Abstract
Targeted hematopoietic irradiation delivered by131I-anti-CD45 antibody has been combined with conventional marrow transplant preparative regimens in an effort to decrease relapse. Before increasing the proportion of therapy delivered by radiolabeled antibody, the myeloablative and immunosuppressive effects of such low dose rate irradiation must be quantitated. We have examined the ability of 131I-anti-CD45 antibody to facilitate engraftment in Ly5-congenic and H2-mismatched murine marrow transplant models. Recipient B6-Ly5a mice were treated with 30F11 antibody labeled with 0.1 to 1.5 mCi 131I and/or total body irradiation (TBI), followed by T-cell–depleted marrow from Ly5b-congenic (C57BL/6) or H2-mismatched (BALB/c) donors. Engraftment was achieved readily in the Ly5-congenic setting, with greater than 80% donor granulocytes and T cells after 0.5 mCi 131I (estimated 17 Gy to marrow) or 8 Gy TBI. A higher TBI dose (14 Gy) was required to achieve engraftment of H2-mismatched marrow, and engraftment occurred in only 3 of 11 mice receiving 1.5 mCi131I delivered by anti-CD45 antibody. Engraftment of H2-mismatched marrow was achieved in 22 of 23 animals receiving 0.75 mCi 131I delivered by anti-CD45 antibody combined with 8 Gy TBI. Thus, targeted radiation delivered via131I-anti-CD45 antibody can enable engraftment of congenic marrow and can partially replace TBI when transplanting T-cell–depleted H2-mismatched marrow.
BONE MARROW transplantation has been used for more than two decades to treat hematologic malignancies and aplastic anemia and, more recently, genetic diseases such as thalassemia and sickle cell anemia.1-6 However, the success of marrow transplantation has been limited by the high relapse rates seen in patients transplanted for advanced malignancies, by the toxicity of preparative regimens, and by the lack of HLA-identical family member donors for most patients. In principle, these limitations could be addressed by the use of radiolabeled monoclonal antibodies (MoAbs) to increase the irradiation to lymphohematopoietic tissues while minimizing the exposure of normal organs. First, delivery of more irradiation to sites of leukemic involvement should decrease the risk of relapse. Second, targeted irradiation of lymphohematopoietic organs should decrease the need for high-dose chemotherapy or total body irradiation (TBI) to prevent rejection of the allogeneic graft. Alternatively, adding targeted irradiation to full-dose systemic therapy could enhance the level of immunosuppression, thereby decreasing the risk of rejection of marrow from HLA-mismatched or unrelated donors, especially when the marrow has been depleted of T cells to prevent graft-versus-host disease (GVHD).
We have shown that infusion of radiolabeled antibodies against CD45 delivers irradiation selectively to lymphohematopoietic tissues.7-9 Significant supplemental doses of radiation have been delivered to bone marrow and spleen in combination with conventional marrow transplant preparative regimens without excessive toxicity, and the relapse rates after these novel preparative regimens have been low in preliminary studies. In the initial clinical studies, treatment with radiolabeled antibody was combined with standard preparative regimens containing cyclophosphamide and either TBI9,10 or busulfan,11 because it is unknown whether the relatively low dose rate radiation delivered by antibody has sufficient antileukemic efficacy to prevent relapse and sufficient immunosuppressive activity to prevent rejection of allogeneic marrow.
Radiolabeled antibody delivers radiation at an exposure rate that is one to two orders of magnitude lower than the rate normally delivered from an external beam source. Several studies have demonstrated the influence of exposure rate on the amount of irradiation required to achieve engraftment.12-15 van Os et al14,15 compared exposure rates ranging from 0.5 to 40 cGy/min in a variety of murine transplant models. In a congenic donor and recipient strain combination that differed by a single nonimmunogenic marker, 5.5 Gy TBI delivered at 40 cGy/min was sufficient to allow 80% engraftment in 50% of the recipients. In the same model with TBI delivered at 2 cGy/min, a 7 Gy exposure was needed to achieve the same degree of engraftment.15 In an H2-compatible strain combination with disparity for multiple minor histocompatibility antigens, 6 Gy TBI delivered at 40 cGy/min was sufficient to allow engraftment. In the same model with TBI delivered at 2 cGy/min, an 8 Gy exposure was needed.14 With T-cell–depleted marrow from H2-mismatched donors, the difference between low and high dose rate was at least 6 Gy, because full engraftment at the high-dose rate (40 cGy/min) occurred after 10 Gy, but long-term engraftment did not occur after 16 Gy (the highest dose tested) delivered at 2 cGy/min.14 These results suggest that hematopoietic stem cells and precursors of cellular immunity have a substantial ability to repair irradiation damage.
Little is known about the relative biologic effects of low dose rate radiation resulting from the administration of radiolabeled antibody. To address this issue, we initiated studies using131I-labeled anti-CD45 antibody, with or without external beam TBI, as a preparative regimen in two models of murine transplantation. To measure marrow ablation, B6-Ly5a recipients were transplanted with marrow from congenic Ly5b donors.16,17 In this model, the donor and recipient disparity is limited to CD45 allotype and does not evoke a cellular immune response.18 In an approach designed to test the immunosuppressive effects of the regimen, the same recipients (H2b) were transplanted with T-cell–depleted marrow from H2-mismatched (H2d) BALB/c donors. Our results demonstrate that irradiation delivered solely by131I-anti-CD45 antibody allows engraftment of marrow in Ly5-congenic recipients but not in H2-mismatched recipients, where both natural killer (NK) cells and T cells are known to cause rejection. However, engraftment of T-depleted H2-mismatched marrow could be accomplished by adding low-dose external beam TBI to the radiation delivered by 131I-anti-CD45 antibody.
MATERIALS AND METHODS
Mice.
Male B6-Ly5a mice were bred at the Fred Hutchinson Cancer Research Center (Seattle, WA) and housed under specific pathogen-free conditions with acidified water and autoclaved chow. C57BL/6 and BALB/c donors were purchased from Jackson Laboratory (Bar Harbor, ME) and were 6 to 12 weeks old at the initiation of each experiment.
MoAbs.
MoAbs GK1.5 (rat IgG2b anti-CD4), 30-H12 (rat IgG2b anti-thy1.2), and 2.34 (rat IgG2banti-CD8) were prepared from culture supernatants of cells lines obtained from ATCC (GK1.5 and 2.34) or Dr Jeffrey Ledbetter (Bristol Meyers Squibb, Seattle, WA; 30-H12). A hybridoma cell line secreting MoAb 30F11 (rat IgG2b), which recognizes all murine CD45 isoforms, was the gift of Dr Ledbetter. Hybridoma cell lines secreting MoAbs A20 (murine IgG2a), which recognizes the Ly5.1 epitope encoded by the Ly5a allotype of murine CD45, and 104 (murine IgG2a), which recognizes the Ly5.2 epitope encoded by the Ly5b allotype of murine CD45, were the gift of Dr Shoji Kimura (Sloan Kettering Institute, New York, NY). Ascites containing each MoAb was produced in BALB/c mice under specific pathogen-free conditions. Batch extraction and purification of antibodies from ascites was performed using Abx exchange resin (J.T. Baker, Phillipsburg, NJ) and high-pressure liquid chromatography (Biosys 510 Beckman, Fullerton, CA).
Iodination and characterization.
Antibodies were iodinated with Na125I or Na131I (ICN, Irvine, CA) using the Iodogen method19 for trace labeling and the chloramine T-labeling method for high specific activity labeling.20 The immunoreactivity (percentage of counts able to bind at antigen excess) of each preparation was determined by incubating antibody at low concentration (5 ng/mL) with 1.0 × 106 to 3 × 107B6-Ly5a splenocytes for 1 hour at room temperature and measuring unbound antibody as previously described.21Preparations with less than 70% immunoreactivity were not used.
Antibody localization and estimation of radiation absorbed doses.
The double isotope labeling method of Pressman22 was used to determine the biodistribution of 30F11 antibody as previously described.8 Animals were injected via tail vein with 200 μL volume containing 100 μg of antibody labeled with131I (specific activity, 1 μCi/μg) and an equal amount of rat IgG (Sigma Immunochemical, St Louis, MO) labeled with125I. Groups of 5 mice were killed at multiple time points from 1 to 96 hours after injection, and multiple tissues were sampled. The 125I and 131I contents of weighed samples and standard samples of the injection mix were determined by multichannel gamma counting (Packard 5000 Autogamma counter; Packard Instrument Co, Downers Grove, IL). Counting data were adjusted for cross-over from the 131I channel to the 125I channel and were corrected for decay.
Time-activity curves were constructed for each organ from the data on the percentage injected dose per gram (% ID/g) at each time point, and the infinite-time integral was calculated for the area under the curve to estimate the total number of disintegrations for dosimetry assuming that both 30F11 antibody and Rat IgG were labeled with131I. Absorbed fractions of β-particle energy for131I were calculated by applying electron transport theory (EGS423) to a dosimetric model for the laboratory mouse.24 25 This model accounts for the size, shape, position, and density of organs, as well as for the overlap and contact from surrounding organs and tissues. The absorbed fractions included contributions from same-organ irradiation as well as from cross-organ irradiation. Contributions to the absorbed dose from 131I penetrating gamma radiation in mouse organs were negligible (<1% of the total dose) and were neglected. The femoral marrow was assumed to be a cylinder of 0.5 mm in radius; therefore, only a proportion (46%) of the radioiodine present in marrow was assumed to deposit its energy inside the marrow. The β-particle absorbed fraction for lymph nodes was estimated to be 73%. Results were expressed as gray (Gy) per millicurie (mCi) 131I.
Bone marrow transplantation.
Four days before marrow infusion, groups of 6 recipient B6-Ly5a mice were treated with 100 μg of 30F11 antibody labeled with varying amounts of 131I at a maximum specific activity of 1.5 mCi 131I/100 μg. Cages were changed every other day for 1 week to decrease the irradiation from excreted urine in the cage bedding. External beam TBI was administered on the day of marrow infusion and was delivered at 0.2 Gy/min from dual60Cobalt sources (J.L. Shepherd Co, San Fernando, CA).
Marrow cells were treated with hemolytic buffer and T cells were depleted by complement-mediated lysis with antibodies against CD4, CD8, and Thy-1, a method that routinely depleted 97% to 99% of T cells (data not shown). A marrow cell dose of 1.0 × 107nucleated cells (counted before T-cell depletion) was injected via tail vein. This moderately high cell dose was selected to be certain that cell dose was not a limiting factor for engraftment, especially considering that some residual radiolabeled antibody remains in hematopoietic tissues at 96 hours after antibody administration when the marrow was infused. The marrow was depleted of T cells in all experiments to allow comparison between Ly5-congenic and H2-mismatched transplants. H2 disparity provides a rigorous test of immunosuppression, and T-cell depletion allows recipients to survive without lethal or debilitating GVHD.
Assessment of engraftment.
Mice were examined daily for survival and for signs of GVHD or other illness. At 4, 8, and 12 weeks posttransplant, orbital blood samples were stained with 50 μL of biotinylated A20 (anti-Ly5.1) antibody or biotinylated 104 (anti-Ly5.2) antibody at 100 μg/mL for 30 minutes followed by phycoerythrin-streptavidin (PharMingen, San Diego, CA) and fluorescein isothiocyanate (FITC)-conjugated CD3-specific antibody (PharMingen). Control samples consisted of cells from unmanipulated mice of both donor and host strains. Samples were analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA). Lymphocyte and granulocyte populations were defined by forward and 90° scatter characteristics. The lymphocyte population was analyzed separately using two colors to distinguish CD3+ T cells and CD3− B cells.
