Abstract
Acquired mutations truncating the C-terminal domain of the granulocyte colony-stimulating factor receptor (G-CSF-R) are found in about 20% of severe congenital neutropenia (SCN) patients, with this cohort of patients predisposed to acute myeloid leukemia (AML). In myeloid cells, such mutations act in a dominant-negative manner leading to hyperproliferation and lack of differentiation in response to G-CSF. However, why these truncated receptors are dominant in function over wild-type receptors has remained unclear. We report that ligand-induced internalization of truncated G-CSF-R is severely impaired compared with the wild-type receptor, which results in sustained activation of STAT proteins. Strikingly, in cells coexpressing both truncated and wild-type forms, the truncated receptors acted dominantly with regard to both internalization and sustained activation. Site-directed mutagenesis of the C-terminus showed that receptor tyrosines in this region were dispensable for internalization, whereas a di-leucine–containing motif in Box B3 played some role. However, loss of the di-leucine motif was not the critical determinant of the sustained activation status of truncated receptors. These data suggest that defective internalization, leading to extended receptor activation, is a major cause of the dominant hyperproliferative effect of truncated G-CSF receptors, which is only partially due to the loss of a di-leucine motif present in the Box B3 region of the full-length receptor.
GRANULOCYTE colony-stimulating factor (G-CSF) is a major regulator of neutrophil production.1-4Its effects are mediated by a receptor of the hematopoietin superfamily, the G-CSF-R, which forms homo-oligomeric complexes upon ligand binding.5 Like other family members, the G-CSF-R lacks intrinsic tyrosine kinase activity but activates cytoplasmic tyrosine kinases.2,5,6 Important signaling pathways activated by the G-CSF-R include those involving various members of the Janus tyrosine kinase (Jak) and signal transducer and activator of transcription (STAT) families of proteins,7-15 the Src kinases p55Lyn and p56/59Hck,16-18and components of the p21ras/Raf/MAPK pathway.11 19-22
Severe congenital neutropenia (SCN) is a heterogeneous disorder characterized by a severe reduction in circulating neutrophils (<0.2 × 109/L). We have previously identified a subset of SCN patients with acquired nonsense mutations in the gene encoding the G-CSF-R. These mutations truncate between 82 and 98 amino acids from the carboxy-terminus of the receptor, a region implicated in maturation induction and growth arrest.23-26 In patients carrying these mutations, a neutrophilic progenitor cell that attains aGCSFR mutation has gained the ability to clonally expand, suggesting hyperproliferation of an early compartment. In addition, such patients have a strong predisposition to acute myeloid leukemia (AML).26 Truncated receptors show normal affinity for G-CSF.23 However, when expressed in myeloid cells, these truncated receptors transduce a strong growth signal but fail to induce maturation.25 Coexpression of wild-type and truncated receptors in myeloid cell lines has shown that truncated receptors act in a dominant-negative manner over wild-type receptors to enhance proliferation at the expense of maturation.25 Similar dominant hyperproliferation is seen in mice heterozygous for a targeted G-CSF-R truncation27 and presumably also in SCN/AML patients, because GCSFR mutations affect just a single allele.24-26 However, the molecular mechanisms responsible for the dominant hyperproliferative function of truncated G-CSF receptors have remained unknown.
To investigate these mechanisms, we studied receptor activation and internalization after exposure to ligand in 32D cells expressing different G-CSF-R forms. We show that activation of STAT complexes by truncated receptors is significantly prolonged due to defective receptor internalization. Importantly, mutant receptors were found to act in a dominant-negative manner over wild-type receptors in both processes. Site-directed mutagenesis of the G-CSF-R C-terminal domain showed that three conserved tyrosines contained within this domain are not essential for efficient receptor internalization or deactivation. In contrast, mutation of a conserved di-leucine– containing motif in Box B3, which was previously shown to be important for internalization of the related gp130 receptor,28 caused delayed internalization and prolonged receptor activation, although not as pronounced as the effect of C-terminal truncation. Moreover, steady-state surface expression and activation status of these di-leucine mutant receptors was only marginally different from wild-type G-CSF-R, which was reflected in a minor enhancement of proliferation, with maturation signaling remaining intact. Together, these data provide a plausible explanation for the dominant hyperproliferative function of truncated G-CSF-Rs in response to G-CSF, mediated by defective receptor internalization after ligand binding, leading to extended growth signals, which is only partially due to the loss of a di-leucine motif in Box B3 of the receptor.
MATERIALS AND METHODS
Cell culture.
32D.cl8.6, a subline of the interleukin-3 (IL-3)–dependent murine myeloid 32D cell line,29 was maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10 ng of murine IL-3 per milliliter at 37°C and 5% CO2.
Site-directed mutagenesis and transfections.
The pLNCX expression clones of human WT (wild-type) G-CSF-R, the C-terminal deletion mutant mDA (Δ715), and the single tyrosine-to-phenylalanine (Y→F) substitution mutants Y704F, Y729F, Y744F, and Y764F have been described previously.23,30 Double Y→F mutants were created from the single mutants using site-directed mutagenesis, as described,30 and from these a triple Y→F mutant, mA (Y729F, Y744F, Y764F), was constructed by recombinant polymerase chain reaction (PCR), using the following oligonucleotide primers: GRRV14 (5′-CCTGGGCTTGTGGGGCTGC), GRFR11 (5′-TGCTGGGCAGCCCCACAAG), LNCXRV (5′-CCCTTACTTTCTGGGGTGGACATC), and GRFR7 (5′-GTCCTCACCCTGATGACC). The 5′ segment of each mutant was amplified using GRFR7 and GRRV14, and the 3′ segment was amplified using GRFR11 and LNCXRV. Products of the primary PCR were isolated, mixed 1:1, and used as a template for a secondary PCR with GRFR7 and LNCXRV. To produce the mutant mLALA (L753A, L754A), which lacks a conserved di-leucine motif in Box B3 of the receptor, a similar strategy was employed, except using GRFR12 (5′-CAGCCCGCTGCAGCGGGCCTCACCCCCAG) in place of GRFR11, using GRRV15 (5′-GCCCGCTGCAGCGGGCTGAGTGGAGTCAC) instead of GRRV14, and with DNA encoding the WT G-CSF-R as a template. In each case, the resultant product was digested with Hpa I and Bgl II and cloned into pLNCX containing WT G-CSF-R, which had also been digested with these enzymes. The authenticity of all mutants was verified by restriction enzyme analysis and DNA sequencing. For stable transfections, parental 32D.cl8.6 cells were electroporated with 10 μg Pvu I-digested pLCNX clones, using a Progenetor II apparatus (Hoefer Scientific Instruments, San Francisco, CA) set at 260 V, 100 μF, and 1 second. After 48 hours of incubation, cells were selected with G418 (GIBCO-BRL, Breda, The Netherlands) at a concentration of 0.8 mg/mL. Multiple clones were expanded for further analysis. In addition, clones from a previous study were used that contain pLNCX expressing WT G-CSF-R in combination with either pBabe alone (32D[WT/vec]) or with pBabe expressing the mDA (32D[WT/mDA]), selected with 0.8 mg/mL G418 and 1 μg/mL puromycin.25
Flow cytometric analysis.
To determine G-CSF-R expression levels in of 32D.cl8.6 transfectants, cells (106) were incubated at 4°C for 1 hour sequentially with 10 μg/mL of biotinylated mouse antihuman G-CSF-R monoclonal antibody LMM741 (PharMingen, San Diego, CA), 5 μg/mL of phytoerythrin-conjugated streptavidin (SA-PE), 5 μg/mL of biotinylated antistreptavidin antibody, and finally 2 μg/mL of SA-PE, with washing between each antibody step. Samples were analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA). At least three independently derived clones of each construct were selected on the basis of homogeneous receptor expression. For internalization experiments, cells (106) were incubated at 4°C for 1 hour with 0.2 μg/mL biotinylated G-CSF and then for various times at 37°C. Subsequently, cells were incubated for 30 minutes at 4°C with SA-PE in the presence of 0.02% NaN3 before flow cytometric analysis.
Measurement of 125I-G-CSF internalization.
Cells (2 to 4 × 106) were incubated for 1 hour at 4°C in 100 μL α-minimal essential medium containing 10% FCS and 1500 pmol/L 125I-G-CSF (Amersham Nederland BV, Den Bosch, The Netherlands), with or without excess nonlabeled G-CSF, and then transferred to 37°C for various times. Cells were washed either in phosphate-buffered saline (PBS; total ligand) or sodium citrate, pH 4 (internalized ligand), before centrifugation through an FCS cushion. Specific binding was determined as the difference in total binding in the absence or presence of unlabeled G-CSF. Internalized ligand was expressed as a percentage of total specific binding at each time point.
Cell proliferation and morphological analysis.
To determine the proliferation and differentiation characteristics of 32D.cl8.6 clones, cells were incubated at an initial density of 1 to 2 × 105 cells/mL in RPMI medium supplemented with 10% FCS with 100 ng/mL of human G-CSF, with 10 ng/mL of murine IL-3, or without growth factors. The medium was replenished every 2 to 4 days, and the cell densities were adjusted to 1 to 2 × 105cells/mL. Viable cells were counted on the basis of trypan blue exclusion. To analyze morphological features, cells were spun onto glass slides and examined after May-Grünwald-Giemsa staining. Without IL-3 or G-CSF, all transfectants died within 1 to 2 days and showed no signs of neutrophilic differentiation, whereas parental 32D.cl8.6 cells also died within 1 to 2 days in G-CSF–containing medium. To quantify the neutrophilic maturation of 32D.cl8.6 transfectants in response to G-CSF, the number of cells showing signs of differentiation (ie, band neutrophils or more mature) was determined and expressed as a percentage of total living cells (percentage of neutrophils).
Preparation of nuclear extracts.
Cells were deprived of serum and factors for 4 hours at 37°C in RPMI 1640 medium at a density of 1 to 2 × 106/mL and then stimulated with either RPMI 1640 medium alone or in the presence of 100 ng/mL human G-CSF. At different time points, 10 vol of ice-cold PBS supplemented with 10 μmol/L Na3VO4 were added to the cells, which were then pelleted and resuspended in ice-cold hypotonic buffer (20 mmol/L HEPES, pH 7.8, 20 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L Na4P2O7, 1 mmol/L dithiothreitol [DTT], 1 mmol/L EDTA, 1 mmol/L EGTA, 0.2% Tween-20, 0.125 μmol/L okadaic acid, 1 mmol/L Pefabloc SC, 50 μg/mL aprotinin, 50 μg/mL leupeptin, 50 μg/mL bacitracin, and 50 μg/mL iodoacetamide).31 Cells were vortexed for 10 seconds and the nuclei were pelleted by centrifugation at 15,000g for 30 seconds. Nuclear extracts were prepared by resuspension of the nuclei in high-salt buffer (hypotonic buffer with 420 mmol/L NaCl and 20% glycerol) followed by rocking for 30 minutes at 4°C. Insoluble materials were removed by centrifugation at 4°C for 15 minutes at 15,000g.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were incubated for 20 minutes at room temperature with 0.2 ng of 32P-labeled double-stranded oligonucleotide and 2 μg of poly(dI-dC) in 20 μL of binding buffer (13 mmol/L HEPES, pH 7.8, 80 mmol/L NaCl, 3 mmol/L NaF, 3 mmol/L NaMoO4, 1 mmol/L DTT, 0.15 mmol/L EDTA, 0.15 mmol/L EGTA, and 8% glycerol).32 The oligonucleotide probes used in this study were m67 (5′-CATTTCCCGTAAATC), a high-affinity mutant of the sis-inducible element (SIE) of the human c-fosgene,33 which binds STAT1 and STAT3, and β-cas (5′-AGATTTCTAGGAATTCAATCC), derived from the 5′ region of the β-casein gene,34 which binds STAT5 and STAT1. The DNA-protein complexes were separated by electrophoresis on 5% polyacrylamide gels containing 5% glycerol in 0.25× TBE. The gels were dried and subsequently analyzed by autoradiography.
RESULTS
STAT activation from truncated G-CSF receptors is prolonged.
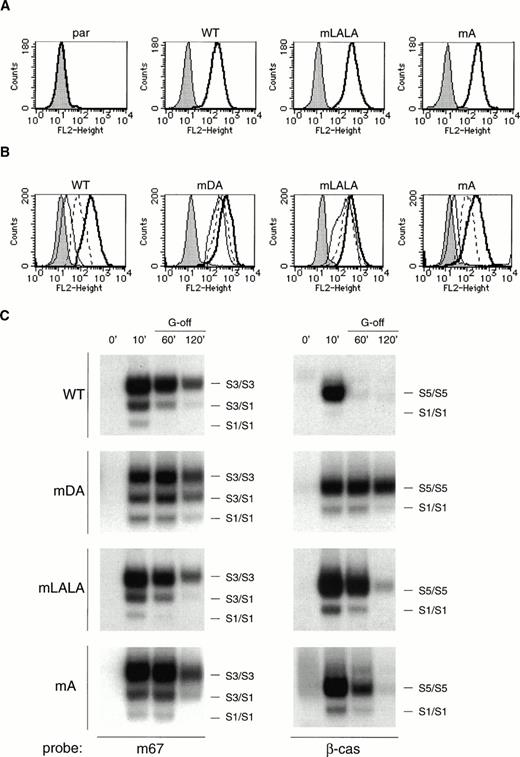
Activation of STAT proteins has been implicated in the control of G-CSF–mediated proliferation and differentiation.35,36 In addition, STAT activation represents a sensitive measure of receptor activation.37 To investigate how stimulation of truncated G-CSF receptors might lead to hyperproliferation at the expense of maturation, we examined the effect of receptor truncation on STAT activation in 32D cells, in which the dominant hyperproliferative function of truncated receptors has been clearly documented.25 32D.cl8.6 clones expressing either wild-type (WT) G-CSF-R, or a truncated form derived from an SCN patient (mDA; Figs 1 and 2A) were stimulated with G-CSF, and the kinetics of STAT activation were examined (Fig 2B). At early time points (up to 15 minutes), G-CSF–induced activation of STAT5 from WT and mDA receptors was similar, whereas STAT3 activation from mDA was reduced. However, at later time points of stimulation, when STAT5 activation from the WT G-CSF-R decreased, activation from mDA persisted. A similar result was seen with STAT3, although not as marked. In addition, activation of STAT1-containing complexes from mDA was greater and more sustained compared with the WT G-CSF-R. IL-3–induced activation of STAT5 complexes was equivalent in cells expressing either receptor type (data not shown).
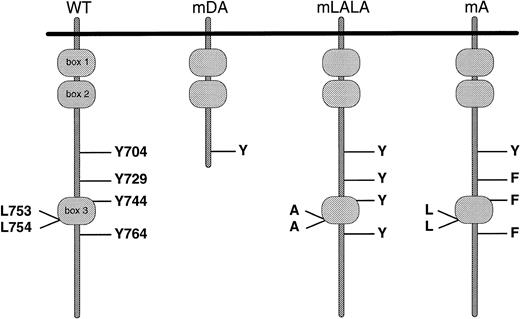
Schematic representation of G-CSF-R proteins studied. Cytoplasmic domains of wild-type and mutant receptors are shown. Boxes B1 and B2 denote subdomains conserved in members of the hematopoietin receptor superfamily, whereas Box B3 is conserved only with a limited number of family members, including gp130.62 63 Y, tyrosine; F, phenylalanine; L, leucine; A, alanine.
Schematic representation of G-CSF-R proteins studied. Cytoplasmic domains of wild-type and mutant receptors are shown. Boxes B1 and B2 denote subdomains conserved in members of the hematopoietin receptor superfamily, whereas Box B3 is conserved only with a limited number of family members, including gp130.62 63 Y, tyrosine; F, phenylalanine; L, leucine; A, alanine.
Activation of STAT complexes by wild-type and truncated receptors. (A) Flow cytometric analysis of G-CSF-R expression on representative 32D.cl8.6 clones expressing wild-type (WT) or truncated (mDA) G-CSF-Rs. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti-G-CSF-R step (shaded). (B) EMSA of nuclear extracts prepared from 32D[WT] and 32D[mDA] cells stimulated with G-CSF for the times indicated, analyzed using m67 and β-cas probes. Supershift analysis with antibodies against various STAT proteins was used to identify which STATs were present in each complex: S1, STAT1; S3, STAT3; S5, STAT5. This is representative of four independent experiments. (C) 32D[WT] and 32D[mDA] cells were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Activation of STAT complexes by wild-type and truncated receptors. (A) Flow cytometric analysis of G-CSF-R expression on representative 32D.cl8.6 clones expressing wild-type (WT) or truncated (mDA) G-CSF-Rs. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti-G-CSF-R step (shaded). (B) EMSA of nuclear extracts prepared from 32D[WT] and 32D[mDA] cells stimulated with G-CSF for the times indicated, analyzed using m67 and β-cas probes. Supershift analysis with antibodies against various STAT proteins was used to identify which STATs were present in each complex: S1, STAT1; S3, STAT3; S5, STAT5. This is representative of four independent experiments. (C) 32D[WT] and 32D[mDA] cells were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
To further examine the activation kinetics of truncated receptors, cells expressing WT or mDA receptors were stimulated with G-CSF for 10 minutes, extensively washed to remove the cytokine, and then analyzed for STAT activation. Under these conditions, extended activation of mDA receptors, as shown by prolonged activation of STATs 1, 3, and 5, was again observed (Fig 2C). This result shows that the truncated receptors that bound ligand during the first 10 minutes of stimulation were responsible for the prolonged STAT activation, and not new receptors recruited to the cell surface, implying that truncated receptors have extended off-rates after ligand binding. Because WT and mDA receptors show equivalent Kd,23 this suggests that receptor deactivation is altered in truncated receptors.
Impaired ligand-mediated G-CSF-R internalization by truncated receptors.
Ligand-induced receptor internalization represents a major mechanism of downmodulating receptor activation.28,38 Therefore, to investigate the extended receptor off-rates, the kinetics of G-CSF-R internalization after exposure to G-CSF were examined by measuring the amount of surface bound biotinylated G-CSF over time (Fig 3A). Significant internalization of the WT G-CSF-R was already seen within 15 minutes, and by 90 minutes most ligand/receptor complexes had been internalized. In contrast, ligand-induced receptor internalization of mDA was delayed, and even after 90 minutes a significant proportion of ligand/receptor complexes were still present on the cell surface. To confirm and quantify this result, we also studied internalization using 125I-labeled G-CSF (Fig 3B). This indicated that, after 2 hours, 88% of ligand was internalized via the WT G-CSF-R, compared with only 33% via mDA. Similarly, the receptor C-terminus of the IL-8 receptor has been shown to greatly influence the cellular responses to IL-8.39
Internalization of ligated G-CSF-R complexes. (A) 32D[WT] and 32D[mDA] cells were allowed to bind biotinylated–G-CSF at 4°C and were subsequently incubated at 37°C for various times before staining with SA-PE to determine surface-bound G-CSF. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of four independent determinations. (B) Rate of internalization of125I-G-CSF by 32D[WT] (▪) and 32D[mDA] (▵) cells, expressed as the percentage of total specific binding that was resistant to acid washing at each time point. Similar results were obtained in a duplicate experiment.
Internalization of ligated G-CSF-R complexes. (A) 32D[WT] and 32D[mDA] cells were allowed to bind biotinylated–G-CSF at 4°C and were subsequently incubated at 37°C for various times before staining with SA-PE to determine surface-bound G-CSF. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of four independent determinations. (B) Rate of internalization of125I-G-CSF by 32D[WT] (▪) and 32D[mDA] (▵) cells, expressed as the percentage of total specific binding that was resistant to acid washing at each time point. Similar results were obtained in a duplicate experiment.
Cytochalasin D can mimic the effect of receptor truncation.
Cytochalasin D specifically inhibits actin polymerization, without affecting other pathways,40-44 and has been used previously to interfere with a range of internalization processes.45-49 32D[WT] cells pretreated with cytochalasin D for 20 minutes before G-CSF stimulation showed both impaired receptor internalization (Fig 4A) and sustained receptor activation (Fig 4B) compared with cells with no pretreatment. Thus, blocking the internalization even of WT G-CSF-R is sufficient to enhance receptor activation, which suggests a causal relationship between these two processes. In addition, this result implicates actin microfilaments as effectors of the ligand-induced internalization of the G-CSF-R.
Effect of cytochalasin D on receptor activation and internalization. (A) Receptor internalization of 32D[WT] cells in the presence or absence of cytochalasin D, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent experiments. (B) 32D[WT] cells, in the presence or absence of cytochalasin D, were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Effect of cytochalasin D on receptor activation and internalization. (A) Receptor internalization of 32D[WT] cells in the presence or absence of cytochalasin D, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent experiments. (B) 32D[WT] cells, in the presence or absence of cytochalasin D, were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Truncated receptors act in a dominant-negative manner with regard to internalization and STAT activation.
Defective internalization and prolonged receptor activation provides a possible explanation for the enhanced proliferation mediated by truncated receptors. However, we have previously shown that truncated receptors exert a dominant hyperproliferative function over wild-type maturation signals in 32D cells coexpressing WT and mDA receptors.25 Therefore, we analyzed these cells (Fig 5A) with regard to receptor internalization and STAT activation after exposure to ligand. Impaired receptor downregulation was observed in coexpressing cells (32D[WT/mDA]) compared with cells expressing only wild-type receptors (32D[WT/vector]) (Fig 5B). Furthermore, 32D[WT/mDA] cells showed more sustained STAT activation in response to G-CSF than 32D[WT/vector] cells (Fig 5C). This clearly shows that mutant receptors exert a dominant effect over wild-type receptors with regard to internalization and activation, as well as proliferation.
Dominant-negative effect of truncated receptors. (A) Flow cytometric analysis of G-CSF-R expression on 32D[WT/vector] and 32D[WT/mDA] cells. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti–G-CSF-R step (shaded). (B) Receptor internalization of 32D[WT/vector] and 32D[WT/mDA] cells, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent determinations. (C) 32D[WT/vector] and 32D[WT/mDA] cells were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Dominant-negative effect of truncated receptors. (A) Flow cytometric analysis of G-CSF-R expression on 32D[WT/vector] and 32D[WT/mDA] cells. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti–G-CSF-R step (shaded). (B) Receptor internalization of 32D[WT/vector] and 32D[WT/mDA] cells, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent determinations. (C) 32D[WT/vector] and 32D[WT/mDA] cells were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Mutational analysis of the G-CSF-R C-terminus.
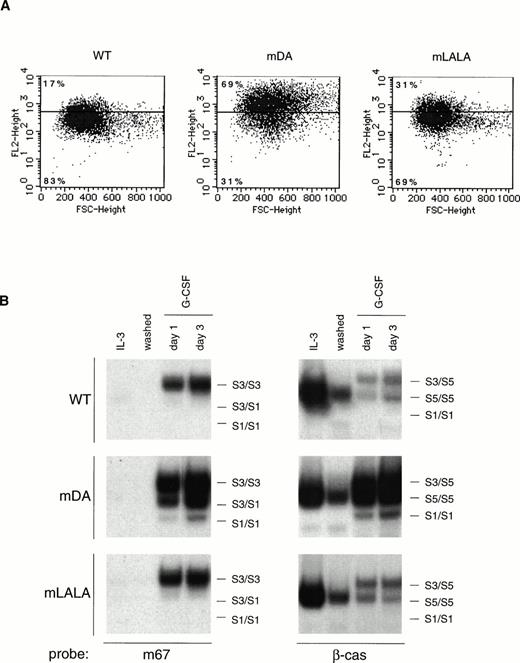
The data presented above suggest that sequences in the C-terminus mediate ligand-induced G-CSF-R internalization that subsequently affects receptor activation and cell proliferation. Because internalization motifs based on di-leucine and tyrosine-containing sequences have been described,50 51 we examined whether such motifs in the G-CSF-R C-terminus were involved in receptor internalization. Two specific mutants were constructed: mLALA, which has a conserved di-leucine motif in Box B3 replaced with a di-alanine, and mA, a triple Y→F mutant that lacks the three tyrosines in the C-terminal region (Fig 1). These mutant receptors were introduced into 32D.cl8.6 cells and clones expressing equivalent levels of each receptor were selected for further analysis (Fig 6A). Mutation of the di-leucine motif resulted in a delay in receptor internalization (Fig 6B), leading to extended STAT activation (Fig 6C), although neither of these effects was as prominent as observed with mDA. In addition, clones expressing the mLALA mutant showed some variability in growth but with a tendency for slightly enhanced proliferation compared with the 32D[WT] cells, although clearly not the sustained exponential growth of 32D[mDA] (Fig 7A). Furthermore, 32D[mLALA] clones showed effective, but delayed, neutrophilic maturation in response to G-CSF (Fig 7B and C), suggesting that the di-leucine motif plays some role in internalization and subsequent growth control. In contrast, receptor internalization kinetics for mA were equivalent to those of the WT G-CSF-R (Fig 6B), whereas STAT activation was similar, albeit marginally extended (Fig 6C). Moreover, all 32D[mA] clones showed comparable growth and differentiation to that of 32D[WT] clones (Fig7). This shows that the tyrosines of the C-terminal region are not required for receptor internalization, growth inhibition, or differentiation.
Receptor internalization and activation in cells expressing mLALA and mA mutants. (A) Flow cytometric analysis of G-CSF-R expression on parental 32D.cl8.6 cells and representative 32D.cl8.6 transfectants. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti–G-CSF-R step (shaded). (B) Receptor internalization of 32D.cl8.6 cells expressing wild-type or mutant G-CSF receptors, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent determinations. (C) 32D.cl8.6 cells, expressing wild-type or mutant G-CSF receptors, were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Receptor internalization and activation in cells expressing mLALA and mA mutants. (A) Flow cytometric analysis of G-CSF-R expression on parental 32D.cl8.6 cells and representative 32D.cl8.6 transfectants. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti–G-CSF-R step (shaded). (B) Receptor internalization of 32D.cl8.6 cells expressing wild-type or mutant G-CSF receptors, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent determinations. (C) 32D.cl8.6 cells, expressing wild-type or mutant G-CSF receptors, were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.
Growth and differentiation of cells expressing mLALA and mA mutants. (A) Cell-proliferation of 32D.cl8.6 clones expressing WT (▴; thick line), mDA (▪; thick dashed line), mLALA (▵; thin line), and mA (□; thin dashed line) receptors. Data represent growth of individual mLALA and mA clones, whereas for WT and mDA the mean growth of three clones is shown as a reference. (B) Maturation of 32D.cl8.6 cells expressing wild-type or mutant G-CSF receptors, as indicated in (A), expressed as the percentage of living cells showing signs of maturation at each time point. Data represent the mean of three clones. (C) Morphological features of 32D[WT] cells in the presence of IL-3 or representative clones expressing wild-type or mutant G-CSF-R after 7 days of exposure on G-CSF.
Growth and differentiation of cells expressing mLALA and mA mutants. (A) Cell-proliferation of 32D.cl8.6 clones expressing WT (▴; thick line), mDA (▪; thick dashed line), mLALA (▵; thin line), and mA (□; thin dashed line) receptors. Data represent growth of individual mLALA and mA clones, whereas for WT and mDA the mean growth of three clones is shown as a reference. (B) Maturation of 32D.cl8.6 cells expressing wild-type or mutant G-CSF receptors, as indicated in (A), expressed as the percentage of living cells showing signs of maturation at each time point. Data represent the mean of three clones. (C) Morphological features of 32D[WT] cells in the presence of IL-3 or representative clones expressing wild-type or mutant G-CSF-R after 7 days of exposure on G-CSF.
The proliferation data for cells expressing the various receptor mutants correlated well with their ability to inhibit short-term receptor internalization and deactivation. To further corroborate these findings, we also examined steady-state G-CSF-R expression (Fig 8A) and STAT activation (Fig 8B) upon continuous exposure to ligand. After 1 day of G-CSF treatment, 32D[mDA] cells showed an increase in G-CSF-R on the cell surface compared with cells cultured in IL-3, whereas both 32D[WT] and 32D[mLALA] cells showed decreased receptor levels, albeit not as severe for mLALA. Upon extended exposure to ligand, the STAT complexes observed changed somewhat in all clones, most notable being the appearance of a STAT3/STAT5 heteromeric complex, similar to what we have previously described in NFS-60 cells.13 However, importantly, total STAT activation remained high in 32D[mDA] cells, whereas it was considerably less for both 32D[WT] and 32D[mLALA] cells, although slightly higher for mLALA than for the WT (Fig 8B). Once again, activation of STAT5 was most affected by the receptor truncation. 32D[mA] cells gave almost identical results to 32D[WT] cells (data not shown). Thus, the proliferation properties of the different clones parallel the steady-state receptor levels and STAT activation, consolidating the correlation between receptor activation status and proliferation.
Steady-state receptor levels and STAT activation in the presence of ligand. (A) Flow cytometric analysis of G-CSF-R levels on 32D.cl8.6 cells expressing wild-type or mutant G-CSF-R either growing on IL-3 (line indicates median FACS signal) or switched to G-CSF for 1 day (dots). Percentages indicate the proportion of G-CSF–stimulated cells with G-CSF-R expression either above or below the median level on IL-3. Similar results were obtained with three independent clones. (B) STAT activation in 32D.cl8.6 cells expressing wild-type or mutant G-CSF-R, either growing on IL-3, washed, or switched to G-CSF for the times indicated, using m67 and β-cas probes. This is a representative of three independent clones.
Steady-state receptor levels and STAT activation in the presence of ligand. (A) Flow cytometric analysis of G-CSF-R levels on 32D.cl8.6 cells expressing wild-type or mutant G-CSF-R either growing on IL-3 (line indicates median FACS signal) or switched to G-CSF for 1 day (dots). Percentages indicate the proportion of G-CSF–stimulated cells with G-CSF-R expression either above or below the median level on IL-3. Similar results were obtained with three independent clones. (B) STAT activation in 32D.cl8.6 cells expressing wild-type or mutant G-CSF-R, either growing on IL-3, washed, or switched to G-CSF for the times indicated, using m67 and β-cas probes. This is a representative of three independent clones.
DISCUSSION
Expression of truncated G-CSF receptors leads to hyperproliferation in response to G-CSF in both mice and myeloid cell lines, with truncated receptors acting dominantly over wild-type receptors.25,27Furthermore, SCN patients with mutation of a single GCSFRallele show clonal expansion of the mutant population and are predisposed to AML,26 suggesting an equivalent effect in these patients. We set out to identify a molecular mechanism(s) that might explain this dominant hyperproliferative function of truncated receptors. We showed that, compared with wild-type receptors, truncated receptors showed prolonged activation, due to a much slower off-rate, which correlated with defective internalization of these receptors. Cytochalasin D could inhibit both internalization and deactivation of wild-type receptors, supporting the notion that the two processes are linked mechanistically. These data suggest that a motif(s) in the C-terminus, absent in truncated receptors, is required for receptor internalization and concomitant deactivation. We have recently confirmed these results using bone marrow cells from mice with a targeted truncation of the G-CSF-R (data not shown).
Both di-leucine and tyrosine-based internalization motifs have been reported.50,51 Indeed, the C-terminal region of G-CSF-R contains a di-leucine motif in Box B3 that is involved in ligand-mediated receptor endocytosis of the closely related gp130 receptor.28 In addition, the C-terminus contains three tyrosines, each of which are candidates for the YXXφ motif (in which X is any amino acid, and φ has a bulky hydrophobic side-chain) implicated in the control of internalization for a number of other receptors.51 Mutational analysis showed that these three tyrosines are not required for receptor internalization or the growth-inhibitory/differentiation function mediated via the C-terminus. On the other hand, mutation of the di-leucine motif delayed receptor internalization, resulting in prolonged STAT activation in the short term. However, the effect of this mutation was relatively minor in the long term, reflected in a modest enhancement of G-CSF–mediated proliferation and a delay in maturation. These findings clearly indicate that another as yet unknown motif(s) in the C-terminus also plays a role in the control of these processes. Such sequences may simply represent other motifs required for efficient internalization. However, the regulation may be more complex than this. For example, in the experiments involving cytochalasin D (Fig 4), the 32D[WT/mDA] cells (Fig 5), or cells expressing the mLALA mutant (Fig 6), in which the receptor C-terminus is present in some form, it was apparent that the effects on internalization were more severe than those on STAT deactivation, if compared with 32D[mDA] cells. Conversely, 32D[mA] cells showed a slightly longer STAT activation than 32D[WT] cells (Fig 6C). Therefore, it is likely that the C-terminus activates alternate inhibitory mechanisms of receptor activation, which may involve receptor tyrosines.
Although multiple mechanisms apparently de-activate the WT G-CSF-R, we showed that truncated receptors acted in a dominant manner over wild-type receptors with regard to both internalization and activation, thereby paralleling their dominant hyperproliferative function and suggesting a causal relationship. This was further supported with studies of different receptor forms in the continuous presence of ligand, in which a correlation between steady-state receptor levels, STAT activation, and proliferation was observed. Thus, truncated receptors may exert their dominant function by interfering with the ligand-induced internalization of wild-type receptors, leading to enhanced proliferative signaling, which suggests that the motifs in the C-terminus required for internalization must be present in more than one copy on a given ligated receptor complex to do so efficiently. One explanation for this is that the molecules that mediate the internalization process may need to be multimeric to function effectively. Alternatively, we cannot rule out the possibility that the presence of active complexes consisting solely of truncated receptors in these cells is sufficient to produce the hyperproliferative effect.
Whatever the precise details, it is clear that truncated receptors, either homo-oligomeric or complexed with wild-type receptors, are capable of generating extended signals in response to G-CSF stimulation, which ultimately leads to hyperproliferation. It is possible that the hyperproliferation may be due to the sustained activation of STAT proteins per se, because STAT proteins are known to be constitutively activated in a number of leukemias,52-55as well as cell lines transformed by viruses56 or oncogenes.57 Indeed, it has been shown that introduction of v-Src into 32D cells leads to both constitutive STAT activation and sustained proliferation with a corresponding block in G-CSF–mediated differentiation, similar to what is observed with mutant mDA.58 In addition, it has recently been shown that STAT3 activation is required for cell transformation by Src,59,60whereas a constitutively active STAT5 mutant has been identified that functions to promote cell proliferation.61 In the case of extended G-CSF-R signaling, the most important contribution for mediating hyperproliferation would presumably be from STAT5, which is known to play a role in G-CSF–mediated growth signaling36and whose activation status is most affected by the receptor truncation. However, because STAT3 has been implicated in G-CSF–mediated differentiation,35 it may be that it is the altered balance between STAT5 and STAT3 that is important. Nevertheless, it is obvious that the sustained activation of other G-CSF-R signaling pathways may also contribute to the observed hyperproliferation.
This report provides a plausible explanation for the dominant hyperproliferative function of truncated G-CSF-Rs in response to G-CSF mediated by extended growth signals after ligand binding due to defective receptor internalization. These data further implicate G-CSF-R truncation as a possible preleukemic event, because cells carrying a GCSFR mutation could be expected to have a growth advantage due to prolonged receptor activation in the presence of ligand. In this light, therefore, because SCN patients are routinely treated with G-CSF, our data would suggest that further examination should be afforded to the effects of chronic G-CSF administration on the incidence of AML in SCN patients with a GCSFR mutation.
ACKNOWLEDGMENT
The authors thank Drs Mirjam Hermans, Marieke von Lindern, and Tania de Koning-Ward for helpful discussions and critical reading of the manuscript and thank Karola van Rooyen for exquisite graphical work.
Supported by an EMBO Long Term Fellowship (A.C.W.) and grants from the N.W.O. and Dutch Cancer Society (I.P.T.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alister C. Ward, PhD, Institute of Hematology, Erasmus University (Room H Ee 1314), PO Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: ward@hema.fgg.eur.nl.

![Fig. 2. Activation of STAT complexes by wild-type and truncated receptors. (A) Flow cytometric analysis of G-CSF-R expression on representative 32D.cl8.6 clones expressing wild-type (WT) or truncated (mDA) G-CSF-Rs. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti-G-CSF-R step (shaded). (B) EMSA of nuclear extracts prepared from 32D[WT] and 32D[mDA] cells stimulated with G-CSF for the times indicated, analyzed using m67 and β-cas probes. Supershift analysis with antibodies against various STAT proteins was used to identify which STATs were present in each complex: S1, STAT1; S3, STAT3; S5, STAT5. This is representative of four independent experiments. (C) 32D[WT] and 32D[mDA] cells were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.447/4/m_blod40237002w.jpeg?Expires=1771327842&Signature=JPEEmNkKNqWy9JL4Vt6exnuYzlzAxOQOGtK2lfahz2KTQAoAKdrfdQjwiL6v~5m8gXm0PyFV2UX-qTPCdCEt86Q0FGu8OCnH9Jxkz8KQxNJd7gaUKje~f4Bqhpp0uCP2O7lX2KQSOaULm7xE7UIc2HxZqTqEuvqufLCu8gWTNdO--DoN7XMzqI0xxZnuau33YXGzjtPmQyo1w0ndlSFjqJ3BP01Ws29iw-lhAshrDRyZq~gxwq4~siesgsCJ1N~lhaFkxTicElvTjp-wRLsZl3uAeEhRKvxNXX8dBZw-~3v~4so9YNQ1hyZOzwS9ZuT1nW3D-0U0pJBDgqBdJL2VDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Internalization of ligated G-CSF-R complexes. (A) 32D[WT] and 32D[mDA] cells were allowed to bind biotinylated–G-CSF at 4°C and were subsequently incubated at 37°C for various times before staining with SA-PE to determine surface-bound G-CSF. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of four independent determinations. (B) Rate of internalization of125I-G-CSF by 32D[WT] (▪) and 32D[mDA] (▵) cells, expressed as the percentage of total specific binding that was resistant to acid washing at each time point. Similar results were obtained in a duplicate experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.447/4/m_blod40237003x.jpeg?Expires=1771327842&Signature=ydpR7b3OUyNA8qW4bmxzKe45YwyBtjE1SwNEfWV5pB1MWWQONE0Fcw32TgCQEzKX9jFdpvs69~F3ROLoQxGWGPobMhjcaDnNcjZ0V5nsRK0d9rmmGqFfL5ia6Hk5Va2Wei0hR8E1yLGKT-gDjlL5XnFx6M2Hh1-Iq2z~7PtSuIFXXeXNyf~CNzmHd~RESBQe9Ouv~w-yDavRs25Hmz2m--RjBqhm-uxj-XHfxf-3LNLY9MkRu8wvAyOceEKDFthts4Q3d2K~aaWB3JZp63S-KNGGKWkMa64JdCMVrrUFnnyBJwghAxkCkEiF83u9OxkGALAIjNrWjKuZt2KxYVBoww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of cytochalasin D on receptor activation and internalization. (A) Receptor internalization of 32D[WT] cells in the presence or absence of cytochalasin D, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent experiments. (B) 32D[WT] cells, in the presence or absence of cytochalasin D, were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.447/4/m_blod40237004w.jpeg?Expires=1771327842&Signature=esuezAUxDWzI5i11flKCszEPv-wQEpePT9ECyUsrsKUxy6bTgglKcl7WfO5K1SQAGIsDI8iz6mg-NYd3IqHgI5i9cLeVPbN02yub3LVVlJo9Ita19OaFd~tXIVOP2vgF4T23RQBXWM2sruQ-5aMs2-Gdng9dZooBKb1WtB7n9D2opqzZTdGdId8KfuKi8iXtvBx9abH0Fi3AQjt83jfSllW16~w5GYhzlu-n2AxyuKSJFZEUKgJk9jkpVVKwM3rdvNH28vIfUdYLpSxilw~k5IHQbibxLwkYxUgDGvXnNzNZ7-t~pCwUP169Tzy~C7Y-6YJUDKBv47dn9BnVFPwQQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Dominant-negative effect of truncated receptors. (A) Flow cytometric analysis of G-CSF-R expression on 32D[WT/vector] and 32D[WT/mDA] cells. Cells were either stained with biotinylated mouse antihuman G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated antistreptavidin, and finally PE-conjugated streptavidin (unshaded), or without the anti–G-CSF-R step (shaded). (B) Receptor internalization of 32D[WT/vector] and 32D[WT/mDA] cells, as indicated. (Bold line) 0 minutes; (dotted line) 30 minutes; (thin line) 90 minutes; (shaded histogram) 0 minutes, with initial binding in the presence of excess nonbiotinylated–G-CSF. This is representative of three independent determinations. (C) 32D[WT/vector] and 32D[WT/mDA] cells were stimulated with G-CSF for 10 minutes, washed extensively, and incubated in media alone for the times indicated (G-off). Nuclear extracts were prepared at the indicated times and assayed by EMSA using the m67 and β-cas probes. This is representative of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.447/4/m_blod40237005w.jpeg?Expires=1771327842&Signature=yn-eXma1qiibEHBaOHYjgwhsIOENK2YrorGtzZ~hfJ8tE2KKLDBPcdnn-ryDiSEm5cseZyLFzL3spA5nDBufN9yhwD8dOH8dhsGLEX2xJyhise3YLPPWWT67E6zzaaZzsej8tfYDzYWI~PIx7Ocg5RsAiWYP7flqt0bXzeVG5e-9bx1~fLk9VuiSLAUol5jIQTzWE7ryEBQq4ErDIqce4dEaebuMU4f4-pbHDNm9s3gQWeUBGHcm6HkiSjOYxlyvk9fp-iO2jd-KDLL6Do2cU3YYaegECMtrBCh8RHcdpiBBGq9W5XbjUcDIYSFjdb9RJ~EYQoa6qj~c6yX7n8SJkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Growth and differentiation of cells expressing mLALA and mA mutants. (A) Cell-proliferation of 32D.cl8.6 clones expressing WT (▴; thick line), mDA (▪; thick dashed line), mLALA (▵; thin line), and mA (□; thin dashed line) receptors. Data represent growth of individual mLALA and mA clones, whereas for WT and mDA the mean growth of three clones is shown as a reference. (B) Maturation of 32D.cl8.6 cells expressing wild-type or mutant G-CSF receptors, as indicated in (A), expressed as the percentage of living cells showing signs of maturation at each time point. Data represent the mean of three clones. (C) Morphological features of 32D[WT] cells in the presence of IL-3 or representative clones expressing wild-type or mutant G-CSF-R after 7 days of exposure on G-CSF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.447/4/m_blod40237007w.jpeg?Expires=1771327842&Signature=wuIUSzu3id~h02n2ktmaKLzzixV55M-SvyD5RRRiJMacJhOzf6NinVDpp8AO9BOakBJ08GHzk91MBby3XoWqGtk4Y~iB25byOz3GxGYiozLAfaKRDj19sNrhF~jp3ihood8Z8yAvZuXj-Vs8dZm9M3S-9VpcgTZh7Himpj9A-kK75ti4RnhlBq32ImYf3GAlBmstPYxyB4hyK0nOwnwS0SCrQNh6duxgxVzdDfll-7mRBKd7BzjaEUySvbFKQh6aelXWfAtc73FU4BrwXrQKdX20f~7sKwuLEcMmv--iTCGPH6ADydV16eJKo3Aw7sAmYTTNi56CutUDH-2PRFUAhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal