Abstract
Dendritic cells (DC) originate from a bone marrow (BM) precursor and circulate via the blood to most body tissues where they fulfill a role in antigen surveillance. Little is known about DC numbers in disease, although the reported increase in tissue DC turnover due to inflammatory stimuli suggests that blood DC numbers may be altered in some clinical situations. The lack of a defined method for counting DC has limited patient studies. We therefore developed a method suitable for routine monitoring of blood DC numbers, using the CMRF44 monoclonal antibody (MoAb) and flow cytometry to identify DC. A normal range was determined from samples drawn from 103 healthy adults. The mean percentage of DC present in blood mononuclear cells (MNC) was 0.42%, and the mean absolute DC count was 10 × 106 DC/L blood. The normal ranges for DC (mean ± 1.96 standard deviation [SD]) were 0.15% to 0.70% MNC or 3 to 17 × 106 DC/L blood. This method has applications for monitoring attempts to mobilize DC into the blood to facilitate their collection for immunotherapeutic purposes and for counting blood DC in other patients. In preliminary studies, we have found a statistically significant decrease in the blood DC counts in individuals at the time of blood stem cell harvest and in patients with acute illnesses, including allogeneic bone marrow transplant (BMT) recipients with acute graft-versus-host disease (aGVHD).
DENDRITIC CELLS (DC) originate from bone marrow (BM) precursors and circulate via the blood to most tissues of the body where they have a sentinel role in immune surveillance. These peripheral DC acquire antigens and migrate via the lymph to regional lymph nodes where they initiate T-lymphocyte responses.1,2DC populations may be heterogeneous and several potential differentiation pathways have been described.2 Myeloid DC in man are best identified by a combination of morphologic, phenotypic, and functional features: there is at present no evidence for a human lymphoid-derived blood DC. No surface marker specific for blood myeloid-derived DC (hereafter DC), which are found among a heterogeneous population of lineage marker negative (lin-) HLA-DR positive cells, generally 1% to 2% of blood mononuclear cells (MNC), has been described to date. As yet, no DC deficiency state has been described in man, and there is little information on blood DC numbers in disease. However, the turnover of DC in the tissues is substantially increased by various inflammatory stimuli,3-6and blood DC numbers might be raised to support this. Alternatively, other BM-derived cells, for example monocyte-derived DC (Mo-DC)7 8 might account for the increased turnover of DC at sites of inflammation.
Previous studies have estimated blood DC numbers by extrapolating from the yield of DC obtained (after multistep purification procedures) from a given volume of blood. These estimates of DC number are dependent on the isolation methods used and the criteria used to define DC, and thus vary considerably. Moreover the enumeration of DC after the use of complex isolation techniques is not applicable to the routine monitoring of DC numbers. Early work estimated blood DC numbers as being in the order of 0.1% to 0.5% of MNC,9 later quoted as 0.3% of MNC.10 Subsequent estimates of blood DC number range from approximately 0.1% of MNC11 to 0.9% to 1.0% of MNC,12,13 although higher estimates (4% of MNC14) have been published. DC numbers appear to be decreased in the blood of acquired immunodeficiency syndrome (AIDS) patients13 and in patients with advanced stages of breast cancer.15 The ability to measure DC numbers readily in patients would encourage further studies of the role DC play in disease and facilitate attempts to mobilize DC into the blood to be collected for immunotherapeutic purposes.
There are precedents for counting rare cell populations by flow cytometry, notably the routine monitoring of blood CD34+cell numbers.16,17 Attempts to measure DC number are complicated by the lack of a suitable specific marker for circulating blood DC.18,19 However, blood DC do express some relatively restricted activation antigens, notably the CD8320,21 and CMRF4422,23 antigens, after a brief period of in vitro culture. The CMRF44+/CD14 and CD19− cells generated in this manner have the morphology, phenotype, and functional attributes of DC.23 The relatively rapid upregulation of the CMRF44 antigen23 indicated that it might be possible to use the CMRF44 monoclonal antibody (MoAb) to measure and monitor blood DC numbers. We now report a flow cytometric method, which uses the CMRF44 MoAb to count the DC, after blood MNC have been activated by a brief period of in vitro culture. The method has been used to determine a normal range for blood DC counts in a healthy adult population. As a preliminary study of the method’s use in a clinical setting, we measured blood DC counts in patients undergoing blood stem cell mobilization before transplantation and monitored DC counts in patients undergoing autologous and allogeneic blood stem cell or bone marrow transplantation (BMT). The resulting data supports the hypothesis that blood DC numbers may be altered in certain disease states.
MATERIALS AND METHODS
Subject selection.
A reference population was established from healthy volunteers aged 19 to 86 years, who had had no recent illness at the time of specimen collection. All samples were collected between 8 am and 2 pm. Patients undergoing autologous or allogeneic stem cell transplantation in this institution were invited to contribute to this study. Blood samples were taken after engraftment (neutrophils >1.0 × 109/L). Other patients with a single disorder were also tested (Table 1). BM was obtained by aspiration from the posterior iliac crest of volunteer normal donors. Cord blood was collected by venipuncture of the umbilical vein of normal full-term deliveries. All specimens were obtained with the approval of the local Ethical Committee after informed consent had been given by the donor (or mother in the case of cord blood collections). The stem cell mobilization protocol involved a single dose of cyclophosphamide (2 g/m2) and granulocyte colony-stimulating factor (G-CSF; Roche, Auckland, New Zealand), 5 to 10 μg/kg/day from day 0 to the end of harvesting, or G-CSF (10 μg/kg/day) alone. Harvests were usually initiated on the day that the CD34+ count rose over 20 × 106/L.
Demographic Details and Diagnoses of the Normal Individuals and Patients Studied
| . | No. . | Male:Female . | Age Range . | Diagnosis . |
|---|---|---|---|---|
| Normals | 103 | 50:53 | 19-86 | NA |
| Stem cell harvest | 20 | 10:10 | 23-62 | NHL (9), MM (5), HD (1), Ca breast (1), other (3)*, normal donor (2) |
| Post-BMT | 35 | 17:18 | 18-58 | MM (8), NHL (5), CML (9), AML (4), ALL (7), AA (1), primary amyloidosis (1) |
| Other* | 8 | 4:4 | 21-80 | Pyelonephritis (3), cellulitis (1), post op (1), MDS (3) |
| . | No. . | Male:Female . | Age Range . | Diagnosis . |
|---|---|---|---|---|
| Normals | 103 | 50:53 | 19-86 | NA |
| Stem cell harvest | 20 | 10:10 | 23-62 | NHL (9), MM (5), HD (1), Ca breast (1), other (3)*, normal donor (2) |
| Post-BMT | 35 | 17:18 | 18-58 | MM (8), NHL (5), CML (9), AML (4), ALL (7), AA (1), primary amyloidosis (1) |
| Other* | 8 | 4:4 | 21-80 | Pyelonephritis (3), cellulitis (1), post op (1), MDS (3) |
Abbreviations: NHL, non-Hodgkins lymphoma; MM, multiple myeloma; HD, Hodgkin’s disease; Ca breast, breast carcinoma; CML, chronic myeloid leukemia; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; AA, aplastic anemia; MDS, myelodysplastic syndrome; NA, not applicable.
Neuroectodermal tumor (1), amyloidosis with plasma cell dyscrasia (1), T-ALL (1).
Preparation of sample.
A total of 5 mL of blood was collected into EDTA and processed within 4 hours of collection as follows: a routine blood count was performed using a Coulter STKS (Coulter Electronics, Hialeah, FL). The absolute number of MNC/L blood was determined from this measurement. The remaining blood was diluted 1:1 with sterile phosphate-buffered saline (PBS) and underlayed with Ficoll-Paque (Pharmacia, Uppsala, Sweden) before centrifugation at 520g for 15 minutes at room temperature. The MNC were recovered from the low-density interface and washed three times before culturing for 24 hours in RPMI-1640/10% fetal calf serum (FCS) (Life Technologies, Auckland, NZ) at 37°C and 5% CO2 in air in Falcon 2054 round bottom polystyrene tubes (Becton Dickinson, Sydney, Australia). Typically, a 5 mL blood sample yielded approximately 5 × 106 MNC, which were cultured at a concentration of 1 × 107 cells/mL (normally 0.25 to 1 mL media). In some experiments, cells from individual donors were cultured for 4, 24, and 48 hours. Granulocyte-macrophage (GM)-CSF (Sandoz, Basel, Switzerland), interleukin-3 (IL-3) (Sandoz), and tumor necrosis factor (TNF)α (Hoffman-La Roche, Basel, Switzerland) were used at the indicated concentration to document the effect of adding cytokines to this culture media. RPMI/10%FCS was chosen as the culture media on the basis of a previous comparison of the ability of different media to support DC in vitro24 and to provide standard culture conditions in individuals in whom drugs or other serum factors may have affected DC activation. After culture, cells were washed and their viability checked using trypan blue exclusion. A repeat blood sample was requested in the rare instances where the viability was <95%. Cells were then resuspended at 107/mL and labelled as described below.
MoAbs.
The MoAb, CMRF44 (IgM)22, was produced in this laboratory, as was the IgM negative control MoAb, CMRF50, which recognizes a keratinocyte antigen and does not react with hematopoeitic cells. Phycoerythrin (PE)-conjugated CD14 (leuM3, IgG2a), CD19 (leuM12, IgG1), anti–HLA-DR (L243, IgG2a), and IgG2a negative control MoAb were purchased from Becton Dickinson. CD83 (HB15a, IgG2b) was purchased from Immunotech (Marseille, France). Fluorescein isothyocyanate-conjugated sheep antimouse immunoglobulin (FITC-SAM) was purchased from Silenus (Hawthorne, Victoria, Australia). Propidium iodide (PI) was purchased from Sigma (St Louis, MO). CMRF31 (CD14, IgG2a, this laboratory), FMC63 (CD19, IgG1, a gift of Prof H. Zola, Adelaide, Australia), HuNK2 (CD16, IgG2a, a gift from Prof I. McKenzie, Melbourne, Australia), OKT3 (CD3, IgG2a, from a hybridoma obtained from the American Type Tissue Collection (Rockville, MD) were used to create a MoAb cocktail against the B and T lymphocyte, monocyte and natural killer (NK) leukocyte lineages. Cells that did not label with this MoAb cocktail were designated lin−.
Labelling procedure.
Cells were labelled using standard techniques. Briefly, 50 μL of cells (107/mL) were placed into five tubes. Two tubes were labelled with the negative control MoAb and three with the CMRF44 MoAb for 20 minutes at 4°C, before being washed twice in PBS/1%bovine serum albumin (BSA) and then labelled with FITC-SAM for another 20 minutes. After further washing and blocking using 10% mouse serum, PE-conjugated CD14 and CD19 MoAb were added to each tube and incubated for 20 minutes at 4°C. A final wash was followed by the addition of 5 μL of 10 μmol/L PI to each tube.
Flow cytometry.
Analysis was performed using a fluorescence-activated cell sorting (FACS) vantage flow cytometer (Becton Dickinson, San Jose, CA) equipped with a 488 nm argon ion laser with fluorescence emission detected at 525, 575, and 675 nm. A total of 50,000 events was acquired from each tube and analyzed using Cellquest software (Becton Dickinson). Live cells were gated on the basis of forward and side light scatter characteristics and PI exclusion. A region, which encompassed CMRF44+ CD14−CD19− events, was copied onto all five dot plots. On the negative control dot plots, this region typically contained <0.1% of total events. The percentage of CMRF44+ CD14−CD19− events was noted and the mean of the two negative control values was subtracted to give triplicate measurements of DC number as a percentage of total MNC. The mean of these triplicate values was taken as the final DC percentage of MNC and used to calculate the absolute DC number.
Statistics.
Statistical analysis (t tests for independent groups and paired data as appropriate) was performed using the Instat statistics package (GraphPad Software, San Diego, CA).
RESULTS
Analysis of blood DC numbers by flow cytometry.
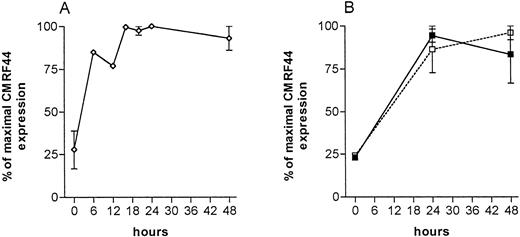
The kinetics of CMRF44 expression on cultured lin−, HLA class II+ cells (enriched by magnetic depletion of lin+ cells) was checked to determine the optimal period of in vitro culture for this test. The CMRF44 antigen is one of the first activation antigens expressed by DC,23 but culture for less than 6 hours was insufficient to allow accurate detection of DC (Fig 1A). Culture times of 12 hours clearly distinguished CMRF44+ cells, but are not convenient for a routine laboratory. The number of CMRF44+ DC was near maximal after 16 to 48 hours. Although the mean fluorescence intensity of the positive cells was slightly higher at 48 rather than 24 hours, the viability of the cultured cells was better at 24 hours and therefore 24 hours in vitro culture was selected as a suitable culture period for routine DC counting.
Time course of CMRF44 antigen expression (normalized, 100% = maximum number of cells labelling with CMRF44) on: (A) lin− (DC enriched) sorted cell populations cultured for the period indicated (n = 6). (B) Blood MNC, cultured as described for routine DC counting (solid line, normal individuals (n = 3), dashed line, patients (n = 3).
Time course of CMRF44 antigen expression (normalized, 100% = maximum number of cells labelling with CMRF44) on: (A) lin− (DC enriched) sorted cell populations cultured for the period indicated (n = 6). (B) Blood MNC, cultured as described for routine DC counting (solid line, normal individuals (n = 3), dashed line, patients (n = 3).
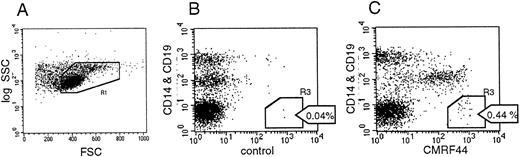
Flow cytometric analysis of the MoAb-labelled cells enabled the percentage of DC present in cultured MNC populations to be measured (see Materials and Methods and Fig 2). Events that were CMRF44+ CD14−CD19− (R3 in Fig 2C) were, after subtraction of the control (background staining cells), counted as DC and expressed as a percentage of the blood MNC. Absolute DC counts were then calculated from the number of MNC/L blood (as determined by the automated cell counter), multiplied by the percentage of DC. Absolute DC counts varied independently of the number of MNC and did not correlate with either the absolute monocyte or lymphocyte counts (r2 = 0.05 and 0.06, respectively).
FACS analysis of labelled cells. (A) MNC were gated (R1) and dead cells excluded by PI labelling (R2 not shown). A total of 50,000 events was aquired from each tube. (B) The number of CD14− and CD19− events occuring with the negative control antibody was measured (R1 and R3). Two negative control tubes were analyzed, and the mean of these two values (eg, 0.04% in the example shown) was used. (C) Events in R1 and R3 (CMRF44+ CD14− and CD19−cells) were counted in three separate tubes. The mean negative control value (B) was subtracted from each value. The mean of these three replicates was used as the DC percentage of MNC. The absolute DC count was calculated as described in the Results.
FACS analysis of labelled cells. (A) MNC were gated (R1) and dead cells excluded by PI labelling (R2 not shown). A total of 50,000 events was aquired from each tube. (B) The number of CD14− and CD19− events occuring with the negative control antibody was measured (R1 and R3). Two negative control tubes were analyzed, and the mean of these two values (eg, 0.04% in the example shown) was used. (C) Events in R1 and R3 (CMRF44+ CD14− and CD19−cells) were counted in three separate tubes. The mean negative control value (B) was subtracted from each value. The mean of these three replicates was used as the DC percentage of MNC. The absolute DC count was calculated as described in the Results.
The precision of the method was tested by performing multiple simultaneous tests on individual donors. The variance of the three replicates of the single tests performed on 97 individuals was 0.004%. When three simultaneous tests of three replicates were performed on three normal donors, the variance of the replicates was 0.004% with an additional component of variance due to the tests of 0.0036%. This suggested that other test factors, as well as the labelling and/or analysis of the replicate tubes, accounted for most of the observed variance, but that other factors also contributed to the variance. Therefore, the effect of varying the MNC concentration during the culture and the effect of titrating conditioned media into the cell culture was studied. Variation between the conditions tested in this manner appeared no greater than that seen between replicates performed in identical conditions (data not shown), suggesting that labelling and/or analysis accounted for most of the observed variation.
However, when normal individuals were tested at intervals of >1 week, considerable variations in relative and absolute DC numbers were seen (Table 2). This may reflect fluctuation within a relatively broad range, as is seen with other leukocytes, or could be due to cyclical variation in blood DC numbers. It was unlikely to be due to circadian variation, as all samples were collected at a similar time of the day.
Variation in DC Numbers Over Time in Normal Subjects
| Donor . | Test 1 . | Test 2 . | Test 3 . |
|---|---|---|---|
| ND 1 | .52 (13.5) | .28 (7.6) | .23 (6.4) |
| ND 2 | .58 (17.4) | .94 (26.3) | .48 (12.0) |
| ND 3 | .46 (10.6) | .33 (6.6) | .47 (10.8) |
| ND 4 | .30 (8.4) | .56 (15.7) | .37 (9.6) |
| ND 5 | .40 (6.4) | .42 (9.2) | .49 (13.7) |
| ND 6 | .61 (11.0) | .20 (4.0) | .53 (11.1) |
| ND 7 | .36 (12.7) | .63 (7.2) | .50 (9.0) |
| Donor . | Test 1 . | Test 2 . | Test 3 . |
|---|---|---|---|
| ND 1 | .52 (13.5) | .28 (7.6) | .23 (6.4) |
| ND 2 | .58 (17.4) | .94 (26.3) | .48 (12.0) |
| ND 3 | .46 (10.6) | .33 (6.6) | .47 (10.8) |
| ND 4 | .30 (8.4) | .56 (15.7) | .37 (9.6) |
| ND 5 | .40 (6.4) | .42 (9.2) | .49 (13.7) |
| ND 6 | .61 (11.0) | .20 (4.0) | .53 (11.1) |
| ND 7 | .36 (12.7) | .63 (7.2) | .50 (9.0) |
Seven individuals had DC counts performed on three separate occasions (the interval between counts was >1 week). Percentage DC of blood MNC and absolute DC counts × 106/L (in parentheses) are shown.
Analysis of the reference population and determination of a normal range.
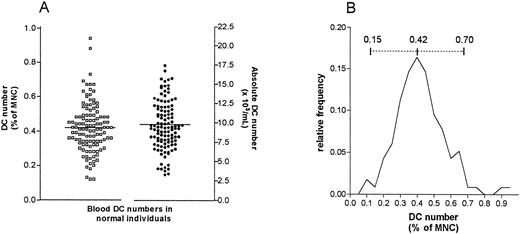
The reference population comprised 103 normal individuals, drawn mainly from a population of hospital and laboratory workers, but also the general public. The age range of this population was 19 to 86 years and the male:female ratio was 50:53. The mean number of DC as a percentage of MNC was 0.42% (95% confidence interval [CI] = 0.39 to 0.45) and the observed range was 0.12 to 0.94% MNC. The mean absolute DC number was 9.8 × 106/L blood (95% CI = 9.1 to 10.4), and the observed range 3 to 26 × 106 DC/L (Fig 3A). These results showed an approximately normal distribution (Fig 3B).
(A) Scatter plot of the distribution of DC numbers in 103 normal individuals, expressed as percentage of blood MNC and as absolute counts. (B) DC numbers appear to follow a normal distribution. The mean DC proportion (0.42% of MNC) and the calculated normal range (0.15% to 0.70% of MNC) are shown (solid and dashed lines, respectively). Absolute DC counts follow a similar distribution (not shown).
(A) Scatter plot of the distribution of DC numbers in 103 normal individuals, expressed as percentage of blood MNC and as absolute counts. (B) DC numbers appear to follow a normal distribution. The mean DC proportion (0.42% of MNC) and the calculated normal range (0.15% to 0.70% of MNC) are shown (solid and dashed lines, respectively). Absolute DC counts follow a similar distribution (not shown).
The mean percentage and absolute DC number did not differ significantly when females and males were analyzed separately, although the range was slightly greater in females aged under 50, raising the possibility that hormonal factors may influence DC number. DC numbers did not vary significantly when the reference population was segregated on the basis of age and appeared to be maintained even in the oldest (13 patients > 60 years) subjects in this study.
A normal range, based on a mean of 0.42 ± 1.96 SD was derived as 0.15 to 0.70% of MNC and 3 to 17 × 106 DC/L blood.
Validation of the method.
It was theoretically possible that in clinical situations, factors such as inflammatory mediators might affect CMRF44 antigen expression, and thus the assay. Indeed, our initial experience was that DC numbers in patients were often low. Therefore, additional experiments, including the use of alternative methods for estimating DC number, were performed to validate the method. Time course experiments were performed on normal subjects and patients with inflammatory conditions to ensure that the kinetics of CMRF44 expression was similar when unseparated MNC were cultured for DC counting. A period of 20 to 24 hours was confirmed in these individuals as suitable for DC counting, based on CMRF44 antigen expression, and the kinetics of its expression appeared similar in both the normal and patient groups (Fig 1B).
Supplementing the culture media with cytokines known to promote DC survival (GM-CSF, TNFα, or IL-3) did not significantly increase DC numbers (Table 3), and the effects were variable between individuals. In some cases these cytokines (particularly the TNFα) increased CRMF-44 and decreased CD14 or CD19 expression on non-DC populations, making clear discrimination of CMRF44+/CD14 and 19− cells difficult.
DC Counts Obtained in Five Individuals After Culture in Media With Additional Cytokine Compared With Those Obtained Without Supplementation
| Cytokine . | DC No.3-150 . | ±SD . |
|---|---|---|
| +GM-CSF | 109% | ±19% |
| +TNFα | 104% | ±27% |
| +IL-3 10 ng/mL | 107% | ±26% |
| +IL-3 50 ng/mL | 106% | ±21% |
| Cytokine . | DC No.3-150 . | ±SD . |
|---|---|---|
| +GM-CSF | 109% | ±19% |
| +TNFα | 104% | ±27% |
| +IL-3 10 ng/mL | 107% | ±26% |
| +IL-3 50 ng/mL | 106% | ±21% |
Normalized, media alone = 100%.
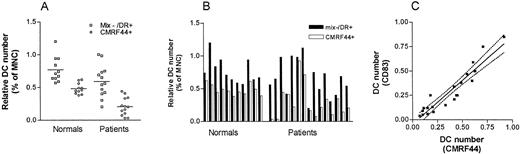
Alternative methods of estimating blood DC number were used in parallel with the CMRF44-based method to obtain some comparative data. The number of lin−/HLA-DR+ cells present before cell culture was tested in 11 normal individuals and 13 patients with a low mean DC count. A lower number of lin−/HLA-DR+ cells (0.60% v 0.85%) paralleled the lower CMRF44+ DC number (0.21% v0.49%) in these patients compared with the normals (Fig 4A). Nonetheless, the relatively constant relationship between the lin−HLA-DR+ cells and CMRF44+ DC seen in the normal individuals was lost for the patients (Fig 4B). This probably reflected the variable cellular composition of the lin−HLA-DR+ population.
Comparision of DC numbers identified as either lin− HLA-DR+ cells (before culture) or as CMRF44+ CD14− CD19− cells (after overnight culture). (A) The mean numbers of lin−HLA-DR+ cells and CMRF44+DC were both reduced in patients (n = 13) compared with normal individuals (n = 11). (B) The relatively constant relationship between the numbers of CMRF44+ DC and lin−HLA-DR+ cells seen in normal individuals (where CMRF44+ DC typically account for 40% to 80% of lin− HLA-DR+ cells) is not seen in the patient group (CMRF44+ DC account for 5% to 95% of the lin− HLA-DR+ cells). (C) The use of CMRF44 or CD83 to identify DC was compared in both normals (n = 5) and patients (n = 16). A strong correlation (r2= 0.89) was found between the DC numbers measured using CMRF44 or CD83.
Comparision of DC numbers identified as either lin− HLA-DR+ cells (before culture) or as CMRF44+ CD14− CD19− cells (after overnight culture). (A) The mean numbers of lin−HLA-DR+ cells and CMRF44+DC were both reduced in patients (n = 13) compared with normal individuals (n = 11). (B) The relatively constant relationship between the numbers of CMRF44+ DC and lin−HLA-DR+ cells seen in normal individuals (where CMRF44+ DC typically account for 40% to 80% of lin− HLA-DR+ cells) is not seen in the patient group (CMRF44+ DC account for 5% to 95% of the lin− HLA-DR+ cells). (C) The use of CMRF44 or CD83 to identify DC was compared in both normals (n = 5) and patients (n = 16). A strong correlation (r2= 0.89) was found between the DC numbers measured using CMRF44 or CD83.
In 21 experiments (5 normal individuals and 16 patients), additional labelling with an alternative DC activation marker, CD83, was performed. The DC number was usually slightly (mean 15%) lower when measured using this antibody, possibly as a result of the different kinetics of CD83 antigen upregulation.23 There was a strong correlation (r2 = 0.89) between CMRF44+ and CD83+ DC numbers (Fig 4C) in both normal individuals and patients. These results indicate that the low DC number seen in some patients was not simply a consequence of altered CRMF44 expression during illness.
Monitoring DC numbers during stem cell mobilization and harvesting.
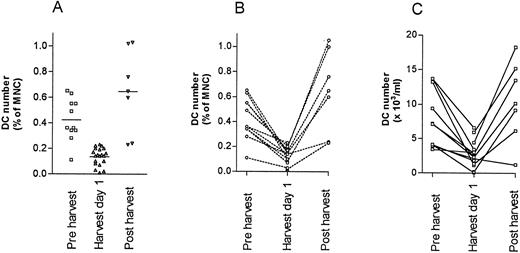
Blood stem cell mobilizations were studied in 20 individuals; in 17 patients cyclophosphamide/G-CSF was used and in one patient and two normal donors, G-CSF alone was used to mobilize stem cells into the blood (Fig 5). DC numbers were assayed on day 1 of the harvest in all patients. Where possible pre- and/or postmobilization counts were done, although due to logistical reasons, not all patients could be tested at all time points. The mean percentage DC and absolute DC counts were significantly lower than normal on day 1 of harvesting (DC percentage 0.14% v 0.42% of MNC, P < .01 and absolute DC counts 2.9 v 9.8 x 106/L, P < .01). Using all possible data points, paired analysis of DC numbers was also performed and again the difference between the pre- and during harvest DC number was significant (n = 11, 0.42 v 0.13% of MNC, two-tailed P < .01: paired Student’s t test) and (8.0 v 2.6 × 106/L, P < .01) (Fig 5B and C). Two normal donors were harvested for allografts after mobilization with G-CSF alone and a similar pattern was seen, with a decrease in the DC blood count at the time of harvest. An increase in DC count (some within the normal range) was noted 1 to 2 weeks after harvesting in all of the donors tested (Fig 5).
Blood DC numbers at the time of stem cell mobilization in 20 subjects. (A) Mean DC percent of blood MNC before, during, and after stem cell harvest. (B) Paired (pre- and during or during and post) percent DC of blood MNC in individual patients. (C) Paired (pre- and during or during and post) absolute DC counts. The differences in DC counts before and during the harvest are statistically significant (see text).
Blood DC numbers at the time of stem cell mobilization in 20 subjects. (A) Mean DC percent of blood MNC before, during, and after stem cell harvest. (B) Paired (pre- and during or during and post) percent DC of blood MNC in individual patients. (C) Paired (pre- and during or during and post) absolute DC counts. The differences in DC counts before and during the harvest are statistically significant (see text).
For comparison, DC numbers in BM aspirates and cord blood were also measured using this method. Normal BM aspirate DC numbers appeared similar to normal blood DC numbers (mean marrow DC number = 0.41%, n = 3). The DC numbers in cord blood were lower than those seen in adult blood (mean = 0.23%, n = 8), as has been reported previously.25
Analysis of blood DC counts after stem cell transplantation.
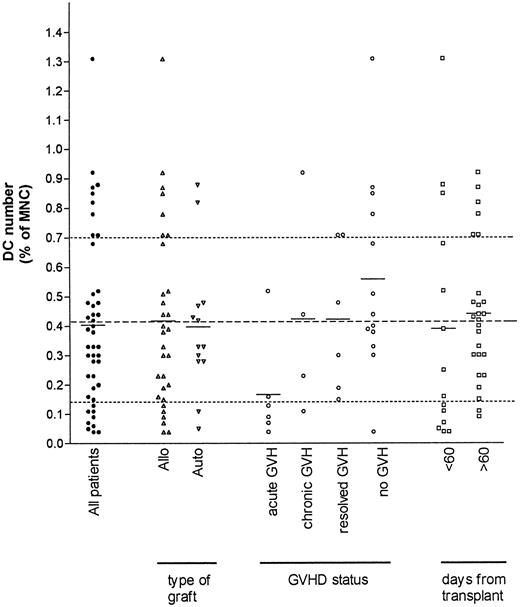
The range of DC counts after stem cell transplantation did not follow a normal distribution (Fig 6). A total of 35 patients were studied, with 11 being tested on more than one occasion. Subdividing patients on the basis of graft type showed a similar mean percentage of DC in both the allogeneic and autologous recipients. In the autografted group, no difference was seen between DC numbers in patients who received blood stem cell autografts (with low DC percentages as above), CD34+ selected blood stem cell autografts (with very low absolute DC numbers), or the patients who had received a BM graft.
Post-BMT DC counts from 35 patients (filled symbols) were subdivided on the basis of the three criteria indicated. The mean DC number for each patient group is shown (solid line). The mean (dashed line) and the limits of the normal range (dotted lines) are shown for comparison. Mean DC numbers were similar after autologous and allogeneic grafts. The mean DC number for the patients with aGVHD was significantly lower than the other postallogeneic stem cell transplant patients (see text).
Post-BMT DC counts from 35 patients (filled symbols) were subdivided on the basis of the three criteria indicated. The mean DC number for each patient group is shown (solid line). The mean (dashed line) and the limits of the normal range (dotted lines) are shown for comparison. Mean DC numbers were similar after autologous and allogeneic grafts. The mean DC number for the patients with aGVHD was significantly lower than the other postallogeneic stem cell transplant patients (see text).
In contrast, the percentage and absolute DC number were low in patients with acute graft-versus-host disease (aGVHD) compared with other allo BMT patients (mean DC number 0.17% v 0.54%, P = .01), the only exception being in one patient with grade 1 skin aGVHD. A low DC number (0.06% of MNC) was also seen in one pediatric patient with aGVHD, but this result was not included in the above analysis due to the lack of a formal normal range for this age group. The low DC number is likely to be related to aGVHD rather than other associated factors, as mean DC counts in the early (28 to 60 days) or later (>60 days) posttransplant period did not differ significantly, and likewise mean DC counts were similar after grouping patients on the basis of cyclosporin A treatment (not shown). As steroids, usually prednisone or dexamethasone, were used to treat aGVHD, these agents may have contributed to the low DC counts in these patients, however chronic GVHD (cGVHD) patients who were also receiving steroids showed no obvious effect on DC numbers. In addition, the effect of high dose steroids on DC number was monitored in two (nontransplant) patients with immune thrombocytopenia, and no change in DC number was seen (not shown).
Some patients with no intercurrent illness (including those with recently resolved aGVHD) had high DC counts in the first 3 months posttransplant, although the low MNC numbers in some patients meant this did not always translate into a high absolute DC count.
DISCUSSION
Ideally, blood myeloid DC number would be determined with the use of a marker specific for circulating DC. Because no such marker is currently available, culture of MNC is required to allow the use of DC activation antigens for positive identification of DC. The decision to use CMRF44 as the reference antibody for this study was based on the rapidity of the upregulation of the CMRF44 antigen and its relative density of expression on DC. These two factors are important in that they allow a short period of in vitro culture with subsequent clear identification of the DC by flow cytometry. CD83 can be used to enumerate blood DC,11 but in our experience, CD83 antigen expression lags behind CMRF44 antigen expression on DC and is not as strongly expressed.23 Both of these factors may contribute to the slightly lower DC numbers we observed when we used CD83 in place of CMRF44. DC enumeration using CD83 after a multistep purification suggested that DC numbers were in the range 0.06% to 0.15% of blood MNC,11 but potential DC loss during purification, together with the factors mentioned above, may account for these values, which are lower than our range of 0.15% to 0.70% of blood MNC. In our experience, DC yields after purification are often only approximately 50% of that expected based on blood DC numbers found in this study.24 An alternative method based on a lin− HLA-DR+ DC phenotype has also been used to directly enumerate blood DC,12 and the numbers of lin− HLA-DR+ we observed in normal individuals (0.85%) correlated closely with the lin−HLA-DR+ cell number reported (0.90%). This approach has the benefit of avoiding the requirement for cell culture, but is less precise in its definition of a DC. The lin−HLA-DR+ population is not homogeneous in its expression of a number of surface markers and has been reported to give rise to a number of different cell populations.26-28Furthermore, expression of HLA-DR on other activated cells during illness may also complicate the issue. Our results indicate that the non-DC, lin− HLA-DR+ populations may vary considerably in number within patients, creating potential for error when this method is used to monitor blood DC.
The need to culture cells introduces a number of potential variables, which may influence the result and certain assumptions are inherent to this method. First, it must be assumed that DC are not over or under-represented in the cell losses, which occur during density gradient separation and culture of the MNC. This method involves minimal cell handling, and we excluded samples with low viability from analysis to limit potential variance from this source. A second assumption made in comparing DC numbers between populations is that the DC number is not affected by the relative composition of the cultured MNC. The relatively minor effect that cytokine supplementation of the culture media had on DC numbers suggests that MNC cultured in RPMI/10% FCS can provide a suitable millieu for DC maturation and survival over the culture period used in this method. Validation of this method is difficult in the absence of a reference standard, but these results appear internally consistent and our mean of 0.42% MNC is similar to 0.47% (n = 8) reported by Olweus et al.29 The potential confounding factors in our method have been identified and eliminated as far as possible. The observed range in DC numbers in normals and patients is relatively small (<1% of blood MNC), therefore we derived a normal range from over 100 individuals to ensure random variation did not unduly influence our results. This will provide a useful reference standard for further refinement of DC counting methods. Such advances are likely to include direct cell counting on the analysis tube using comparative standards and direct labelling of the DC with improved CMRF44 conjugates.
The clinical relevance of altered blood DC numbers is not yet clear and, given the difficulty in determining the cause of the alterations in blood DC number we documented, it is important not to overinterpret these results. Variation in DC blood transit time may be important in regulating the supply of DC to the tissues, yet may not alter the number of DC present in the blood at a single point in time. The information on blood DC kinetics needed to analyze these results is not yet available, although studies on blood monocyte kinetics suggest that normal monocyte blood transit times (half-life in circulation of 8 hours) are reduced during acute illness.30,31 The short circulation time of monocytes and lymphocytes32 and the rather low number of DC (approximately 5 × 107) in the blood at any one time (balanced against the relatively large estimated daily turnover of tissue DC5 6) all suggest that the DC blood transit time is likely to be short.
Several factors may contribute to the low blood DC counts we observed during acute inflammation. DC production and release into the blood may be exceeded by an increased rate of egress of DC from the blood, either at the site of inflammation or generally due to DC activation in the bloodstream. Production of other myeloid cells during acute inflammation may occur at the expense of DC production, either as a direct consequence of a bipotential precursor cell becoming committed to non-DC pathways33,34 or by inhibition of DC development by other cell types or cytokines.35 These factors may explain the fact that we found low blood DC counts in situations similar to those in which other investigators (working mainly with animal models) have found increased tissue DC turnover. Alternatively, tissue DC turnover may be supported from an alternative source, perhaps akin to the in vitro generation of Mo-DC.7,8 We did not find DC counts to drop with age or steroids despite suggestions from animal studies that these might affect DC numbers in the tissues.36 37
This method will be useful for monitoring attempts to mobilize DC into the blood. The numbers of other leukocyte types are generally reported to remain constant or increase in G-CSF–mobilized blood.38,39 Our data suggests that DC numbers are reduced at the time that CD34+ cells are harvested after cyclophosphamide/G-CSF or G-CSF stimulation and augments previous data on the composition of blood stem cell grafts (reviewed in To et al40). This observation may explain, in part, the previously reported finding that after G-CSF mobilization, blood MNC are less allostimulatory on a cell for cell basis.41However, the harvested product still contains a substantial number of DC. There was no reason, apart from the precedent of the dramatic but unexpected effect of G-CSF on stem cells, to suspect that DC numbers would be altered by this commonly used stem cell mobilization protocol. Interestingly, there appears to be little difference in DC numbers when GM-CSF is used instead of G-CSF in the mobilization protocol (R. Brown, personal communication, November 1992), despite the well-documented effect of GM-CSF on in vitro DC development.42 43 The kinetics of blood DC production in this situation may be slower than other leukocytes, however, it is also possible that G-CSF either directly or indirectly influences DC production or transit times. These influences may also explain the apparent increase in DC counts subsequent to harvesting.
A number of cytokines (principally combinations involving GM-CSF, IL-4, and TNFα, but also other factors8,43-46) are known to promote the in vitro development of DC from several blood precursor cell populations. As yet, stimuli, which may specifically mobilize DC into the blood, have not been described. The ability to harvest significant numbers of DC directly from the blood may offer a useful alternative to the in vitro generation of these cells for clinical use. In mice, flt3 ligand has been reported to increase DC numbers in several organs, including the blood.47 Flt3 ligand effects on human DC are now being examined and early results suggest DC are increased in the blood.48 As experience with CD34+ stem cell mobilization has shown, testing regimens specifically designed to increase blood DC number will require a practical and reproducible method of monitoring the cells in question, and the methods used are likely to evolve over time. The method reported here is practical and the small volume of blood required makes daily testing feasible. These results may be a useful basis for comparison as interest in DC mobilization for cancer immunotherapy increases.
Previous studies have shown that DC repopulation occurs rapidly after BMT.49 Normal DC numbers were found in patients grafted with CD34+ selected cells, providing evidence that CD34+ selected cells are capable of repopulating the DC lineage. The fact that relatively high DC numbers were observed in patients who were otherwise well after stem cell mobilization or transplantation could be seen as a rebound phenomenon and may relate to the previously reported finding that DC precursors (colony-forming unit-dendritic Langerhans cell [CFU-DL]) are more numerous in blood as counts recover after chemotherapy.50 In some of the allogeneic BMT cases, skin biopsy material was available and our preliminary data suggest that blood and skin DC numbers are both low in aGVHD (Mckenzie, manuscript in preparation). Taking the low DC numbers seen in aGVHD at face value, it may be that the initial low DC number is due to increased turnover and is later depressed as a result of decreased production of DC, possibly as part of a normal immunoregulatory process. As we have also found low blood DC numbers in four of five non-BMT patients with acute inflammation due to infections or trauma, it is possible that this may be a general response to inflammation and not an aGVHD specific effect.
The availability of this routine method will allow blood DC counts to be correlated with clinical outcomes, possibly providing data that can address the question of whether or not variations in DC number relate to disease pathogenesis. The method and normal range we report here represent an initial step towards achieving this goal. The initial results obtained using this method suggest that monitoring DC counts may be clinically useful, as well as providing data, which improves our understanding of basic DC biology. The test has immediate application for monitoring the production of DC for experimental immunotherapy protocols.
ACKNOWLEDGMENT
We wish to thank the volunteers and patients who provided blood samples and acknowledge the contribution of clinical colleagues and Alison Inder in collecting patient specimens. Valuable statistical advice was provided by Dr Elisabeth Wells. We thank Jan Merry-Martin for typing the manuscript.
Supported by the New Zealand Health Research Council, Canterbury Medical Research Foundation, Cancer Society of New Zealand, Lottery Health Research Council, McClelland Trust, Leukemia and Blood Foundation of New Zealand, Loch Maben Trust, University of Otago, and others.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Prof D.N.J. Hart, PhD, MD, Director, Mater Medical Research Institute, Raymond Terrace, Brisbane, Queensland, 4101 Australia; e-mail: dhart@mater.org.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal