Abstract
Interleukin-15 (IL-15) is an important lymphokine regulating natural killer (NK) activity, T-cell proliferation, and T-cell cytotoxic activities. We hypothesized that the reduced expression and production of IL-15 from cord blood (CB) may contribute to the immaturity of CB immunity and potentially delay immune reconstitution after CB transplantation. We compared the expression and production of IL-15 from activated cord versus adult mononuclear cells (MNCs) and the regulatory mechanisms associated with IL-15 expression in CB MNCs. We have also studied the effect of exogenous IL-15 stimulation on CB and adult peripheral blood (APB) MNCs in terms of NK and lymphokine-activated killer (LAK) activities and cytokine induction. Lipopolysaccharide (LPS)-stimulated CB and APB MNCs were used to determine IL-15 expression and protein production by Northern analysis and Western immunoblot analysis. IL-15 mRNA expression and protein accumulation in CB MNC were 25% ± 2.0% (12 hours, n = 4, P < .05) and 30% ± 2.5% (12 hours, n = 3, P < .05), respectively, when compared with APB MNCs. Nuclear run-on assays showed no differences between CB and APB MNCs during basal levels of transcription and after transcriptional activation. However, the half-life of IL-15 mRNA was approximately twofold lower in activated CB MNCs than in activated APB MNCs (CB: 101 ± 5.8 minutes v APB: 210 ± 8.2 minutes, n = 3, P < .05). Exogenous IL-15 significantly enhanced CB NK and LAK activities up to comparable levels of APB (P < .05). IL-15 also significantly induced interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) protein production (days 1, 3, and 6, P < .05, n = 3) in CB MNCs. IL-15–stimulated LAK cells induced a significant lytic response against two acute lymphoblastic cell lines and two pediatric neuroblastoma cell lines. Both NK and LAK activities were augmented by the combination of IL-12 and IL-15, and the low-dose combination of IL-12 and IL-15 achieved similar levels of in vitro NK and LAK cytotoxicity compared with higher doses of either lymphokine. The present study suggests that IL-15 mRNA and protein expression is decreased in activated CB, secondary, in part, to altered posttranscriptional regulation. The reduced production of IL-15 from CB MNCs in response to stimulation may contribute to the decrease in IFN-γ and TNF-α production and CB cellular immunity. However, exogenous IL-15 enhanced IFN-γ and TNF-α production and NK and LAK cytotoxicities in CB MNCs. The reduced production of IL-15 from activated CB may contribute to the immaturity of CB cellular immunity and delayed immune reconstitution after unrelated CB transplantation. Exogenous IL-15 administration may compensate for the immaturity of CB immunity. The synergistic in vitro effects of low-dose IL-12 and IL-15 also implies the possible use of low doses each of IL-12 and IL-15 for enhancing immune reconstitution and/or possibly as a form of antitumor immunotherapy after CB transplantation.
WE HAVE RECENTLY shown that umbilical cord blood (CB) could be used successfully as an alternative source of hematopoietic stem cells after myeloablative therapy for both malignant and nonmalignant disorders.1 Recent results after unrelated CB transplantation have suggested a significant delay in immune reconstitution.2,3 CB hematopoiesis and cellular immunity are developmentally immature when compared with adult peripheral blood (APB). This immaturity may be due, in part, to defects in CB cellular lymphokine and cytokine production.4 Neonatal lymphocytes from CB have reduced T-cell5,6 and natural killer (NK) cell function7,8 and decreased type-specific antibody production.9,10 Harris et al11,12 and others have also reported that CB is composed of phenotypically and functionally immature lymphocytes that are associated with decreased allo-antigen–specific cytotoxic T lymphocytes (CTLs).13,14 We have previously shown reduced mRNA expression and protein production of specific cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF ), granulocyte colony-stimulating factor (G-CSF ), interleukin-3 (IL-3), macrophage colony-stimulating factor (M-CSF ), and IL-12 from stimulated human CB versus APB mononuclear cells (MNCs).15-18 Decreased production of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) from human CB versus APB MNCs has also been reported.19-21 These defects in CB T-cell immune function and specific cytokine production may contribute to the delayed immune reconstitution in patients after unrelated CB transplantation.
IL-15 was recently identified as a novel cytokine that was originally cloned from a simian kidney epithelial cell line (CV-1/EBNA).22 The IL-15 gene has 8 exons and is located in the human genome on chromosome 4q31.23 Human IL-15 cDNA encodes a 162 amino acid peptide with a long leader sequence of 48 amino acids yielding a 114 amino acid mature protein with a size of 13 to 14 kD.22 IL-15 mRNA is expressed by a wide variety of tissues, including placenta, skeletal muscle, kidney, lung, heart, fibroblasts, epithelial cells, and most abundantly by activated monocytes. IL-15 shares many biologic properties with IL-2, including induction of T-cell, B-cell, and NK cell proliferation.22,24-28 Comparatively, IL-2 is predominantly expressed and produced by activated T cells.22,29 IL-15 binds to only the β and γ subunits of the IL-2 receptor complex without requiring the use of the α subunit to exert its biologic activities.22,30-32 IL-15 has been reported to enhance nonspecific NK and lymphokine-activating killer (LAK) cytotoxic activities.22,26-28 IL-15 also induces NK cell production of IFN-γ, TNF-α, and GM-CSF.28 In vitro and in vivo studies have shown IL-15 to induce a variety of antitumor effects, including induction of CTL and LAK activities.33 34
We hypothesize that the reduced production of IL-15 from activated CB MNCs compared with APB MNCs may contribute, in part, to immaturity of CB cellular immunity and potentially the delay in immune reconstitution after unrelated CB transplantation. Furthermore, exogenous IL-15 administration may enhance CB cellular immunity and has the potential for enhancing immune reconstitution and for immunotherapy after unrelated CB transplantation. In this study, therefore, we investigated the expression and production of IL-15 and regulatory mechanisms associated with IL-15 expression in CB compared with APB MNCs. We sought to determine if IL-15 could enhance CB IFN-γ and TNF-α production and CB NK and LAK cytotoxicities compared with APB and also to determine if IL-15 would be additive or synergistic with IL-12 with regard to in vitro antitumor immunity.
MATERIALS AND METHODS
Isolation of MNCs from CB and APB. Peripheral blood was obtained by venipuncture from healthy adult volunteers in accordance with the principles of the Declaration of Helsinki. Blood samples were also obtained from the umbilical cords of the placentas of normal, full-term, nonstressed infants immediately after scheduled cesarean section. The samples were collected in heparinized syringes. CB and APB MNCs were isolated from whole blood by density gradient separation on Ficoll-Hypaque gradients (density = 1.007 g/mL; Sigma Chemical Co, St Louis, MO) for 30 minutes. The MNCs at the interface were collected, washed twice, and resuspended in RPMI-1640 (GIBCO, Grand Island, NY) culture medium supplemented with 10% heat-inactivated human AB serum (Sigma). MNCs isolated by this density gradient separation were purified to greater than 98% homogeneity, and cell viability as measured by trypan blue exclusion was more than 99%. There was no difference in the MNC differential between CB and APB (CB: 82% ± 8.0% lymphocytes and 8.8% ± 4.0% monocytes; APB: 86% ± 4.0% lymphocytes and 7.2% ± 3.0% monocytes). The cells were cultured at a density of 1 × 106 cells/mL in culture medium for the following assays.
RNA isolation and Northern blotting. To determine IL-15 mRNA expression, CB and APB MNCs (60 × 106 cells) were stimulated with lipopolysaccharide (LPS; from Escherichia coli 0127:B8 at 10 μg/mL; Sigma) for up to 48 hours. Total cellular RNA was extracted from stimulated and unstimulated cells by the method of Chomczynski and Sacchi.35 Polyadenylated (A+) RNA from cytoplasmic total RNA was then purified with oligo (dT) cellulose column (mRNA purification kit from Pharmacia Biotech Inc, Piscataway, NJ) and electrophoresed on 1% agarose and 5% formaldehyde gels. The samples were heated in 40% formamide and 14% formaldehyde at 65°C for 15 minutes and then cooled before the addition of 1 μg/mL ethidium bromide. RNA was transferred to nitrocellulose and baked for 2 hours. Hybridization with an antisense probe made by transcription of a human cDNA (kindly provided by Dirk Anderson, Immunex, Seattle, WA) was performed at 63°C overnight in 50% formamide, 5× sodium chloride sodium citrate (SSC), 1× Denhardt's, 50 mmol/L sodium phosphate (pH 6.5), 0.1% sodium dodecyl sulfate (SDS), 250 μg/mL salmon sperm DNA, and 10% dextran sulfate. Blots were washed with 2× SSC at room temperature and with 0.1× SSC at 68°C for 1 hour. Blots were exposed to Kodak XAR-5 film (Eastman Kodak, Rochester, NY). The hybridization signals were quantified by densitometry of autoradiographs. Blots were then rehybridized with glyceride-3 phosphate dehydrogenase (GAPDH) probe, 775-bp Pst I/Xba I fragment from phcGAP (ATCC, Rockville, MD), as an internal standard. The levels of IL-15 mRNA were calculated by normalizing signal optical densities to those of GAPDH mRNA. Cord and adult Northern blot analyses were performed simultaneously under identical hybridization conditions and with the same amount of exposure time of the blot.
Immunoblot analysis. Plastic adherent monocytes (10 to 20 × 106 cells, >95% CD14+ monocytes) were obtained from MNC culture (5 × 106 cell/mL) after being incubated at 37°C for 1 hour according to the method of D'Andrea et al.36 Stimulated (LPS at 10 μg/mL for 12 hours) and adhered (without LPS) monocyte cultures were gently washed twice in phosphate-buffered saline (PBS). Whole cells were then lysed in PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA, 1 mmol/L phenylmethyl sulfonyl fluoride,37 and 20 μg/mL pepstatin A for 30 minutes on ice. Lysates were spun in a microfuge at 12,000 rpm for 15 minutes. Supernatant was collected and stored at −70°C for Western blot analysis. Lysates were thawed, and quantitation of total protein from each sample was performed in duplicate, twice before loading, using the BCA protein assay (Pierce, Rockford, IL). Equal amounts (20 μg) of protein were loaded per lane, and proteins were separated by 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were electrophoretically transferred to nitrocellulose membranes and blocked with 5% nonfat dry milk solubilized in PBS for 1 hour at room temperature. Blots were then incubated in 10 μg equivalent of goat polyclonal antibody (AB-247-NA; R & D Systems, Minneapolis, MN) directed against human IL-15 protein in 2% milk (vol/vol, solubilized in PBS) overnight at 4°C. Incubation with 0.2% (vol/vol) horseradish peroxidase-conjugated swine antigoat IgG (BioSource International, Camarillo, CA) for 1 hour at room temperature followed. IL-15 protein bands were visualized by development with luminol/peroxide chemiluminescent substrate (Pierce) for 1 minute and exposed to x-ray film for 30 seconds. Two-dimensional densitometry of film was performed using the Bio-Image Model 50S (Millipore, Bedford, MA) automated scanning system. Immunoblot analyses were performed with three different samples.
Nuclear run-on transcription assay. Cultures of three different samples were stimulated with LPS (10 μg/mL; Sigma) for 12 hours to obtain maximal induction of IL-15. Nuclei isolation and nuclear run-on assays were performed as previously described38 and involved modification procedures described by Weber et al39 and Groudine et al.40 Nuclear run-on assays were performed with cDNA (IL-15, 0.48 kb cDNA; GAPDH, 775-bp Pst I/Xba I fragment from phcGAP) as targets. The amount of target DNA per slot was 1 μg. The hybridization mixture contained 2.5 to 5 × 107 cpm/5 mL. Run-on signal strengths were determined by densitometry of autoradiographs. The density of the bands was calculated by normalizing values with respect to the signals of internal standards (GAPDH).
mRNA half-life. Cord and adult MNCs (60 × 106 cells) were stimulated with LPS (10 μg/mL) for 12 hours before exposure to the transcriptional inhibitor, actinomycin D (10 μg/mL), as described previously.17 Cells were harvested at intervals of 0, 60, 120, 240, and 360 minutes. Cytoplasmic RNA was extracted, poly (A)+ RNA was purified, and Northern blot analysis was performed as described above for the IL-15 mRNA expression. The amount of IL-15 mRNA was normalized to the amount of GAPDH mRNA in each sample and then expressed as a percentage, setting the amount of mRNA at time 0 equal to 100%. The data were plotted against the time after addition of actinomycin D, and the half-life of each transcript was calculated based on the resultant graphs.
Induction of cytokine production and NK and LAK cytotoxicity. Recombinant simian IL-15 (specific activity on CTLL proliferation, 3.33 × 105 U/μg) was kindly provided by Dr T. Troutt (Immunex), and the recombinant human IL-12 (specific activity on phytohemagglutinin blast proliferation, 5.26 × 106 U/μg) was kindly provided by Dr S. Wolf (Genetics Institute, Cambridge, MA). MNCs at 5 × 106 cells/mL were placed into a plastic petri dish for 1 hour of incubation at 37°C to remove monocytes. Monocyte-depleted MNCs (MD MNCs) were adjusted to 1 × 106 cells/mL, seeded in 24-well flat-bottom plates containing varying concentrations of cytokines, and incubated at 37°C in a 5% CO2 humidified incubator. NK activity was measured against K562 target cells (NK-sensitive, a human erythroleukemic cell line; ATCC) after 18 hours of stimulation, and LAK activity was measured against Daudi target cells (LAK-sensitive, a human Burkitt's lymphoma; ATCC) after 72 hours of stimulation. At the end of the incubation, the effector cells were harvested, washed, and resuspended in appropriate concentrations based on the ratios of effector to target cells (E:T ratio) for the cytotoxicity assays.
Cytotoxicity assay. A standard 3-hour 51Cr-release assay41 was performed to measure the cytotoxicity. Briefly, the target cells were labeled with 100 μCi of Na2CrO4 , washed twice, and resuspended in a concentration of 5 × 104 cells/mL. One hundred microliters of each target and effector cell suspension with E:T ratios (20:1, 10:1, and 5:1) was added to a V-bottom 96-well culture plate. The mixture was centrifuged briefly and incubated at 33°C for 3 hours. At the end of the incubation, 150 μL of cell-free supernatant was collected from each well. The radioactivity was measured in a Beckman LS 1800 Liquid Scintillation Counter (Beckman, Fullerton, CA). All of the samples were run in triplicate. The percentage of lysis was calculated at each E:T ratio using the formula ([Experimental − Spontaneous Release]/[Maximum − Spontaneous Release]) × 100% and then converted to lytic units (LU; 30% target cell killing in 107 effector cells) using a computer-assisted program.42 To determine the tumoricidal spectrum of CB nonspecific cytotoxicity, two acute lymphoblastic leukemia cell lines, CCRF-CEM (T cells; ATCC) and CCRF-SB (B cells; ATCC), and two neuroblastoma cell lines, NB-100 (ATCC) and SK-N-MC (ATCC), were used as target cells.
The study of effects of IL-15 and IL-12 in combination on CB NK and LAK activities. A method previously described by DeBlaker-Hohe et al43 was used to determine if IL-15 and IL-12 in combination induces a synergistic or additive NK and LAK activity from CB MNCs. To avoid a deviation of using controls twice while comparing the cytolytic response from the combination of two cytokines with the sum of that from each single cytokine, the method was modified by subtracting the control from each single data before being applied to the calculation. Each cytotoxicity result of 17 combinations from 5 doses of IL-15 (0.1, 0.5, 1.0, 5.0, and 10 ng/mL) and 4 doses of IL-12 (0.1, 0.5, 1.0, and 10 U/mL) was compared with the sum of that from the same dose of each single cytokine using the following formula: [(Cytotoxicity in Combination − Sum of Each Cytokine Alone)/(Sum of Each Cytokine Alone)] × 100%. Based on the calculated results, the synergistic effect was arbitrarily defined as the cytolytic response from the combination of two cytokines exceeding the sum of that of each single cytokine by more than 10%, additive as 0% to 10% and nonadditive as less than 0%.
TNF-α and IFN-γ enzyme-linked immunosorbent assay (ELISA). CB and APB MNCs at a concentration of 1 × 106 cells/mL were stimulated by IL-15 (50 ng/mL) for 24, 72, and 144 hours. The supernatant was collected and the protein level was measured by ELISA (Biosource) following the manufacturer's protocol. All of the samples were run in duplicate and data were presented as the mean ± SEM. The sensitivity of the assay was 7.8 pg/mL.
Statistical analysis. Results from cytotoxicity studies and ELISA were presented as the mean ± SEM of three or more samples. Student's t-test was used for determining significant differences between two groups, and Kruskal-Wallis nonparametric ANOVA test was used for comparing multiple groups with Bartlett's test as the posttest for determining specific significant subgroups (Instat Graph Pad Software, San Diego, CA). A P value <.05 was considered significant.
RESULTS
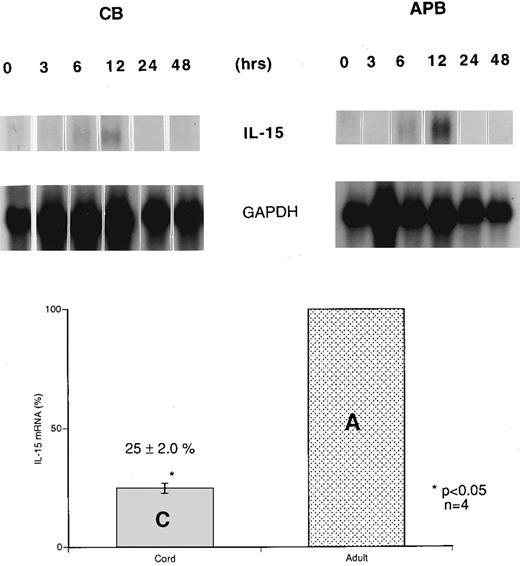
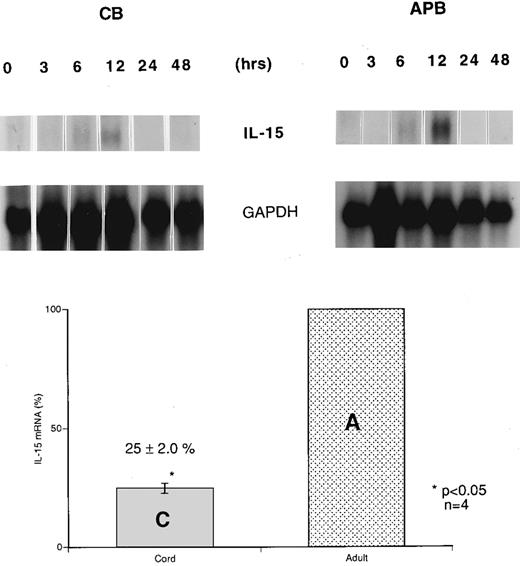
Reduced IL-15 mRNA expression in stimulated cord versus adult MNCs. Northern blot analyses of CB and APB MNCs were performed simultaneously under identical conditions to compare the IL-15 mRNA expression before and after LPS stimulation. Unstimulated MNCs from both CB and APB had an undetectable expression of IL-15 mRNA. In a time course study of IL-15 mRNA expression after LPS (10 μg/mL) stimulation, IL-15 mRNA expression was induced within 6 hours upon LPS stimulation and reached a peak level at 12 hours in both CB and APB MNCs, returning to basal level after 24 hours. However, IL-15 mRNA expression in CB MNCs was only 25% ± 2.0% (mean ± SEM, n = 4, P < .05) compared with the level in APB MNCs after 12 hours of stimulation with LPS (Fig 1).
Time course of induction of IL-15 mRNA expression in cord (CB) and adult (APB) MNCs. CB and APB MNCs were isolated, cultured in RPMI, and stimulated with 10 μg/mL LPS for 0, 3, 6, 12, 24, and 48 hours. Cells were harvested and poly (A)+ RNA samples were analyzed by Northern blot analysis of IL-15 mRNA (1.5 kb). Results shown are representative of three different CB and APB RNA blot hybridizations, normalized to GAPDH signal. The lower panel bar graph represents comparative IL-15 mRNA expression from LPS-stimulated (12 hours) CB and APB MNCs. Four different CB and APB samples were analyzed after 12 hours of LPS stimulation (10 μg/mL) for IL-15 mRNA expression by Northern blot analysis. The amount of IL-15 mRNA was expressed as a percentage, setting the amount of APB mRNA equal to 100% (APB v CB, 100% v 25% ± 2.0%, P < .05, n = 4).
Time course of induction of IL-15 mRNA expression in cord (CB) and adult (APB) MNCs. CB and APB MNCs were isolated, cultured in RPMI, and stimulated with 10 μg/mL LPS for 0, 3, 6, 12, 24, and 48 hours. Cells were harvested and poly (A)+ RNA samples were analyzed by Northern blot analysis of IL-15 mRNA (1.5 kb). Results shown are representative of three different CB and APB RNA blot hybridizations, normalized to GAPDH signal. The lower panel bar graph represents comparative IL-15 mRNA expression from LPS-stimulated (12 hours) CB and APB MNCs. Four different CB and APB samples were analyzed after 12 hours of LPS stimulation (10 μg/mL) for IL-15 mRNA expression by Northern blot analysis. The amount of IL-15 mRNA was expressed as a percentage, setting the amount of APB mRNA equal to 100% (APB v CB, 100% v 25% ± 2.0%, P < .05, n = 4).
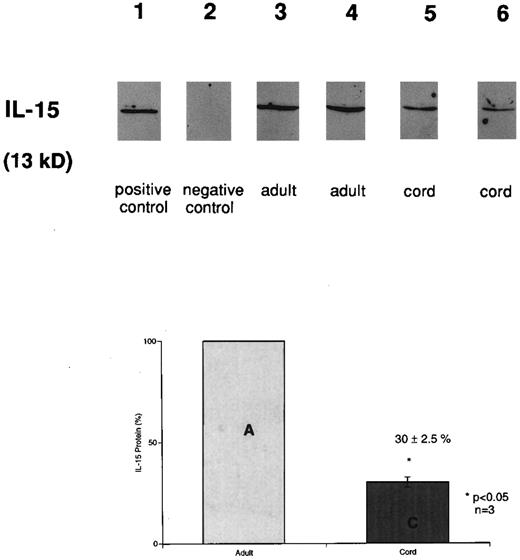
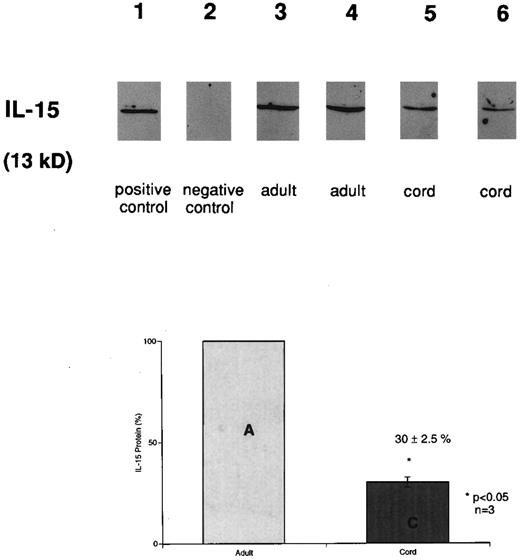
Decreased IL-15 protein accumulation in cord versus adult MNCs. IL-15 protein production from whole cell lysates was determined by Western immunoblot analysis with a goat polyclonal anti–IL-15 antibody (Fig 2). Equal amounts of protein (20 μg) were loaded per lane after being quantitated in duplicate twice. Bands with a molecular size of 13 kD were detected from stimulated cord and adult cells. IL-15 was not detected in adhered cells without LPS. IL-15 protein production in LPS-stimulated CB monocytes (lanes 5 and 6) was only 30% ± 2.5% (mean ± SEM) of the level in APB monocytes (lanes 3 and 4; n = 3, P < .05) after 12 hours of LPS stimulation.
Detection of IL-15 in stimulated cord (CB) and adult (APB) monocytes. Immunoblot analysis of LPS (12 hours) stimulated CB and APB monocytes using anti–huIL-15 goat antibody. Equal amounts of protein (20 μg) were loaded per lane. Before loading, quantitation of total protein from each sample was performed in duplicate, twice using BCA protein assay. Lane 1, rhIL-15; lane 2, adhered monocytes; lanes 3 and 4, LPS-stimulated adult monocytes; lanes 5 and 6, LPS-stimulated cord monocytes. The lower panel bar graph represents comparative IL-15 protein production from three different LPS-stimulated (12 hours) CB and APB monocytes. The amount of IL-15 protein was expressed as a percentage, setting the amount of APB level equal to 100% (APB v CB, 100% v 30% ± 2.5%, P < .05, n = 3).
Detection of IL-15 in stimulated cord (CB) and adult (APB) monocytes. Immunoblot analysis of LPS (12 hours) stimulated CB and APB monocytes using anti–huIL-15 goat antibody. Equal amounts of protein (20 μg) were loaded per lane. Before loading, quantitation of total protein from each sample was performed in duplicate, twice using BCA protein assay. Lane 1, rhIL-15; lane 2, adhered monocytes; lanes 3 and 4, LPS-stimulated adult monocytes; lanes 5 and 6, LPS-stimulated cord monocytes. The lower panel bar graph represents comparative IL-15 protein production from three different LPS-stimulated (12 hours) CB and APB monocytes. The amount of IL-15 protein was expressed as a percentage, setting the amount of APB level equal to 100% (APB v CB, 100% v 30% ± 2.5%, P < .05, n = 3).
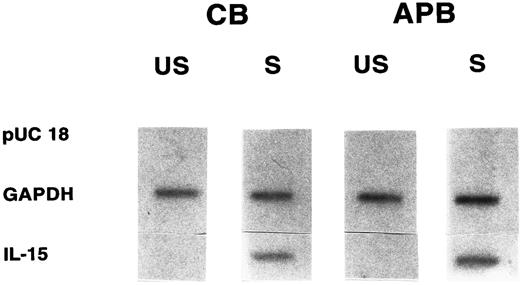
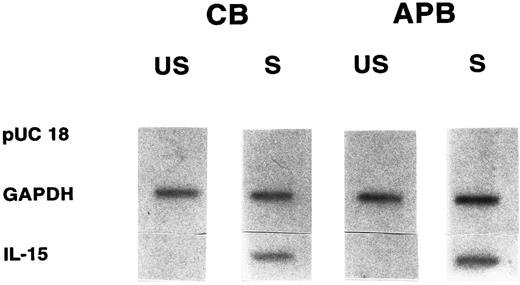
Comparable transcriptional rate of the IL-15 gene in cord versus adult MNCs. Nuclear run-on transcriptional analysis was performed to determine if the low amount of IL-15 mRNA in stimulated CB MNCs was due to a decreased transcription rate of the IL-15 gene. Nuclear run-on transcripts from nuclei isolated from CB MNCs and stimulated with LPS for 12 hours were compared with run-on transcripts isolated from similarly treated APB MNCs. As shown in Fig 3, unstimulated CB and APB MNCs showed low basal level signals of IL-15 transcript (optical density [OD], < 0.1), which was approximately the same in both cord and adult. After stimulation with LPS (12 hours), the transcriptional rate of the IL-15 gene was significantly increased in both CB and APB MNCs (cord, 20- ± 2.5-fold; adult, 22- ± 1.7-fold; n = 3, P < .05). However, there was no appreciable difference between activated CB and APB MNC in the degree of transcriptional activation (P = not significant [NS]).
Nuclear run-on analyses of IL-15 transcription in cord (CB) versus adult (APB) MNCs stimulated with LPS (10 μg/mL for 12 hours). Equivalent amounts of radioactive labeled RNA were hybridized to filters containing the indicated target DNA. Results shown are representative of three different experiments. US, unstimulated; S, stimulated; pUC 18, control vector; GAPDH, 775-bp Pst I/Xba I fragment from phc GAP; IL-15, 480-bp cDNA (CB 20- ± 2.5-fold v APB 22- ± 1.7-fold, P = NS, n = 3).
Nuclear run-on analyses of IL-15 transcription in cord (CB) versus adult (APB) MNCs stimulated with LPS (10 μg/mL for 12 hours). Equivalent amounts of radioactive labeled RNA were hybridized to filters containing the indicated target DNA. Results shown are representative of three different experiments. US, unstimulated; S, stimulated; pUC 18, control vector; GAPDH, 775-bp Pst I/Xba I fragment from phc GAP; IL-15, 480-bp cDNA (CB 20- ± 2.5-fold v APB 22- ± 1.7-fold, P = NS, n = 3).
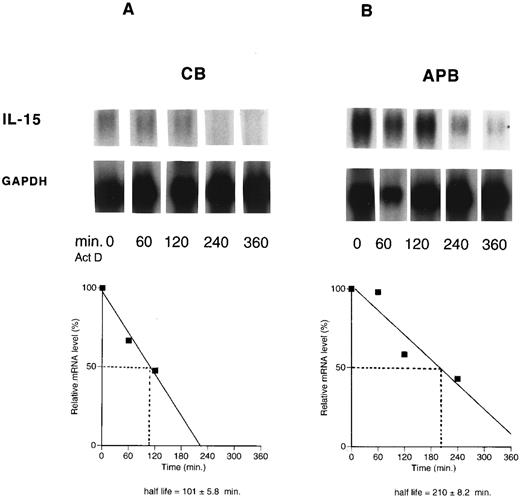
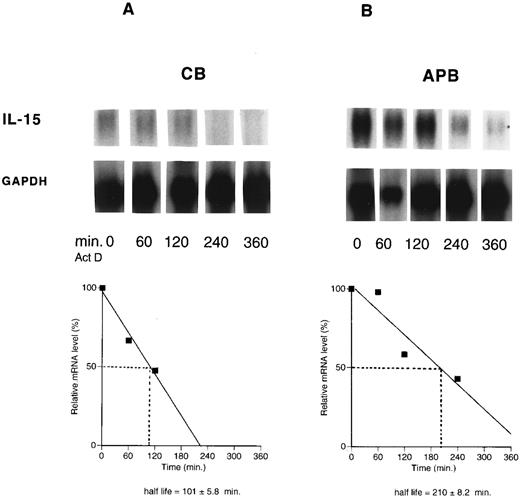
Decreased IL-15 mRNA half-life in cord versus adult. Because the transcriptional rate of the IL-15 gene was virtually the same for both CB and APB MNCs, the stability of IL-15 mRNA was compared in stimulated CB and APB MNCs by blocking mRNA synthesis with actinomycin D to determine whether the differential regulation was occurring at the posttranscriptional level. CB and APB MNCs were stimulated with LPS (10 μg/mL) for 12 hours before actinomycin D (10 μg/mL) was added for various time periods (0 to 360 minutes.). Northern blots of poly (A)+ RNA were hybridized with an antisense riboprobe made by transcription of human cDNA. The levels of IL-15 mRNA progressively decreased during actinomycin D exposure in both CB and APB MNCs. Transcripts were quantitated by densitometric scanning of the autoradiographs. As shown in Fig 4, the measured mRNA half-life of IL-15 from stimulated CB MNCs was approximately twofold lower than that from stimulated APB MNCs (t1/2 : 101 ± 5.8 minutes v 210 ± 8.2 minutes, CB v APB, mean ± SEM, n = 3, P < .05).
The half-life of IL-15 mRNA in cord (CB) (A) versus adult (APB) (B) MNCs. Actinomycin D (10 μg/mL) was added for the indicated times to cells from cord (A) and adult (B), stimulated with LPS (10 μg/mL) for 12 hours. Poly (A)+ RNA was analyzed by Northern blotting for the presence of IL-15 transcript (1.5 kb). The data were plotted against the time after the addition of actinomycin D. Results shown are representative of three different experiments (t1/2 : 101 ± 5.8 minutes v 210 ± 8.2 minutes, CB v APB, P < .05, n = 3).
The half-life of IL-15 mRNA in cord (CB) (A) versus adult (APB) (B) MNCs. Actinomycin D (10 μg/mL) was added for the indicated times to cells from cord (A) and adult (B), stimulated with LPS (10 μg/mL) for 12 hours. Poly (A)+ RNA was analyzed by Northern blotting for the presence of IL-15 transcript (1.5 kb). The data were plotted against the time after the addition of actinomycin D. Results shown are representative of three different experiments (t1/2 : 101 ± 5.8 minutes v 210 ± 8.2 minutes, CB v APB, P < .05, n = 3).
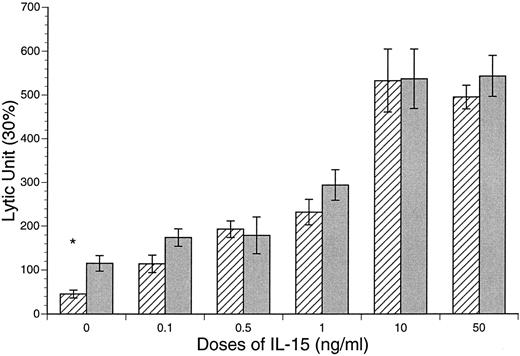
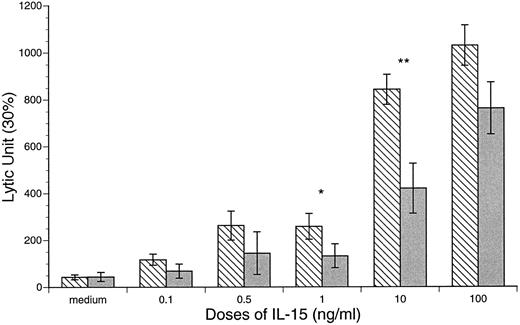
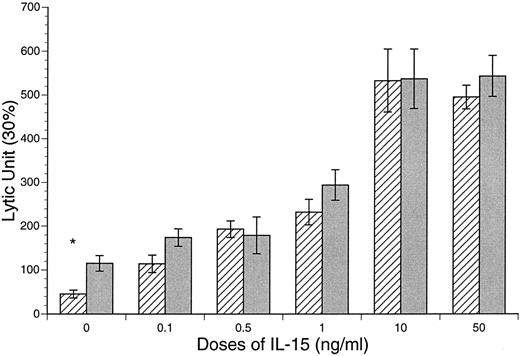
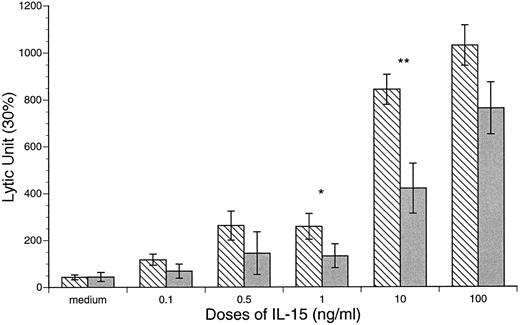
Enhanced CB and APB NK and LAK cytotoxic activities after IL-15 stimulation. The baseline NK and LAK cytotoxicities were determined after 18 and 72 hours of incubation in the absence of cytokines. The NK activity of CB against K562 was significantly lower than that of APB MD MNCs (CB v APB: 60 ± 9 v 115 ± 18 LU, P < .05, n = 10; Fig 5). However, the LAK activity of CB was similar to APB (CB v APB: LAK, 43 ± 10 v 44 ± 19 LU, P = NS, n = 10; Fig 6). After incubation of MD MNCs with IL-15 (10 ng/mL), both CB and APB NK activities (18 hours) were significantly increased over control (control v IL-15 at 10 ng/mL: CB, 45 ± 9 v 533 ± 72 LU, P < .01, n = 10; APB, 115 ± 18 v 537 ± 68 LU, P < .05, n = 6). CB NK activity reached a comparable level of APB (IL-15 at 10 ng/mL: CB v APB, 533 ± 72 v 539 ± 68 LU, P = NS). IL-15 (10 ng/mL) also induced a significant increase of both CB and APB LAK activities against Daudi target cells (control v IL-15 at 10 ng/mL: CB, 43 ± 10 v 843 ± 64 LU, P < .01, n = 12; APB, 44 ± 19 v 420 ± 107 LU, P < .01, n = 6; Fig 6). However, CB MD MNCs was more responsive to IL-15 stimulation than APB for induction of LAK activity (CB v APB: IL-15 at 1.0 ng/mL, 262 ± 62 v 144 ± 91, P < .05; IL-15 at 10 ng/mL 843 ± 64 v 420 ± 107 LU, P < .01; Fig 6).
IL-15 induction of CB and APB MNC NK cytotoxicity against K562 line. *CB v APB, 45 ± 9 v 115 ± 18 LU, P < .05, n = 10; (▨) CB; () APB.
IL-15 induction of CB and APB MNC NK cytotoxicity against K562 line. *CB v APB, 45 ± 9 v 115 ± 18 LU, P < .05, n = 10; (▨) CB; () APB.
IL-15 induction of CB and APB MNC LAK activity against Daudi cell line. CB v APB: *IL-15 at 1 ng/mL, 258 ± 55 v 132 ± 51, P < .05; **IL-15 at 10 ng/mL, 843 ± 64 v 420 ± 107 LU, P < .01. (▧) CB; () APB.
IL-15 induction of CB and APB MNC LAK activity against Daudi cell line. CB v APB: *IL-15 at 1 ng/mL, 258 ± 55 v 132 ± 51, P < .05; **IL-15 at 10 ng/mL, 843 ± 64 v 420 ± 107 LU, P < .01. (▧) CB; () APB.
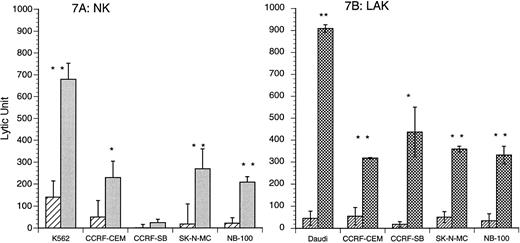
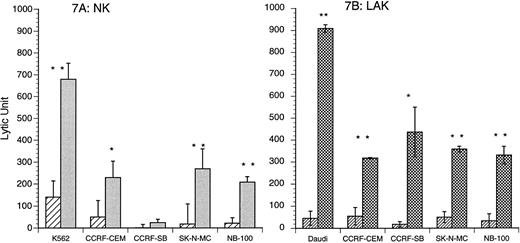
IL-15 induced nonspecific tumoricidal NK and LAK activities. Four cell lines, CCRF-CEM, CCRF-SB, NB-100, and SK-N-MC, were used to examine in vitro antitumor activity of CB NK and LAK cells after IL-15 stimulation. IL-15 (10 ng/mL) stimulation resulted in enhanced CB cytotoxicity against all of these tumor cell lines. As shown in Fig 7A, three cell lines, CCRF-CEM, SK-N-MC, and NB-100, became more sensitive to CB NK cytotoxicity induced by IL-15 (control v IL-15 stimulated: CCRF-CEM, 50 ± 34 v 229 ± 34 LU, P < .05; SK-N-MC, 18 ± 17 v 269 ± 91 LU, P < .01; NB-100, 22 ± 4 v 208 ± 25 LU, P < .01, n = 4). IL-15–induced CB LAK cytotoxicity produced an enhanced lytic response against all four tumor cell lines over controls (control v IL-15 stimulated: CCRF-CEM, 55 ± 39 v 318 ± 3 LU, P < .01; CCRF-SB, 18 ± 12 v 436 ± 114 LU, P < .05; SK-N-MC, 50 ± 25 v 358 ± 13 LU, P < .01; NB-100, 33 ± 31 v 330 ± 40 LU, P < .01, n = 3; Fig 7B).
(A and B) IL-15 (10 ng/mL) induction of CB NK and LAK cytotoxicity against several tumor cell lines. Acute lymphoblastic leukemia cell lines: CCRF-CEM (T-lymphoblastoid cells) and CCRF-SB (B-lymphoblastoid cells); neuroblastoma cell lines: SK-N-MC and NB-100. (A) The tumoricidal spectrum of CB NK activity: IL-15 (10 ng/mL) v unstimulated: CCRF-CEM*, 229 ± 75 v 50 ± 34 LU; SK-N-MC**, 269 ± 91 v 18 ± 17 LU; NB-100**, 208 ± 25 v 22 ± 4 LU; n = 4. (▨) Medium; () IL-15 (10 ng/mL). (B) The tumoricidal spectrum of CB LAK activity: IL-15 (10 ng/mL) v unstimulated: CCRF-CEM**, 318 ± 3 v 55 ± 39 LU; CCRF-SB*, 436 ± 114 v 18 ± 12 LU; SK-N-MC**, 358 ± 13 v 50 ± 25 LU; NB-100**, 330 ± 40 v 33 ± 31 LU; n = 3. (▨) Medium; (▩) IL-15 (10 ng/mL). *P < .05; **P < .01.
(A and B) IL-15 (10 ng/mL) induction of CB NK and LAK cytotoxicity against several tumor cell lines. Acute lymphoblastic leukemia cell lines: CCRF-CEM (T-lymphoblastoid cells) and CCRF-SB (B-lymphoblastoid cells); neuroblastoma cell lines: SK-N-MC and NB-100. (A) The tumoricidal spectrum of CB NK activity: IL-15 (10 ng/mL) v unstimulated: CCRF-CEM*, 229 ± 75 v 50 ± 34 LU; SK-N-MC**, 269 ± 91 v 18 ± 17 LU; NB-100**, 208 ± 25 v 22 ± 4 LU; n = 4. (▨) Medium; () IL-15 (10 ng/mL). (B) The tumoricidal spectrum of CB LAK activity: IL-15 (10 ng/mL) v unstimulated: CCRF-CEM**, 318 ± 3 v 55 ± 39 LU; CCRF-SB*, 436 ± 114 v 18 ± 12 LU; SK-N-MC**, 358 ± 13 v 50 ± 25 LU; NB-100**, 330 ± 40 v 33 ± 31 LU; n = 3. (▨) Medium; (▩) IL-15 (10 ng/mL). *P < .05; **P < .01.
Additive and synergistic effects of low doses of IL-15 and IL-12 on CB NK and LAK cytotoxicity. The effects of the combination of IL-15 and IL-12 on NK and LAK cytotoxicity was examined by comparing the cytolytic response from two cytokines in combination with the sum of that from each single cytokine. The results in Table 1 show that using lower doses of IL-15 (0.1, 0.5, and 1.0 ng/mL) and IL-12 (0.1, 0.5, and 1.0 U/mL) concomitantly generated either synergistic or additive effects on CB NK cytotoxicity against K562 targets. The synergy from these lower dose combinations induced a comparable NK cytotoxicity compared with single higher doses of each cytokine individually. Specifically, IL-15 (1.0 ng/mL) and IL-12 (0.5 U/mL) induced a lytic response comparable to that induced by 10 ng/mL of IL-15 alone or 10 U/mL of IL-12 alone (532 ± 64 v 473 ± 70 LU, P = NS, and v 410 ± 48 LU, P = NS). Conversely, higher dose combinations of IL-12 and IL-15 induced only nonadditive effects on CB NK cytotoxicity. Table 2 summarizes the combined effects of IL-15 and IL-12 on CB LAK cytotoxicity against Daudi targets. The synergistic effect of IL-12 and IL-15 is seen at two combinations of lower doses of IL-15 and IL-12 (0.1 or 0.5 ng/mL of IL-15 + 0.1 U/mL of IL-12) and the lytic response from the latter combination is comparable to that of a single higher dose of IL-12 (10 U/mL), but much lower than that of IL-15 (10 ng/mL) (IL-15 + IL-12 v IL-12 alone v IL-15 alone: 512 ± 48 LU v 501 ± 54 LU, P = NS; and v 823 ± 68 LU, P < .01). The higher dose combinations of IL-15 and IL-12 induced a suppressive effect on LAK cytotoxicity when compared with the single higher dose of IL-12 and IL-15 (10 ng/mL IL-15 + 10 U/mL IL-12 v 10 ng/mL IL-15 alone v 10 U/mL IL-12 alone, 259 ± 77 v 823 ± 68 LU, P < .01; 259 ± 77 v 501 ± 54, P < .05, n = 10).
IL-15 enhancement of IFN-γ and TNF-α production from CB MNCs. IL-15 (50 ng/mL) stimulation induced a significant increase of IFN-γ protein production in CB MNCs (IL-15 stimulated v control: day 1, 112 ± 13 v 3.3 ± 1.2 pg/mL, P < .01; day 3, 480 ± 75 v 8 ± 4 pg/mL, P < .01; day 6, 670 ± 20 v 135 ± 52 pg/mL, P < .01, n = 3) However, the IFN-γ levels in IL-15–stimulated CB were still significantly lower than stimulated APB (IL-15–stimulated CB v IL-15–stimulated APB: day 1, 112 ± 13 v 567 ± 44 pg/mL, P < .01; day 3, 480 ± 75 v 866 ± 30 pg/mL, P < .05; day 6, 670 ± 20 v 830 ± 20 pg/mL, P < .05, n = 3; Fig 8A). Similarly, IL-15 (50 ng/mL) stimulation also significantly increased TNF-α protein production in CB MNCs (IL-15 stimulated v control: day 1, 308 ± 81 v 50 ± 26 pg/mL, P < .01; day 3, 423 ± 76 v 70 ± 30 pg/mL, P < .01; day 6, 358 ± 44 v 128 ± 51 pg/mL, P < .05, n = 3). The TNF-α production in CB MNCs was still significantly lower than APB (IL-15–stimulated CB v stimulated APB: day 1, 308 ± 46 v 676 ± 88 pg/mL, P < .01; day 3, 423 ± 76 v 983 ± 179 pg/mL, P < .05; day 6, 358 ± 44 v 1,125 ± 108, P < .01, n = 3; Fig 8B).
(A and B) IL-15 (50 ng/mL) induction of IFN-γ and TNF-α production in CB MNCs. (A) Time course of IFN-γ production in CB and APB MNCs; (▨) CB-control, (▨) CB–IL-15, (▧) APB-control, (▧) APB–IL-15. (B) Time course of TNF-α production in CB and APB MNCs; (▩) CB-control, () CB–IL-15, ()]) APB-control, ()]) APB–IL-15.
(A and B) IL-15 (50 ng/mL) induction of IFN-γ and TNF-α production in CB MNCs. (A) Time course of IFN-γ production in CB and APB MNCs; (▨) CB-control, (▨) CB–IL-15, (▧) APB-control, (▧) APB–IL-15. (B) Time course of TNF-α production in CB and APB MNCs; (▩) CB-control, () CB–IL-15, ()]) APB-control, ()]) APB–IL-15.
DISCUSSION
Numerous studies have demonstrated the success of hematopoietic growth factors to enhance hematologic reconstitution after stem cell transplantation.44-51 Vowels et al52 reported that the use of GM-CSF post-CB transplantation resulted in rapid engraftment and mild graft-versus-host disease. However, the delay in immune reconstitution post-CB transplantation may be due to the defects in CB cellular immunity and cytokine production.2-4 We have recently reported that the reduced expression and production of IL-12 from activated CB may contribute to the immaturity in CB cellular immunity.18 The number and functionality of donor-derived lymphocytes in patients after CB transplantation remains to be determined. The use of exogenous cytokines post-CB transplantation that enhance CB immune function may compensate for the immaturity of CB cellular immunity and enhance immune reconstitution and CB tumor immunity post-CB transplantation.
Although there are many similar biologic activities between IL-2 and IL-15, the regulation of IL-15 expression differs markedly from that of IL-2. IL-15 mRNA is expressed in a variety of tissues, including placenta, skeletal muscle, kidney, and activated monocytes/macrophages but not normal T cells.22 Enhancement of IL-15 protein production from monocytes occurs in response to a wide variety of agonists including LPS, IFN-γ, Bacillus Calmette-Guerlin (BCG), Mycobacterium tuberculosis, Toxoplasma gondii, or Salmonella cholersesuis.53-55 During states of increased demand (stimulation), mononuclear phagocytes contribute significantly to the production of IL-15 in both CB and APB. However, our present results suggest that CB MNCs express and produce less IL-15 in response to bacterial stimulation (Figs 1 and 2). The decreased IL-15 mRNA expression in CB versus APB MNCs is not secondary to alteration in IL-15 gene transcription (Fig 3). In comparison, alterations in posttranscriptional stability appear to account, in part, for the decrease in IL-15 mRNA expression in CB versus APB MNCs (Fig 4).
Control of mRNA stability is not well understood, but the process is thought to involve various factors interacting with specific mRNA sequences.56,57 The adenosine + uridine (AU)-rich or AUUUA-repeat elements (ARE) in the 3′ untranslated regions (UTR) of many cytokine and protooncogene transcripts are known to be the targets of a pathway for selective processing and mRNA degradation.58-62 Several AUUUA repeats of the 3′ UTR of IL-15 sequence may contribute to the instability of IL-15 mRNA.
We previously reported the complex posttranscriptional regulatory mechanisms associated with several cytokines. The reduced accumulation of M-CSF, GM-CSF, and IL-12 mRNA in CB MNCs is associated with a reduction in mRNA half-life compared with APB MNCs, whereas the rate of gene transcription remains comparable in CB and APB MNCs.15,17,18 The 3′ UTR of these cytokines have multiple copies of AUUUA-elements.63,64 A reduced mRNA half-life and comparable transcription rates for GM-CSF, M-CSF, and IL-12 in CB versus APB MNCs indicate that these ARE-containing transcripts may also be less stable in CB MNCs. Translational inhibition by cycloheximide after stimulation of cells causes a superinduction of GM-CSF, as well as M-CSF mRNA, which is approximately 2.5-fold greater in CB versus APB MNCs.17,65 Increased transcript stabilization in stimulated CB MNCs after CHX treatment suggests that, before translational inhibition, higher levels of a translational-dependent nuclease may be present in CB MNCs. An ARE-directed endonuclease66 and several ARE-binding factors61,62,67-71 have been identified. One of these is a 37-kD protein designated AUF1. We have recently reported that the decreased GM-CSF mRNA stability in CB versus APB MNCs was inversely correlated with AUUUA-element binding activity and with the levels of AUF1 binding factor.65 It seems likely that various protein factors interacting with specific mRNA sequences exist in vivo and are involved in the regulation of AU-rich mRNA decay. Any alteration in the expression and/or biologic activities of these various protein factors in stimulated CB MNCs could contribute to the reduction of IL-15 mRNA expression. Further studies are required to test these possibilities.
The immaturity of CB immunity, which is associated with decreased production of IL-2, IL-12, IL-15, IFN-γ, and TNF-α, may contribute to diminished CB NK, LAK, and CTL cytotoxicities. Seki et al7 and others have reported that NK cytotoxicity is decreased in CB compared with APB. IL-2 can enhance the NK cytotoxicity of CB MNCs to the level of APB MNC activity.11 We have recently18 reported that IL-12 can enhance CB NK cytotoxicity up to levels of APB MNC activity. IL-15 not only enhances T-cell function, but also enhances cytolytic function of both CD56dim NK and CD8+ T cells.22,28,32 Carson et al28 reported that activation of CD56dim NK cells by IL-15 was similar to that of IL-2. However, the IL-15–enhanced NK cytotoxic activity is completely IL-2 independent. Our present studies suggested that IL-15 also enhanced CB NK and LAK activities up to the adult level. CB LAK activity appeared to be more sensitive to exogenous IL-15 compared with APB (Fig 5). Tumoricidal studies showed that IL-15 induced significant CB NK and LAK activities against a broad range of neuroblastoma, leukemia, and lymphoma cell lines (Fig 7).
Although IL-2 has been shown to have therapeutic benefits for some cancer patients,72 the substantial toxicities associated with high doses of IL-2 have limited its use clinically.73 IL-12 has also experienced dose-limiting toxicities.74 Recently, IL-15 has been shown to mimic the antitumor activities of IL-2 with potentially less toxicity in an in vivo animal model.33 Further studies are required to evaluate this aspect. Combinations of lower doses of IL-15 and IL-12 might have the potential of augmenting in vivo antitumor immune function and minimizing toxicity. DeBlaker-Hoke et al43 showed a synergistic effect on inducing APB NK and LAK activities by the combination of lower doses of IL-2 and IL-12. Carson et al28 suggested that the combination of IL-12 and IL-15 had a synergistic effect on augmenting APB NK cytolytic activity and IFN-γ production. In the present study, we demonstrated that low-dose combinations of IL-12 (0.1 U/mL) and IL-15 (0.1 to 1.0 ng/mL) induced a synergistic or additive effect on CB NK cytotoxicity, except for the combination of IL-12 (1.0 U/mL) and IL-15 (1.0 ng/mL). The synergistic NK activity reached the same levels as a single high dose (IL-12, 10 U/mL; IL-15, 10 ng/mL) of either individual cytokine. Although no synergistic or additive effects from high-dose combinations of IL-12 (5 to 10 U/mL) and IL-15 (5 to 10 ng/mL) on CB NK activity are seen, the cytotoxicity levels were still higher than that induced by the single dose of individual cytokine (Table 1). Similarly, low-dose combinations of IL-12 (0.1 U/mL) and IL-15 (0.1 to 0.5 ng/mL) had a synergistic effect on CB LAK activity that is comparable to the level as a single high dose of IL-12 (10 U/mL), but lower than a single high dose of IL-15 (10 to 100 ng/mL). However, the high-dose combination of IL-12 (1.0 to 10 U/mL) and IL-15 (10 to 100 ng/mL) had a suppressive effect on CB LAK activity (Table 2). This suppression may be due to NK cell apoptosis in which decreased numbers of killer cells resulted in a low level of LAK activity. This observation is consistent with the report by Ross and Caligiuri75 in which costimulation of IL-12 and IL-15 or IL-12 and IL-2 induced NK cell proliferation and IFN-γ production initially, followed by NK cell apoptosis and a decline in IFN-γ production.
The IFN-γ and TNF-α production in IL-15–stimulated CB MNCs was significantly induced and increased up to the unstimulated APB level, but far lower than the IL-15 stimulated APB level (Fig 8). This result is consistent with our earlier observation on IFN-γ production in IL-12–stimulated CB and APB MNCs.18 This partial compensation after IL-15 stimulation suggests that the decreased production of IFN-γ and TNF-α by CB MNCs may be due to at least two factors: defective CB IFN-γ and TNF-α production and defective CB IFN-γ– and TNF-α–inducing cytokines, such as IL-15, IL-12, and IL-2. Interestingly, the effect of IL-15 on CB NK activity and IFN-γ production showed that IL-15 alone is capable of inducing CB NK activity up to the APB level; however, IL-15 alone cannot compensate for IFN-γ production up to the stimulated APB level (Figs 5 and 8). This disparity indicates the important role of intrinsically deficient cytokine production such as IL-12, IL-15, and IFN-γ in the pathogenes of the immaturity of CB cellular immunity. Further studies are required to verify these observations.
In summary, the present study showed that IL-15 mRNA and protein production is decreased in activated CB compared with APB MNCs. This discrepancy in IL-15 production is secondary, at least in part, to altered posttranscriptional regulation. The reduced production of IL-15 from activated CB MNCs might contribute to the immaturity in CB cellular immunity. However, exogenous IL-15 stimulation may compensate for the immaturity in CB immunity by enhancing NK and LAK activities and by inducing IFN-γ and TNF-α production. The additional synergistic effects of lower doses of IL-15 in combination with IL-12 suggests the potential benefit of the combination of each cytokine to increase CB antitumor immunity and potentially decrease toxicity compared with higher doses of either of the cytokines alone. Further studies are needed to define the clinical implications of these findings and the potential use of IL-15 to enhance CB cellular immunity and/or accelerate immune reconstitution after CB transplantation.
ACKNOWLEDGMENT
The authors thank Sally Anderson, Renee Dulak, and Linda Rahl for their editorial assistance in the preparation of this manuscript.
J.X.Q., S.m.L., and Y.S. contributed equally to the manuscript.
Supported by grants from the Pediatric Cancer Research Foundation and the Walden W. and Jean Young Shaw Foundation.
Presented in part at the American Society of Hematology, December 1996, Orlando, FL.
Address reprint requests to Mitchell S. Cairo, MD, Director, Hematology/Oncology Research and Blood and Marrow Transplantation, Children's Hospital of Orange County, 455 S Main St, Orange, CA 92868.

![Fig. 8. (A and B) IL-15 (50 ng/mL) induction of IFN-γ and TNF-α production in CB MNCs. (A) Time course of IFN-γ production in CB and APB MNCs; (▨) CB-control, (▨) CB–IL-15, (▧) APB-control, (▧) APB–IL-15. (B) Time course of TNF-α production in CB and APB MNCs; (▩) CB-control, () CB–IL-15, ()]) APB-control, ()]) APB–IL-15.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3106/4/m_bl_0021f8.jpeg?Expires=1769344312&Signature=b6j53nTQrlXV37sqp0Mgv-D102e5C8CA5h8lvTkPkE3fE6OvJlN3e6zRx09dNmz3IWi8KZkhovYrBkygm7yW9laStz3vyCxHJlrBI5ig~xNh0c7nK08HmuDKjlIbCKzhOweEkspaLe~JCFvv5M9Icak~bl04kfB~Ge0gj7022hEzE-mYbZ3-pdzYjEOLy1OSLMywipXP6oyeMIIaGIlvR-Ximzqae7pOXnJJXL170mosaAIT-jvZ76Az0AsEkmuUXuOhV0X23ahIUxK6TLBqRmvUCwOk6woLzVACY2ITFXV97rFpP91i1SSBf1jxiogDwDzIw6pYLikpkg7c2uVyVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 8. (A and B) IL-15 (50 ng/mL) induction of IFN-γ and TNF-α production in CB MNCs. (A) Time course of IFN-γ production in CB and APB MNCs; (▨) CB-control, (▨) CB–IL-15, (▧) APB-control, (▧) APB–IL-15. (B) Time course of TNF-α production in CB and APB MNCs; (▩) CB-control, () CB–IL-15, ()]) APB-control, ()]) APB–IL-15.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3106/4/m_bl_0021f8.jpeg?Expires=1769430503&Signature=Znqr654JPqtL65ajvjhoy9nR3oy12gl7Ey17AJwweTaaKnzqPvNwEMNgzB4N9l1wzyMaPd~JGpkt8hfU7dVPw1s-3rJl38GEPKtIWyiA86zcpNR1kt3vAl6rkV2xWpYe9N6Rz~d9kY-5ywAyxAJAbSLAzVLleSOaFxy9KpDxJTnQWW0pm-4D4pkcgqD7l49yddJcPZT~nl0ITIGRSpBJSZ8xn40S64cJ3ToPJ8cOlf5nGZQsZN7V5LibNAcy~T2iD-69GPvGhfR4~3RpEoMfj8XlvVwbAR7~OEZRUrtlpurFGDK-nhC6lQhJjdNgWz1CzC8~NPB~wj1ScGpRuTAuKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)