those of patients no. 1, 2, and 3, respectively. A blood sample from patient no. 1 after BMT (patient post-BMT) was collected in the separate examination.
However, what was unexpected in the present study was a finding that every sample from the patients reacted with anti-WASP antibody exhibited fluorescence peaks somewhat shifting from those of negative controls with mouse IgG1 control (dashed lines in Fig 1). The proportion of cells belonging to M1 area (positive fluorescence) was calculated to be 2.5%, 21.9%, and 2.2% in patients no. 1, 2, and 3, respectively, and the proportion of positive cells in patient no. 2 was apparently higher than those in patients no. 1 and 3. As shown in Table 1, the mutation in patient no. 2 is missense of exon 1, whereas those in patients no. 1 and 3 are nonsense mutation in exon 7 and premature termination in exon 10, respectively. Thus, it is possible to assume that in patient no. 2 some WASP might be expressed and detectable by the present method, although we could not detect it using Western blot analysis. It might be due to differences in sensitivity between the two methods, or unstable WASP in patient no. 2 might be destroyed during the process of Western blotting, although this is not proven in the present study. On the other hand, the fluorescence shift seen in patient no. 1 might be due to binding of the anti-WASP antibody with non-WASP components in lymphocytes or to thoroughly nonspecific binding, because epitopes of the anti-WASP antibody, 3F3-A5, reside in a.a. 202-302 of WASP and WASP of the patient no. 1 is truncated from a.a. 211, being defective in most parts of the epitope. This might be also applied to the results observed in patient no. 3. Nevertheless, it is apparent that the present flow cytometric analysis of the lymphocytes is capable of differentiating WAS patients from normal or carrier states. Findings of normal WASP expressions in lymphocytes from carrier mothers were consistent with nonrandom X-inactivation reported for WAS carriers.5
The present procedure is similar to that used for detection of BTK, a cytoplasmic protein deficient in X-linked agammaglobulinemia. Futatani et al6 reported a limitation of the procedure in which, among 41 cases tested, normal BTK expression was demonstrated in 1 patient with a missense mutation in BTK gene.6 Thus, a similar limitation should exist in the present procedure. Accumulation of data on this procedure together with mutation analysis is being performed in our laboratory.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal