Abstract
Human cytomegalovirus (HCMV) infections are commonly associated with a generalized immunologic hyporesponsiveness. The present study was designed to evaluate the potential mechanisms of HCMV-associated immunosuppression. In our initial experiments, monocytes in peripheral blood mononuclear cells (PBMCs) exposed to cell-free HCMV appeared morphologically less differentiated than monocytes in PBMCs exposed to a mock preparation. These morphologic changes were closely correlated with a decrease in monocyte oxidative activity and occurred under noncytopathic conditions. HCMV-associated suppression of monocyte differentiation did not require virus replication, occurred in PBMCs from either HCMV seropositive or seronegative donors, and required HCMV interaction with the nonadherent cells. An HCMV-induced soluble factor was found to not only reproduce the identical changes in purified monocytes but to inhibit the phagocytic activity of these cells. Additionally, the HCMV-induced factor accounted for a generalized defect in the ability of PBMCs to proliferate in response to mitogens and recall antigens. In subsequent experiments, interferon-α (IFN-α) was identified as the soluble factor involved in these immunosuppressive effects. Thus, PBMCs, when exposed to HCMV, produce a soluble factor, identified as IFN-α, that appears to be an important mediator of immunosuppression associated with HCMV infection.
HUMAN cytomegalovirus (HCMV) is a ubiquitous herpesvirus commonly associated with immunosuppression. Immunocompromised hosts such as neonates, transplant recipients, and patients with acquired immunodeficiency syndrome (AIDS) are susceptible to severe viral infection. In nonimmunosuppressed hosts, during mononucleosis, acute cytomegalovirus infection results in a transient immunosuppression followed by a carrier state, with minor or no symptomatology.1
HCMV infection has been previously associated with suppression of both cell-mediated and humoral immune responses. Several investigators have attributed HCMV suppressive effects to virus-induced dysfunction of monocytes/macrophages. Both monocytes from patients with HCMV mononucleosis and normal monocytes infected in vitro have an impaired ability to support mitogen-driven T-cell responses.2,3 Others have confirmed the role of adherent mononuclear cells in the suppression of other mononuclear cell functions such as natural killer (NK) cell activity.4,5 A reduction in interleukin-1 (IL-1) activity in the supernatant of HCMV-infected monocytes was proposed to partially account for the HCMV suppressive effects.6,7 This abrogation of IL-1 activity required infectious virus and was associated with the release of an inhibitor of IL-1 activity.6 However, these results were refuted later by the same investigators and others who found that HCMV-associated immunosuppression was largely caused by mycoplasma present in virus preparations. Mitogen-mediated lymphoproliferative response, accessory cell function, and IL-1 production or activity were not suppressed when monocytes were infected in vitro with mycoplasma-free HCMV isolates.8 9 These results emphasize the importance of continually monitoring the purity of viral cultures for mycoplasma, and cast some doubt on the ability of HCMV to affect directly monocyte/macrophage functions.
In reconstitution experiments, HCMV infection of peripheral blood lymphocytes (PBLs) resulted in a greater suppression of T-cell proliferation in response to phytohemagglutinin (PHA) than HCMV infection of monocytes alone.10,11 The proliferative defect was accompanied by a reduction in IL-2 and IL-1 production or activity as well as an impaired ability to respond to these cytokines, which was more pronounced in HCMV-infected PBLs than HCMV-infected monocyte cultures. Recently, in HCMV-infected transplant recipients, reduction of T-cell proliferation has been shown to be an accessory-cell independent phenomenon associated with an increased incidence of apoptosis in PBLs.12 According to these findings, immature lymphocytes of mice infected with murine CMV (MCMV) have shown an increased sensitivity to apoptosis induction by anti-CD3 monoclonal antibody (MoAb).13 Additionally, extensive immunosuppressive effects of HCMV have been demonstrated in vitro with no evidence of productive infection.5,14 15 Taken together, these data suggest that the effect of HCMV infection on PBMCs is a generalized metabolic depression of cell activity, and support the hypothesis that HCMV-induced immunosuppression may be caused by an indirect effect of the virus acting on the monocytes as well as the lymphocytes.
In the present study, we investigated how HCMV, through a common mechanism, could induce changes in monocyte maturation and function, and impair the lymphocyte proliferative responses to mitogen and recall antigen without replicating in these cells. PBMCs, when exposed to HCMV, produce a soluble factor, which appears to be interferon-α (IFN-α), that largely accounts for HCMV-associated immunosuppression.
MATERIALS AND METHODS
Cells.Human foreskin fibroblasts (HFF ) were maintained in Dulbecco's modified Eagle's medium (DMEM; Flow Laboratory, Inc, McLean, VA) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gemini Bio-Products, Inc, Calabasas, CA), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. PBMCs from healthy donors were collected in heparin and separated by Ficoll-Hypaque (Pharmacia Biotech, Alameda, CA) density centrifugation. Cells were cultured in RPMI 1640 supplemented with 5% heat-inactivated human serum (Gemini Bio-Products, Inc), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Adult human elutriated monocytes (ABI, Inc, Columbia, MA) were grown in RPMI 1640 containing 20% heat-inactivated human serum supplemented with 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Elutriated cells were composed of greater than 96% monocytes. All the cells were cultured at 37°C in a humified atmosphere of 5% CO2 .
Preparation of cell-free virus.The HCMV laboratory-adapted strains, AD169 and Towne, and the nonlaboratory-adapted strain, Toledo, were obtained from the American Type Culture Collection (ATCC; Rockville, MD). A low passage patient-derived strain of HCMV was isolated from urine of a patient with AIDS diagnosed with HCMV retinitis. Virus stocks used were cell-free virus passaged between four and eight times and expanded on HFF. Supernatants were harvested from HFF-infected cells when cytopathic effects (CPE) reached 100%, clarified by centrifugation (1,200g for 10 minutes), and filtered with 0.45-μm filters. HCMV was then pelleted by ultracentrifugation (154,325g for 30 minutes at 4°C) and the pellet was resuspended in RPMI 1640. Supernatant from mock-infected HFF treated in a similar manner was used as control. Viral stocks were titrated on HFF by standard plaque assay.16 HCMV was inactivated with psoralen (AMT, 4'-aminomethyltrioxalen) (Calbiochem-Novabiochem Corp, San Diego, CA) at 10 μg/mL in combination with long-wavelength UV light (320 to 380 nm) for 12 minutes. Such treatment renders the virus noninfectious while preserving its antigenic structure. The mock preparation was treated in an identical manner. The efficiency of inactivation was confirmed by the absence of virus plaques following the inoculation of HFF cultures with AD169-treated virus.
All virus strains used were tested and were negative for the presence of mycoplasma contamination. Testing was done independently by the UCSD Core Mycoplasma Facility (University of California, San Diego) using the Gen-Probe hybridization assay (Gen-Probe, Inc, San Diego, CA), which allows for the detection of all species that commonly infect tissue cultures. All virus preparations and media used were tested for the presence of endotoxin with the E TOXATE assay (Sigma Corp, St Louis, MO). The concentrations were less than 0.06 Endotoxin unit (EU)/mL or 0.012 ng/mL.
Oxidative activity assay.The MTS/PMS assay was used to assess the cellular oxidative activity. The MTS/PMS assay is a colorimetric assay derived from the methotrexate (MTT) assay17 composed of solutions of a tetrazolium compound (MTS) and an electron coupling reagent (PMS). MTS is bioreduced by cells into a formazan that is soluble in tissue culture medium. It measures the mitochondrial dehydrogenase activity that is proportional to the cell number and the intrinsic metabolic capacity of the cells. Infectious or inactivated cell-free HCMV or the mock preparation was added concurrently to PBMCs (1 × 105 cells) in a flat-bottomed 96-well plate (Costar, Cambridge, MA) in a total volume of 200 μL. The MTS/PMS assay was performed after variable periods of time depending on the experiment. Briefly, at the time of the assay, 100 μL of media was removed and 20 μL of MTS/PMS (Promega Corp, Madison, WI) was added to each well for 4 hours. All assays were performed in triplicate and absorbance was measured at 492 nm using a microplate spectrophotometer (Titertek Multiscan MCC; Labsystems, Inc, Morton Grove, IL).
CMV conditioned medium (CMV CM) activity on purified monocytes.To assess the role of a soluble factor in CMV-induced suppression, purified monocytes were incubated with CM. Supernatants of PBMCs from seronegative donor (2 × 106 cells/mL) incubated for 2 days with cell-free inactivated Toledo virus at 0.002 multiplicity of infection (MOI) equivalent or with the mock preparation were harvested. The cells and the virus were removed by centrifugation (1,200g for 10 minutes and 154,325g for 30 minutes at 4°C, respectively). Virus-free conditioned media were aliquoted and frozen at −70°C. Elutriated monocytes (8 × 104 cells/well) were first cultured in flat-bottomed 96-well plates for 3 hours at 37°C to allow cell adherence. The nonadherent cells were removed and 50 μL of the day 2 CMV and mock CM were added together with 150 μL of fresh medium. Every other day, 100 μL was removed from each well and replaced by 50 μL of CM and 50 μL of fresh medium. The oxidative assay was performed in triplicate, after 2, 4, 6, and 8 days. In the same way, to evaluate the kinetics ability of HCMV-induced factor(s) to suppress monocyte oxidative activity, CM were collected after increasing periods of PBMCs exposure to HCMV or mock preparation (inactivated Toledo virus, 0.001 MOI equivalent) and added to purified monocytes. The MTS/PMS assay was performed after 4 days.
Phagocytosis assay.Elutriated monocytes (5 × 105 cells/well) were cultured with the day 2 CMV and mock CM in a 12-well plates for 4 days as described previously. Measurement of the phagocytic activity of purified monocytes was assessed by a modification of the method of Steinkamp et al.18 At day 4, the cells were incubated for 30 minutes at 4°C in phosphate-buffered saline (PBS) and detached by scraping. 2.5 × 107 of 1.8-μm fluorescent microspheres (Polysciences, Warrington, PA) in complete medium was added to each tube, vortexed, and incubated at 37°C for 45 minutes under agitation. The cell suspension was then layered over 1.5 mL fetal bovine serum (FBS) and centrifuged at 250g for 10 minutes at room temperature. Serum and nonphagocytosed beads were removed, and the cells were washed twice with PBS containing 2% FBS and fixed in PBS 1% paraformaldehyde. Cells were analyzed for fluorescence (phagocytized spheres) and light scatter (cell size) as they flowed through a flow cytometer (EPICS V cytometer, Miami, FL). Cells found to have ingested 7 or more beads were scored as positive for phagocytic activity.
Cytokine quantification, IFN-α, antibodies, and neutralizing assays.Cytokine quantification was performed by enzyme-linked immunosorbent assay (ELISA) per the manufacturer's instructions. The following ELISA kits were used: IFN-α, IFN-γ, IL-4, IL-10, and tumor necrosis factor-α (TNF-α) (Biosource, Camarillo, CA) and TNF-β (Bender MedSystems, Vienna, Austria). Purified human natural IFN-α was purchased from The Purdue Frederick Co (Norwalk, CT). The first anti–IFN-α and anti–IFN-β neutralizing polyclonal antibodies used were purchased from Boehringer Mannheim Corp (Indianapolis, IN). Both are horse polyclonal antibody to human IFN-α or IFN-β. The second anti–IFN-α neutralizing antibody used was a sheep IFN-α polyclonal antiserum (Biosource). Normal nonimmune sheep serum (Sigma Corp) was used as a negative control. Neutralizing assays were performed with 1 × 105 PBMCs in flat-bottomed 96-well plates, in a final volume of 200 μL. Anti–IFN-α or anti–IFN-β horse neutralizing polyclonal antibody (100 U/mL) and inactivated cell-free HCMV (Toledo virus, 0.002 MOI equivalent) or the mock preparation were added to the PBMCs culture at day 0. The absorbances were determined after 3 days.
Lymphocyte proliferation assays.Lymphocyte proliferation was measured in two different groups of experiments. We first compared the proliferative response of PBMCs incubated with cell-free virus with the proliferative response of PBMCs incubated with the CMV CM. The inactivated Toledo virus (0.002 MOI equivalent) and the mock preparation or the day 2 CMV CM and the mock CM, 50 μL of each per well, were added at day 0 and maintained in culture. PBMCs (1.5 × 105 cells/well) from three HCMV seronegative donors were cultured in triplicate in RPMI 1640 supplemented with 10% heat-inactivated human serum (Gemini Bio-Products, Inc), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in flat-bottomed 96-well plates in a total volume of 200 μL. The following activators were used: PHA, 2 μg/mL (Sigma Corp); pokeweed mitogen (PWM), 1 μg/mL (Sigma Corp); and the tetanus toxoid (TT), 25 μg/mL (Connaught Laboratories, Inc, Swiftwater, PA). The plates were incubated at 37°C during 3 days for the mitogen proliferative response and 6 days for the recall antigen proliferative response. The cells were pulse-labeled with 3H-thymidine (3H-TdR), 1 μCi/well, 6.7 Ci/mmol (Dupont, Boston, MA) during the last 12 hours of culture, harvested onto glass fiber filters, and counted in a Beckman LS 6000SC (Beckman, Fullerton, CA).
In another set of experiments, PBMCs (1 × 106 cells/mL) were incubated with cell-free inactivated Toledo strain at 0.002 MOI equivalent or with the mock preparation in the absence or in the presence of anti–IFN-α neutralizing antibody. IFN-α neutralizing sheep antiserum (2,000 U/mL) and nonimmune sheep serum (used at the same dilution) were added at the begining of the culture and after 6, 15, and 21 hours. The CM were obtained after 24 hours and treated as described previously to be tested in the proliferative assay (50 μL/well).
Statistical analysis.Student's t-tests were used to compare normally distributed data assessing HCMV effects on monocyte oxidative activity. The P values are two-tailed unpaired t-tests unless otherwise indicated.
RESULTS
HCMV-associated alterations of monocyte maturation.In studies designed to evaluate the potential mechanisms of immunosuppression associated with HCMV, PBMCs were incubated with HCMV. Monocytes in PBMCs exposed to the mock preparation, as well as control medium, appeared to differentiate in culture. The cells increased in size, flattened, and spread. In contrast, monocytes in PBMCs exposed to the cell-free HCMV remained undifferentiated, small, rounded, and poorly attached to the plastic.
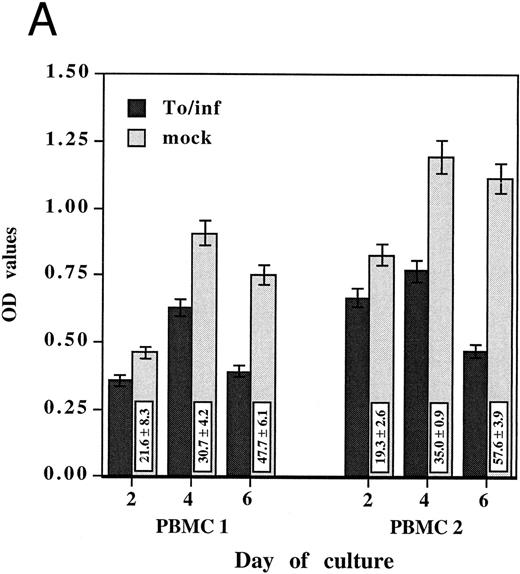
Because increased oxidative activity is commonly associated with monocyte activation and differentiation,19 the oxidative activity of PBMCs exposed to either HCMV or the mock preparation was compared. Oxidative activity, as measured by the MTS/PMS assay, was assayed after 2, 4, and 6 days of culture in two different PBMCs (Fig 1A). The oxidative activity of PBMCs incubated with the mock preparation increased with time of culture and was not different from the control medium only, whereas its remained low in PBMCs incubated with HCMV. Inhibition of oxidative activity after 2 days was 21.6% ± 8.3% for PBMCs 1 (P = .006) and 19.3% ± 2.6% for PBMCs 2 (P < .001). Inhibition of oxidative activity was more pronounced after 4 days, 30.7% ± 4.2% for PBMCs 1 (P = .003) and 35.0% ± 0.9% for PBMCs 2 (P < .001) and increased at 6 days to 47.7% ± 6.1% for PBMCs 1 (P < .001) and 57.6% ± 3.9% for PBMCs 2 (P < .001). The monocyte-free samples showed an oxidative activity similar to those of the background control while unseparated PBMCs and adherent monocytes in the same assay exhibited measurable oxidative activity, demonstrating that adherent monocytes were responsible for producing the oxidative activity measured in this system (data not shown).
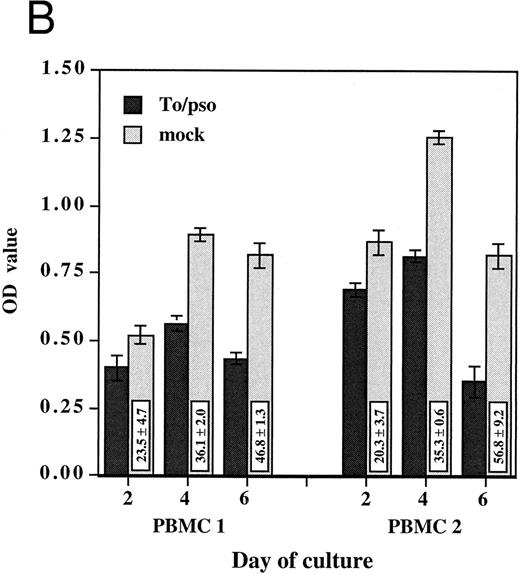
HCMV-associated suppression of monocyte oxidative activity. PBMCs from two HCMV seronegative donors were incubated for 2, 4, and 6 days with infectious cell-free HCMV, To/inf (A) or psoralen/UV-inactivated cell-free HCMV, To/pso (B). Infectious or inactivated cell-free HCMV, 0.002 MOI equivalent, and the respective mock preparations, used at the same dilutions, were added concurrently to the cells and maintained in culture. The monocyte oxidative activity, was measured using the MTS/PMS assay. Assays were performed in triplicate and results are presented as the mean ± SD of the optical density (OD) values. The boxes contain the percentage of oxidative activity inhibition (mean ± SD) corresponding to the ratio between the OD value obtained with HCMV and the mock preparation multiplied by 100.
HCMV-associated suppression of monocyte oxidative activity. PBMCs from two HCMV seronegative donors were incubated for 2, 4, and 6 days with infectious cell-free HCMV, To/inf (A) or psoralen/UV-inactivated cell-free HCMV, To/pso (B). Infectious or inactivated cell-free HCMV, 0.002 MOI equivalent, and the respective mock preparations, used at the same dilutions, were added concurrently to the cells and maintained in culture. The monocyte oxidative activity, was measured using the MTS/PMS assay. Assays were performed in triplicate and results are presented as the mean ± SD of the optical density (OD) values. The boxes contain the percentage of oxidative activity inhibition (mean ± SD) corresponding to the ratio between the OD value obtained with HCMV and the mock preparation multiplied by 100.
To address the question of whether or not this HCMV-induced effect on monocytes required infectious virus, these experiments were repeated with psoralen/UV-inactivated cell-free HCMV. Inactivated HCMV had a markedly suppressive effect on monocyte oxidative activity. As shown in Fig 1B, the percentages of inhibitions obtained with psoralen/UV-inactivated HCMV were of the same extent as those obtained with the infectious virus. Furthermore, monocytes in PBMCs incubated with inactivated HCMV showed the same morphologic characteristics of undifferentiated monocytes. In subsequent experiments, psoralen/UV-inactivated viruses were used.
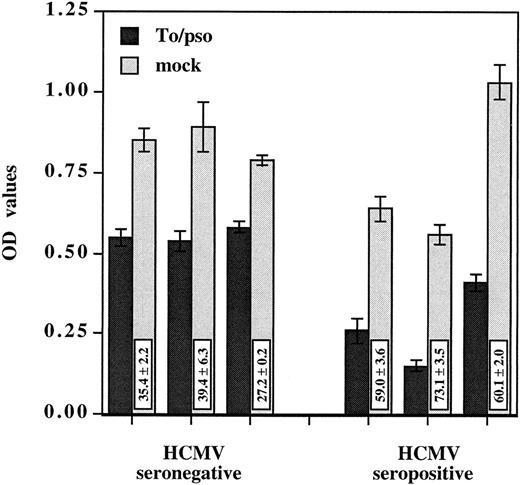
HCMV-induced suppressive effect was observed using PBMCs from either HCMV seronegative or seropositive donors (Fig 2). The suppression was significantly greater with the HCMV immune versus nonimmune PBMCs, 64.1% ± 7.3% and 33.7% ± 6.7% of inhibition, respectively (P < .001). Several laboratory adapted strains as well as a clinical isolate shared the ability to suppress monocyte oxidative activity (Table 1). The extent of oxidative activity suppression was dose dependent and varied among the viral strains. AD169 was least efficient in suppressing oxidative activity when compared with the other isolates. At 0.02 MOI, AD169 inhibited the monocyte oxidative activity by only 29.6% ± 6.1% and the virus had no effect when used at 0.002 MOI. Taken together, these results indicate that HCMV-induced suppressive effects on monocytes do not require virus replication and occur in PBMCs from HCMV seropositive, as well as seronegative, donors.
HCMV-induced suppression of monocyte oxidative activity occurs in PBMCs from seropositive as well as seronegative donors. Inactivated cell-free HCMV, To/pso 0.002 MOI equivalent, was incubated as described previously with three PBMCs from seronegative donors and three PBMCs from seropositive donors. The monocyte oxidative activity was measured after 4 days. Results are presented as the mean ± SD of the OD values. The boxes contain the percentage of oxidative activity inhibition (mean ± SD) corresponding to the ratio between the OD value obtained with HCMV and the mock preparation multiplied by 100.
HCMV-induced suppression of monocyte oxidative activity occurs in PBMCs from seropositive as well as seronegative donors. Inactivated cell-free HCMV, To/pso 0.002 MOI equivalent, was incubated as described previously with three PBMCs from seronegative donors and three PBMCs from seropositive donors. The monocyte oxidative activity was measured after 4 days. Results are presented as the mean ± SD of the OD values. The boxes contain the percentage of oxidative activity inhibition (mean ± SD) corresponding to the ratio between the OD value obtained with HCMV and the mock preparation multiplied by 100.
HCMV Laboratory and Clinical Strains Suppress Monocyte Oxidative Activity
| . | Multiplicity of Infection (MOI) Equivalent (%) . | ||
|---|---|---|---|
| . | 0.02 . | 0.002 . | 0.0002 . |
| Laboratory strains | |||
| AD169 | 29.6 ± 6.1 | −2.3 ± 5.1 | 3.0 ± 4.1 |
| Towne | 44.8 ± 6.2 | 21.9 ± 9.1 | 3.1 ± 6.6 |
| Toledo | 43.4 ± 3.0 | 42.4 ± 2.8 | 21.6 ± 5.6 |
| Clinical strain | |||
| Hei | ND | 42.5 ± 4.1 | 12.1 ± 3.2 |
| . | Multiplicity of Infection (MOI) Equivalent (%) . | ||
|---|---|---|---|
| . | 0.02 . | 0.002 . | 0.0002 . |
| Laboratory strains | |||
| AD169 | 29.6 ± 6.1 | −2.3 ± 5.1 | 3.0 ± 4.1 |
| Towne | 44.8 ± 6.2 | 21.9 ± 9.1 | 3.1 ± 6.6 |
| Toledo | 43.4 ± 3.0 | 42.4 ± 2.8 | 21.6 ± 5.6 |
| Clinical strain | |||
| Hei | ND | 42.5 ± 4.1 | 12.1 ± 3.2 |
Monocyte oxidative activities were compared between PBMC incubated with different strains of inactivated HCMV at different MOI equivalents (see legend to Fig 1). Assays were performed in triplicate at day 4. Results are presented as the percentage of inhibition (mean ± SD) corresponding to the ratio between the mean of the OD values obtained with HCMV and the mock preparation multiplied by 100.
To determine whether the suppressive effect on monocytes could be induced directly by HCMV, purified monocytes (adult human elutriated monocytes) were incubated for 4 days with inactivated Toledo strain at 0.02 MOI equivalent and AD169 at 0.02, 0.2, and 2 MOI equivalent. No difference in the oxidative activity or cell appearance was observed compared to cells incubated with the mock preparation (data not shown). These results suggest that the HCMV-induced suppressive effect requires an interaction with the nonadherent cells in the PBMCs.
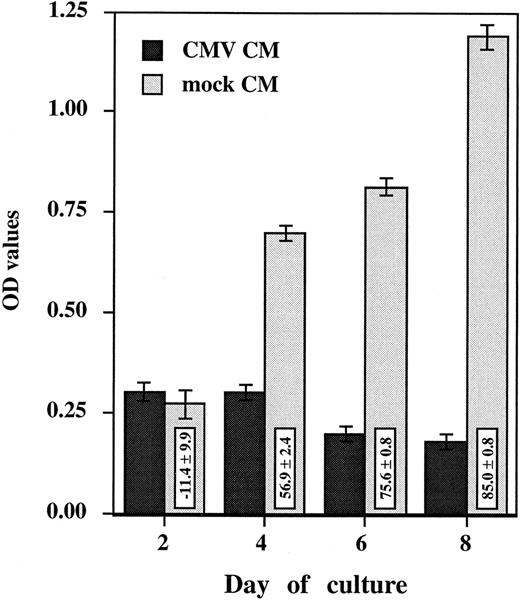
HCMV-induced suppressive effect is mediated by a soluble factor.To investigate the role of a soluble factor responsible for HCMV-induced suppressive effects, purified monocytes were incubated with the day 2 virus-free CM (see Materials and Methods). Monocyte oxidative activity and morphologic changes were assessed after 2, 4, 6, and 8 days of culture (Figs 3 and 4). Purified monocytes treated with the mock CM showed a time-dependent increase in oxidative activity (from 0.272 ± 0.036 at day 2 to 1.190 ± 0.031 at day 8) (Fig 3) that was closely correlated with the occurrence of morphologic changes characterizing in vitro monocyte differentiation (Fig 4). The cells flattened and spread, increasing greatly in size and developing a higher cytoplasm to nucleus ratio. After 6 days, obvious membrane ruffles and an increased number of cytoplasmic granules and lipid inclusions were observed. In contrast, the oxidative activity of purified monocytes treated with the CMV CM remained low, under 0.301 (Fig 3), and was consistantly associated with an undifferentiated phenotype. The monocytes remained small, rounded with prominent membrane ridges and folds (Fig 4). The inhibition of oxidative activity in cells treated with CMV CM ranged from 56.9% ± 2.4% at day 4 to 85.0% ± 0.8% at day 8. The observed changes were not caused by a general cytopathic or toxic effect because no difference in cell viability was seen (as measured by trypan blue exclusion) and the total cell numbers were similar. These results suggest that HCMV induced the production of a soluble factor that is responsible for a block in monocyte differentiation.
Virus-free CMV conditioned medium (CMV CM) suppresses monocyte oxidative activity. Purified primary monocytes were incubated with day 2 virus-free CMV and mock conditioned medium (CM). The day 2 CMV and mock CM (50 μL) was added together with 150 μL of medium. Every 2 days, 100 μL was removed from each well and replaced by 50 μL of CM and 50 μL of fresh medium. The oxidative assay was performed in triplicate, after 2, 4, 6, and 8 days. Results, presented as the mean ± SD of the OD values, are representative of two independent experiments. The boxes contain the percentage of oxidative activity inhibition (mean ± SD) corresponding to the ratio between the OD value obtained with HCMV and the mock preparation multiplied by 100.
Virus-free CMV conditioned medium (CMV CM) suppresses monocyte oxidative activity. Purified primary monocytes were incubated with day 2 virus-free CMV and mock conditioned medium (CM). The day 2 CMV and mock CM (50 μL) was added together with 150 μL of medium. Every 2 days, 100 μL was removed from each well and replaced by 50 μL of CM and 50 μL of fresh medium. The oxidative assay was performed in triplicate, after 2, 4, 6, and 8 days. Results, presented as the mean ± SD of the OD values, are representative of two independent experiments. The boxes contain the percentage of oxidative activity inhibition (mean ± SD) corresponding to the ratio between the OD value obtained with HCMV and the mock preparation multiplied by 100.
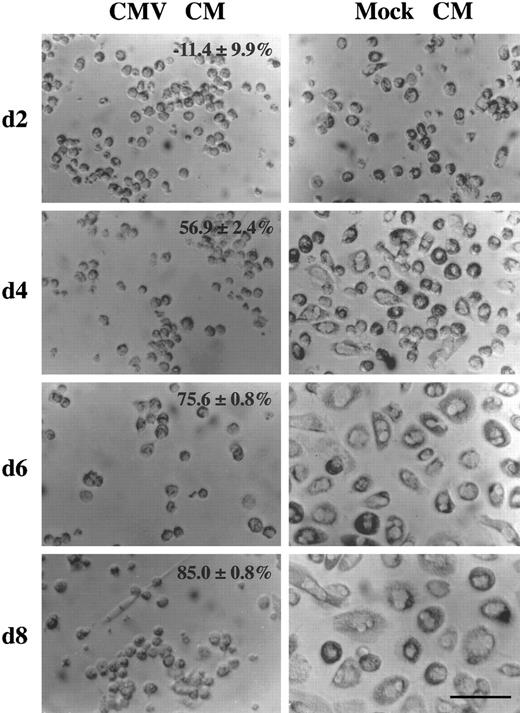
HCMV-induced factor alters monocyte differentiation. Purified human primary monocytes were incubated with the day 2 virus-free CMV CM (picture on the left) or the mock CM (picture on the right) (see legend to Fig 3). The percentage of oxidative activity inhibition (mean ± SD) are shown. The scale is shown by the bar which is equal to 80 μm. These results are representative of two independent experiments. After 2 days of culture, approximatly 20% of the monocytes incubated with the mock CM appeared to be slightly bigger in size and some of them had started to spread. However, monocytes incubated with the CMV CM remain small, rounded with prominent membrane ridges. At this time, no significant difference in monocyte oxidative activity is detected (see Fig 3). After 4 days, morphologic changes were obvious, with the majority of the monocytes flattened and spread, increased greatly in size, and showing a higher cytoplasm to nucleus ratio. In contrast, cells treated with the CMV CM maintained the phenotype of undifferentiated monocytes. In correlation, the oxidative activity of mock CM treated cells dramatically increased from 0.272 ± 0.036 to 0.698 ± 0.011, whereas it remained low, 0.301 ± 0.019, in CMV CM–treated monocytes (Fig 3). After 6 days, the cell size increased and the cell spreading was more pronounced. The cytoplasm to nucleus ratio was significantly augmented and membrane ruffles and numerous cytoplasmic granules and lipid inclusions were observed. In parallel, the oxidative activity of mock CM treated cells increased to 0.815 ± 0.022 but remained low, 0.199 ± 0.002, in CMV CM–treated monocytes (Fig 3). After 8 days, mock CM–treated monocytes presented similar morphologic characteristics as day 6 with increasing cell size and increasing oxidative activity (Fig 3), whereas these parameters remained unchanged in CMV CM–treated monocytes.
HCMV-induced factor alters monocyte differentiation. Purified human primary monocytes were incubated with the day 2 virus-free CMV CM (picture on the left) or the mock CM (picture on the right) (see legend to Fig 3). The percentage of oxidative activity inhibition (mean ± SD) are shown. The scale is shown by the bar which is equal to 80 μm. These results are representative of two independent experiments. After 2 days of culture, approximatly 20% of the monocytes incubated with the mock CM appeared to be slightly bigger in size and some of them had started to spread. However, monocytes incubated with the CMV CM remain small, rounded with prominent membrane ridges. At this time, no significant difference in monocyte oxidative activity is detected (see Fig 3). After 4 days, morphologic changes were obvious, with the majority of the monocytes flattened and spread, increased greatly in size, and showing a higher cytoplasm to nucleus ratio. In contrast, cells treated with the CMV CM maintained the phenotype of undifferentiated monocytes. In correlation, the oxidative activity of mock CM treated cells dramatically increased from 0.272 ± 0.036 to 0.698 ± 0.011, whereas it remained low, 0.301 ± 0.019, in CMV CM–treated monocytes (Fig 3). After 6 days, the cell size increased and the cell spreading was more pronounced. The cytoplasm to nucleus ratio was significantly augmented and membrane ruffles and numerous cytoplasmic granules and lipid inclusions were observed. In parallel, the oxidative activity of mock CM treated cells increased to 0.815 ± 0.022 but remained low, 0.199 ± 0.002, in CMV CM–treated monocytes (Fig 3). After 8 days, mock CM–treated monocytes presented similar morphologic characteristics as day 6 with increasing cell size and increasing oxidative activity (Fig 3), whereas these parameters remained unchanged in CMV CM–treated monocytes.
To determine whether the undifferentiated phenotype could have consequences on monocyte phagocytic function, the phagocytic capacity of purified monocytes incubated for 4 days with either the CMV CM or the mock CM was compared. As measured by a flow cytometric assay that quantifies the phagocytosis of fluorescent microspheres, we found a lower phagocytic capacity of monocytes incubated with the CMV CM than the mock CM, 8.9% versus 21.8% of the cells presented more than 7 fluorescent microspheres per cell.
HCMV-induced suppressive effects are mediated through IFN-α.To identity the HCMV-induced factor responsible for the inhibition of monocyte differentiation, the CMV CM and the mock CM cytokine profile were compared. From the results presented in Table 2, those obtained for IFN-α were found to be unique. IFN-α was present in CMV CM from HCMV seropositive and seronegative PBMCs but not in the mock CM. The observations that the level of IFN-α was similar in immune and nonimmune PBMCs and that HCMV-associated suppression of monocyte oxidative activity was more pronounced in HCMV immune PBMCs suggested that other cytokines, induced in a secondary immune response to HCMV, may play a role in HCMV-associated suppression. To address the mechanisms of HCMV-induced immunosuppression reflected in a primary infection, we focused on results obtained in the absence of preexisting immunity (ie, PBMCs from HCMV seronegative donors). Therefore, in subsequent experiments, PBMCs from seronegative donors were used.
Cytokine Profile of the CMV CM Compared With the Mock CM
| Cytokines . | . | CMV CM . | Mock CM . |
|---|---|---|---|
| IL-4 | − | <2 | <2 |
| + | 7.6 | <2 | |
| IL-10 | − | 9.9 | 8.6 |
| + | 68.9 | 17.6 | |
| TNF-α | − | 74.8 | 158.7 |
| + | 408.4 | 251.4 | |
| TNF-β | − | <8 | <8 |
| + | ND | ND | |
| IFN-α | − | 48.5 | <8 |
| + | 55.8 | <8 | |
| IFN-γ | − | 6.4 | <4 |
| + | 207.2 | <4 |
| Cytokines . | . | CMV CM . | Mock CM . |
|---|---|---|---|
| IL-4 | − | <2 | <2 |
| + | 7.6 | <2 | |
| IL-10 | − | 9.9 | 8.6 |
| + | 68.9 | 17.6 | |
| TNF-α | − | 74.8 | 158.7 |
| + | 408.4 | 251.4 | |
| TNF-β | − | <8 | <8 |
| + | ND | ND | |
| IFN-α | − | 48.5 | <8 |
| + | 55.8 | <8 | |
| IFN-γ | − | 6.4 | <4 |
| + | 207.2 | <4 |
Inactivated HCMV (To/pso, 0.002 MOI equivalent) or the mock preparation were added to either PBMC from a HCMV seronegative (−) or seropositive (+) donor. Cytokines present in CM were quantified by ELISA, results are presented in pg/mL.
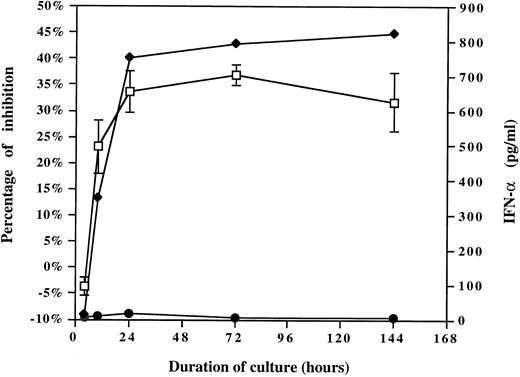
To evaluate the role of IFN-α, the kinetics of IFN-α release were compared with the kinetics ability of HCMV-induced factor(s) to suppress monocyte oxidative activity. Supernatants were collected after increasing periods of PBMCs exposure to HCMV or mock preparation (inactivated Toledo virus, 0.001 MOI equivalent) and either added to the purified monocytes or tested by ELISA to determine the quantity of IFN-α. The MTS/PMS assay was performed after 4 days. As shown in Fig 5, an excellent correlation was observed between the ability of HCMV-induced factor(s) to suppress monocyte oxidative activity and the kinetics of IFN-α release. IFN-α was rapidly produced in the first 4 to 10 hours, the time at which the suppressive activity was detected in the supernatant of HCMV-treated PBMCs. IFN-α concentration in correlation with the extent of monocyte oxidative inhibition increased to reach a plateau after 1 day through 3 days. These results support IFN-α as the cytokine responsible for the suppression of monocyte maturation.
The kinetics of HCMV-induced factor(s) ability to suppress monocyte oxidative activity correlates with the kinetics of IFN-α release. The kinetics of HCMV-induced factor(s) activity (left part of the graph) were compared with the kinetics of IFN-α release (right part of the graph). CM were collected after increasing periods of HCMV or mock preparation exposure to PBMCs (inactivated Toledo virus, 0.001 MOI equivalent) and either added to the purified monocytes or tested by ELISA to determine the quantity of IFN-α. The CM was added to the purified monocytes, and after 4 days the MTS/PMS assay was performed. (♦), Quantity of IFN-α present in the CMV CM; (•), quantity of IFN-α present in the mock CM; (□), percentage of monocyte oxidative activity inhibition (mean ± SD).
The kinetics of HCMV-induced factor(s) ability to suppress monocyte oxidative activity correlates with the kinetics of IFN-α release. The kinetics of HCMV-induced factor(s) activity (left part of the graph) were compared with the kinetics of IFN-α release (right part of the graph). CM were collected after increasing periods of HCMV or mock preparation exposure to PBMCs (inactivated Toledo virus, 0.001 MOI equivalent) and either added to the purified monocytes or tested by ELISA to determine the quantity of IFN-α. The CM was added to the purified monocytes, and after 4 days the MTS/PMS assay was performed. (♦), Quantity of IFN-α present in the CMV CM; (•), quantity of IFN-α present in the mock CM; (□), percentage of monocyte oxidative activity inhibition (mean ± SD).
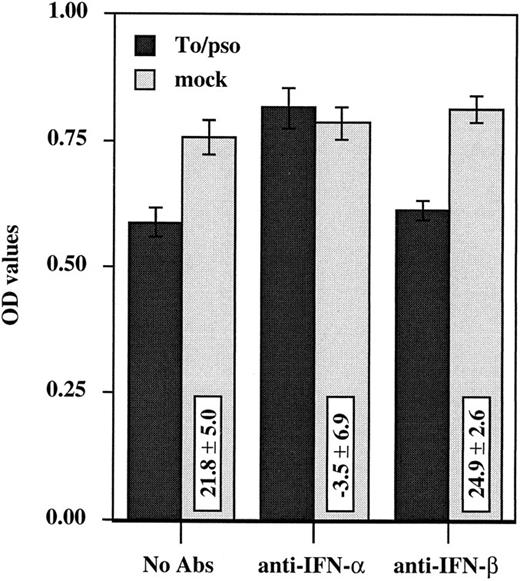
To further determine the role of IFN-α in this system, anti–IFN-α neutralizing antibody was used in an attempt to block the suppressive activity. HCMV-induced suppression of monocyte oxidative activity was blocked by the addition of anti–IFN-α neutralizing antibody, −3.5% ± 6.9% of inhibition versus 21.8% ± 5.0% with no antibody (P = .001, one-tailed unpaired t-test), whereas anti–IFN-β neutralizing antibody had no effect on HCMV-induced inhibition, 24.9% ± 2.6% versus 21.8% ± 5.0% with no antibody (P = .455, one-tailed unpaired t-test) (Fig 6). These two polyclonal antibodies presented no enhancing or suppressive effect on monocyte oxidative activity of the mock CM-treated cells, confirming the specificity of the blocking capacity of the anti–IFN-α antibodies. Furthermore, anti–IFN-α neutralizing antibody was able to restore monocyte differentiation as determined by cell morphologic appearence. Finally, purified human natural IFN-α could reproduce the identical changes on the morphologic characteristics and the oxidative activity of purified monocytes. Increasing amounts of purified IFN-α were added to the cells, and the oxidative activity was measured after 4 days of culture. A dose-dependent response was observed: 3.4% ± 5.9%, 13.2% ± 1.0%, 27.3% ± 2.7%, 46.7% ± 3.3%, and 71.0% ± 0.8% of inhibition corresponding to 10-fold dilutions ranging from 50 pg/mL to 500,000 pg/mL. Taken together, these data identify IFN-α as the cytokine responsible for HCMV-induced suppressive effects on monocytes.
Anti–IFN-α neutralizing antibody blocks HCMV-induced suppression of monocyte oxidative activity. Anti–IFN-α or anti–IFN-β neutralizing polyclonal antibodies (100 U/mL) and inactivated cell-free HCMV (Toledo virus, 0.002 MOI equivalent) (dark bars) or the mock preparation (light bars) were added simultaneously to PBMCs cultures at day 0. Assays were performed in triplicate and the oxidative assay was performed at day 3. Three independent experiments were performed and results are presented as the mean ± SD of the OD values.
Anti–IFN-α neutralizing antibody blocks HCMV-induced suppression of monocyte oxidative activity. Anti–IFN-α or anti–IFN-β neutralizing polyclonal antibodies (100 U/mL) and inactivated cell-free HCMV (Toledo virus, 0.002 MOI equivalent) (dark bars) or the mock preparation (light bars) were added simultaneously to PBMCs cultures at day 0. Assays were performed in triplicate and the oxidative assay was performed at day 3. Three independent experiments were performed and results are presented as the mean ± SD of the OD values.
To confirm that it was the interaction of HCMV with the nonadherent cells in the PBMCs that was responsible for IFN-α production, we compared the quantity of IFN-α present in the CM after 1 day of HCMV exposure in the following cultures: the adherent cell fraction (mainly monocytes), the nonadherent cell fraction (mainly lymphocytes), and the reconstituted cell fractions. IFN-α was present in the supernatant of the nonadherent cell fraction and to the same extent in the reconstituted cell fractions, but not within the first 4 days in the adherent cell fraction (data not shown). These results indicate that HCMV-induced IFN-α production requires HCMV interaction with the nonadherent cells in the PBMCs.
CMV-induced factor impairs the lymphocyte proliferative response to mitogen and recall antigen.To evaluate the role of HCMV-induced soluble factor in HCMV-associated inhibition of lymphocyte proliferation, we compared the proliferative response of PBMCs incubated with cell-free inactivated virus or CMV CM with their respective controls, mock preparation and mock CM. As shown in Table 3, lymphocyte proliferative response to either mitogens (PHA and PWM) or recall antigen (TT) was significantly decreased in the presence of cell-free HCMV as well as CMV CM. The percentages of inhibition observed with cell-free HCMV (PHA: 29% ± 1%, PWM: 23% ± 5%, and TT: 51% ± 9%) were close to those obtained with CMV CM (PHA: 31% ± 5%, PWM: 32% ± 4%, and TT: 35% ± 6%). After 6 days, a significant decrease of lymphocyte proliferation was also noticed in nonstimulated PBMCs. These results show that the HCMV-induced factor accounts for the proliferative defect observed in PBMCs incubated with HCMV.
CMV-Induced Factor(s) Impairs the Lymphocyte Proliferative Response to Mitogens and Recall Antigen
| . | Mock Preparation . | Cell-Free HCMV . | Inhibition (%) . | P Value . | Mock CM . | CMV CM . | Inhibition (%) . | P Value . |
|---|---|---|---|---|---|---|---|---|
| Mitogens (day 3) | ||||||||
| Control medium | 2.2 ± 1.23-150 | 1.7 ± 0.83-150 | 2.4 ± 0.63-150 | 2.0 ± 1.03-150 | ||||
| PHA | 193.9 ± 16.1 | 137.2 ± 11.7 | 29 ± 13-151 | <.0013-152 | 189.2 ± 19.8 | 130.5 ± 22.8 | 31 ± 53-151 | <.0013-152 |
| PWM | 58.7 ± 15.5 | 45.1 ± 11.2 | 23 ± 5 | .026 | 63.0 ± 16.5 | 43.3 ± 12.5 | 32 ± 4 | .005 |
| Recall antigen (day 6) | ||||||||
| Control medium | 33.9 ± 18.0 | 15.2 ± 9.2 | 45.4 ± 19.4 | 11.2 ± 6.5 | ||||
| TT | 124.4 ± 15.1 | 61.5 ± 16.9 | 51 ± 9 | <.001 | 121.9 ± 18.6 | 80.1 ± 16.5 | 35 ± 6 | <.001 |
| . | Mock Preparation . | Cell-Free HCMV . | Inhibition (%) . | P Value . | Mock CM . | CMV CM . | Inhibition (%) . | P Value . |
|---|---|---|---|---|---|---|---|---|
| Mitogens (day 3) | ||||||||
| Control medium | 2.2 ± 1.23-150 | 1.7 ± 0.83-150 | 2.4 ± 0.63-150 | 2.0 ± 1.03-150 | ||||
| PHA | 193.9 ± 16.1 | 137.2 ± 11.7 | 29 ± 13-151 | <.0013-152 | 189.2 ± 19.8 | 130.5 ± 22.8 | 31 ± 53-151 | <.0013-152 |
| PWM | 58.7 ± 15.5 | 45.1 ± 11.2 | 23 ± 5 | .026 | 63.0 ± 16.5 | 43.3 ± 12.5 | 32 ± 4 | .005 |
| Recall antigen (day 6) | ||||||||
| Control medium | 33.9 ± 18.0 | 15.2 ± 9.2 | 45.4 ± 19.4 | 11.2 ± 6.5 | ||||
| TT | 124.4 ± 15.1 | 61.5 ± 16.9 | 51 ± 9 | <.001 | 121.9 ± 18.6 | 80.1 ± 16.5 | 35 ± 6 | <.001 |
The proliferative response of PBMC incubated with cell-free inactivated HCMV was compared with the proliferative response of PBMC incubated with CMV CM. The inactivated HCMV and the mock preparation or the day 2 CMV CM and the mock CM were added at day 0 and maintained in culture.
3H-thymidine incorporation assays are shown as the mean cpm/well ± SD multiplied per 103, obtained with 3 different PBMC cultures from HCMV seronegative donors. Each assay was done in triplicate.
Percentage of inhibition = 100 minus (value obtained with HCMV or CMV CM/its mock control multiplied by 100).
P value, comparing the proliferative response obtained with cell-free HCMV or CMV CM with their respective mock control.
To further investigate the role of IFN-α as the cytokine responsible for HCMV-associated inhibition of lymphocyte proliferation, the ability of anti–IFN-α neutralizing antibody to reverse the HCMV-induced inhibition of lymphocyte proliferation was examined. The proliferative response of PBMCs incubated with the CM obtained either from PBMCs incubated for 1 day with HCMV or the mock preparation alone, or in the presence of anti–IFN-α neutralizing antibody or the control nonimmune sheep serum was examined (Table 4). The addition of anti–IFN-α neutralizing antibody restored the proliferative response of PBMCs incubated with HCMV in variable ranges depending on the activator used. When nonimmune sheep serum was added to the cultures, the extent of HCMV-associated inhibition was not significantly different from what was observed in the absence of antibody (24% ± 5% v 26% ± 1% for PHA-driven proliferation and 23% ± 7% v 27% ± 9% for PWM-driven proliferation and inhibition was more pronounced in response to TT (70% ± 2% v 53% ± 7%) (data not shown). Thus, reductions in the inhibition of proliferation observed with the IFN-α neutralizing antibody were specific for IFN-α. The PHA and TT-driven proliferative responses were restored in a significant manner when anti–IFN-α neutralizing antibody was added to HCMV cultures. This was demonstrated by the significant difference between the inhibition of the proliferative response obtained in the presence of anti–IFN-α neutralizing antibody compared with the control nonimmune sheep serum (12% ± 1% inhibition v 23% ± 7%, P = .039 and 35% ± 7% v 70% ± 2%, P < .001, respectively). The addition of anti–IFN-α neutralizing antibody also resulted in a trend toward reduced HCMV-associated suppression of PWM-driven lymphocyte proliferation from 24% ± 5% inhibition of lymphocyte proliferation in the presence of nonimmune sheep serum to 14% ± 4% inhibition (P = .07). These results show that IFN-α plays an important role in HCMV-associated suppression of lymphocyte proliferation in response to PHA and recall Ag. In addition, IFN-α may be involved, albeit to a lesser extent, in reduced proliferation after PWM stimulation.
Anti–IFN-α Neutralizing Antibody Restores the Lymphocyte Proliferative Response to Mitogen and Recall Antigen
| . | IFN-α Neutralizing Antiserum . | Nonimmune Sheep Serum . | P Value . | ||||
|---|---|---|---|---|---|---|---|
| . | Mock CM . | CMV CM . | Inhibition (%) . | Mock CM . | CMV CM . | Inhibition (%) . | . |
| Mitogens (day 3) | |||||||
| Control medium | 1.2 ± 0.44-150 | 0.9 ± 0.44-150 | 1.7 ± 0.44-150 | 0.8 ± 0.24-150 | |||
| PHA | 155.4 ± 31.2 | 130.5 ± 23.0 | 14 ± 44-151 | 153.0 ± 27.2 | 112.8 ± 16.0 | 24 ± 54-151 | .039‡ |
| PWM | 28.6 ± 11.6 | 24.4 ± 8.6 | 12 ± 1 | 24.6 ± 7.8 | 19.0 ± 4.7 | 23 ± 7 | .073 |
| Recall antigen (day 6) | |||||||
| Control medium | 25.6 ± 7.5 | 11.0 ± 7.4 | 32.6 ± 0.4 | 6.8 ± 1.9 | |||
| TT | 48.8 ± 4.1 | 31.8 ± 5.8 | 35 ± 7 | 54.5 ± 8.6 | 16.8 ± 3.2 | 70 ± 2 | <.001 |
| . | IFN-α Neutralizing Antiserum . | Nonimmune Sheep Serum . | P Value . | ||||
|---|---|---|---|---|---|---|---|
| . | Mock CM . | CMV CM . | Inhibition (%) . | Mock CM . | CMV CM . | Inhibition (%) . | . |
| Mitogens (day 3) | |||||||
| Control medium | 1.2 ± 0.44-150 | 0.9 ± 0.44-150 | 1.7 ± 0.44-150 | 0.8 ± 0.24-150 | |||
| PHA | 155.4 ± 31.2 | 130.5 ± 23.0 | 14 ± 44-151 | 153.0 ± 27.2 | 112.8 ± 16.0 | 24 ± 54-151 | .039‡ |
| PWM | 28.6 ± 11.6 | 24.4 ± 8.6 | 12 ± 1 | 24.6 ± 7.8 | 19.0 ± 4.7 | 23 ± 7 | .073 |
| Recall antigen (day 6) | |||||||
| Control medium | 25.6 ± 7.5 | 11.0 ± 7.4 | 32.6 ± 0.4 | 6.8 ± 1.9 | |||
| TT | 48.8 ± 4.1 | 31.8 ± 5.8 | 35 ± 7 | 54.5 ± 8.6 | 16.8 ± 3.2 | 70 ± 2 | <.001 |
PBMC were incubated with cell-free inactivated HCMV or with the mock preparation in the absence or in the presence of anti–IFN-α neutralizing antibody or nonimmune sheep serum. Antibody was added at the beginning of the culture and after 6, 15, and 21 hours. The CM were obtained after 24 hours, treated to remove the virus, and then tested in the proliferative assay.
3H-thymidine incorporation assays are shown as the mean cpm/well ± SD multiplied per 103, obtained with 3 different PBMC cultures from CMV seronegative donors. Each assay was done in triplicate.
Percentage of inhibition = 100 minus (value obtained with CMV CM/its mock control multiplied by 100).
P value = one-tailed unpaired t-test, the proliferative responses obtained with CM from PBMC incubated with HCMV in the presence of anti-IFN-α neutralizing antibody was compared with the nonimmune sheep serum.
DISCUSSION
Viral infections have long been associated with immunosuppression. A variety of mechanisms have been identified by which viruses can directly or indirectly suppress immunity.20 Included in the category of host suppressor factors is the family of IFNs. In addition to their antiviral effects, the IFN family of proteins has been shown to mediate a wide range of activities comprising inhibition of cell growth, the involvement in hematopoiesis, and regulatory effects on cellular and humoral immune responses.21 We report in this paper that IFN-α is an important mediator of HCMV-associated immunosuppression. During secondary HCMV infection or exposure, other cytokines (eg, IFN-γ) may play a role in the suppression observed. However, during primary exposure, IFN-α appeared to be the only cytokine responsible for an apparent block in monocyte maturation and function which is demonstrated by an inhibition of monocyte differentiation, a low oxidative activity, and an inhibition of phagocytic activity. In addition, we present evidence that IFN-α accounts for the HCMV-associated inhibition of the lymphocyte proliferative responses to mitogen and recall antigen.
IFNs are produced in response to a variety of stimuli including viral, bacterial, mycoplasmal, and tumor cell agents. As has been described for herpes simplex virus type 1,22 we have shown that PBMCs are efficient producers of IFN-α in response to live as well as inactivated cell-free HCMV, indicating that HCMV replication is not required for IFN-α induction in this system. As indicated by the apparent low number of viral particles per cell used in our experiments (0.02 to 0.0002 MOI equivalent), HCMV, with the exception of AD169, appeared to be a powerful IFN-α inducer. In our experiments, AD169 exposure of PBMCs (0.002 MOI) resulted in 40-fold less IFN-α production when compared with Toledo strain (10.4 pg/mL v 432.8 pg/mL, day 2). Furthermore, levels of IFN-α induced by those two viruses correlated with their ability to suppress monocyte oxidative activity (12.2% ± 1.8% v 42.8% ± 7.1%, respectively). This is in agreement with AD169 being a poorly immunosuppressive virus, as shown in previous studies.4,5,7,15,23,24 However, a considerable proportion of HCMV particles present in virus stocks may be either defective particles or not infectious in human foreskin fibroblasts.25 Thus, the ability of different HCMV strains to alter monocyte maturation may be dependent on factors other than their ability to induce IFN-α.
We have found that HCMV-associated suppression of monocyte maturation and function is the result of an indirect mechanism that requires HCMV interaction with the nonadherent cells in the PBMCs, that are the IFN-α–producing cells. There has been much conflicting data reported in the literature regarding the cellular origin of IFN-α/β. Most recently, attention has focused on a nonadherent population of cells within the peripheral blood identified as low-density HLA-DR+ cells but not T cells, B cells, NK cells, stem cells, or macrophages. These cells, presenting several characteristics of dendritic cells, have been termed “natural interferon producing cells” or NIPC. Although many cells in the body are able to produce IFNs under appropriate stimulus, the NIPC along with the monocytes have emerged as the most important IFN-α–producing populations in the peripheral blood.22 Our data would support the NIPC population as the cells responsible for HCMV-stimulated IFN-α production.
Clinical data suggest that HCMV-associated alterations in human alveolar macrophage function may contribute to morbidity from pulmonary superinfection in CMV-infected patients.26,27 In addition, MCMV infection of alveolar macrophages and alterations in their phagocytic and oxidative activity have been described in experimental primary MCMV infection in animals that develop concomitant MCMV interstitial pneumonia.28,29 In vitro, incubation of PBMCs with infectious HCMV but not with ether-inactivated virus markedly suppressed monocyte phagocytic activity, as determined by suppressing phagocytosis of radiolabeled erythrocytes and the attendant monocyte respiratory burst, as shown by a chemiluminescence assay.5 Using the same cellular system, with inactivated virus, we observed that IFN-α is responsible for a similar HCMV-induced suppression as demonstrated by reduced monocyte maturation that was correlated with oxidative activity and a lower phagocytic activity. The importance of the envelope component of viruses to induce IFN-α production in PBMCs22 may explain the discrepancy between the nonsuppressive property of either inactivated HCMV5 (that disrupts the virus envelope) and the suppressive property of psoralen/UV inactivated HCMV (that preserves the viral particle structure). Our results are in agreement with the known immunomodulating property of IFN-α on monocytes/macrophages.21 Virus-induced IFN-α production may be a general mechanism by which viruses affect monocyte/macrophage function. For example, suppression of the monocyte respiratory burst of normal PBMCs in response to PMA stimulation has been associated with HHV-6, a herpesvirus closely related to HCMV.30 The suppressive effect was mediated by a soluble factor and was specific to the protein kinase C (PKC) pathway which is known to be involved in the mechanism of IFN-α signal transduction.31
IFN-α also accounts for the HCMV-associated suppression of the lymphocyte proliferative response to mitogens and recall antigens. The addition of anti–IFN-α neutralizing antibody results in a significant but uncomplete restoration of lymphocyte proliferation, suggesting that other cytokines may be involved. Whether or not the antiproliferative effect of IFN-α is the result of a direct effect on the lymphocytes or an indirect effect on the monocyte/macrophage accessory cell function remains to be established. Previous reports have attributed HCMV-associated immunosuppression to a generalized immunologic hyporesponsiveness referred to as anergy, involving both lymphocyte and monocyte function. Among the different mechanisms that can be proposed to explain HCMV suppressive properties, the virus-induced production of IFN-α is very attractive.
Extensive immunosuppressive effects of HCMV have been shown with no evidence of productive infection5,14,15 and IFN-α production is induced with no virus gene expression. In agreement with the increased suppression of PHAdriven proliferative responses observed after HCMV infection of PBL rather than monocytes alone,10,11 we demonstrated that HCMV does not directly affect the monocyte/macrophage function that requires virus interaction with the nonadherent cells in the PBMCs, which are the IFN-α–producing cells. The HCMV-associated proliferative defect has been associated with a reduced production and/or activity of IL-2 and IL-16,7,11 and with the induction of an IL-1 inhibitor.6 In this regard, IFN-α can modulate the synthesis of several cytokines, especially the proinflammatory cytokines (IL-1, TNF-α, IL-6, and IL-8), which are downregulated.32-35 Furthermore, IFN-α is a potent inducer of the IL-1 receptor antagonist,36 which could be the previously reported IL-1 inhibitor. HCMV-associated immunosuppression like the IFN-α production is a transient phenomenon, with early profound dysfunctions that improve to normal responsiveness over a period of weeks or months. Furthermore, preculture of mononuclear leukocytes from HCMV mononucleosis patients significantly increases their response to Con A, and was related to a loss of suppressor cell function as well as the release of a soluble suppressor factor.2,3 Finally, IFN-α has been proposed to be part of a general mechanism by which viruses can cause bone marrow suppression or bone marrow failure.21 HCMV and HHV6 are known to suppress bone marrow progenitor cells growth and differentiation.24,37 A soluble factor has been implicated in both HCMV and HHV6-associated myelosuppression30,38,39 and Knox and Carrigan38 have shown that suppression is largely mediated by IFN-α.
IFN-α induction and immunosuppression are common features in the early course of viral infection. The biologic significance of this transient state in terms of advantage or disadvantage for the infected host remains unclear. The establishment of antiviral state, growth inhibition, and immunomodulation appear to be nonspecific mechanisms that may contribute to the antiviral response. IFN-α–treated monocytes may have been directed toward an alternate pathway of macrophage differentiation that maintains the monocyte cytotoxic state.40 This is an important aspect of the primary natural immune defense and may constitute a control mechanism to limit virus infection. On the other hand, this state may promote the immunologic disorders frequently associated with viral infections20 and favor the occurrence of opportunistic infections.
Supported by Public Health Service Grants No. AI28270, AI27670, and AI36214 to the UCSD Center for AIDS Research, and MH45294.
Address reprint requests to Stephen A. Spector, MD, University of California, San Diego, Clinical Sciences Building, 0672, 9500 Gilman Dr, La Jolla, CA 92093-0672.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal