Abstract
Activated interleukin-2 (IL-2)–dependent T cells express high levels of Bcl-2 protein. On cytokine withdrawal, Bcl-2 expression decreases and the cells die rapidly by apoptosis. We have previously shown that the survival of IL-2–deprived T cells can be promoted by factor(s) secreted by fibroblasts. Here we report that reduced glutathione (GSH), but not its oxidized counterpart GSSG, also enhances the in vitro survival of these cells. Exogenous GSH mediates its effect intracellularly, as (1) endogenous glutathione concentrations are increased up to fivefold in the presence of GSH, and (2) acivicin, an inhibitor of transmembrane GSH transport, abrogates GSH-dependent survival. The GSH-rescued T cells do not proliferate and express only low levels of Bcl-2, resembling WI38 fibroblast-rescued T cells. We, therefore, investigated a role for GSH in fibroblast-promoted T-cell survival. We show that WI38-promoted survival results in elevated GSH levels in surviving T cells and is abrogated by buthionine sulfoximine (BSO), an inhibitor of GSH synthesis. Furthermore, both WI38-promoted T-cell survival and GSH upregulation are associated with large molecular weight molecules (<30 kD). Thus, the upregulation of GSH by WI38 fibroblasts appears to be crucial in their ability to enhance the survival of cytokine-deprived activated T cells in vitro.
THE LIFE AND DEATH of T lymphocytes in vivo are precisely regulated. Many T cells die in the thymus at the early stages of their development, leading to the elimination of autoreactive and nonfunctional T cells.1,2 Similarly, the majority of activated T cells generated during an immune response die upon resolution of the disease, thus re-establishing overall T cell numbers.3-5 In both cases, cell death occurs by apoptosis, a common physiological process for the removal of unwanted cells,6,7 which is recognizable by a variety of characteristic morphological changes.8,9 T-cell apoptosis may result passively from the absence of appropriate survival signals (death by neglect) or actively by the ligation of certain cell surface receptors including Fas/Apo-1 (CD95) and the tumor necrosis factor receptor (TNF-R; activation-induced death).10 11
The removal of the cytokine interleukin-2 (IL-2) from activated T cells in vitro leads to apoptosis associated with the downregulation of Bcl-2.12 We have previously shown that the survival of these activated, but cytokine-deprived, T cells can be enhanced by two distinct sets of soluble factors in vitro. The first induces the reexpression of Bcl-2 and subsequent cell proliferation and includes the cytokines IL-2, IL-4, IL-7, and IL-15, each of which signals via the common γ-chain of the IL-2 receptor.13 The second involves a factor (or factors) secreted by a variety of fibroblast, epithelial, and endothelial cell lines.12,14,15 Although fibroblast-derived factor(s) effectively promote the survival of activated T cells, neither Bcl-2 upregulation nor cell proliferation are induced.15 Therefore, unlike T-cell survival promoted by the common γ-chain cytokines, fibroblast-promoted T-cell survival does not appear to depend on enhanced Bcl-2 expression.
Recent reports suggest that Bcl-2 may function in an antioxidant pathway,16,17 and several studies have shown that the overexpression of Bcl-2 in various cell types can, at least partially, inhibit lipid peroxidation.16-19 In T cells, many studies have demonstrated the importance of the intracellular redox status in functions such as activation,20,21 proliferation,22-25 and differentiation.26 The cellular redox status is determined by the production and removal of reactive oxygen species (ROS), the obligatory by-products of aerobic metabolism which, when present in excess, damage cellular macromolecules including DNA, protein, and lipids. To combat oxidative damage, cells have evolved a variety of antioxidant defense systems.27-29 Of particular interest is the small molecule antioxidant glutathione (GSH; γ-glutamylcysteinylglycine), the most prevalent intracellular thiol ubiquitous to all cell types30 that is pivotal in protecting cells from lipid peroxidation.31 GSH has been shown to influence a variety of T-cell functions including the binding, internalization, and degradation of IL-2,32 activation-induced death,33 and protection against γ-irradiation.34 GSH is, therefore, critical for the maintenance of proper T-cell function. Because fibroblast-promoted T-cell survival occurs in the presence of low Bcl-2,12 we investigated the possibility that intracellular GSH may substitute for Bcl-2 in the fibroblast-enhanced survival of activated human T cells in vitro. Here we report that exogenous GSH enhances the survival of both resting and activated human T cells in vitro and does so by alteration of intracellular GSH levels. Furthermore, we demonstrate a role for intracellular GSH in the fibroblast-promoted survival of activated T cells, an observation that may explain the survival of T cells expressing low levels of Bcl-2 in some tissues in vivo.35 36
MATERIALS AND METHODS
Chemicals and cytokines.GSH exists in two forms, reduced (GSH) and oxidized (GSSG), and the amino acid precursor of GSH biosynthesis, L-cysteine, similarly has an oxidized derivative (L-cystine). Acivicin irreversibly inhibits γ-glutamyl transpeptidase, a membrane-bound enzyme responsible for the transport of GSH across the cell membrane.37 Buthionine sulfoximine (BSO) and diethyl maleate (DEM) both deplete (and therefore inhibit) intracellular GSH, BSO by blocking GSH synthesis and DEM by directly binding to GSH.38 39 All chemicals were purchased from Sigma (Dorset, UK). Recombinant human IL-2 (2 × 106 IU/mg) was purchased from Pepro Tech EC Ltd (London, UK) and used at a pretitrated optimal concentration (5 ng/mL).
Preparation and culture of activated T cells.Cell culture was performed in RPMI-1640 supplemented with L-glutamine, benzyl-penicillin, streptomycin (GIBCO Ltd, Paisley, UK) and 10% fetal calf serum (FCS; M.B. Meldrum Ltd, Bourne End, Bucks, UK). Heparinized peripheral blood mononuclear cells (PBMC) were isolated from healthy volunteers and IL-2–dependent activated T cells generated as previously described.12 Briefly, Ficoll-Hypaque (Nycomed, Oslo, Norway) separated PBMC were activated with 1 μg/mL phytohemagglutinin (PHA; Wellcome Ltd, High Wycombe, Bucks, UK) for 3 to 6 days. The cells were then washed and maintained in the presence of IL-2 for at least 10 days before use. Activated T cells prepared in this way were generally greater than 90% CD3+. For experimental purposes, the cells were washed to remove IL-2 and immediately transferred to fresh medium containing relevant supplements. All experiments were performed in the absence of serum unless otherwise indicated.
Antibodies and cell staining.For flow cytometric studies of intracellular Bcl-2, T-cell lines were permeabilized using Permeafix (Ortho Diagnostic Systems Ltd, High Wycombe, Bucks, UK) before immunophenotyping. Bcl-2 expression was measured using a fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (Dako Ltd, High Wycombe, Bucks, UK). FITC-conjugated mouse-IgG1 (Dako Ltd) was used as a negative control for staining. Data were acquired on a FACScan (Becton Dickinson Ltd, Oxford, UK) using forward and side scatter profiles to exclude dead cells and debris. Membrane CD3 expression was determined directly using an FITC-conjugated monoclonal antibody (OKT3; ATTC, Rockville, MD).
Generation and fractionation of fibroblast-derived supernatant.The human embryonic lung fibroblast cell line, WI38, was a gift from P. Beverley (ICRF, London, UK). The cells were maintained in RPMI-1640 medium supplemented with L-glutamine, antibiotics and 10% FCS (as before). WI38-conditioned medium was generated in the absence of serum by incubation of just-confluent cells for 4 to 5 days with RPMI alone. The conditioned medium was harvested and clarified by centrifugation and filtration through 0.2-μm sterile Acrodisc filters (Gelman Sciences, Ann Arbor, MI) and was stored at −20°C for up to 1 month.
WI38-derived supernatant was crudely fractionated using Centriplus-30 centrifugal concentrators (Amicon Ltd, Gloucestershire, UK). This yielded fractions containing molecules of greater and less than 30 kD. The fractions were made up to their original volume with RPMI-1640 before testing for their ability to promote T-cell survival.
Assay for T-cell survival.The number of viable T cells remaining after incubation under various conditions was used as a measure of cell survival. Viable cell numbers were counted using a Cytoron absolute flow cytometer (Ortho Diagnostic Systems Ltd). Cells were fixed in 1% paraformaldehyde and the absolute number of viable cells determined within the lymphoid gate, using the forward and 90° side scatter profiles to exclude dead cells and debris. Survival was expressed as a percentage of the original cell input.
Assay for total GSH.The assay used to determine the intracellular total GSH content of the cells was modified from Schick et al.40 GSH accounts for most intracellular nonprotein thiols and can be sequentially oxidized by 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) and reduced by nicotinamide adenine dinucleotide phosphate (NADPH) in the presence of GSH reductase. The cells (2 to 4 × 106) were washed three times in phosphate-buffered saline (PBS) to remove exogenous GSH, followed by the addition of 10 μL per 1 × 106 cells solution A (5% trichloroacetic acid [TCA]/0.1 N HCl/10 mmol/L EDTA). Before lysis, the level of extracellular GSH was negligible. After vortexing for 1 minute, precipitated protein and any residual membrane-bound GSH was removed by centrifugation at 10,000 rpm for 1 minute. The TCA supernatants, containing acid soluble thiols, were then transferred to 96-well plates and solution A added to a final volume of 50 μL per well. Alternatively, the TCA supernatants were stored at −20°C. To each well was added 140 μL 0.3 mmol/L NADPH and 100 μL 1 U/mL GSH reductase, both in stock buffer (125 mmol/L sodium phosphate pH 7.5/ 6.3 mmol/L EDTA). Lastly, 20 μL 6 mmol/L DTNB (in stock buffer) was added per well. The rate of change in absorbance at 405 nm was recorded over a 10-minute time period, and the total GSH (ie, reduced and oxidized) content of the cells estimated by comparison to a GSH standard. Total cell numbers (viable and nonviable) were determined using the Cytoron absolute, as above, and results expressed as nmol of total GSH per 1 × 106 cells. Although the method does not distinguish between the reduced and oxidized forms of GSH, GSH is present in most cells at approximately 500-fold greater concentrations than GSSG. All reagents were purchased from Sigma.
Proliferation assay.Cell proliferation was assayed by 3H-thymidine incorporation. Experiments were set up in 96-well plates and 25 kBq 3HTdR (2 Ci/mmol/L; Amersham Plc, Amersham, Bucks, UK) added per well 18 hours before harvesting. The cells were collected onto filter paper (Printed Filtermat A; Wallac Oy Turku, Finland) using an automatic cell harvester (Tomtec; Wallac) and the radioactivity incorporated into the cells measured using a liquid scintillation counter (Betaplate 1205; Wallac). The results were expressed as counts per minute.
RESULTS
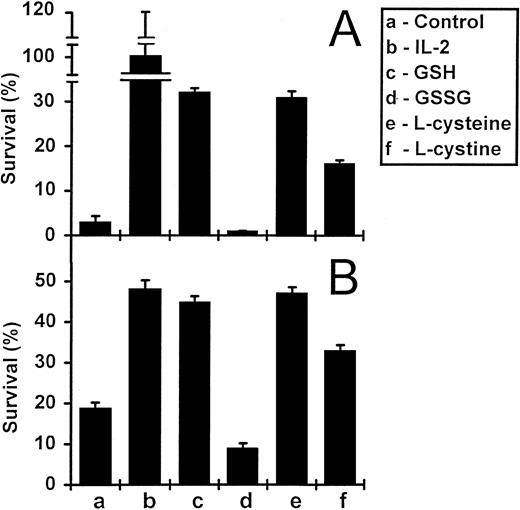
Reduced glutathione and L-cysteine enhance the survival of activated T cells in vitro.The withdrawal of IL-2 from activated T-cell lines leads to apoptosis, a process associated with the downregulation of Bcl-2 expression.13 The re-addition of IL-2 leads to upregulation of Bcl-2 expression, cell proliferation, and cell survival.13 We tested the ability of exogenously added reduced GSH and related compounds to promote the survival of IL-2–deprived T cells. Survival was determined as the number of viable cells recovered as a percentage of original input. The results of a typical experiment are shown in Fig 1A. After a 5-day incubation, GSH at a concentration of 3 mmol/L, increased the survival of IL-2–deprived T cells from 3% (in the absence of GSH) to 32%. In contrast, the oxidized form of GSH (GSSG), did not enhance the survival of these cells. Both the reduced form of the amino acid precursor of GSH (L-cysteine) and its oxidized derivative (L-cystine) also promoted T-cell survival, although L-cystine was less effective. We also tested GSH and L-cysteine on the in vitro survival of freshly isolated PBMC (Fig 1B). Again, both GSH and L-cysteine significantly enhanced the survival of these cells, whereas GSSG and L-cystine either did not (GSSG) or were less effective (L-cystine).
Survival of PBMC and IL-2–dependent activated T cells in the presence of GSH and L-cysteine. IL-2–deprived activated T cells (A) and freshly isolated PBMC (B) were incubated under various conditions for 5 and 8 days, respectively. Cells were incubated alone, with IL-2 (5 ng/mL), or with GSH, GSSG, L-cysteine, and L-cystine, each at concentrations of 3 mmol/L (A) or 1 mmol/L (B). Values are means ± standard error of mean (SEM) (n = 3 or 4). The results are representative of three independent experiments.
Survival of PBMC and IL-2–dependent activated T cells in the presence of GSH and L-cysteine. IL-2–deprived activated T cells (A) and freshly isolated PBMC (B) were incubated under various conditions for 5 and 8 days, respectively. Cells were incubated alone, with IL-2 (5 ng/mL), or with GSH, GSSG, L-cysteine, and L-cystine, each at concentrations of 3 mmol/L (A) or 1 mmol/L (B). Values are means ± standard error of mean (SEM) (n = 3 or 4). The results are representative of three independent experiments.
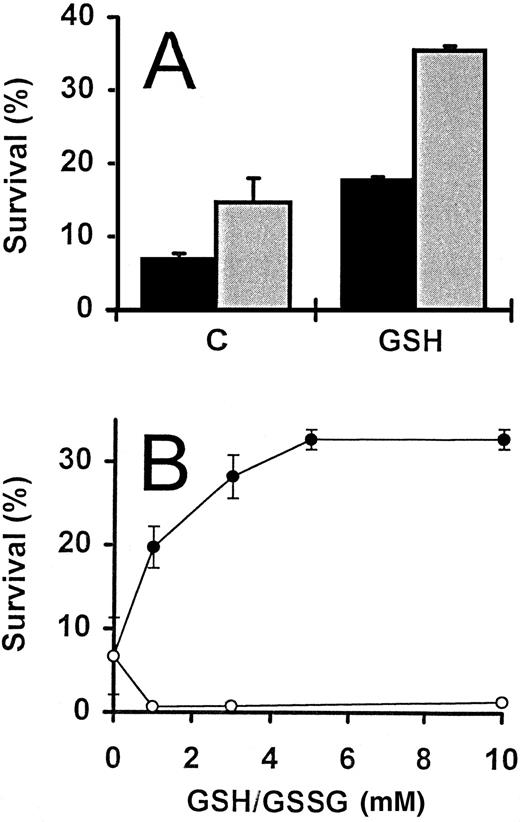
During the course of the study described above, serum was routinely omitted from the culture medium. The inclusion of 5% FCS, however, had no effect on the increase of activated T-cell survival promoted by exogenous GSH, although survival values were consistently greater when serum was included (Fig 2A). In addition, the survival-promoting effect of GSH was titratible and a plateau reached with concentrations of 5 mmol/L and above (Fig 2B).
GSH-promoted survival of activated T cells. (A) IL-2–deprived T cells were incubated in the presence () and absence (▪) of FCS (5%), alone (C) or in the presence of GSH (3 mmol/L). Percent survival was determined following incubation for 4 days. (B) Activated T cells deprived of IL-2 were incubated with increasing concentrations of either GSH (•) or GSSG (○), and survival determined after 3 days. For both experiments, the values represent means ± SEM of triplicate cultures, and results are representative of two independent experiments.
GSH-promoted survival of activated T cells. (A) IL-2–deprived T cells were incubated in the presence () and absence (▪) of FCS (5%), alone (C) or in the presence of GSH (3 mmol/L). Percent survival was determined following incubation for 4 days. (B) Activated T cells deprived of IL-2 were incubated with increasing concentrations of either GSH (•) or GSSG (○), and survival determined after 3 days. For both experiments, the values represent means ± SEM of triplicate cultures, and results are representative of two independent experiments.
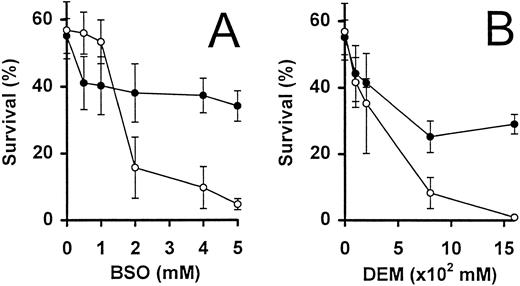
Intrinsic glutathione levels are important for the survival of activated T cells.The inhibitor, BSO, was used to specifically and irreversibly inhibit γ-glutamyl–cysteine, an enzyme essential for the biosynthesis of GSH39 (Fig 3A). Activated T cells deprived of IL-2 were incubated for 24 hours in the presence of increasing concentrations of BSO. Concentrations of BSO greater than 1 mmol/L were found to dramatically decrease the survival of these cells, by threefold to fivefold, as compared with cells incubated in the absence of BSO (Fig 3A). Because in a parallel experiment, the addition of 5 mmol/L BSO reduced the intracellular total GSH content from 0.12 nmol/106 cells to undetectable levels (<0.001 nmol/106 cells), the reduction in cell survival could presumably be due to intracellular GSH depletion. This effect was confirmed when the simultaneous addition of 3 mmol/L GSH largely abrogated the toxic effect of BSO (Fig 3A).
Activated T-cell survival in the presence of GSH inhibitors. IL-2–deprived T cells were incubated with increasing concentrations of BSO (A) and DEM (B), in the presence (•) and absence (○) of GSH (3 mmol/L). Percent surviving cells (means ± SEM of triplicate cultures) was determined following incubation for 24 hours. Similar results were obtained in a further independent experiment.
Activated T-cell survival in the presence of GSH inhibitors. IL-2–deprived T cells were incubated with increasing concentrations of BSO (A) and DEM (B), in the presence (•) and absence (○) of GSH (3 mmol/L). Percent surviving cells (means ± SEM of triplicate cultures) was determined following incubation for 24 hours. Similar results were obtained in a further independent experiment.
The use of a second inhibitor diethyl maleate (DEM), which binds directly to GSH, confirmed the observations made above. Addition of 0.16 mmol/L DEM decreased cell survival from 57% (in the absence of additional factors) to less than 1% after 24 hours (Fig 3B). As in the case of BSO, the presence of GSH (3 mmol/L) could partially abrogate the toxic effect of DEM on these cells.
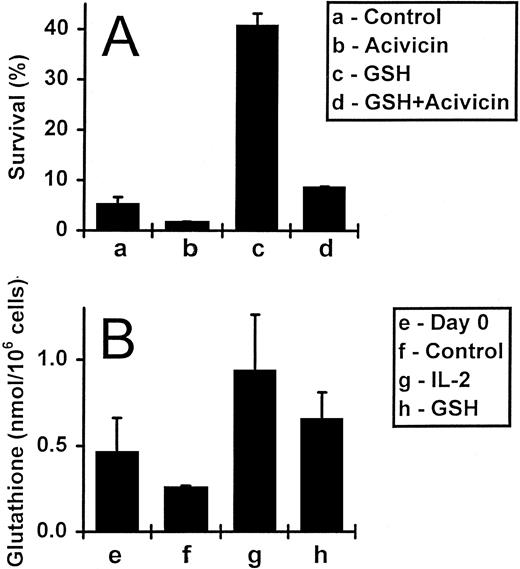
Exogenously added GSH raises intracellular glutathione levels, but does not affect proliferation or Bcl-2 levels.The GSH depletion experiments described above implied that exogenously added GSH might enhance cell survival by influencing intracellular GSH levels, rather than by acting at the cell surface. To clarify this issue, we first incubated IL-2–deprived activated T cells with 5 mmol/L acivicin, which inhibits GSH uptake, in the presence and absence of 3 mmol/L GSH (Fig 4A). As expected, after a 5-day incubation, T-cell survival increased from 5% to 41% with the addition of GSH alone. Importantly, the simultaneous addition of acivicin blocked this survival effect. Acivicin per se, in titrated doses from 1 to 10 mmol/L, had little effect on the survival of activated T cells (data not shown).
The uptake of exogenous GSH by activated T cells. (A) Activated T cells (deprived of IL-2) were incubated for 5 days either alone, with acivicin (5 mmol/L) or GSH (3 mmol/L), or with acivicin and GSH together. Values are means ± SEM of triplicate cultures, and the results are representative of four independent experiments. (B) Activated T cells incubated alone, with IL-2 (5 ng/mL) or with GSH (3 mmol/L) for 2 days were assessed for their total GSH content (expressed as nmol/106 cells; see Materials and Methods). The initial GSH content, at day 0, was also determined. Values represent means ± SEM for three independent experiments.
The uptake of exogenous GSH by activated T cells. (A) Activated T cells (deprived of IL-2) were incubated for 5 days either alone, with acivicin (5 mmol/L) or GSH (3 mmol/L), or with acivicin and GSH together. Values are means ± SEM of triplicate cultures, and the results are representative of four independent experiments. (B) Activated T cells incubated alone, with IL-2 (5 ng/mL) or with GSH (3 mmol/L) for 2 days were assessed for their total GSH content (expressed as nmol/106 cells; see Materials and Methods). The initial GSH content, at day 0, was also determined. Values represent means ± SEM for three independent experiments.
Secondly, we directly assessed the effect of exogenous GSH on intracellular GSH levels measured using an enzymic GSH recycling assay.40 Results obtained from three different experiments are shown in Fig 4B. IL-2–deprived activated T cells incubated either alone or in the presence of GSH (3 mmol/L) were assayed for total GSH content on days 0 and 2. Culture of cells without exogenous GSH for 2 days led to a decrease in the cellular GSH content compared with the starting population from 0.46 to 0.26 nmol/106 cells, respectively. The addition of GSH increased the intracellular GSH content relative to the starting population from 0.46 to 0.65 nmol/106 cells, respectively, and also induced a 2.5-fold increase over cells cultured for the same length of time, but without this molecule (0.26 to 0.65 nmol/106 cells). In addition, the intracellular GSH level of the cells was also elevated by the presence of IL-2, to 0.93 nmol/106 cells.
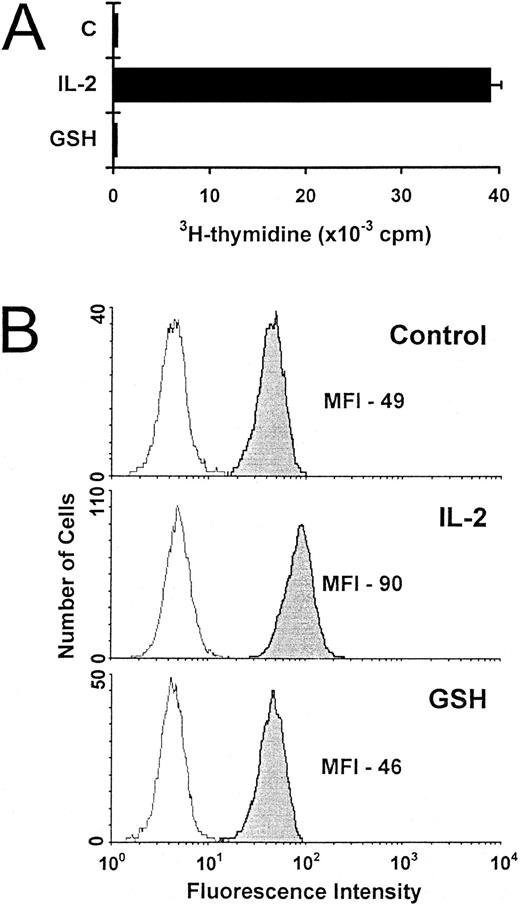
Increased T-cell survival due to exogenous GSH could not be explained by enhanced proliferation, as 3H-thymidine incorporation was not induced in cells exposed to GSH (Fig 5A). Neither could survival be explained in terms of Bcl-2 expression, which was found to be significantly lower in IL-2–deprived T cells rescued by GSH than in IL-2–supplemented cells (Fig 5B).
Proliferation and Bcl-2 expression of activated T cells incubated with exogenous GSH. (A) The proliferation of IL-2–deprived activated T cells incubated alone (C), with IL-2 (5 ng/mL) or with GSH (3 mmol/L) was determined after 3 days. Values are expressed as mean 3H-thymidine incorporation (± SEM) of quadruplicate cultures from a representative experiment. (B) Activated T cells incubated for 3 days either alone (control), with IL-2 (5 ng/mL), or with GSH (3 mmol/L) were stained for Bcl-2 expression. The results are representative of three independent experiments.
Proliferation and Bcl-2 expression of activated T cells incubated with exogenous GSH. (A) The proliferation of IL-2–deprived activated T cells incubated alone (C), with IL-2 (5 ng/mL) or with GSH (3 mmol/L) was determined after 3 days. Values are expressed as mean 3H-thymidine incorporation (± SEM) of quadruplicate cultures from a representative experiment. (B) Activated T cells incubated for 3 days either alone (control), with IL-2 (5 ng/mL), or with GSH (3 mmol/L) were stained for Bcl-2 expression. The results are representative of three independent experiments.
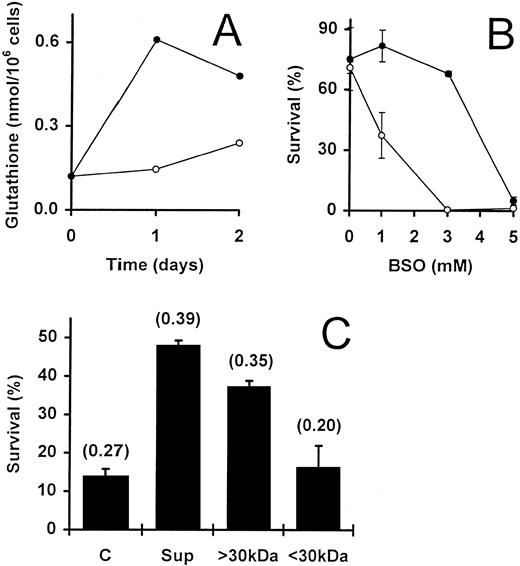
Fibroblast-derived supernatant promotes T-cell survival via upregulation of intracellular GSH levels.We and others have previously demonstrated that the survival of IL-2–dependent activated T cells can be promoted by a variety of different fibroblast lines and their supernatants.12,14,15 IL-2–deprived T cells rescued from apoptosis by fibroblast-conditioned medium are essentially noncycling cells that express only low levels of Bcl-2.15 These observations were confirmed for T cells rescued from apoptosis by the human embryonic lung fibroblast cell line WI38 (data not shown). Thus, the survival of T cells promoted by fibroblasts or by GSH appear to share common features. We, therefore, directly investigated whether WI38-derived supernatant was able to influence the total GSH levels in activated T cells. Supernatant generated in the absence of serum (see Materials and Methods) was added to IL-2–deprived T cells to a concentration of 50%. At the start of the culture period (day 0), the GSH content of the cells was found to be 0.12 nmol/106 cells. In the absence of additional factors, this level had not significantly altered by day 2 (Fig 6A). In contrast, the addition of WI38-derived supernatant to the cells for 1 day resulted in a fivefold elevation of GSH (0.12 to 0.61 nmol/106 cells) and, after 2 days, there was still a fourfold elevation of the intracellular GSH (0.12 to 0.48 nmol/106 cells) as compared with the starting population on day 0 (Fig 6A). When cells cultured with W138 supernatants were compared with control cells cultured alone for the same time, a fourfold and twofold increase was observed on day 1 and day 2, respectively. The upregulation of GSH by W138 supernatants relative to controls cultured for the same periods was confirmed in an additional experiment on day 2 (Fig 6C) and two further experiments analyzed after 5 days of culture (see legend to Fig 6).
Involvement of intrinsic GSH in WI38 supernatant-promoted activated T-cell survival. (A) Activated T cells, deprived of IL-2, were incubated in the presence (•) and absence (○) of WI38-derived supernatant (50%). The GSH content of the cells was determined initially (day 0) and after culture for 1 and 2 days. Two further independent experiments gave similar results. (B) IL-2–deprived T cells were incubated for 2 days with (•) and without (○) WI38-derived supernatant (50%) in the presence of increasing concentrations of BSO. Values are means ± SEM of triplicate cultures. (C) WI38-derived supernatant was crudely fractionated using Amicon C30 ultrafiltration units (see Materials and Methods). This yielded fractions containing either low molecular weight proteins (<30 kD) or higher molecular weight proteins (<30 kD). IL-2–deprived T cells were incubated alone (C), with unfractionated supernatant (Sup; 50%) or with the appropriate fractions (<30 kD and <30 kD; 50%). The data shows the percentage survival at day 5 in a representative experiment, and the values are means ± SEM of triplicate cultures. Numbers in brackets show the total GSH content of the cells (nmol/106 cells) as determined after a 2-day incubation. In two separate experiments, the GSH content after 5 days in the controls compared with the cultures to which W138 supernatants were added was 0.89 versus 1.87 nmol/106 cells and 0.64 versus 1.51 nmol/106 cells, respectively.
Involvement of intrinsic GSH in WI38 supernatant-promoted activated T-cell survival. (A) Activated T cells, deprived of IL-2, were incubated in the presence (•) and absence (○) of WI38-derived supernatant (50%). The GSH content of the cells was determined initially (day 0) and after culture for 1 and 2 days. Two further independent experiments gave similar results. (B) IL-2–deprived T cells were incubated for 2 days with (•) and without (○) WI38-derived supernatant (50%) in the presence of increasing concentrations of BSO. Values are means ± SEM of triplicate cultures. (C) WI38-derived supernatant was crudely fractionated using Amicon C30 ultrafiltration units (see Materials and Methods). This yielded fractions containing either low molecular weight proteins (<30 kD) or higher molecular weight proteins (<30 kD). IL-2–deprived T cells were incubated alone (C), with unfractionated supernatant (Sup; 50%) or with the appropriate fractions (<30 kD and <30 kD; 50%). The data shows the percentage survival at day 5 in a representative experiment, and the values are means ± SEM of triplicate cultures. Numbers in brackets show the total GSH content of the cells (nmol/106 cells) as determined after a 2-day incubation. In two separate experiments, the GSH content after 5 days in the controls compared with the cultures to which W138 supernatants were added was 0.89 versus 1.87 nmol/106 cells and 0.64 versus 1.51 nmol/106 cells, respectively.
To determine if the increased GSH concentration was due to accumulation of existing GSH or upregulation of GSH synthesis, the cells were exposed to the GSH inhibitors, BSO and DEM. Again, the experiments were performed both in the presence and absence of fibroblast supernatant. As previously shown (see Fig 3A), by day 2, the highest concentration of BSO (5 mmol/L) had dramatically reduced the survival of the T cells from 71% to almost 0% (Fig 6B). The simultaneous addition of 50% supernatant did not prevent the cell induced by this concentration of BSO. Similar observations were obtained with DEM when used at a concentration of 0.16 mmol/L (data not shown). Thus, intracellular GSH appears to be essential for the supernatant-dependent survival of these cells. However, the supernatant was able to partially block the destructive effect of both inhibitors when they were used at lower (suboptimal) concentrations (Fig 6B and data not shown). Because BSO blocks the synthesis of GSH, this observation may be due to an upregulation of GSH synthesis by factor(s) present in the supernatant.
Several reports have demonstrated the in vitro generation of extracellular GSH, L-cysteine, and derivative compounds by different cell types including macrophages, which can potentially act upon lymphocytes in coculture.41-44 To test the possibility that fibroblast-secreted GSH or L-cysteine induced the upregulated GSH levels while promoting the survival of the IL-2–deprived T cells, we fractionated the WI38-conditioned medium. Crude fractionation using Amicon Centricon units gave rise to fractions containing either molecules of less than 30 kD (the filtrate) or larger than 30 kD (the retentate). Both survival of activated T cells and increased intracellular GSH content were associated with the fraction containing molecules with molecular weights of greater than 30 kD (Fig 6C). The filtrate did not promote survival and had no effect on the GSH content of the cells. Because GSH and L-cysteine are both small molecules that would be expected to reside in the filtrate, the latter observation eliminates the possibility of their direct involvement in fibroblast-promoted T-cell survival.
DISCUSSION
This study demonstrates that (1) exogenously added GSH promotes the survival of IL-2–deprived activated T cells by influencing the GSH content of the cells; (2) intrinsic intracellular GSH levels are critical for activated T-cell survival; and (3) WI38-promoted survival of activated T cells leads to a concomitant rise in the intracellular GSH level of the cells, associated with factor(s) of greater than 30 kD. Taken together, these observations implicate GSH as a possible mediator in fibroblast-enhanced survival of activated T cells.
The apoptosis induced in activated T cells by the withdrawal of IL-2 in vitro is associated with a decrease in Bcl-2 expression12 (this study). As we have previously demonstrated, the continued presence of cytokines such as IL-2, IL-4, IL-7, and IL-15 (all of which signal via the γ-chain of the IL-2 receptor) promotes the survival of these cells via upregulation of Bcl-2 and subsequent cell proliferation.13 In contrast, fibroblast-promoted T-cell survival appears to be independent of Bcl-2, as Bcl-2 is not re-expressed in the rescued cells.12 This is also true for activated T cells rescued from apoptosis by the addition of exogenous GSH in vitro (this study). Thus, T-cell survival promoted by fibroblasts or GSH appear similar to one another, while both are fundamentally different from cytokine-promoted survival.
GSH is a potent antioxidant usually associated with the removal of intracellular H2O2 and the prevention of membrane damage.27,29 Our observation that GSH can promote IL-2–deprived activated T-cell survival implies that these cells are dying in vitro, primarily as a result of oxidative stress (this study). There has been much debate about the involvement of pro and antioxidants in the control of apoptosis. Although many studies have implicated ROS in the induction of apoptotic cell death, ROS are not an absolute requirement because apoptosis can be induced under hypoxic conditions where cellular ROS production is minimized.45,46 A recently published study has demonstrated that the opening of mitochondrial permeability transition (PT) pores is a crucial event in the induction of apoptosis.47 PT is modulated by multiple factors, including ROS. Bcl-2 appears to protect against the induction of PT by a number of agents, and the protection afforded by Bcl-2 correlates with its antiapoptotic function.47 Thus, it seems that both Bcl-2 and the redox status of the cell can influence the same cellular event, namely the induction of mitochondrial PT. In our system, the addition of GSH or the upregulation of endogenous GSH by WI38-derived factor(s), may compensate for low Bcl-2 expression by limiting oxidative damage and perhaps PT induction.
Understanding the mechanisms that govern activated T-cell apoptosis and the factors involved may help to explain a number of different aspects of T-cell biology. For instance, immune stimulation leads to a huge expansion of activated T cells that are reactive towards the stimulating antigen.48-50 Following resolution of the disease, the vast majority of these cells die by apoptosis allowing cellular homeostasis to be re-established.12,51 Previous studies from our laboratory have demonstrated that activated T cells circulating in the peripheral compartment are predisposed to die, expressing only low levels of Bcl-2 along with high levels of the Fas/Apo-1 receptor (CD95).12 However, some antigen-specific activated T cells survive to become memory-type T cells that protect individuals from recurrent disease.4,46 The persistance of some of the original priming antigen has been shown to be required for the maintenance of the T-cell memory pool in some systems, inducing the proliferation of previously activated T cells.52,53 Antigen-nonspecific mechanisms for the persistance of T-cell memory have also been invoked54-56 and may include the delay of apoptosis as described in this study, whereby activated T cells prone to die are rescued in appropriate tissue microenviroments. This possibility is supported by circumstantial evidence. In lymph nodes taken from human immunodeficiency virus (HIV)-positive individuals, where T cells are in close association with tissue stroma, the T cells express significantly lower levels of Bcl-2 than circulating T cells from the same patients, yet little evidence for apoptosis can be found.35 The present study suggests that T-cell survival in such conditions may be due to increased intracellular GSH levels.
In addition to the prevention of apoptosis caused by cytokine withdrawal, recent data supports the idea that GSH may also prevent actively induced death due to the ligation of CD95, as Fas-mediated apoptosis of human leukemic T cells can be abrogated by the addition of N-acetyl–cysteine (NAC).57 This effect was shown to be due to enhanced intracellular GSH levels. In this context, it has been shown that patients with HIV infections are extremely susceptible to apoptosis following CD95-ligation.58,59 Interestingly, the GSH content of the T cells in these patients is low, even before the onset of symptoms.60 It is not known if both phenomena are related, but clinical trials have been initiated in the use of NAC to boost cell numbers in patients with HIV.60 The identification of ubiquitous natural tissue-derived factor(s) that can prevent apoptosis by upregulating intracellular GSH may be of considerable significance. It is tempting to speculate that one consequence of HIV infection may be the perturbation of the tissue-survival signal pathway leading to GSH upregulation in T cells.
In summary, our data suggest that signals from tissue microenvironments may have a profound influence on the survival of activated T cells after immune stimulation and that the upregulation of intracellular GSH is central in this process. This may be of particular relevance in the pathogenesis of certain diseases such as rheumatoid arthritis, where the synovial microenvironment appears to contribute to the generation and persistance of T-cell infiltrates in chronically inflamed synovia.36 Although the identity of the fibroblast-secreted factor(s) is currently unknown, recent data suggests that the factor(s) may be integrin-binding36 consistent with a molecular weight of greater than 30 kD (this study). Investigations into the identification of the fibroblast-derived factor(s) are currently ongoing.
Supported by the Medical Research Council, Grant No. G9218555MA and G9319116MA and by the Arthritis and Rheumatism Council, Grant No. SO185.
Address reprint requests to Arne N. Akbar, PhD, Department of Clinical Immunology, Royal Free Hospital School of Medicine, Pond St, Hampstead, London NW3 2QG, UK.