RESULTS
Biodistribution of 131I-anti-CD45 antibody and estimation of radiation absorbed doses.
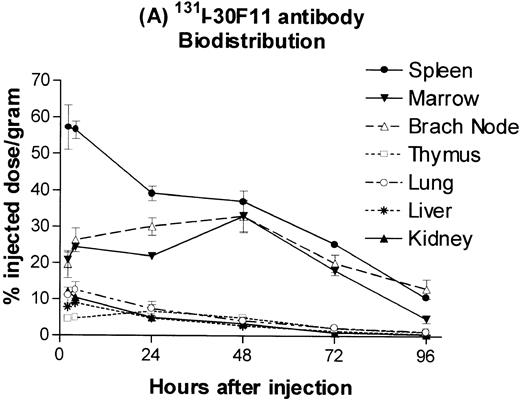
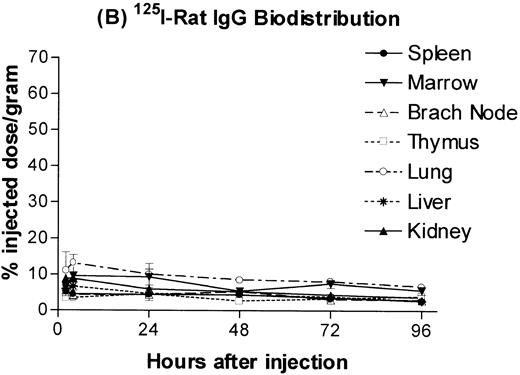
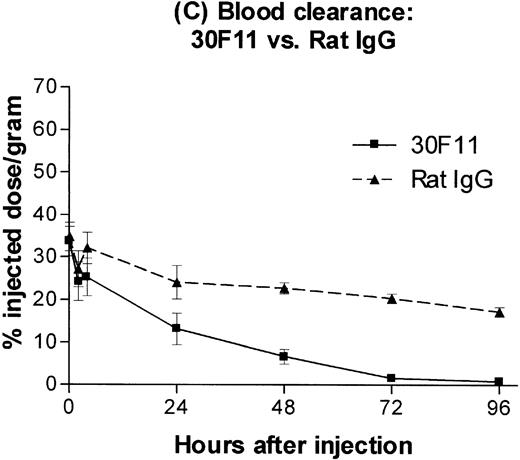
B6.Ly5a mice were injected with a 100 μg dose of trace 131I-labeled anti-CD45 MoAb 30F11 or125I-labeled rat IgG and the amount of isotope in major organs was determined. The resulting time-activity curves demonstrated greater uptake and retention of radiolabeled anti-CD45 antibody in spleen, lymph nodes, and marrow as compared with the lung, the normal nontarget organ with the highest concentration (Fig 1A). The corresponding time-activity curves for 125I-labeled rat IgG (ie, IgG purified from sera of normal rats), used as a nonspecific control, did not show any specific retention in spleen, lymph nodes, or marrow (Fig 1B). Anti-CD45 antibody was cleared from the blood far more rapidly than control rat IgG, presumably by rapid binding of circulating antibody to CD45 on readily accessible cells in spleen and marrow (Fig 1C).
Biodistribution of 100 μg dose of trace-labeled 30F11 antibody (A) and polyclonal rat IgG (B). Data points represent the mean percentage of injected dose per gram ± SD for groups of 5 animals. (C) Whole blood concentrations of 30F11 and polyclonal rat IgG over 96 hours after injection.
Biodistribution of 100 μg dose of trace-labeled 30F11 antibody (A) and polyclonal rat IgG (B). Data points represent the mean percentage of injected dose per gram ± SD for groups of 5 animals. (C) Whole blood concentrations of 30F11 and polyclonal rat IgG over 96 hours after injection.
Estimates of absorbed irradiation doses delivered to target and normal organs by 30F11 antibody or polyclonal rat IgG, assuming they were labeled with 131I, were calculated using the time-activity curves for each tissue (Table 1). The estimated absorbed irradiation delivered to spleen, lymph nodes, and bone marrow by a 100 μg dose of antibody 30F11 labeled with 1 mCi131I was, respectively, 6.5-, 4.0-, and 2.1-fold greater than that delivered to lung. When 131I was delivered from polyclonal rat IgG, the spleen, bone marrow, and lymph nodes all absorbed less irradiation than the lung and absorbed approximately the same amount of irradiation as the liver and kidney. The higher estimated irradiation absorbed by nontarget organs after administration of 131I-rat IgG as compared with 131I-30F11 antibody reflects the slower clearance of 131I-rat IgG from the blood.
Estimated Radiation Absorbed Doses (Gy/mCi131I) for 30F11 Antibody and Rat IgG
| Tissue . | 30F11 (Gy/mCi) . | Rat IgG (Gy/mCi) . |
|---|---|---|
| Spleen | 107.7 | 23.7 |
| Marrow | 34.8 | 25.4 |
| Axillary lymph node | 65.5 | 21.7 |
| Brachial lymph node | 70.3 | 24.3 |
| Thymus | 12.8 | 33.3 |
| Lung | 16.7 | 44.8 |
| Liver | 10.7 | 19.9 |
| Kidney | 12.5 | 24.5 |
| Stomach | 10.4 | 15.2 |
| Total body | 5.9 | 18.7 |
| Tissue . | 30F11 (Gy/mCi) . | Rat IgG (Gy/mCi) . |
|---|---|---|
| Spleen | 107.7 | 23.7 |
| Marrow | 34.8 | 25.4 |
| Axillary lymph node | 65.5 | 21.7 |
| Brachial lymph node | 70.3 | 24.3 |
| Thymus | 12.8 | 33.3 |
| Lung | 16.7 | 44.8 |
| Liver | 10.7 | 19.9 |
| Kidney | 12.5 | 24.5 |
| Stomach | 10.4 | 15.2 |
| Total body | 5.9 | 18.7 |
Estimates of radiation absorbed doses calculated from time-activity curves of trace-iodinated 30F11 antibody and rat IgG from experiment shown in Fig 1, assuming that each was labeled with131I. Radiation doses are expressed as grays delivered per millicurie of 131I administered.
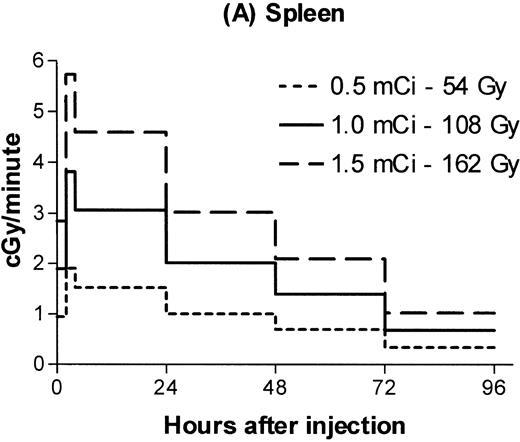
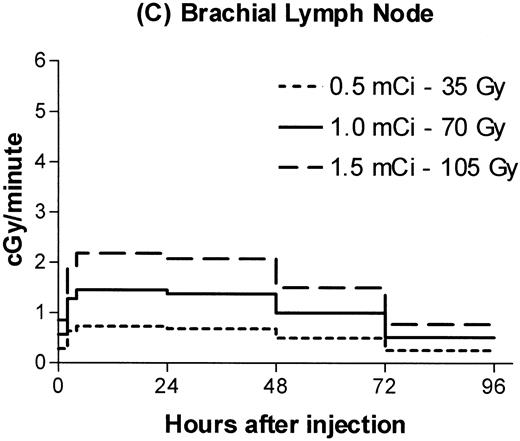
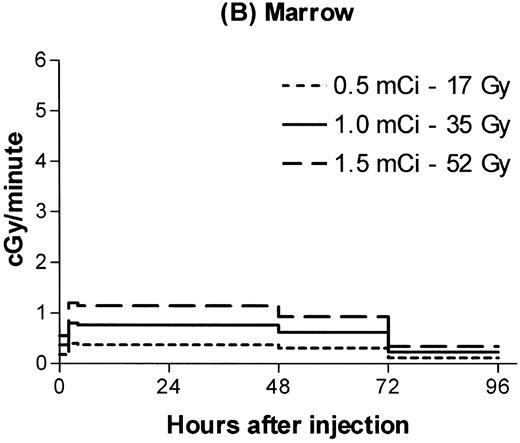
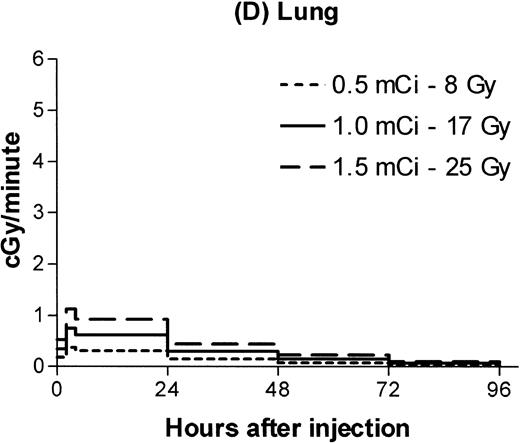
Estimated absorbed irradiation rates varied between tissues and over time for a given tissue, as shown in Fig 2for 0.5, 1.0, and 1.5 mCi of 131I. For antibody labeled with 1.0 mCi of 131I, the highest irradiation rate for a hematopoietic tissue was 3 cGy/min in the spleen between 4 and 24 hours after infusion. Maximum lymph node dose rates ranged from 1.3 to 1.5 cGy/min. For brachial lymph nodes, 75% of the total estimated irradiation was absorbed at a rate of at least 1 cGy/min. For marrow, 86% of the total dose was absorbed at an estimated rate of at least 0.6 cGy/min.
Estimated radiation dose rate and total Gy delivered by 100 μg 30F11 antibody labeled with three different 131I doses for spleen (A), bone marrow (B), brachial lymph node (C), and lung (D). The estimated irradiation delivered to each organ between experimental time points was divided by the length of the time interval to calculate dose rates.
Estimated radiation dose rate and total Gy delivered by 100 μg 30F11 antibody labeled with three different 131I doses for spleen (A), bone marrow (B), brachial lymph node (C), and lung (D). The estimated irradiation delivered to each organ between experimental time points was divided by the length of the time interval to calculate dose rates.
Engraftment of congenic marrow after 131I-30F11 antibody or TBI.
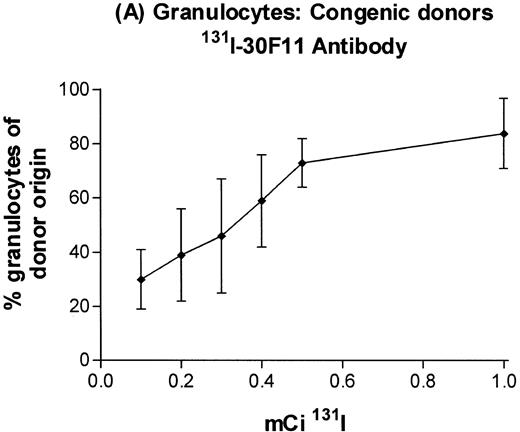
In the initial experiments with C57BL/6 donors and congenic B6-Ly5a recipients, we administered a 100 μg dose of 30F11 antibody labeled with 0 (ie, unlabeled antibody), 0.5, 1.0, 1.25, and 1.5 mCi of 131I, followed 4 days later by injection of T-cell–depleted marrow or medium alone. All mice receiving marrow survived, whereas 2 of 6 mice treated with 1.25 mCi131I and 4 of 6 treated with 1.5 mCi 131I and no marrow died between 11 and 15 days after radiolabeled antibody injection (data not shown). Although postmortem examinations were not performed, death at these time points after delivery of radiation is consistent with death from marrow aplasia. All mice receiving at least 0.5 mCi 131I-anti-CD45 antibody demonstrated successful engraftment (Table 2). Myeloid engraftment occurred within 4 weeks posttransplant, but full T-cell engraftment did not occur until 12 weeks, presumably reflecting delayed disappearance of recipient T cells.
Engraftment of Congenic Marrow in Mice After131I-Anti-CD45 Antibody
| Administered 131I Activity . | % Donor Granulocytes (mean ± SD) . | % Donor T Cells (mean ± SD) . | ||
|---|---|---|---|---|
| 28 d After BMT . | 113 d After BMT . | 28 d After BMT . | 113 d After BMT . | |
| 0 (Ab only) | 4.9 ± 5.6 | 1.6 ± 0.9 | 0 ± 0 | 0.1 ± .2 |
| 0.5 mCi | 81.3 ± 7.5 | 86.6 ± 7.0 | 34.7 ± 8.1 | 80.4 ± 7.6 |
| 1.0 mCi | 92.7 ± 3.5 | 91.5 ± 3.7 | 38.5 ± 9.4 | 87.6 ± 3.5 |
| 1.25 mCi | 96.0 ± 2.9 | 95.8 ± 3.2 | 44.9 ± 9.8 | 89.1 ± 4.9 |
| 1.5 mCi | 98.3 ± 1.4 | 94.9 ± 7.7 | 38.1 ± 14.8 | 89.8 ± 4.3 |
| Administered 131I Activity . | % Donor Granulocytes (mean ± SD) . | % Donor T Cells (mean ± SD) . | ||
|---|---|---|---|---|
| 28 d After BMT . | 113 d After BMT . | 28 d After BMT . | 113 d After BMT . | |
| 0 (Ab only) | 4.9 ± 5.6 | 1.6 ± 0.9 | 0 ± 0 | 0.1 ± .2 |
| 0.5 mCi | 81.3 ± 7.5 | 86.6 ± 7.0 | 34.7 ± 8.1 | 80.4 ± 7.6 |
| 1.0 mCi | 92.7 ± 3.5 | 91.5 ± 3.7 | 38.5 ± 9.4 | 87.6 ± 3.5 |
| 1.25 mCi | 96.0 ± 2.9 | 95.8 ± 3.2 | 44.9 ± 9.8 | 89.1 ± 4.9 |
| 1.5 mCi | 98.3 ± 1.4 | 94.9 ± 7.7 | 38.1 ± 14.8 | 89.8 ± 4.3 |
Engraftment of T-cell–depleted C57BL/6 marrow in B6-Ly5a recipients. Values are the mean for 3 (antibody only) to 5 or 6 mice per group.
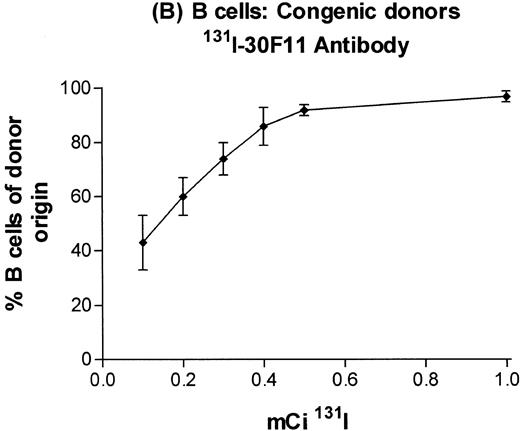
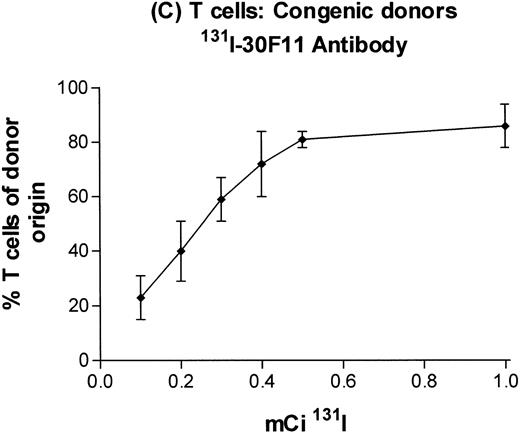
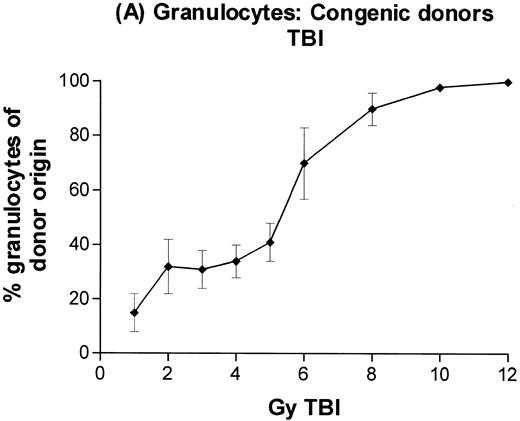
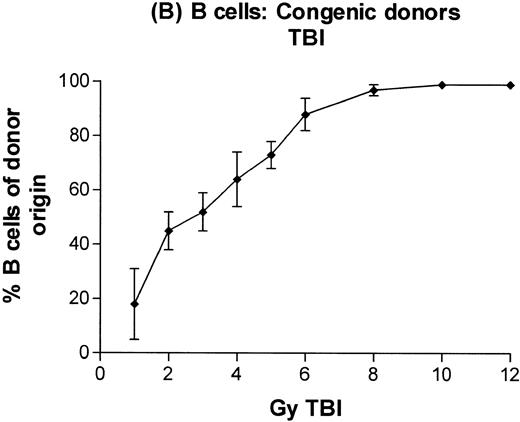
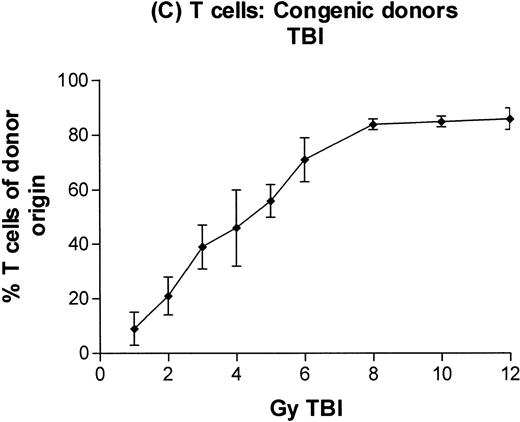
In subsequent experiments, mice received varying doses of TBI or a broader range of 131I doses before infusion of T-cell–depleted congenic marrow. A near-linear relationship between radiation dose and engraftment of donor marrow was found for131I doses of up to 0.5 mCi (Fig 3) and TBI doses of up to 8 Gy (Fig 4). Fifty percent engraftment of granulocytes, B cells, and T cells was observed after treatment with 0.2 to 0.3 mCi of 131I-labeled antibody, which corresponded to an estimated marrow radiation dose of 7 to 10 Gy. Engraftment after 8 Gy TBI (dose rate, 20 cGy/min) was equivalent to engraftment after 0.5 mCi of 131I delivered via 30F11 antibody, with 80% donor cells. The estimated irradiation absorbed by marrow after treatment with 0.5 mCi of 131I on 100 μg 30F11 was approximately 17 Gy, more than twice the 8 Gy required when the irradiation was delivered by external beam.
Engraftment of T-cell–depleted C57BL/6 marrow in B6-Ly5a recipients treated with 100 μg of 30F11 antibody labeled with 0.1 to 1.0 mCi 131I. Proportion of granulocytes (A), B cells (B), and T cells (C) of donor origin (mean ± SD) 3 months after transplantation.
Engraftment of T-cell–depleted C57BL/6 marrow in B6-Ly5a recipients treated with 100 μg of 30F11 antibody labeled with 0.1 to 1.0 mCi 131I. Proportion of granulocytes (A), B cells (B), and T cells (C) of donor origin (mean ± SD) 3 months after transplantation.
Engraftment of T-cell–depleted C57BL/6 marrow in B6-Ly5a recipients treated with 1 to 12 Gy TBI. Proportion of granulocytes (A), B cells (B), and T cells (C) of donor origin (mean ± SD) 3 months after transplantation.
Engraftment of T-cell–depleted C57BL/6 marrow in B6-Ly5a recipients treated with 1 to 12 Gy TBI. Proportion of granulocytes (A), B cells (B), and T cells (C) of donor origin (mean ± SD) 3 months after transplantation.
Engraftment of T-cell–depleted H2-mismatched marrow after131I-anti-CD45 antibody alone or with external beam TBI.
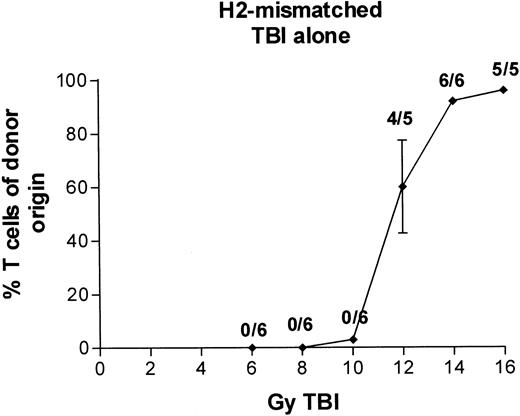
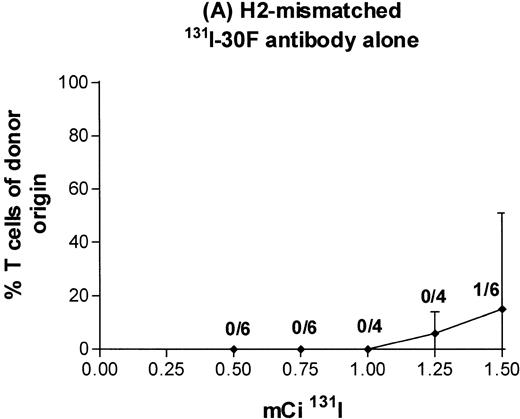
In the initial experiments with BALB/c donors and MHC-mismatched B6-Ly5a recipients, we administered either a 100 μg dose of antibody 30F11 labeled with 0.5 to 1.5 mCi of131I on day −4 or 2 to 16 Gy TBI on day 0, followed by infusion of T-cell–depleted marrow or medium alone. With external beam TBI and no marrow infusion, the LD50 was approximately 10 Gy, and no recipients survived exposures of ≥14 Gy (data not shown). With infusion of T-cell–depleted H2-incompatible marrow, engraftment (>80% donor T cells at 3 months posttransplant) was observed in all recipients prepared with ≥14 Gy TBI delivered by external beam (Fig 5). With131I-labeled antibody and no marrow infusion, the LD50 was between 0.5 and 1.0 mCi, and no recipients survived after administration of 1.5 mCi (data not shown). With infusion of T-cell–depleted H2-incompatible marrow, 1.5 mCi of131I-labeled antibody was not sufficient to permit engraftment. Only 1 of 6 mice receiving the highest isotope dose had more than 80% T cells of donor origin (Fig6A).
Engraftment of T-cell–depleted BALB/c marrow in B6-Ly5a recipients treated with 6 to 16 Gy TBI. Proportion of T cells of donor origin (mean ± SD) 3 months after transplantation. Data point labels indicate the number of mice with ≥80% donor T cells of total surviving mice (of 6 treated mice per group).
Engraftment of T-cell–depleted BALB/c marrow in B6-Ly5a recipients treated with 6 to 16 Gy TBI. Proportion of T cells of donor origin (mean ± SD) 3 months after transplantation. Data point labels indicate the number of mice with ≥80% donor T cells of total surviving mice (of 6 treated mice per group).
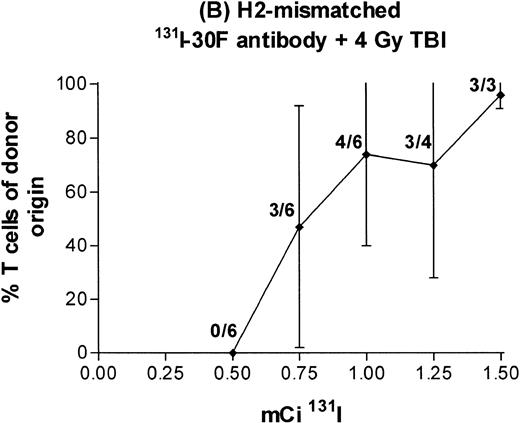
Engraftment of T-cell–depleted BALB/c marrow in mice treated with 100 μg 30F11 antibody labeled with 0.5 to 1.5 mCi131I alone (A) or combined with 4 Gy TBI (B). Proportion of T cells of donor origin (mean ± SD) 3 months after transplantation. Data point labels indicate the number of mice with ≥80% donor T cells of total surviving mice (of 6 treated mice per group).
Engraftment of T-cell–depleted BALB/c marrow in mice treated with 100 μg 30F11 antibody labeled with 0.5 to 1.5 mCi131I alone (A) or combined with 4 Gy TBI (B). Proportion of T cells of donor origin (mean ± SD) 3 months after transplantation. Data point labels indicate the number of mice with ≥80% donor T cells of total surviving mice (of 6 treated mice per group).
It was not possible to deliver more irradiation with the use of131I-30F11 antibody alone, because labeling the antibody to a specific activity higher than 15 mCi/mg impaired immunoreactivity of the antibody, and antibody doses higher than 100 μg delayed clearance from both blood and marrow, causing the infused donor marrow to be damaged by isotope persisting for more than 4 days (data not shown). To circumvent this problem, we tested the immunosuppressive effects of radiation from 131I-30F11 antibody combined with 4 Gy external beam TBI. This combined preparative regimen resulted in engraftment in most recipients treated at 131I doses of at least 1.0 mCi 131I (Fig 6B). In two experiments, 5 of 12 mice receiving 1.5 mCi survived and demonstrated donor engraftment, but the remaining 7 mice treated with 1.5 mCi died between 9 and 14 days after marrow transplant. These results suggest that the margin between immunosuppressive and toxic doses of radiation is small when high doses of radioisotope are combined with 4 Gy TBI.
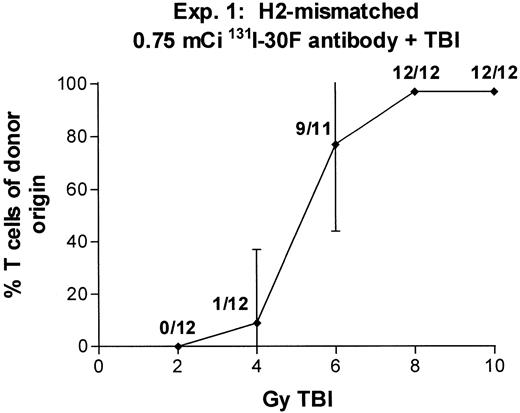
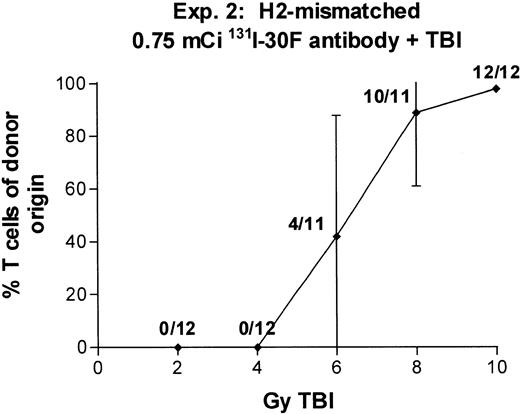
In two experiments we combined 0.75 mCi of 131I-anti-CD45 antibody with TBI doses varying between 2 and 10 Gy (Fig 7). With 6 Gy TBI, engraftment was observed in 9 of 11 recipients (experiment no. 1) and 4 of 11 recipients (experiment no. 2), and with 8 Gy TBI, engraftment was observed in 12 of 12 recipients (experiment no. 1) and 10 of 11 recipients (experiment no. 2). These results suggest that the immunosuppressive effect of 131I-anti-CD45 antibody labeled with 0.75 mCi 131I can replace 6 to 8 Gy TBI in producing engraftment equivalent to that seen with 14 Gy TBI alone. A dose of 0.75 mCi 131I is estimated to deliver approximately 25 Gy to marrow, 50 Gy to lymph nodes, and 80 Gy to spleen.
Engraftment of T-cell–depleted BALB/c marrow in mice treated with 100 μg 30F11 antibody labeled with 0.75 mCi131I combined with 2 to 10 Gy TBI. Proportion of T cells of donor origin (mean ± SD) 3 months after transplantation. Data point labels indicate the number of mice with ≥80% donor T cells of total surviving mice (of 12 treated mice per group).
Engraftment of T-cell–depleted BALB/c marrow in mice treated with 100 μg 30F11 antibody labeled with 0.75 mCi131I combined with 2 to 10 Gy TBI. Proportion of T cells of donor origin (mean ± SD) 3 months after transplantation. Data point labels indicate the number of mice with ≥80% donor T cells of total surviving mice (of 12 treated mice per group).
DISCUSSION
We asked if radiation delivered by 131I-labeled anti-CD45 antibody could enable engraftment of congenic marrow and of H2-incompatible marrow. Using congenic marrow,131I-anti-CD45 antibody alone enabled engraftment, with ≥80% engraftment when antibody was labeled with ≥0.5 mCi131I, and thus could replace TBI. When donor and recipient were H2-incompatible, 131I-anti-CD45 antibody could partially replace TBI, demonstrating its potential to permit reduction of external beam TBI and possibly provide a less toxic marrow transplant preparative regimen.
Several factors influence the relationship between radiation dose and successful engraftment, including recipient variables such as strain, health status, and presensitization to donor alloantigens26,27 and graft variables such as histocompatibility, stem cell source, cell dose, and number of T cells.12,28-36 Engraftment is also influenced by the exposure rate of irradiation and by the fractionation schedule.12-15 37-39 Delivering radiation with131I-anti-CD45 antibody enabled engraftment of donor cells in congenic mice. All B6-Ly5a recipients treated with 0.75 mCi 131I and 3 of 6 treated with 1.0 mCi131I conjugated to anti-CD45 antibody survived without infusion of donor marrow, demonstrating that the radiation delivered by these doses of isotope was not myeloablative and did not have fatal nonhematopoietic toxicity. Nonetheless, recipients treated with a much lower dose of 131I delivered by anti-CD45 antibody (0.3 mCi) had 60% T-cell, 70% B-cell, and 45% myeloid engraftment after infusion of Ly5-congenic marrow. Eighty percent engraftment required 8 Gy TBI or 0.5 mCi of 131I. The 17 Gy estimated irradiation absorbed by marrow delivered by antibody labeled with 0.5 mCi131I supports the concept that radiation delivered at the low continuous dose rate and low linear energy transfer of β-particles such as 131I has a lower relative biological efficacy (RBE) than radiation delivered at the much more rapid rate used for TBI.
Previous studies have shown that when the external beam TBI dose rate is decreased from 25 to 1 cGy/min, the LD50 for early gastrointestinal toxicity is increased from 12 to 21 Gy and the LD50 for late nonhematopoietic deaths occurring by 1 year is increased from 10 to 21 Gy in BALB/c mice supported with syngeneic marrow transplants.40 In that study, no mice treated with 20 Gy at 1 cGy/min had detectable histologic changes in the lung or kidney. In CBA mice treated with thoracic irradiation at high dose rate (180 cGy/min), the minimum dose required to produce a detectable elevation in breathing rate at 28 weeks after treatment increased from 13.8 Gy rad when the irradiation was delivered as a single fraction to 30.4 Gy when the irradiation was delivered in 7 fractions.41 In a study with β-emitting radionuclide90Y administered as inhaled fused aluminosilicate particles, estimated lung radiation doses up to 27 Gy did not decrease survival as compared with untreated controls.42 These results are consistent with our observations of lower RBE of radiation delivered by 131I-labeled antibody compared with equivalent doses delivered as conventional TBI.
In a transplant model demanding profound immunosuppression, engraftment of T-cell–depleted H2-mismatched marrow could not be achieved using131I-CD45 antibody alone. Engraftment occurred in only a minority of mice receiving the highest 131I dose delivered (1.5 mCi). Several factors might explain the inability to achieve engraftment after treatment with radiolabeled antibody, despite the delivery of estimated radiation doses of almost 100 Gy to lymph nodes, 50 Gy to marrow, and 160 Gy to spleen. First, a blood-thymus barrier impeded antibody localization in the thymus, with a dose of approximately 19 Gy delivered by 1.5 mCi 131I. Second, the absorbed radiation actually delivered to cells that cause graft rejection might be less than estimated from the biodistribution studies. The estimates of absorbed dose in hematopoietic tissues represent the average for cells that are assumed to reside in that tissue for at least the first 96 hours after radiolabeled antibody infusion and do not account for heterogeneity within the tissue. T cells or NK effectors that circulate during some or all of the period of radiation delivery may thus receive a lower radiation dose. A cell that circulates during the entire period of radiation delivery will receive at least the radiation dose estimated for total body (5.9 Gy/mCi 131I). It is not possible to quantitate the additional radiation received by a circulating cell because it involves not only radiation delivered by antibody bound to the cell and to neighboring cells in circulation as well as that present in plasma (which depends on geometry of blood vessels in relationship to the path length of the isotope), but also radiation delivered during the time the cell traffics in tissues such as the spleen where greater radiation effects are present. Within lymph nodes, radiation delivery may be heterogeneous, and the amount of irradiation delivered to individual nodes could vary. Finally, cellular repair mechanisms might be able to keep pace with the rate of damage inflicted by the low radiation dose rate delivered from 131I, thereby avoiding cell death.
Our results with CD45-congenic donor/recipient pairs suggest that treatment with 131I-anti-CD45 antibody alone is sufficient to allow engraftment of donor cells at 131I doses that are well tolerated. This raises the possibility that131I-labeled anti-CD45 antibody might provide a less toxic method for selective ablation of marrow and might enable reconstitution with autologous hematopoietic cells modified by gene therapy. Furthermore, the finding that engraftment of T-cell–depleted H2-mismatched marrow reliably occurred when 0.75 mCi of131I-anti-CD45 antibody was combined with 8 Gy TBI suggests that targeted radiation may be able to replace nearly half of the TBI ordinarily administered in such transplants. Such an approach holds the potential for enhanced antileukemic efficacy while inducing less systemic toxicity, thereby improving the outcome after marrow transplantation for acute leukemia.
ACKNOWLEDGMENT
The authors are indebted to Minna Zheng, Jennifer Smith, and Carol Dean for their expert technical assistance.
Supported by National Institutes of Health Grant No. CA01690, the Adler Foundation, and the Parker-Hughes Trust.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dana C. Matthews, MD, Fred Hutchinson Cancer Research Center D1-100, 1100 Fairview Ave N, PO Box 19024, Seattle, WA 98109; e-mail: dmatthew@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal