Abstract
The binding of CD2, present on T cells, to its counterreceptor CD48 facilitates adhesion, signaling, alloantigen-induced cytokine production, and cytotoxic T-lymphocyte responses. Because these T-cell functions have been implicated in graft-versus-host disease (GVHD) pathogenesis, we have analyzed the effects of the CD2:CD48 pathway on GVHD mediated by CD4+ and CD8+ T cells infused into sublethally irradiated recipients. CD4+ T-cell–mediated, and to a lesser extent, CD8+ T-cell–mediated GVHD was inhibited by CD2 + 48 monoclonal antibody (MoAb) infusion. To assess the effects of combined MoAb infusion on alloengraftment, two different alloengraftment bone marrow transplantation (BMT) models were used. In both, MoAb infusion markedly inhibited alloengraftment and hematopoietic recovery post-BMT. To determine if the adverse effects on lymphohematopoiesis in the allogeneic BMT recipients were caused by an immune or nonimmune mechanism, studies were performed in congenic BMT recipients to preclude an immune mechanism as the cause for delayed recovery post-BMT. MoAb infusion resulted in impaired lymphohematopoietic recovery in congenic BMT recipients and markedly reduced day 12 colony-forming unit–spleen formation in syngeneic BMT recipients, consistent with a nonimmune mediated mechanism. Because the spleen is a site of early hematopoietic recovery post-BMT, studies were performed using adult splenectomized syngeneic BMT recipients. MoAb infusion delayed recovery in both nonsplenectomized and splenectomized recipients post-BMT, indicating that the delayed hematopoietic recovery was not the consequence of an abnormal homing pattern of hematopoietic progenitors to the spleen early post-BMT. CD48 MoAb was necessary and sufficient for the inhibition of GVHD lethality and delayed lymphohematopoietic effects of the combined MoAb regimen. CD48 MoAb was found to induce a profound modulation of CD48 antigen expression on BM cells, suggesting that the CD48 antigen may have an important function in hematopoiesis in the BM compartment. Taken together, these data provide evidence that the CD48 antigen plays a critical role in regulating hematopoiesis in post-BMT.
CD2, FIRST DESCRIBED as the sheep erythrocyte receptor, is a 55- to 60-kD glycoprotein expressed on the surface of T cells, B cells, natural killer (NK) cells, and some antigen-presenting cells (APC).1-5 The multimeric binding of murine CD2 to natural ligand facilitates the adhesion between T cells and APC and delivers a costimulatory signal for initiating T-cell responses.6-10 The in vivo administration of αCD2 monoclonal antibody (MoAb) has been shown to inhibit CD4+ and CD8+ T-cell alloresponses, leading to prolonged allograft survival.9,11,12 CD48, a 45-kD glycosyl phosphatidylinositol-anchored glycoprotein member of the immunoglobulin supergene family, is expressed on almost all T cells and B cells, and is the natural ligand for CD2. CD48 participates in T-cell activation.10 αCD48 MoAb has been shown to inhibit interleukin-2 (IL-2) production, IL-2 receptor expression, T-cell proliferation,13 and IL-4 production.14αCD2 + CD48 MoAb have been shown to have synergistic effects in inhibiting alloresponses including T-cell priming to alloantigen, IL-2 production, and T-cell help for allo–cytotoxic T-lymphocyte (allo-CTL) generation.14
Although the in vivo administration of αCD2 + CD48 MoAb has been shown to result in the indefinite survival of cardiac allografts,14 the proinflammatory response generated by cardiac allografting is predominantly local, whereas that of irradiation used to condition bone marrow transplantation (BMT) recipients as well as the graft-versus-host disease (GVHD) process itself is systemic. Therefore, the current study was undertaken to determine whether αCD2 and αCD48 MoAb were capable of inhibiting GVHD lethality. In the process of performing these studies, we identified a critical role for CD48 in regulating alloengraftment as well as lymphohematopoietic recovery in both allogeneic and congenic BMT recipients. These data have significant implications for human BMT studies.
MATERIALS AND METHODS
Mice.
For BMT experiments, B10.BR/SgSnJ (H2k), B6.C.H2bm1 (termed bm1), B6.C.H2bm12 (termed bm12), C57BL/6-severe combined immunodeficiency disorder (scid)/(termed B6-SCID) (H2b), and DBA/1 (H2q) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 (termed B6) (H2b; CD45.1), B6-Ly 5.2 (B6-CD45.2), and BALB/c (H2d) mice were purchased from the National Institutes of Health (Bethesda, MD). Female donor and recipient mice were 8 to 10 weeks old at the time of BMT. All mice were housed in a specific pathogen-free facility in microisolater cages.
Anti-CD2 + 48 MoAb preparation and administration.
Anti-CD2 MoAb (hybridoma RM2-2, rat IgG) and αCD48 (hybridoma HM48-1, hamster IgG) were generated as described.10,15 Irrelevant rat IgG and irrelevant hamster IgG were purchased from Rockland Laboratories (Gilbertsville, PA). Anti-CD2 and αCD48 MoAb were generated from ascites fluid, purified by ammonium sulfate precipitation, and dialyzed before injection in vivo. Mice were injected intraperitoneally (IP) with αCD2, αCD48, or both MoAb (300 μg/dose each) or irrelevant IgG (300 or 600 μg/dose, as appropriate) on days −1 and +2, and then twice weekly until day +21 post-BMT. The maximal immune suppressive αCD2 MoAb dose is 100 μg/dose administered on days 0 and 1.11
GVHD induction.
For examining the effects of CD4+ or CD8+ T cells on the recognition of isolated major histocompatibility complex (MHC) disparities, bm12 or bm1 recipients were sublethally irradiated (6.0 Gy total body irradiation [TBI] from a 137Cesium source at a dose rate of 85 cGy/minute) and injected with highly purified lymph node (LN) CD4+ (1.0 × 105cells) or CD8+ (1 × 106 cells) B6 T cells, respectively, as described.16 In other experiments, purified CD4+ LN bm12 T cells (106 cells) were infused into nonirradiated B6-SCID recipients to assess whether αCD2 + 48 MoAb was preventing GVHD lethality by blocking the interaction of donor T cells with host B cells. These latter experiments were used to quantify the degree of donor T-cell engraftment of combined MoAb treatment in a system in which the only effect of treatment would be on the immune response of donor T cells to host alloantigens. Five to eight mice per group per experiment were given allogeneic donor T cells.
To purify LN cells, single-cell suspensions of axillary, mesenteric, and inguinal LN cells were obtained (as a source of GVHD-causing effector cells) by passing minced LN through a wire mesh and collecting them into RPMI 1640. Cell preparations were depleted of NK cells and either CD8+ (hybridoma 2.43, rat IgG2b; provided by Dr David Sachs, Charlestown, MA) or CD4+ (hybridoma GK1.5, rat IgG2b; provided by Dr Frank Fitch, Chicago IL) T cells by the appropriate MoAb coating and passaged through a goat α-mouse and goat α-rat Ig-coated column (Biotex, Edmonton, Canada). The final composition of T cells in the donor graft was determined by flow cytometry and was always found to be 94% CD4+ or CD8+ T cells. For sublethally irradiated recipients of donor MHC disparate T cells, hematocrit values were obtained at periodic intervals as an indicator of the possible BM destructive effects of infused T cells.16
Allogeneic and congenic engraftment experiments.
For alloengraftment experiments involving BALB/c donors, B6 recipients were administered 6.0 or 6.5 Gy TBI. BALB/c donor BM was treated with αThy1.2 (hybridoma 30-H12, rat IgG2b; provided by Dr David Sachs) plus complement. After treatment, donor cell suspensions were washed, resuspended, and 107 treated BM cells were injected via the caudal vein. For studies involving DBA/1 donors, B6 recipients were administered 6.0 Gy TBI and relevant MoAb as described above. Ten to 15 mice per experiment were analyzed.
For congenic BMT experiments, B6 recipients were administered 8.0 Gy TBI and cohorts of mice were infused with 0.3, 1.0, or 3.0 × 106 nonmanipulated B6-CD45.2 BM and either αCD48 MoAb or irrelevant IgG (300 μg/dose) IP as described above. Mice were analyzed for survival and either peripheral blood hematologic recovery or donor cell engraftment and cellularity in the BM and spleen at periodic intervals post-BMT, as indicated in the tables and figures.
To determine whether αCD48 MoAb altered the homing of hematopoietic progenitor cells to the spleen and thereby compromised lymphohematopoietic recovery, cohorts of B6 mice were adult splenectomized 2 weeks before reconstitution with B6-CD45.2 congenic BM (3 × 106/recipient) and either αCD48 or irrelevant MoAb.
Day 12 colony-forming unit–spleen (CFU-S) formation.
To determine the effect of MoAb on early BM-derived progenitor cell repopulation in vivo, syngeneic CFU-S formation was assessed. B6 recipients were irradiated with 7.5 Gy TBI (via 137Cesium source) on day −1 and then administered nonmanipulated B6 BM cells (105/recipient) on day 0. Mice were injected with αCD2, αCD48, and MoAb and/or irrelevant rat IgG as described above through day 9 post-BMT. Twelve days post-BMT, mice were killed and spleens were placed into Bouin’s solution to facilitate enumeration of colonies. Eight mice per group were transplanted.
Ex vivo and in vivo effects of αCD48 MoAb on lymphohematopoietic cells in non-BMT controls.
To determine the effect of αCD48 MoAb on lymphohematopoiesis in the BM and spleen of non-BMT controls, B6 mice were administered irrelevant or αCD48 MoAb (300 μg) IP as described above through day 7 post-BMT. On day 9, mice were electively killed for flow cytometric analysis of BM and spleen.
To assess the effect of αCD48 MoAb on modulation or coating of the CD48 antigen on spleen cells or BM cells, single-cell suspensions of each were obtained from non-BMT B6 controls and then incubated with αCD48 MoAb (20 μg/mL) for 30 minutes on ice. Cells were washed and then incubated with goat anti-hamster fluorescein isothiocyanate (FITC) or αCD48-FITC (Pharmingen, San Diego, CA). As a positive control for splenocyte analysis, αCD3ε MoAb (hamster 145-2C11; provided by Dr Jeffrey Bluestone, University of Chicago) was used under the same conditions.
Flow cytometry.
The T-cell, B-cell, and granulocyte/macrophage constituency of splenocytes or BM cells was measured using MoAb directed toward CD4 or CD8, CD45R/B220, and CD11a, respectively. Anti-CD2 and αCD48 fluorochromes also were used to quantify changes in the expression of CD2 or CD48 antigens in MoAb-treated or control mice. Fluorochrome-labeled MoAbs all were obtained from Pharmingen. Chimerism of peripheral blood mononuclear cells was analyzed at an earlier time point (7 weeks post-BMT) and at later time points (days 110 to 145 post-BMT). For quantification of donor alloengraftment, αH2d (hybridoma 34-5-8S, mouse IgG2a) or αH2q (hybridoma 66-3.5, mouse IgG; both provided by Dr David Sachs) were directly conjugated to FITC. For host-cell quantification, αH2b (hybridoma EH144, mouse IgG; provided by Dr T.V. Rajan, Farmington, CT) was directly conjugated to phycoerythrin (PE).
For congenic BMT experiments, the expression of CD45 alleleic determinants on BM and splenocytes was monitored using αCD45.1 (clone 104-2, rat IgG2a) and αCD45.2 (clone A20-1.7, rat IgG2a; both provided by Dr U. Hammerling, New York, NY). For all flow cytometry studies, single-cell suspensions were incubated with 2.4G2 to block Fc receptors, and then incubated with an optimal concentration of fluorochrome-labeled MoAb for 45 minutes at 4°C. Cells were washed three times and resuspended for analysis. An anti-human CD7 (hybridoma 3A1E, mouse IgG; provided by Dr Barton Haynes, Duke University, Durham, NC) was conjugated to FITC or PE to determine control (background) staining. Background values were subtracted from values obtained with relevant antibodies as previously described.17 Flow cytometry was performed on a FACScalibur (Becton Dickinson, Mountain View, CA). Ten thousand events (determined by forward- and side-light scatter) were analyzed.
Statistical analyses.
Group comparisons of continuous data were made by Student’st-test. Survival data were analyzed by lifetable methods using the Mantel-Peto-Cox summary of chi-square.18 Actuarial survival rates (the proportion of mice surviving on each day post-BMT) are shown. Hematologic and chimerism data were analyzed as individual values with data presented as mean ± 1 standard error of the mean (SEM). Probability (P) values <.05 were considered significant.
RESULTS
The in vivo infusion of αCD2 + 48 MoAb is partially effective in delaying CD8+ T-cell–mediated lethality but is highly effective in preventing CD4+ T-cell–mediated lethality.
To investigate the effect of CD2:CD48 blockade on CD8+T-cell–mediated GVHD, αCD2 + 48 or irrelevant MoAb was administered to sublethally irradiated bm1 recipients of 106 B6 CD8+ T cells (Fig 1A). Cumulative data pooled from two replicate experiments indicate that αCD2 + 48 MoAb–treated recipients had a significantly (P = .002) higher actuarial survival rate as compared with controls (n = 16/group), although there was only one long-term survivor treated with combined MoAb. CD8+ T-cell dose titration experiments indicated that although combined MoAb treatment was associated with a significant prolongation in time to mortality, none of the recipients of as few as 3 × 105 CD8+ T cells survived beyond day 22 posttransfer (data not shown). Therefore, combined MoAb infusion was only partially and modestly effective in reducing CD8+ T-cell alloresponses in vivo.
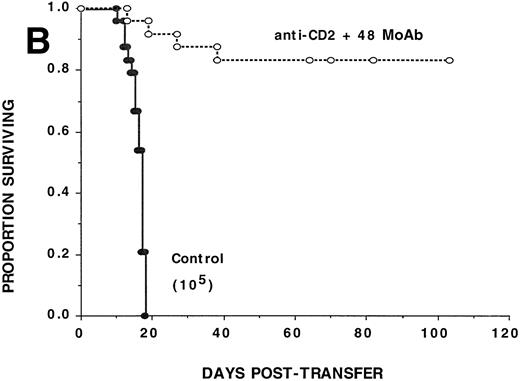
Anti-CD2 + 48 MoAb is ineffective in preventing GVHD-induced lethality by donor CD8+ T cells infused into sublethally irradiated recipients. Sublethally irradiated bm1 (A) or bm12 (B) recipients were administered the indicated number of highly purified CD8+ or CD4+B6 LN cells as shown in parentheses on day 0. Mice received either irrelevant IgG or CD2 + 48 MoAb (300 μg/dose each) IP twice weekly from days −1 through +21 posttransfer. On the x-axis are the days posttransfer and on the y-axis is the proportion of mice surviving. The survival data are plotted. Results from two (A, n = 16/group) or three (B, n = 24/group) replicate experiments with similar results are pooled. Mice administered CD2 + 48 MoAb had a significantly (P = .002) higher actuarial survival rate as compared with controls.
Anti-CD2 + 48 MoAb is ineffective in preventing GVHD-induced lethality by donor CD8+ T cells infused into sublethally irradiated recipients. Sublethally irradiated bm1 (A) or bm12 (B) recipients were administered the indicated number of highly purified CD8+ or CD4+B6 LN cells as shown in parentheses on day 0. Mice received either irrelevant IgG or CD2 + 48 MoAb (300 μg/dose each) IP twice weekly from days −1 through +21 posttransfer. On the x-axis are the days posttransfer and on the y-axis is the proportion of mice surviving. The survival data are plotted. Results from two (A, n = 16/group) or three (B, n = 24/group) replicate experiments with similar results are pooled. Mice administered CD2 + 48 MoAb had a significantly (P = .002) higher actuarial survival rate as compared with controls.
To determine whether CD2:CD48 blockade could inhibit CD4+T-cell–mediated GVHD, αCD2 + 48 or irrelevant MoAb was administered to sublethally irradiated bm12 recipients of B6 CD4+ T cells (105/mouse) (Fig 1B). Cumulative data pooled from three replicate experiments (n = 24/group) indicate that αCD2 + 48 MoAb–treated recipients had a significantly (P = .0009) higher actuarial survival rate as compared with controls (82% v 0%, respectively). Day 14 mean hematocrit values were significantly (P = 5.6 × 10−11) higher in αCD2 + 48 MoAb–treated recipients as compared with controls (33% v19%, respectively). Anti-CD2 + 48 MoAb–treated recipients were not GVHD free as shown by splenic B-cell lymphopenia found on days 82 and 109 posttransfer and confirmed by acute GVHD histology of the lung, liver, skin, and colon (n = 3 mice per time point each for flow cytometry and histology). Although only 105CD4+ T cells were infused on day 0, 0.96 ± 0.41 × 106 and 0.23 ± 0.10 × 106 donor CD4+ T cells were detected at these time points, respectively, in the spleen of anti-CD2 + 48 MoAb–treated recipients, indicating that combined MoAb treatment did not completely prevent donor T-cell engraftment from occurring. Despite the presence of GVHD in these recipients, the severity of the GVHD response in combined MoAb-treated recipients was insufficient to cause mortality in most recipients. Together, these data indicate that αCD2 + 48 MoAb treatment impairs donor CD4+ T-cell alloresponses in vivo.
Because CD2 is expressed on 85% of murine B cells15 and CD48 on virtually all B cells,10 we asked to what extent the binding of αCD2 or αCD48 MoAb on host B cells, which can express MHC class II antigen, was required for the protective effects of these MoAbs on GVHD. For this purpose, we used B6-SCID mice as B-cell– (and T-cell–) deficient recipients (Fig 2). B6-SCID recipients of 106 bm12 CD4+ T cells had a 12% actuarial survival rate, with most succumbing to GVHD-induced mortality by 3 weeks posttransfer. In contrast, recipients administered αCD2 + 48 MoAb had a 100% actuarial survival rate, exceeding their pre-BMT body weights by 2 months posttransfer (data not shown). Long-term survivors (n = 4) had a mean of 4.6 × 106 donor CD4+ T cells present in the spleen on day 78 posttransfer, indicating at least a 4.6-fold expansion of T cells as compared with the number infused. In addition, recipients had histological evidence of acute GVHD involving the lung, colon, skin, and liver. Thus, αCD2 + 48 MoAb did not completely eliminate the GVHD potential of the inoculum of 106 donor CD4+ T cells.
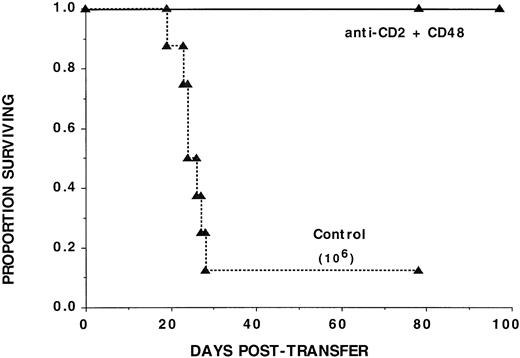
The anti-GVHD effect of CD2 + 48 MoAb is not dependent on interference with T:B cognate interaction. Nonirradiated B-cell–deficient B6-SCID recipients were administered 106highly purified bm12 CD4+ LN cells. Mice receiving CD2 + 48 MoAb were treated as in Fig 1. The survival data are plotted. The days posttransfer are plotted on the x-axis and the proportion of mice surviving on the y-axis. Mice administered CD2 + 48 MoAb had a significantly (P = .00038) higher actuarial survival rate as compared with controls (n = 8 per group).
The anti-GVHD effect of CD2 + 48 MoAb is not dependent on interference with T:B cognate interaction. Nonirradiated B-cell–deficient B6-SCID recipients were administered 106highly purified bm12 CD4+ LN cells. Mice receiving CD2 + 48 MoAb were treated as in Fig 1. The survival data are plotted. The days posttransfer are plotted on the x-axis and the proportion of mice surviving on the y-axis. Mice administered CD2 + 48 MoAb had a significantly (P = .00038) higher actuarial survival rate as compared with controls (n = 8 per group).
Anti-CD48 MoAb inhibits the alloengraftment of T-cell–depleted BM administered to irradiated recipients.
Because αCD2 + 48 MoAb was effective in reducing GVHD lethality and donor T cells are required for optimal alloengraftment, studies were performed to determine whether combined MoAb would have a negative impact on engraftment. B6 recipients were irradiated and then administered T-cell–depleted BALB/c BM. In this strain combination and under these conditions, both T cells and NK cells of the host are involved in graft resistance.19 Anti-CD2 + 48 MoAb impaired the engraftment of T-cell–depleted BALB/c (H2d) donor BM in each of three replicate experiments when recipients were analyzed early (6 to 8 weeks) or late (3.5 to 5 months) post-BMT (Table 1). To determine whether combined MoAb administration would inhibit engraftment in a system in which only host T cells were capable of resisting the donor graft,19B6 recipients were administered T-cell–depleted DBA/1 (H2q, hematopoietic histocompatibility [Hh-1] null) donor BM. Similar to that observed in the BALB/c → B6 system, αCD2 + 48 MoAb markedly reduced the engraftment of donor BM when measured early (7 weeks) and late (3 months) post-BMT (Table 1). αCD48 MoAb alone was responsible for the impairment of alloengraftment in B6 recipients of T-cell–depleted DBA/1 donor BM grafts.
In both alloengraftment systems, the combined administration of αCD2 + 48 MoAb had no effect on the recovery of neutrophils but did significantly reduce the absolute lymphocyte counts in the peripheral blood as measured on day 14 (Table 2). In the DBA/1 → B6 system, total leukocyte and lymphocyte numbers as well as hematocrit values also were significantly, albeit modestly, lower in the αCD2 + 48 MoAb–treated group on day 14. In both systems, a more pronounced effect on each of these parameters was evident on day 28 post-BMT, a time when recipients of irrelevant MoAb but not αCD2 + 48 MoAb had a more vigorous lymphohematopoietic recovery. On day 28 post-BMT, recipients of combined MoAb had a recovery inferior to control recipients analyzed on day 14 post-BMT.
Anti-CD48 MoAb inhibits engraftment of congenic marrow and syngeneic day 12 CFU-S formation in vivo.
Although αCD2 + 48 MoAb infusion inhibited the engraftment of T-cell–depleted allogeneic BM, the mechanism(s) responsible for the inhibitory effect are unknown. To discriminate between an immune and nonimmune mechanism, experiments were performed in a congenic BMT setting, thereby precluding an alloimmune mechanism (Table 3). Lethally irradiated B6 recipients were administered congeneic BM cells (1.0 or 3.0 × 106) along with irrelevant MoAb or αCD48 MoAb. At both cell doses, αCD48 MoAb infusion was associated with a significant reduction in the day 14 post-BMT mean numbers of peripheral blood (PB) leukocytes and PB lymphocytes, and at the lower BM cell dose (106), hematocrit values were significantly, albeit modestly, reduced as well.
Mice administered B6-CD45.2 BM at a cell dose of 3 × 106 cells/recipient were studied for lymphohematopoietic reconstitution in the spleen and BM on days 7, 10, 14, 20, (Tables 4 and5; Figs 3 and4) and day 47 post-BMT (not shown). In the BM compartment, CD48 antigen is expressed in a trimodal fashion with low, medium, or high density (Fig 3A) in contrast to CD48+ splenocytes which uniformly express a medium density of the CD48 antigen (Fig 3B). CD48 antigen surface expression in the BM (Fig 3A) and spleen (Fig 3B) was markedly reduced in αCD48 MoAb–treated recipients at all time points examined between days 7 and 20. Anti-CD48 MoAb–treated mice experienced a delay in the recovery of BM (Table 4 and Fig 4) and splenic (Table 5) cellularity. Moreover, the total number of BM cells CD48hi and CD48med were significantly lower and the numbers of CD48neg BM cells higher in MoAb-treated recipients (Table 4). Splenic flow cytometric analysis indicated that MoAb infusion significantly decreased donor cell recovery for at least 10 days post-BMT (Table 5).
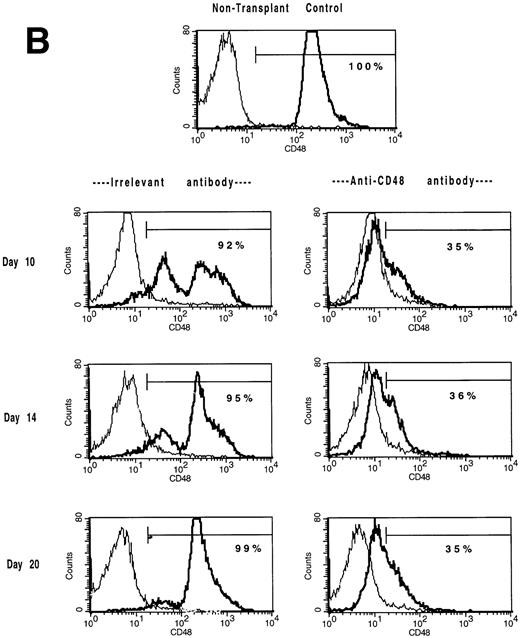
The in vivo infusion of CD48 MoAb in irradiated recipients of congenic BM shifts BM and splenic populations toward a CD48low or CD48neg phenotype. Single-cell suspensions of BM (A) or spleen (B) were obtained from mice undergoing congenic BMT from the experiment shown in Tables 4 and 5 on days 10, 14, and 20 post-BMT as listed. Mice received irrelevant MoAb or CD2 + 48 MoAb according to the regimen listed in Fig 1. Cells were stained for CD48 expression and then analyzed by fluorescence-activated cell sorting (FACS). Isotype-matched control MoAb was used to set gates. For BM (A), CD48 expression was segregated into three antigen density levels. The percent of cells falling within each antigen density level is listed. The isotype-matched control MoAb is shown in the thin line and the CD48 MoAb staining in the heavy line. A representative overlay histogram from three individually analyzed mice per group and a non-BMT control analyzed at each time point are shown. The percentages indicate the percent positive cells in the gate shown.
The in vivo infusion of CD48 MoAb in irradiated recipients of congenic BM shifts BM and splenic populations toward a CD48low or CD48neg phenotype. Single-cell suspensions of BM (A) or spleen (B) were obtained from mice undergoing congenic BMT from the experiment shown in Tables 4 and 5 on days 10, 14, and 20 post-BMT as listed. Mice received irrelevant MoAb or CD2 + 48 MoAb according to the regimen listed in Fig 1. Cells were stained for CD48 expression and then analyzed by fluorescence-activated cell sorting (FACS). Isotype-matched control MoAb was used to set gates. For BM (A), CD48 expression was segregated into three antigen density levels. The percent of cells falling within each antigen density level is listed. The isotype-matched control MoAb is shown in the thin line and the CD48 MoAb staining in the heavy line. A representative overlay histogram from three individually analyzed mice per group and a non-BMT control analyzed at each time point are shown. The percentages indicate the percent positive cells in the gate shown.
Anti-CD2 + 48 MoAb infusion slows the recovery of lymphohematopoiesis in the BM and splenic compartments in lethally irradiated recipients of congenic BM grafts. B6 recipients were lethally irradiated on day −1 and then administered 3.0 × 106 BM from B6-CD45.2 congenic donors on day 0. Mice were treated with irrelevant IgG or CD2 + 48 MoAb as described in Fig1. At the indicated time periods listed on the x-axis, three mice per group were analyzed for reconstitution of the BM compartment as shown in Table 4. The absolute number of cells is listed on the y-axis. Data, mean values ± 1 standard deviation of the mean, are depicted. Comparisons between the groups which were significant (*P < .05; **P < .01) are shown. Representative histograms from these mice are shown in Fig 3A. These data were replicated in a second experiment as shown in Table 7.
Anti-CD2 + 48 MoAb infusion slows the recovery of lymphohematopoiesis in the BM and splenic compartments in lethally irradiated recipients of congenic BM grafts. B6 recipients were lethally irradiated on day −1 and then administered 3.0 × 106 BM from B6-CD45.2 congenic donors on day 0. Mice were treated with irrelevant IgG or CD2 + 48 MoAb as described in Fig1. At the indicated time periods listed on the x-axis, three mice per group were analyzed for reconstitution of the BM compartment as shown in Table 4. The absolute number of cells is listed on the y-axis. Data, mean values ± 1 standard deviation of the mean, are depicted. Comparisons between the groups which were significant (*P < .05; **P < .01) are shown. Representative histograms from these mice are shown in Fig 3A. These data were replicated in a second experiment as shown in Table 7.
To determine whether αCD48 MoAb was depleting CD48hi and CD48med cells, αCD48 MoAb or hamster IgG (300 μg/dose) was injected into B6 mice on days 0, 3, and 7. On day 9, three mice per group were killed for determination of BM and splenic cellularity and phenotype. A representative experiment of two experiments that were performed with similar results is shown in Table 6. Anti-CD48 MoAb did not reduce the total number of BM cells or splenocytes and analysis of the T-cell, B-cell, and myeloid series did not show any adverse effect of αCD48 MoAb on these cell types. The only difference in the number and composition of the BM and splenocyte compartments in these two groups of mice was in the level of detectable CD48 antigen expression, in that all mice receiving αCD48 MoAb had a marked reduction in the level of cell surface CD48 antigen expression (Fig 5and Table 6). Therefore, the infusion of αCD48 MoAb did not result in a physical depletion of CD48+ cells.
The in vivo infusion of CD48 MoAb in non-BMT B6 mice shifts the BM and splenic populations toward a CD48low or CD48neg phenotype. Non-BMT B6 control mice received irrelevant MoAb or CD48 MoAb (300 μg) on days 0, 3, and 7. On day 9, cells were stained for CD48 expression and then analyzed by FACS. Isotype-matched control MoAb was used to set gates. For BM, CD48 expression was segregated into three antigen density levels. The percent of cells falling within each antigen density level is listed. For spleen, the isotype-matched control MoAb is shown in the thin line and the CD48 MoAb staining in the heavy line. A representative overlay histogram from three individually analyzed mice per group at each time point are shown. The percentages indicate the percent of positive cells in the gate shown. The complete data are tabulated in Table 6.
The in vivo infusion of CD48 MoAb in non-BMT B6 mice shifts the BM and splenic populations toward a CD48low or CD48neg phenotype. Non-BMT B6 control mice received irrelevant MoAb or CD48 MoAb (300 μg) on days 0, 3, and 7. On day 9, cells were stained for CD48 expression and then analyzed by FACS. Isotype-matched control MoAb was used to set gates. For BM, CD48 expression was segregated into three antigen density levels. The percent of cells falling within each antigen density level is listed. For spleen, the isotype-matched control MoAb is shown in the thin line and the CD48 MoAb staining in the heavy line. A representative overlay histogram from three individually analyzed mice per group at each time point are shown. The percentages indicate the percent of positive cells in the gate shown. The complete data are tabulated in Table 6.
The lack of CD48hi cells in αCD48 MoAb–treated mice could have been caused by MoAb coating or CD48 antigen modulation. Single-cell suspensions of BM and spleen were incubated with αCD48 MoAb (20 μg/mL) for 30 minutes on ice. Cells were washed and then incubated with goat anti-hamster FITC or αCD48-FITC. As a positive control for splenocyte analysis, αCD3ε MoAb was used under the same conditions. The proportion of CD48+ splenocytes (Fig 6, top) and CD48hi BM cells (Fig 6, bottom) was markedly reduced after αCD48 MoAb incubation (Fig 6) but not αCD3ε MoAb incubation (data not shown). As with the in vivo experiment, only a low proportion of BM or spleen cells exposed to αCD48 MoAb bound goat anti-hamster IgG FITC (Fig 6, right-hand panels), in contrast to the 45% binding noted for αCD3ε MoAb–exposed splenocytes. Collectively, these data would indicate that αCD48 MoAb induces a rapid modulation of the CD48 epiptope and would exclude depletion of CD48hi or coating of the CD48 antigen as the primary reason for the lack of CD48hi cells in the BM and spleen in αCD48 MoAb–treated recipients.
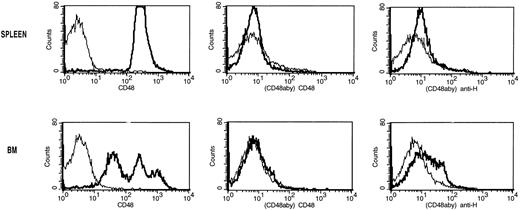
Analysis of modulation and coating of the CD48 antigen induced by the ex vivo incubation of splenocytes or BM cells with CD48 MoAb. Single-cell suspensions of spleen (top panels) and BM (bottom panels) from non-BMT B6 control mice were incubated with CD48 MoAb (CD48aby) (20 μg/mL) for 30 minutes on ice. Cells were washed and then incubated with goat anti-hamster FITC (anti-H) or CD48-FITC (CD48). The thin lines are the isotype-specific negative control MoAb staining and the dark lines are the staining with either CD48-FITC or anti-hamster FITC. The proportion of CD48hiBM cells and splenocytes was markedly reduced after CD48 MoAb. Only a low proportion of BM or spleen cells exposed to CD48 MoAb bound goat anti-hamster IgG FITC.
Analysis of modulation and coating of the CD48 antigen induced by the ex vivo incubation of splenocytes or BM cells with CD48 MoAb. Single-cell suspensions of spleen (top panels) and BM (bottom panels) from non-BMT B6 control mice were incubated with CD48 MoAb (CD48aby) (20 μg/mL) for 30 minutes on ice. Cells were washed and then incubated with goat anti-hamster FITC (anti-H) or CD48-FITC (CD48). The thin lines are the isotype-specific negative control MoAb staining and the dark lines are the staining with either CD48-FITC or anti-hamster FITC. The proportion of CD48hiBM cells and splenocytes was markedly reduced after CD48 MoAb. Only a low proportion of BM or spleen cells exposed to CD48 MoAb bound goat anti-hamster IgG FITC.
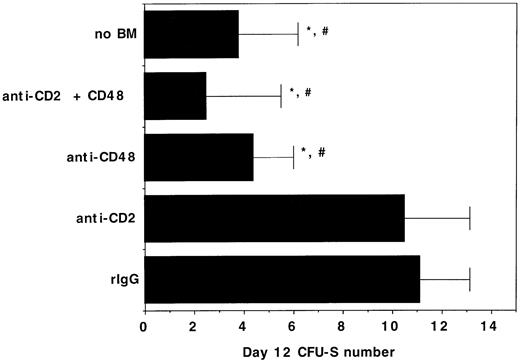
To determine whether the negative effects of αCD2 + 48 MoAb infusion on engraftment may be related to in vivo inhibition of BM-derived progenitor cell growth, day 12 CFU-S formation was quantified in syngeneic BMT recipients of αCD2, αCD48, or αCD2 + 48 MoAb. The mean colony number in irrelevant IgG–treated control recipients was 11 versus ≤4 that were present in recipients of no BM or recipients of BM plus αCD48 MoAb (Fig 7). Moreover, the size of the colonies was much smaller in the latter groups, consistent with a marked reduction in colony cellularity caused by αCD48 MoAb infusion. Cumulatively, the alloengraftment and syngeneic engraftment studies are most consistent with a negative effect of αCD48 MoAb on hematopoiesis in irradiated, transplanted mice and indicate that αCD48 MoAb affects engraftment through nondepletionary mechanism(s).
The in vivo infusion of CD48 MoAb or CD2 + 48 MoAb in irradiated recipients of syngeneic BM inhibits day 12 CFU-S formation. B6 recipients were irradiated with 7.5 Gy total body irradiation via 137Cesium source on day −1 and then administered 105 B6 BM cells on day 0. Mice (n = 8 per group) were injected with CD2 MoAb, CD48 MoAb, CD2 + 48 MoAb, or irrelevant IgG, as indicated on the y-axis at a dose of 300 μg each, administered IP on days -1, 2, 6, and 9 post-BMT. Twelve days post-BMT, mice were killed and spleens were placed into Bouin’s solution to facilitate enumeration of colonies. The mean ± 1 SEM number of colonies is listed on the x-axis. *P < .001 as compared with irrelevant IgG control. #Colonies are all smaller in size than observed in irrelevant MoAb–treated controls.
The in vivo infusion of CD48 MoAb or CD2 + 48 MoAb in irradiated recipients of syngeneic BM inhibits day 12 CFU-S formation. B6 recipients were irradiated with 7.5 Gy total body irradiation via 137Cesium source on day −1 and then administered 105 B6 BM cells on day 0. Mice (n = 8 per group) were injected with CD2 MoAb, CD48 MoAb, CD2 + 48 MoAb, or irrelevant IgG, as indicated on the y-axis at a dose of 300 μg each, administered IP on days -1, 2, 6, and 9 post-BMT. Twelve days post-BMT, mice were killed and spleens were placed into Bouin’s solution to facilitate enumeration of colonies. The mean ± 1 SEM number of colonies is listed on the x-axis. *P < .001 as compared with irrelevant IgG control. #Colonies are all smaller in size than observed in irrelevant MoAb–treated controls.
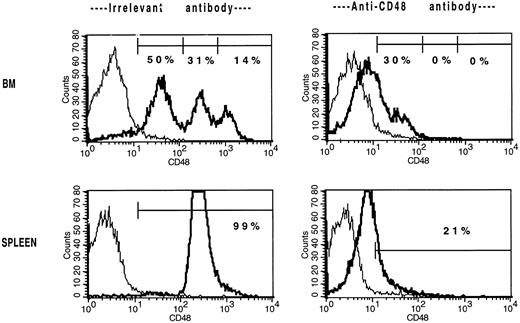
Because αCD48 MoAb administration led to a marked reduction in day 12 CFU-S formation, we considered the possibility that αCD48 MoAb–coated BM progenitor cells had an impaired ability to traffic to the spleen, a major site of early post-BMT hematopoiesis. To exclude the effect of αCD48 MoAb infusion on splenic homing, cohorts of mice were splenectomized 2 weeks before congenic BMT. At all time points examined (days 7, 10, and 14), αCD48 MoAb administration was associated with a marked reduction in the absolute number of donor lymphohematopoietic cells in the BM (Table7). Both the lymphoid and myeloid lineage cells were affected. Interestingly, the absolute number of residual host BM cells was approximately twofold lower in αCD48 MoAb–treated recipients, suggesting that αCD48 MoAb had a direct effect on inhibiting the production of BM hematopoietic cells. Splenectomized mice had similar responses to αCD48 MoAb infusion as nonsplenectomized controls. Although combined MoAb infusion provided a more pronounced and consistent delay of donor BM engraftment than in the previous study (Table 4), in both studies combined MoAb infusion was associated with a delay in recovery at one or more time periods post-BMT.
DISCUSSION
Although we have shown that αCD48 MoAb infusion is highly effective in inhibiting CD4+ and to a far lesser extent CD8+ T-cell–mediated GVHD lethality, αCD48 MoAb infusion was associated with a pronounced decrease in the alloengraftment of T-cell–depleted BM grafts. Because congenic lymphohematopoietic recovery and syngeneic day 12 CFU-S formation also were impaired by αCD48 MoAb infusion, the adverse effects on engraftment do not seem to be related to the effects of these MoAbs on the immune system. Splenectomized recipients treated with aCD48 MoAb also had decreased donor and host lymphohematopoiesis, indicating that αCD48 MoAb–coated donor BM cells were not being redirected into unfavorable sites for hematopoiesis post-BMT. Thus, the CD48 antigen seems to have a critical role in regulating lymphohematopoiesis post-BMT. These findings have significant implications for the extrapolation of this approach to human BMT recipients.
An intriguing and important aspect of our study was the profound deleterious effect of αCD48 MoAb on engraftment. Anti-CD48 MoAb infusion alone or in combination with αCD2 markedly inhibited the engraftment of two types of allogeneic pan-T-cell–depleted BM in irradiated B6 recipients. In one strain combination (BALB/c → B6), depletion of either NK cells or T cells from the host enhance engraftment, whereas in the other (DBA/1 → B6), only host T cells are involved in graft resistance.19 Because αCD48 MoAb binds T cells and NK cells,4-7,10 20-22 it was possible that engraftment might be enhanced or unimpaired if CD8+ T cells were the major effectors of graft rejection. Because donor cell engraftment was suppressed in recipients of αCD48 MoAb–containing regimens, we needed to distinguish between an immunologic effect unique to an allogeneic environment or a primary hematopoietic effect which would be independent of the effects of these MoAbs on allorecognition. Experiments performed in non-BMT mice did not show any evidence of suppressed hematopoiesis under normal physiological conditions. Specifically, as compared with recipients of irrelevant IgG, non-BMT mice administered αCD2, αCD48, or both MoAbs and individually studied did not have significant differences in absolute numbers of BM or splenocytes or the proportion of Mac1+, B220+, CD4+, or CD8+ cells when analyzed 2 days after discontinuing MoAb administration.
To uncover a more subtle contribution by αCD48 MoAb infusion, experiments were performed in irradiated, congenic BMT mice experiencing stress hematopoiesis. As compared with recipients of irrelevant IgG, mice administered αCD48 MoAb had a substantial reduction in the absolute number of donor BM-derived cells localized in the spleen on day 7 post-BMT and in the BM compartment as assessed on days 10 and 14 post-BMT (few donor cells were detectable in the BM of either group on day 7 post-BMT). A striking finding was the absence of CD48hi-expressing cells in the spleen and CD48hi and CD48med cells in the BM compartment. Because there was a proportional increase in the number of CD48neg cells that mostly compensated for the loss of CD48med/hi cells in the BM, the effects in the BM compartment seemed to be caused by CD48 modulation as has been previously described in non-BMT mice administered αCD48 MoAb.23 Our in vitro and in vivo results directly show that αCD48 MoAb is a potent modulator of CD48 antigen expression.
Because the absolute number of donor BM-derived cells localized to the spleen on day 7 post-BMT was markedly reduced, whereas the BM compartment was relatively intact, we considered the possibility that the spleen, the major site of early post-BMT hematopoiesis and of allogeneic graft resistance, was preferentially affected by αCD48 MoAb infusion. In syngeneic BMT recipients, the infusion of regimens containing αCD48 MoAb reduced the number of day 12 CFU-S colonies, an indicator of multipotential progenitor cell engraftment, to the levels observed in irradiated mice that were not administered any BM cells. Because donor BM cells are infused under the cover of αCD48 MoAb while recipient hematopoiesis is already occurring in the BM at the time of MoAb adminstration, it is possible that αCD48 MoAb was affecting the homing and migration of donor BM-derived cells to the spleen early post-BMT. However, splenectomized recipients of congenic BM grafts were equally susceptible to the inhibitory effect of αCD48 MoAb on BM lymphohematopoiesis. Moreover, αCD48 MoAb infusion also reduced the residual host hematopoiesis. Therefore, αCD48 MoAb infusion did not inhibit hematopoietic recovery and engraftment by altering the trafficking of intravenously infused donor BM progenitor cells. Rather, we favor the hypothesis that αCD48 MoAb has a direct effect on lymphohematopoiesis by affecting the necessary signals for hematopoiesis provided by the host microenvironment.
In addition to our observations on combined MoAb-induced inhibition of lymphohematopoietic recovery post-BMT, we also have shown that αCD2 + 48 MoAb infusion is an effective approach to downregulating GVHD induced by CD4+ T cells infused into either sublethally irradiated or nonirradiated SCID MHC class II disparate recipients. Thus, combined MoAb infusion has a direct immune suppressive effect on GVHD-induced mortality. Anti-CD48 MoAb infusion was necessary and sufficient for the GVHD protective effect of the combined MoAb regimen and the biological effect of αCD2 MoAb infusion was only uncovered at low CD4+ T-cell doses (data not shown). The explanation for the lower efficacy of this combined MoAb regimen on CD8+T-cell–mediated GVHD is unknown. The inferior efficacy of combined MoAb in the setting of CD8+ T-cell–mediated GVHD was unexpected for several reasons. The in vivo administration of αCD48 or αCD2 MoAb has been shown to inhibit the in vivo priming and in vitro generation of allospecific CTL.9,11,12,14,23-26Although αCD2 + 48 MoAb inhibits the generation of allospecific CTL, it is possible that there is a differential effect on the generation or function of CTL derived from CD4+ as compared with CD8+ CTL. We do not have any direct evidence to support this hypothesis. However, it is interesting to note that CD2 ligation has been shown to upregulate Fas ligand (CD95L).27 Recent studies by Graubert et al28 have indicated that CD4+ T cells can use CD95L to cause GVHD lethality in MHC class II disparate recipients, whereas CD8+ T cells are unable to use CD95L to mediate GVHD lethality in MHC class I disparate recipients. Therefore, αCD2 + 48 MoAb infusion theoretically may inhibit the development of CD4+CD95L+ CTL effectors in vivo that are responsible for GVHD in the B6 → bm12 system.
Another possibility is that αCD48 MoAb affects lymphokine production differently in CD8+ and CD4+ T cells and this difference leads to a more effective inhibition of CD4+T-cell–mediated lethality as compared with CD8+ T cells. For example, the lymphokines triggered by phytohemagglutinin but not concanavalin A are substantially inhibited by the in vivo infusion of αCD48 or αCD2 MoAb and mixed lymphocyte reaction responses are unaffected by either MoAb.10,11,14 23 These data would indicate that lymphokine responses of some subsets of T cells are more susceptible than others to MoAb inhibition. Collectively, these data are most consistent with the interpretation that there is a qualitative difference between the inhibitory effects of αCD48 MoAb on CD4+ and CD8+T-cell–mediated GVHD. Regardless of the explanation for the differential GVHD inhibitory effects of αCD2 + 48 MoAb infusion, it is clear that the suppression of lymphohematopoiesis conferred by this combined MoAb regimen will dampen the enthusiasm for the clinical trials of αCD2 + 48 MoAb in humans undergoing GVHD unless it can be determined that our findings are either species specific or dependent on the particular epitope recognized by the αCD48 MoAb we have studied.
Supported in part by National Institues of Health Grants No. R01 AI 34495, R01 HL56067, R29 AI32655, R01 AI41428, P60 AR20557, and P01 AI-35296
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bruce R. Blazar, MD, Box 109 UMHC, 420 SE Delaware St, Minneapolis, MN 55455; e-mail: blaza001@maroon.tc.umn.edu.