Abstract
Myelodysplastic syndromes (MDS) and myeloproliferative syndromes (MPS) of childhood are a heterogeneous group of clonal disorders of hematopoiesis with overlapping clinical features and inconsistent nomenclature. Although a number of genetic conditions have been associated with MDS and MPS, the overall contribution of inherited predispositions is uncertain. We report a retrospective study examining clinical features, genetic associations, and outcomes in 167 children with MDS and MPS. Of these patients, 48 had an associated constitutional disorder. One hundred one patients had adult-type myelodysplastic syndrome (A-MDS), 60 had juvenile myelomonocytic leukemia (JMML), and 6 infants with Down syndrome had a transient myeloproliferative syndrome (TMS). JMML was characterized by young age at onset and prominent hepatosplenomegaly, whereas patients with A-MDS were older and had little or no organomegaly. The most common cytogenetic abnormalities were monosomy 7 or del(7q) (53 cases); this was common both in patients with JMML and those with A-MDS. Leukemic transformation was observed in 32% of patients, usually within 2 years of diagnosis. Survival was 25% at 16 years. Favorable prognostic features at diagnosis included age less than 2 years and a hemoglobin F level of less than 10%. Older patients tended to present with an adult-type MDS that is accommodated within the French-American-British system. In contrast, infants and young children typically developed unique disorders with overlapping features of MDS and MPS. Although the type and intensity of therapy varied markedly in this study, the overall outcome was poor except in patients with TMS.
IN ADULTS, PRELEUKEMIC conditions have been subdivided into myelodysplastic syndromes (MDS) and myeloproliferative syndromes (MPS; Table1).1,2 The French-American-British (FAB) group classifies MDS into refractory anemia (RA), refractory anemia with ring sideroblasts (RARS), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEBt), and chronic myelomonocytic leukemia (CMML).2 Myeloproliferative disorders are subdivided into chronic myeloid leukemia, polycythemia vera, primary thombasthenia, and myelofibrosis/agnogenic myeloid metaplasia.1 As the FAB system classification has gained acceptance, several groups have attempted to apply these criteria to pediatric disorders, usually by placing the disorder juvenile chronic myeloid leukemia (JCML) within the FAB category of CMML.3-5However, as emphasized by Wegelius,6 many children show overlapping features of MPS and MDS, particularly patients with JCML and infant monosomy 7 syndrome. This group of patients does not satisfy criteria for the FAB CMML classification group, because most have white blood cell counts (WBC) greater than 13,000/μL,2,6-8 as well as unique biologic characteristics.9 The classification of pediatric MDS is complicated further by the fact that some children develop MDS in the context of inherited predispositions such as neurofibromatosis type 1 (NF1), Fanconi anemia, severe congenital neutropenia, Down syndrome, or Noonan syndrome.10-14 An International Working Group15-17 recently proposed that the term juvenile myelomonocytic leukemia (JMML) be added to the FAB MDS system to accommodate children previously classified as having JCML or infant monosomy 7 syndrome. We report here a retrospective analysis of a large unselected group of children with MPS and MDS in which we define clinical features, associated genetic conditions, and outcome.
PATIENTS AND METHODS
Patients.
Seven tertiary institutions with large pediatric hematology/oncology referral bases participated in this study: University of California, San Francisco (San Francisco, CA); Children’s Hospital of Los Angeles (Los Angeles, CA); Children’s Hospital of Philadelphia (Philadelphia, PA); Children’s Hospital Medical Center of Cincinnati (Cincinnati, OH); Memorial Sloan-Kettering Cancer Center (New York, NY); Fred Hutchinson Cancer Research Center (Seattle, WA); and British Columbia Children’s Hospital (Vancouver, British Columbia, Canada). Some of these patients have been reported previously.3,12 One investigator (S.L.-F.) reviewed the medical records of all patients less than 21 years of age with the diagnoses MDS, MPS, JCML, monosomy 7, and/or preleukemia who presented between January 1975 and April 1995. Children with Philadelphia chromosome-positive chronic myeloid leukemia were excluded from the analysis. A computerized search using the terms “preleukemia,” “MDS,“ “JCML,” and “monosomy 7” identified 245 patients. Seventy-eight of these were excluded for the following reasons: 30 had Fanconi anemia (FA) without evidence of MDS; 14 had acute myeloblastic leukemia (AML) with monosomy 7 without antecedent MDS; 5 had Blackfan-Diamond anemia without MDS; 8 had treatment-related leukemia; 1 had NF1 and AML; 1 had severe congenital neutropenia and AML without preleukemia; 7 had aplastic anemia followed by MDS or leukemia; and 12 had insufficient data for analysis. MDS with clinical and marrow manifestations of the FAB MDS subtypes were classified as adult-type MDS (A-MDS). In accordance with the recent International Working Group recommendations,15-17 children who fulfilled the diagnostic criteria shown in Table 1 were classified as having JMML. This group included patients previously diagnosed with JCML as well as most children with monosomy 7 syndrome. Patients with a myeloproliferative disorder that resolved spontaneously and did not fulfill the criteria for either A-MDS or JMML were classified as having transient myeloproliferative syndrome (TMS).
Records were reviewed to ascertain the history and physical examination at diagnosis of MDS and MPS. Patients were classified as having a constitutional predisposition if they had a previously diagnosed genetic syndrome such as Down syndrome, NF1, Fanconi anemia, and severe congenital neutropenia or a familial myeloid disorder (defined as a first degree relative with MDS, AML, or bone marrow monosomy 7).
The complete blood count (CBC), differential, and hemoglobin F level (HgF) at diagnosis were recorded. Available marrow aspirates and biopsies were assessed blindly by S.K.A. for adequacy of material, percentage of blasts, and percentage of dysplastic cells in each lineage. Biopsy sections were evaluated for cellularity, morphology, blast percentage, myeloid:erythroid ratio, megakaryocyte number, and fibrosis.2 18 Where there was discordance between S.K.A. and institutional marrow findings, the institutional diagnosis was used provided that there were sufficient clinical, hematologic, and cytogenetic data to support a diagnosis of MDS. Cytogenetic reports were reviewed for number of mitoses, numerical and structural abnormalities, and complexity of karyotype. The clinical course was examined for transformation into leukemia, treatment during the course of the disease, and causes of death. The date of last follow-up was used to compute outcome.
Statistical analysis.
Data were collected by S.L.-F. using a data capture tool adapted into the relational database 4th-Dimension 3.0 (4D, San Jose, CA) and then imported into Statistica (StatSoft, Tulsa, OK) for analysis. Descriptive statistics were calculated to characterize the study population overall and within subsets based on age, percentage of HgF, FAB MDS subset or JMML, karyotype, and therapy. Survival probabilities were estimated using the Kaplan-Meier product limit method19 and differences between survival distributions were compared using the log-rank test.20 No multiple comparison adjustment was performed due to the retrospective design of the study.
RESULTS
Demographic and clinical features.
Table 2 shows the distribution of cases in each diagnostic category. A-MDS was the most common diagnosis and accounted for 101 patients (60%). Of 50 patients with A-MDS who were classified by the institution according to the FAB system, 11 had RA, 27 had RAEB, and 12 had RAEBt. The other 43 were classified as MDS not otherwise specified (NOS) at the referring institution based on ineffective hematopoiesis, dysplasia, and exclusion of other diseases. There were 60 patients with JMML (36%), including 48 initially diagnosed with JCML and 12 classified as having monosomy 7 syndrome at the referring institutions. Eight patients with monosomy 7 syndrome did not fulfill the diagnostic criteria for JMML shown in Table 1 and were assigned to the A-MDS group on the basis of marrow dysplasia, absence of organomegaly, and peripheral cytopenias. Six patients with Down syndrome had a TMS.
Overall, 125 patients (75%) were Caucasian, 14 (8.5%) were Hispanic, 12 (8%) were African-American, 6 (3.5%) were Asian, and for 8 (5%) the background of origin was unknown. There was no significant racial predilection. Although the number of African Americans was low, our population approaches the racial population distribution of pediatric malignancies in the United States.21
The 48 patients with a known constitutional predisposition to leukemia are summarized in Table 2. Down syndrome (16 cases) was the most common associated diagnosis, followed by Fanconi anemia (12 cases), familial leukemia (11 cases), Kostmann’s syndrome (1 case), and NF1 (8 cases). The 11 patients with familial leukemia included 3 pairs of affected siblings with bone marrow monosomy 7. All 12 patients with Fanconi anemia and 10 of 16 with Down syndrome developed A-MDS, whereas 7 of 8 patients with NF1 had JMML. As shown in Table 2, children with A-MDS were more likely to have an underlying genetic disorder than patients with JMML (34% v 13%, P = .004).
Table 3 summarizes the clinical features of our patients. The median age at diagnosis was 3.2 years; children with JMML were significantly younger than those with diagnosis of A-MDS (median age at diagnosis, 1.3 v 8 years; P = .001). A male preponderance was seen in both groups, but was more pronounced in JMML (male:female ratio = 1.7:1). Hepatomegaly and splenomegaly were detected in the majority of patients with JMML (75% and 87%, respectively). In contrast, only 22% and 26% of A-MDS cases had either hepatomegaly or splenomegaly. Adenopathy and rashes were only common in JMML.
Laboratory data.
The hematologic findings at diagnosis are shown in Table 3. Children with JMML had prominent myeloproliferative features with a median WBC of 32 × 109/μL. Although patients with A-MDS tended to present with low or normal WBC counts, there was considerable variability. Median hemoglobin values and platelet counts were reduced in both groups but with a great deal of variability. HgF values were available for 62 patients. Of 40 JMML patients tested, 20 (50%) had an HgF greater than 10%. HgF values were measured in only 22 patients without JMML, 5 of whom had a value higher than 10%. As expected, monocytosis was most prominent in JMML.
Bone marrow preparations were available for review from 67 patients, including 23 with both aspirate smears and biopsy sections, 39 with aspirate smears only, 4 with biopsy sections only, and 1 with only a biopsy touch preparation. Seventy-seven percent of the samples showed bilineage or trilineage dysplasia, with the erythroid lineage affected most often (61% of cases). Eighteen others (23%) appeared normal or had insufficient material or information to establish a diagnosis based on marrow findings alone. Twelve (18%) of the institutional diagnoses were discordant with our central review, including 6 patients with acute leukemia and 6 others with a different subtype of MDS. We reviewed the clinical courses of patients in whom central review did not confirm the institutional diagnoses and found that their outcomes were generally consistent with the original diagnosis. Data from these patients were therefore included in the overall analysis according to the diagnosis established at the referring institution. Our experience with central review of bone marrow preparations emphasizes the difficulties in confirming the diagnosis of MDS using a single specimen.
Cytogenetics.
Table 4 summarizes the cytogenetic findings. Original cytogenetics reports were available for review from 126 patients (75%). Of these, 25 (20%) were normal and 101 (80%) were abnormal. Monosomy 7 and/or del(7q) was detected in 53 specimens (52%), including 34 (64%) in which monosomy 7/del(7q) was the sole abnormality. Clonal cytogenetic abnormalities were seen in bone marrow samples from 67 of 73 patients (93%) with A-MDS, including 37 that showed monosomy 7/del(7q). Of these, monosomy 7 was the only abnormality present in 22 cases. Cytogenetic data were available from 48 of 60 patients with JMML. Abnormalities were identified in 29 specimens (60%), of which 16 showed isolated monosomy 7/del(7q).
Trisomy 21 was present in 16 specimens, including 9 patients who had constitutional abnormalities due to Down syndrome. Importantly, the translocations commonly associated with AML, such as t(8;21), t(15;17), t(6;9), and inv(16), were absent.22 Monosomy 5 or del(5q) were only detected in 3 specimens and 1 patient showed trisomy 8. Thirteen marrows were pseudodiploid, 4 were hyperdiploid, and 11 were hypodiploid. No patients had t(5;12).23
Cytogenetic reports were available for 36 of the 48 patients with a known genetic predisposition to myeloid leukemia and showed clonal abnormalities in 25 cases (69%). Monosomy 7/del(7q) occurred in bone marrows from 5 of 9 patients with familial leukemia, in 5 of 9 with Fanconi anemia, in 2 of 14 with Down syndrome, and in 1 of 5 with NF1.
Outcome and treatment.
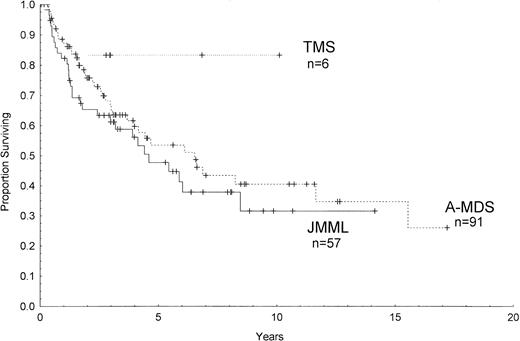
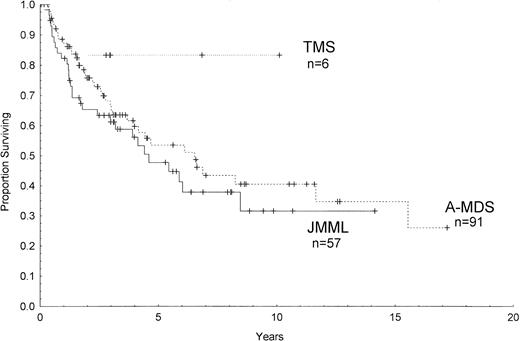
Outcome data were available in 154 patients with a median follow-up from the time of diagnosis of 5.6 years. Figure 1 shows the estimated survival among all patients of 36% ± 5% (percentage of survival ± standard error) at 10 years and 25% ± 9% at 16 years by Kaplan-Meier analysis. The median survival was 6.3 years in A-MDS, 4.4 years in JMML, and has not yet been reached for the 6 patients with TMS (Fig 2). There were no significant differences between the 3 groups or between each group by pairwise comparison (P = .11, log-rank). Among children with constitutional predispositions, only those with Down syndrome fared relatively well (69% ± 19% survival at 6 years).
Kaplan-Meier survival curve of patients with complete follow-up data (n = 154). Survival at 10 years is 36% (confidence interval [CI], 29% to 49%). Survival at 16 years is 25% (CI, 12% to 44%).
Kaplan-Meier survival curve of patients with complete follow-up data (n = 154). Survival at 10 years is 36% (confidence interval [CI], 29% to 49%). Survival at 16 years is 25% (CI, 12% to 44%).
Kaplan-Meier survival curves of children with complete follow-up who were diagnosed with TMS, JMML, or A-MDS. Ticks on the lines represent censored cases.
Kaplan-Meier survival curves of children with complete follow-up who were diagnosed with TMS, JMML, or A-MDS. Ticks on the lines represent censored cases.
Fifty-four patients (32%) developed acute leukemia usually within 2 years of diagnosis (Table 5). Of these, 48 transformed to AML and 1 to ALL. In 2 of 4 patients with Fanconi anemia, transformation occurred more than 5 years after diagnosis of MDS. Forty-one of 101 children (41%) with A-MDS developed acute leukemia compared with 8 of 60 (13%) of patients with JMML (P= .0001, log-rank). Within the A-MDS subgroups, 15 of 39 patients with RAEB or RAEBt developed acute leukemia versus none of 11 with RA. All myeloid leukemia subtypes occurred except promyelocytic leukemia.
Treatment varied among institutions and generally became more intensive in later years. Fifty patients received low-dose chemotherapeutic regimens such as 6-mercaptopurine, hydroxyurea, busulfan, prednisone, or low-dose cytarabine; 22 patients received high-dose AML-like chemotherapy; and 12 received both low- and high-dose chemotherapy. Seven children with Fanconi anemia also received oxymethalone. There was no apparent superiority of one approach over another (data not shown).
One hundred sixteen patients also received a bone marrow transplant (BMT); in 55 it was the first therapy. Of patients who received BMT, 86 were transplanted from an HLA-matched sibling, 23 from an unrelated donor (URD), and 7 received an autologous marrow. Nineteen patients had a second BMT after failure to engraft or relapse (18 of 19 of these were HLA-matched). Survival data were available for 110 patients. Survival for the patients with JMML at 10 years by Kaplan-Meier analysis was similar with or without BMT (31% ± 9% and 34% ± 16%, respectively). Similarly, the actuarial survival for children with A-MDS who received BMTs was not different from those who did not (survival, 39% ± 7% v 43% ± 17% at 10 years, respectively).
Twenty-two patients initially received no cytotoxic therapy, of whom 17 had long-term follow-up. Four of these patients were infants with Down syndrome and TMS. Nine had A-MDS and 4 had JMML. Ten patients were less than 2 years old at diagnosis, and 13 of 14 had a WBC count less than 6.5 × 109/μL. Eleven progressed to AML within 3.5 years and then received intensive multiagent chemotherapy and/or BMT. The other two children are surviving greater than 5 years from diagnosis without therapy or clinical progression. One child presented with A-MDS at 11 years of age, had a WBC count of 2.9 × 109/μL, and hepatosplenomegaly. The other patient has NF1; she presented at birth with neutropenia (WBC count, 3 × 109/μL) and anemia (Hg, 8.2 g/dL) but subsequently developed a myeloproliferative disorder that fulfills diagnostic criteria for JMML. She remains well with elevated neutrophil and monocyte counts. Interestingly, molecular analysis showed loss of the normal parental NF1 allele in her peripheral blood leukocytes.24
Prognostic factors.
Age less than 2 years (P = .002, log-rank) and HgF less than 10% (P = .02, log-rank) were the only clinical or laboratory findings present at diagnosis found to be significantly favorable for survival. When children with Down syndrome were excluded, the results did not change (P = .002 and P = .02, respectively). The clinical diagnosis did not predict outcome (P = .18, log-rank). A low platelet count, the existence of chromosomal abnormalities (none, single, or multiple), and the presence of monosomy 7/del(7q) did not impact negatively on survival. Similarly, the presence of hepatosplenomegaly, lymphadenopathy, rash, initial low or high WBC count, initial low Hg, initial high mean corpuscular volume (MCV), infections, or bleeding had no consistent effect on outcome (data not shown).
Passmore et al14 analyzed clinical and laboratory findings present at diagnosis in a series of children with MDS seen at a large pediatric referral center and found that low platelet count, an HgF greater than 10%, and the presence of complex cytogenetic abnormalities in the bone marrow correlated with a poor outcome. They developed a cumulative scoring system based on these factors that we applied to the 44 patients in our series who had all 3 studies performed at diagnosis. This analysis did not confirm the prognostic value of the Passmore score; however, the number of cases we were able to analyze was relatively small.
DISCUSSION
We describe the largest cohort of pediatric MDS/MPS patients reported to date. The retrospective methodology, absence of some clinical and laboratory data, and the work-in-progress nature of classification systems may weaken the study. However, the large number of cases and the use of one reviewer who examined all primary data have provided a number of new insights.
The 60% of children in this study with A-MDS had disorders that could be accommodated within the standard FAB MDS classification system. Sixty other patients (36%) had a disorder that meets the recent International Working Group’s definition of JMML,17 and 6 children (4%) had Down syndrome and TMS. Patients with A-MDS were more likely to be older children whose disease was characterized by trilineage dysplasia, functional and absolute cytopenias, and little or no extramedullary disease. In contrast, the JMML patients were younger, had extensive myeloid proliferation, had extramedullary hematopoiesis, had a frequently elevated HgF, and showed less obvious morphologic dysplasia. Children with JMML had unique clinical and biologic characteristics that distinguished them from patients with CMML, including young age and WBC counts greater than 13,000/μL.8 The (5;12) TEL/PDGFR β translocation and variant translocation that exists in a subset of older children and adults with CMML has not been observed in JMML.23,25,26Finally, blood and bone marrow from JMML patients uniformly show hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) in in vitro culture assays9,15; this finding has not been described in CMML. Deregulated Ras signaling is common in JMML and may result from either oncogenic RAS mutations or inactivation of the NF1 tumor-suppressor gene in the leukemic clone.24 27-30
Although associations between childhood MDS and disorders such as Down syndrome, NF1, Fanconi anemia, and severe congenital neutropenia are known, the high prevalence of one of these underlying conditions among children with MDS has only been appreciated recently. Bader-Meunier et al31 reported constitutional disorders in 22 of 49 pediatric MDS patients. Similarly, 29% of our patients had a constitutional predisposition to malignant myeloid disorders. This probably represents a conservative estimate due to the combined effects of underreporting and underdiagnosis. The latter possibility is supported by a recent study that found NF1 mutations in bone marrows of 3 of 20 children with JMML without clinical stigmata of NF1.32 Different constitutional disorders were associated with distinct types of MDS/MPS. For example, TMS and MDS in Down syndrome have an excellent outcome.33 In Fanconi anemia, MDS is relatively indolent, but the long-term prognosis is poor.34 In severe congenital neutropenia, somatic mutations in the granulocyte colony-stimulating factor receptor,35,36activating RAS mutations, and monosomy7/del(7q) have been associated with progression to MDS or AML.37 Children with inherited predispositions to MDS provide an extraordinary resource for discovering genes and biochemical pathways that are involved in both normal myelopoiesis and in leukemic transformation.
In this study, clonal cytogenetic abnormalities were identified in 80% of the marrows analyzed, a percentage that is higher than reported in most previous studies.5,10,16,31 Common nonrandom AML translocations were absent and the high frequency of monosomy 5/del(5q) and trisomy 8 seen in adults with MDS was not found in children.38 Monosomy 7/del(7q) was the most common abnormality by far and was detected in 33% of JMML and 51% of A-MDS patients. There was considerable overlap between the occurrence of monosomy 7 and the constitutional disorders. Monosomy 7 has previously been associated with myeloid disorders among patients with Fanconi anemia,34 NF1,39 familial leukemia,40,41 severe congenital neutropenia,42and Down syndrome.33 We have previously proposed that monosomy 7/del(7q) is a cytogenetic opportunist, ie, an abnormality that occurs in patients who are already susceptible to myeloid leukemia either because of a genetic predisposition or medical exposure to mutagenic compounds.13 Molecular and cytogenetic analysis of leukemic marrows with partial deletions of 7q have defined commonly deleted segments, an important first step toward positional cloning of putative myeloid leukemia tumor-suppressor genes on chromosome 7.43-46 The observation that activating RASmutations (or inactivation of NF1) coexists with monosomy 7/del(7q) in the bone marrows of many children with MDS suggests that these alterations cooperate in leukemogenesis (reviewed in Luna-Fineman et al13).
In addition to patients with Down syndrome and TMS, we identified 2 patients who are long-term survivors with supportive care only. The other 11 children who received supportive care developed AML within a few years of diagnosis. A French multicenter study recently describes spontaneous remissions in a child with RA and another with RAEB.31 More recently, Bader-Meunier et al11described remissions with little or no therapy in three of four neonates with CMML and Noonan syndrome. They proposed that MDS in these children may not be malignant disease. Our data from a large unselected population of children with MDS indicate that prolonged survival without therapy is uncommon except in infants with Down syndrome and TMS. Further clinical and molecular investigation of children who do well without therapy are required to elucidate the nature of these unusual cases.
The outcome for children with MDS is poor in this series and in most reports.5,14,16 We and others have found no differences between high-dose chemotherapy and low-dose chemotherapy.47The optimal treatment of MDS in both children and adults has not been defined.8 There is some evidence that patients with monosomy 7 and MDS have outcomes similar to those with de novo AML if they receive AML therapy while they have a low blast count.48 In contrast, patients with monosomy 7 and AML have a poor outcome.49 Recent studies in adults suggest that patients with RAEBt may be more responsive to AML therapy than others.48 However, it is not possible to argue strongly for AML therapy in the majority of these patients. Therapeutic recommendations await results of prospective trials, some of which are now underway.
BMT has been proposed as the treatment of choice for children with MDS.5,47,50-52 Our data and most published series show that only about 30% of patients with JMML who receive allogeneic BMT will become long-term survivors.5,52 However, the European Working Group on MDS in Children (EWOG-MDS) recently reported a 55% 5-year event-free survival (EFS) among 33 patients with MDS after BMT.50 Patients with A-MDS or JMML in our study had a similar prognosis with or without marrow transplantation. Our transplant data must be interpreted with caution, because the patients were treated over two decades of rapidly evolving transplantation technology. When to transplant children with MDS, the ideal preparative regimen, and the role of elective splenectomy in patients with massive splenomegaly all remain controversial. These questions can only be adequately addressed in a multicenter trial that uses a uniform classification system and a consistent treatment plan. Two such trials are anticipated, one in North America and another in Europe.
Data from this large series emphasize the importance of a uniform classification system to assess the biology, prognosis, and therapy of children with MDS and MPS. The presence or absence of a genetic predisposition, the clinical findings, and the bone marrow morphology and cytogenetic data should be considered together in evaluating individual patients. Given our present incomplete state of knowledge, we advocate the addition of JMML as a new subtype to the FAB MDS system to encompass a group of young children with novel clinical and biologic features. However, most children with MDS fulfill FAB criteria, and we believe that these cases should be classified into one of the specific FAB subsets. It is likely that the criteria used to classify children with MDS and MPS will improve as molecular genetic basis of this heterogeneous group of disorders is elucidated further.
ACKNOWLEDGMENT
The authors gratefully acknowledge the help provided by each participating institution through their clinical research associates and research nurses.
Supported in part by National Institutes of Health (NIH) Grants No. M01-RR01271 and R01-CA72614 and by the Frank A. Campini Foundation and facilitated by a collaboration with the Children’s Cancer Group (CCG). CCG is supported by NIH Grant No. CA13539.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sandra Luna-Fineman, MD, Department of Pediatrics, Box 0106, University of California, San Francisco, San Francisco, CA 94143.

![Fig. 1. Kaplan-Meier survival curve of patients with complete follow-up data (n = 154). Survival at 10 years is 36% (confidence interval [CI], 29% to 49%). Survival at 16 years is 25% (CI, 12% to 44%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.459/4/m_blod40219001x.jpeg?Expires=1771337238&Signature=zk116-klQSz2GLb1aUGt20jm-ndmjMR4nxPnybrpRryW1bWLa6z6yjx15bJyLY~MdzEFMjhDvEGo1ftbRSHu8A-zm69njg1h1~YuzeUhI4M15cV6BzJrnjVO63NenBLmbk6qiDC8QjsVlAuKsoENz12aRBzfG24x9wHe6zOr0Uuda6pJchk0c-1zn9tCoHpSwg3q9zQIOyIPwipFlJhiHlapNYKpWrTY9AKpXdMRCbwmRu6v8Indip7RNCKWo~qX0M413VWVjKr~uYvwGec6zTuDjjGlwKf6Ww531pQoSP2vksZLU4tjbI82JMzhPpM3WakBRNWsyuHBcnt7WH9GNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Kaplan-Meier survival curve of patients with complete follow-up data (n = 154). Survival at 10 years is 36% (confidence interval [CI], 29% to 49%). Survival at 16 years is 25% (CI, 12% to 44%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.459/4/m_blod40219001x.jpeg?Expires=1771337239&Signature=uJBFI5NYVkQ-XiL8VX73kujsOXe4GuJAZd-hRkyeyvLI8EmFUPhL0h-yjX4fXPG-oD~3ME90mgPKjqBQMTxMW20tQqgOCXHpG9yFt33krJI0F~jTS2UnFlClJxximUVbWQ6PZuc1LLs1B80~YHD0-VzKcedkx4mFckQbkPaoZbqFlG5m1EYvKNITlM7CzlFqXde6bwRSr-EApZUow-yWVWF0cLbkd94GIVLidDHglqfoeicDieFMKpg6CmcBQP3ld2O23b99a7Dw7OAJOAONSSPMdyU0WMl2z5ozuKTsEDj4hzjbmcLJWdsEAr6YdEs6UexklVKkuFHIo6jWNdZe8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)