Abstract
There is evidence to suggest that elevated plasma levels of lipoprotein (a) [Lp(a)] represent a risk factor for the development of atherosclerotic vascular disease, but the mechanism by which this lipoprotein localizes to involved vessels is only partially understood. In view of studies suggesting a link between inflammation and atherosclerosis and our previous finding that leukocyte defensin modulates the interaction of plasminogen and tissue-type plasminogen activator with cultured human endothelial cells, we examined the effect of this peptide on the binding of Lp(a) to cultured vascular endothelium and vascular smooth muscle cells. Defensin increased the binding of Lp(a) to endothelial cells approximately fourfold and to smooth muscle cells approximately sixfold. Defensin caused a comparable increase in the amount of Lp(a) internalized by each cell type, but Lp(a) internalized as a consequence of defensin being present was not degraded, resulting in a marked increase in the total amount of cell-associated lipoprotein. Abundant defensin was found in endothelium and in intimal smooth muscle cells of atherosclerotic human cerebral arteries, regions also invested with Lp(a). These studies suggest that defensin released from activated or senescent neutrophils may contribute to the localization and persistence of Lp(a) in human vessels and thereby predispose to the development of atherosclerosis.
ATHEROSCLEROSIS is a multifactorial disease process in which no single risk factor correlates with its incidence or predicts for its severity in all individuals. However, there is considerable epidemiological evidence to implicate lipoprotein(a) [Lp(a)] as one of the factors that predisposes to the premature development of coronary artery and cerebrovascular disease.1-4 Furthermore, Lp(a) is found in human atherosclerotic vessels and in experimental, diet-induced atherosclerosis,5-7 and transgenic mice expressing human Lp(a) develop extensive atherosclerotic lesions in their aortae.8,9 A putative mechanism by which Lp(a) promotes atherosclerosis has been proposed based on its similarity in structure to plasminogen. In vitro, Lp(a) competes with plasminogen for binding to cell surfaces and to fibrin, thereby impeding plasmin formation which, in turn, may retard clot lysis.10-13 Binding of Lp(a) may also impair plasmin-mediated conversion of transforming growth factor-β to an active form required to inhibit smooth muscle cell proliferation.9,14 15
Despite these observations, the relationship between the plasma level of Lp(a) and the development of human atherosclerosis continues to be a subject of controversy.16,17 This uncertainty may reflect the involvement of additional factors that regulate the tissue deposition or atherogenic effect of Lp(a)18 that are heterogeneously expressed or that occur sporadically in the population. For example, there is evidence to suggest that infection and vascular inflammation may predispose to the development of atherosclerosis in some individuals. A correlation between the prevalence of atherosclerosis and the number of circulating granulocytes has been identified in several population studies.16,19-26 Furthermore, cardiac allograft rejection,27 recent bacterial infection,28 hemodialysis,29,30 and autoimmune vascular disease29,30 have all been implicated as factors that may accelerate the development of atherosclerosis. More recently, persistent vascular infection with various microorganisms, including DNA viruses31,32 and chlamydia,33-37 has been implicated based on in situ analysis of involved tissue.
One link between inflammation and the development of atherosclerosis may lie in the effect of leukocyte defensins on vascular cells. Leukocyte defensins comprise a family of closely related cationic peptides38-42 found in the granules of neutrophils, where they constitute approximately 5% of the total cellular protein.41 Current evidence suggests that, during phagocytosis, defensins become incorporated into the cell membranes of eukaryotic organisms, disrupting ion fluxes and eventually inducing target cell lysis.43 During this process, defensins are released into the circulation, as a result of which the plasma concentration, which is normally less that 15 nmol/L, may approach 50 μmol/L during severe infection.44
The release of defensins into the extracellular environment may contribute to some of the pathophysiological consequences of inflammation. For example, we recently reported that defensins promote the binding of plasminogen to fibrin and to cultured human endothelial cells but inhibit fibrinolysis mediated by tissue-type plasminogen activator (tPA),45,46 effects reminiscent of those reported for Lp(a).47 The mechanism of this inhibition appears to involve kringle-kringle like interactions.45,47,48 In light of the fact that there is a similarity between the kringle structures of the apo(a) portion of Lp(a) and that of plasminogen,49 we asked whether defensins can modulate the interaction of Lp(a) with cultured human vascular cells as well and whether defensins are found in human atherosclerotic lesions at sites where Lp(a) is deposited. The outcome of this study suggests a potential role for defensin in the uptake and retention of Lp(a) in human atherosclerotic vessels.
MATERIALS AND METHODS
Materials and cell culture.Defensin (human neutrophil peptide-2) and Lp(a) were prepared, iodinated, and characterized as previously described.38,43,46,50 Cultures of human umbilical vein endothelial cells (HUVEC) and umbilical vein smooth muscle cells (SMC) were prepared and characterized as previously described.51,52 Human smooth muscle cells from descending abdominal aorta were from Clonetics (San Diego, CA). Rabbit polyclonal and murine monoclonal antidefensin antibodies were prepared against highly purified HNP-1 as described.38,53 Both antibodies also recognize HNP-2 and HNP-3, but not HNP-4,5 and 6.38,53 Monoclonal antibodies to nonoverlapping determinants on apo(a) were prepared and characterized as previously described.54 Platelet factor 4 (kindly provided by Drs G. Arepally and M. Poncz, University of Pennsylvania, Philadelphia, PA) was prepared as described.55
Binding of defensin to HUVECs and SMCs.Cells were grown to confluence in 48-well Falcon Multiwell tissue culture dishes (Becton Dickinson, Lincoln Park, NJ) to a final density of approximately 5 × 104 cells/well. To measure binding, the cells were prechilled to 4°C for 30 minutes and washed twice with prechilled binding buffer composed of phosphate-buffered saline (PBS) and 1% bovine serum albumin. 125I-Lp(a) was added alone or in the presence of defensin in the same buffer for 90 minutes at 4°C in the absence of unlabeled competitor to determine total binding or in the presence of 100-fold molar excess unlabeled ligand to determine nonspecific binding. In each case, unbound ligand was removed, the cells were washed four times with binding buffer, the cell surface-associated radioactivity was released with 50 mmol/L glycine HCl, pH 2.8, and the residual cell-associated radioactivity was counted after adding 1 N NaOH. Specific binding was defined as the difference between total and nonspecific binding. In other experiments, HUVECs were preincubated with 20 μmol/L defensin for 1 hour at 4°C, and the cells were washed twice with in PBS and incubated with 30 nmol/L 125I-Lp(a). To examine the role of apo(a), Lp(a) was preincubated with a combination of monoclonal apo(a) antibodies to nonoverlapping determinants54 (final concentration) or with the same concentration of control murine IgG before being added to HUVECs and the binding of Lp(a) was then measured. Similar experiments were performed in the presence or absence of 6 mmol/L 6-aminohexanoic acid (6-AHA; Sigma, St Louis, MO).
Two sets of experiments were also performed to determine whether defensin and Lp(a) form complexes in solution. First, 125I-defensin (200 nmol/L) was incubated with Lp(a) (200 nmol/L), albumin (200 nmol/L) in PBS, or PBS alone (200 μL final volume of each) for 60 minutes at 22°C and then loaded on a Sephacryl S-100 column (Pharmacia Biotech, Piscataway, NJ). The radioactivity in successive 0.5-mL fractions was measured and the protein concentration in each fraction was estimated by measuring the optical density (OD) at 280 nm. Second, 125I-defensin or 125I-defensin-Lp(a) complexes isolated from the Sephacryl S-100 column were incubated with a 1:500 dilution of rabbit IgG anti-apoprotein(a) [apo(a)] overnight at 4°C in radioimmunoprecipitation assay (RIPA) buffer composed of 50 mmol/L Tris HCl, pH 7.5, 0.1% sodium deoxycholate, 100 mmol/L NaCl, and 1 mmol/L EDTA. Protein A-Sepharose CL-4B (Pharmacia) beads (30 μL) prewashed with RIPA buffer were added to each sample, mixed well, and incubated at 22°C for 1 hour on a rotator. The beads were centrifuged, washed three times with RIPA buffer, and counted for radioactivity.
Effect of defensin on the binding, internalization, and degradation of Lp(a).Binding of 125I-Lp(a) to HUVECs and to SMCs was determined in a similar manner to that described above for defensin. In this case, the cells were incubated at 37°C for 60 minutes in the presence of various concentration of 125I-Lp(a) alone or 125I-Lp(a) added in the presence of the indicated concentrations of defensin. Cell-associated radioactivity was eluted with 50 mmol/L glycine HCl, pH 2.8. In some experiments, total cell-associated radioactivity was then measured in replicate wells after adding 1 N NaOH. The difference, acid inelutable radioactivity, was taken as a measure of internalization. To measure degradation, 15 nmol/L 125I-Lp(a) was added to SMCs at 37°C for 18 hours in the presence or absence of defensin. The supernatant was removed, trichloroacetic acid (10% final concentration) was added, and the nonprecipitable counts were measured. The results of a similarly treated cell-free control were subtracted in each case to determine cell-specific degradation.
Immunohistochemical analysis of atherosclerotic vessels.Specimens of cerebral and systemic vessels were collected during autopsies performed at the Hospital of the University of Pennsylvania. The clinical summary of the autopsy report of each patient was reviewed independently from the immunohistologic data to identify risk factors for atherosclerosis (hypertension, diabetes mellitus, cigarette use, lipid disorders, and whole brain irradiation) and for evidence of clinical atherosclerosis (coronary artery disease, myocardial infarction, ischemic cerebral infarction, and peripheral vascular disease; see Tables 1 and 2). The presence of gross or microscopic atherosclerosis in the coronary arteries and the aorta, derived from the autopsy reports, was graded as none, minimal, moderate, or severe by the reporting pathologist.
The specimens were analyzed approximately 2 weeks after fixation in 10% buffered formalin as described.7 The specimens were embedded in paraffin, cut in 5-μm sections, and mounted on Micro-Probe slides for immunohistochemical study. Two slides per patient were also stained with hematoxylin and eosin and evaluated by light microscopy. The extent of atherosclerosis in the sections was graded as follows: 0, no evidence of atherosclerosis, defined as normal and consistent thickness and diameter of the vessel without intimal or medial thickening; 1, minimal change and/or fatty streaks, defined as subendothelial proliferation and/or focal accumulation of foam cell macrophages causing slight increase in the vessel wall thickness, usually without affecting the lumen; 2, fibromuscular atherosclerotic plaque with more extensive thickening of the intima as a result of smooth muscle cell proliferation and/or deposition of collagen; 3, advanced atherosclerotic plaque with fibrous deposition, intracellular (lipid-laden macrophages) and/or intercellular lipid deposition (lipid crystals and clefts); 4, complex plaque with necrosis, ulceration, calcification, thrombosis, or hemorrhage superimposed on the features of the advanced plaque.
The UltraProbe Universal Immunostaining Kit from Biomeda Corp (Foster City, CA) was used to evaluate the presence of defensin and apo(a) in vessels. The procedure uses avidin biotin complex (ABC) with alkaline phosphatase as the marker enzyme and Fast Red/Naphthol phosphate as the substrate-chromogen reagent. The kit was used in the MicroProbe system (Fisher Scientific, Pittsburgh, PA) with the capillary gap technology. Rabbit polyclonal and mouse monoclonal antidefensin antibodies were used at a concentration of 8 μg/mL. The purified anti-apo(a) monoclonal antibody was used as previously described.7 56 Secondary antibodies were prepared in 2% normal human serum. To control for nonspecific binding, replicate sections were incubated with PBS, secondary antibody only, and irrelevant Igs (normal rabbit serum and isotype-matched murine monoclonal Ig).
RESULTS
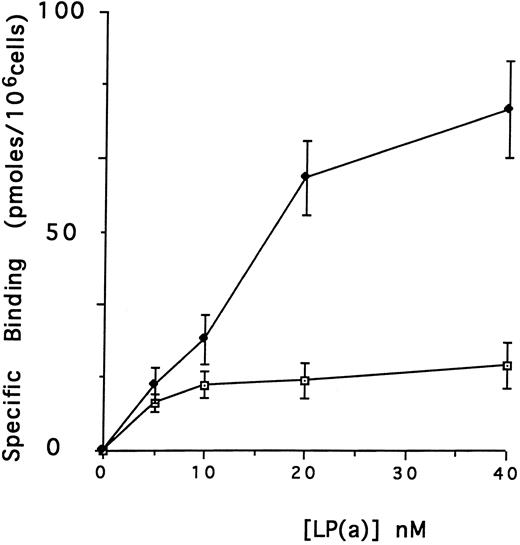
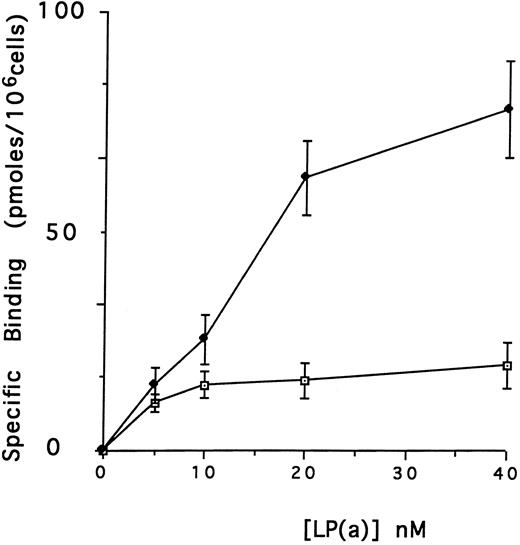
Effect of defensin on the association of Lp(a) with human vascular cells in vitro.The first goal of this study was to examine the effect of defensin on the binding, internalization, and degradation of Lp(a) by human vascular cells. Initial studies were performed using HUVECs, which have been reported previously to bind defensin.46 125I-Lp(a) bound specifically to HUVECs added in the presence or in the absence of defensin (Fig 1A). In each case, binding was specific, dose-dependent, and saturable. In the absence of defensin, half maximal binding of Lp(a) was achieved at a concentration of 5 nmol/L. Defensin stimulated the binding of Lp(a) to HUVECs. The Bmax for Lp(a) was increased 4.2-fold in the presence of defensin, consistent with an increase in the total number of binding sites. In the presence of defensin, half maximal binding was achieved at a concentration of 16.5 nmol/L, consistent with the generation of addition binding sites with slightly reduced affinity for the ligand.
Defensin stimulates the binding of Lp(a) to vascular endothelial cells. HUVECs were incubated with 0 to 40 nmol/L 125I-Lp(a) alone or in the presence of 3 μmol/L defensin for 4 hours at 4°C and the specific binding was determined. Binding of Lp(a) in the absence (⊡) and in the presence (♦) of defensin. The mean ± SD of three experiments is shown.
Defensin stimulates the binding of Lp(a) to vascular endothelial cells. HUVECs were incubated with 0 to 40 nmol/L 125I-Lp(a) alone or in the presence of 3 μmol/L defensin for 4 hours at 4°C and the specific binding was determined. Binding of Lp(a) in the absence (⊡) and in the presence (♦) of defensin. The mean ± SD of three experiments is shown.
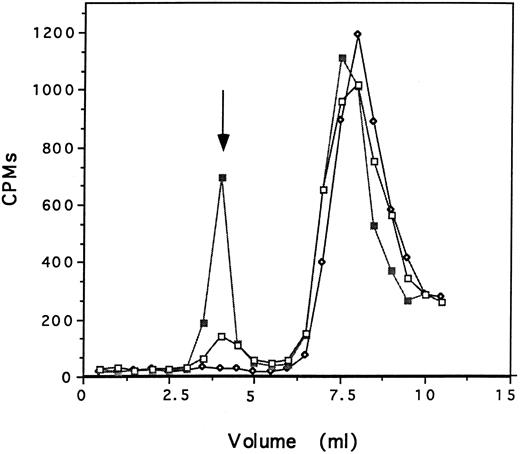
The specificity of the interaction of defensin and Lp(a) with HUVECs was examined in several ways. First, the capacity of 125I-defensin to form complexes with Lp(a) was examined in two ways. In the first set of experiments, 125I-defensin (200 nmol/L), preincubated with Lp(a) (200 nmol/L) or with albumin (200 nmol/L) or with buffer alone was loaded onto a Sephacryl S-100 column and the formation of complexes was examined by measuring the radioactivity and protein content of successive 0.5-mL fractions. 125I-defensin formed complexes with Lp(a) when the reactants were added at equimolar concentrations as shown by the presence of high molecular weight radioactive peaks. Little complex formation was seen when defensin was incubated with comparable concentrations of albumin and no complexes were seen in the absence of added protein (Fig 2). There was no change in the migration of Lp(a) as a result of this incubation, excluding the formation of higher molecular weight aggregates under the experimental conditions. In the second set of experiments, fractions from this column containing 125I-defensin (fraction 16) and 125I-defensin-Lp(a) complexes (fraction 8) were incubated with anti-apo(a) and immunoprecipitated using Staph protein A. In the absence of Lp(a), 0.7% of the available counts were precipitated nonspecifically, whereas 36% of the total counts were precipitated when antibody was added to 125I-defensin-Lp(a) complexes; antidefensin antibody immunoprecipitated 54% of the counts measured in parallel. Taken together, these experiments show that defensin and Lp(a) can form complexes in solution.
Defensin and Lp(a) form complexes in solution. 125I-defensin (200 nmol/L) was incubated with either Lp(a) (200 nmol/L), albumin (200 nmol/L) in 200 μL PBS, or PBS alone for 60 minutes at 22°C. The mixture was loaded onto a Sephacryl S-100 column and the radioactivity and the OD at 280 nm in each 0.5-mL fraction was measured. 125I-defensin alone (⋄); 125I-defensin plus Lp(a) (▪); 125I-defensin plus albumin (□). The arrow denotes the peak OD280 for Lp(a) in the presence and absence of defensin. One experiment representative of two so performed is shown.
Defensin and Lp(a) form complexes in solution. 125I-defensin (200 nmol/L) was incubated with either Lp(a) (200 nmol/L), albumin (200 nmol/L) in 200 μL PBS, or PBS alone for 60 minutes at 22°C. The mixture was loaded onto a Sephacryl S-100 column and the radioactivity and the OD at 280 nm in each 0.5-mL fraction was measured. 125I-defensin alone (⋄); 125I-defensin plus Lp(a) (▪); 125I-defensin plus albumin (□). The arrow denotes the peak OD280 for Lp(a) in the presence and absence of defensin. One experiment representative of two so performed is shown.
Second, experiments were then performed to determine whether defensin and Lp(a) must form a soluble complex before binding to the cells. To do this, HUVEC were preincubated with defensin (20 μmol/L) and washed before adding 125I-Lp(a) (30 nmol/L). Cells preincubated with defensin bound 5.5-fold more 125I-Lp(a) than did cells incubated in the absence of defensin, an increment comparable to that seen when both ligands were added simultaneously. Experiments were then performed to determine whether defensin stimulated the binding of numerous kringle containing proteins and whether the effect was mediated exclusively though its cationic charge. Defensin (20 μmol/L) had no effect on the binding of tPA46 or thrombin (up to 200 nmol/L) to HUVECs, and human PF4, which is of comparable size and cationic charge to defensin, had no effect on the binding of Lp(a) (50 nmol/L) even at the maximal concentrations of defensin used in this study (20 μmol/L; not shown). Fourth, experiments were performed to examine the contribution of the apo(a) moiety to the binding induced by defensin. The capacity of defensin to stimulate the binding of plasminogen is sensitive to 6-AHA.46 In contrast, 50 mmol/L 6-AHA had no effect on the binding of 125I-Lp(a) induced by defensin (not shown). Binding of Lp(a) (30 nmol/L) by defensin (20 μmol/L) was inhibited 65% by a combination of monoclonal antibodies to nonoverlapping determinants on apo(a) compared with the same concentration of murine IgG (n = 2).
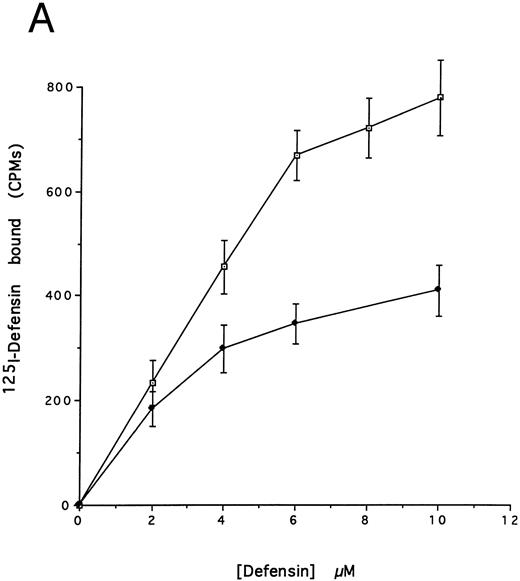
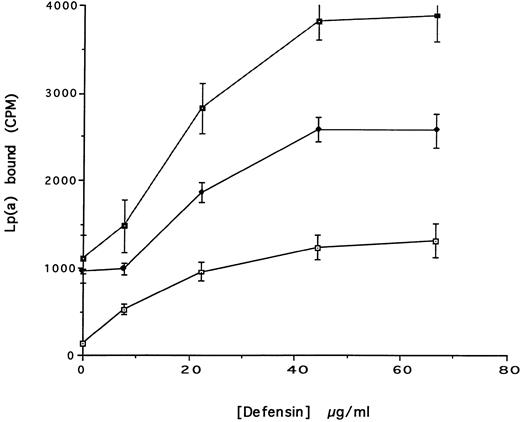
(A) Binding of defensin to vascular smooth muscle cells. 125I-defensin was incubated with HVSMCs alone or in the presence of 100-fold molar excess unlabeled defensin and the cell-associated radioactivity was determined. (⊡) Total binding; (♦) specific binding. The mean ± SD of three experiments is shown. (B) Defensin stimulates the binding of Lp(a) to HVSMCs. Cells were incubated with the indicated concentrations of 125I-Lp(a) alone or in the presence of 3 μmol/L defensin for 4 hours and the specific binding was determined. Binding of Lp(a) in the absence (⊡) and in the presence (♦) of defensin. The mean ± SD of three experiments is shown.
(A) Binding of defensin to vascular smooth muscle cells. 125I-defensin was incubated with HVSMCs alone or in the presence of 100-fold molar excess unlabeled defensin and the cell-associated radioactivity was determined. (⊡) Total binding; (♦) specific binding. The mean ± SD of three experiments is shown. (B) Defensin stimulates the binding of Lp(a) to HVSMCs. Cells were incubated with the indicated concentrations of 125I-Lp(a) alone or in the presence of 3 μmol/L defensin for 4 hours and the specific binding was determined. Binding of Lp(a) in the absence (⊡) and in the presence (♦) of defensin. The mean ± SD of three experiments is shown.
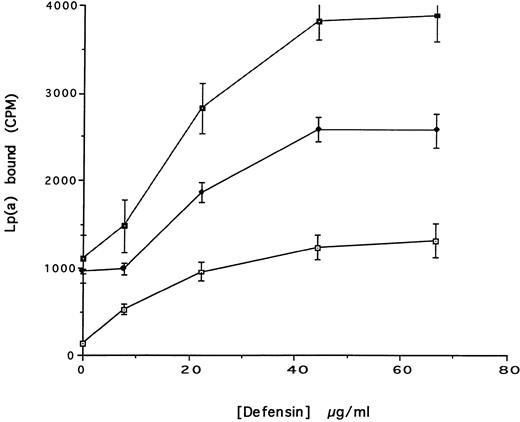
Defensin stimulates the internalization of 125I-Lp(a) by vascular smooth muscle cells. 125I-Lp(a) (15 nmol/L) was incubated with HVSMC for 4 hours at 37°C in the presence of the indicated concentrations of defensin. The cells were washed and cell surface bound and internalized radioactivity was measured as described. (▪) Total cell-associated Lp(a); (♦) internalized Lp(a); (⊡) surface-bound Lp(a). The mean ± SD of three experiments is shown.
Defensin stimulates the internalization of 125I-Lp(a) by vascular smooth muscle cells. 125I-Lp(a) (15 nmol/L) was incubated with HVSMC for 4 hours at 37°C in the presence of the indicated concentrations of defensin. The cells were washed and cell surface bound and internalized radioactivity was measured as described. (▪) Total cell-associated Lp(a); (♦) internalized Lp(a); (⊡) surface-bound Lp(a). The mean ± SD of three experiments is shown.
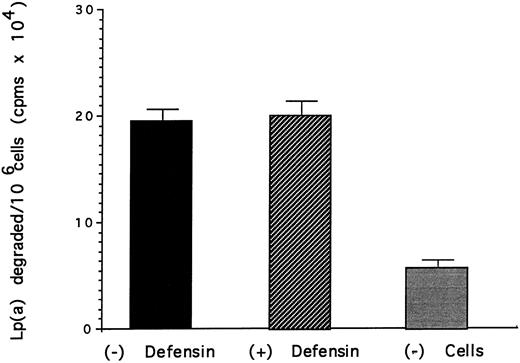
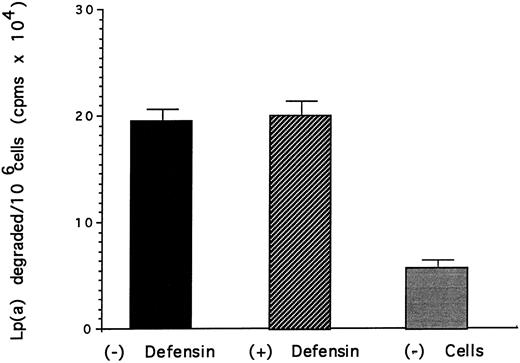
Effect of defensin on the degradation of Lp(a) by vascular smooth muscle cells. HVSMC was incubated with 125I-Lp(a) (15 nmol/L) alone or in the presence of the indicated concentrations of defensin for 24 hours at 37°C. The media was removed and the TCA-soluble counts were measured as a marker of degradation. The mean ± SD of three experiments is shown.
Effect of defensin on the degradation of Lp(a) by vascular smooth muscle cells. HVSMC was incubated with 125I-Lp(a) (15 nmol/L) alone or in the presence of the indicated concentrations of defensin for 24 hours at 37°C. The media was removed and the TCA-soluble counts were measured as a marker of degradation. The mean ± SD of three experiments is shown.
We then asked whether defensin had a similar effect on the binding of Lp(a) to human vascular smooth muscle cells. Defensin bound in a specific, dose-dependent, and saturable manner to SMC (Fig 3A). Half maximal binding was attained at a defensin concentration of 3.2 μmol/L. The presence of defensin had an even more marked effect on the binding of Lp(a) to SMC than to HUVECs causing an approximately sixfold increase in the observed Bmax (Fig 3B), consistent with the higher number of defensin binding sites expressed by this cell type. Defensin (20 μmol/L) stimulated the binding of Lp(a) to human SMC derived from the descending aorta to the same extent as to SMCs from the umbilical vein (∼5-fold, n = 2).
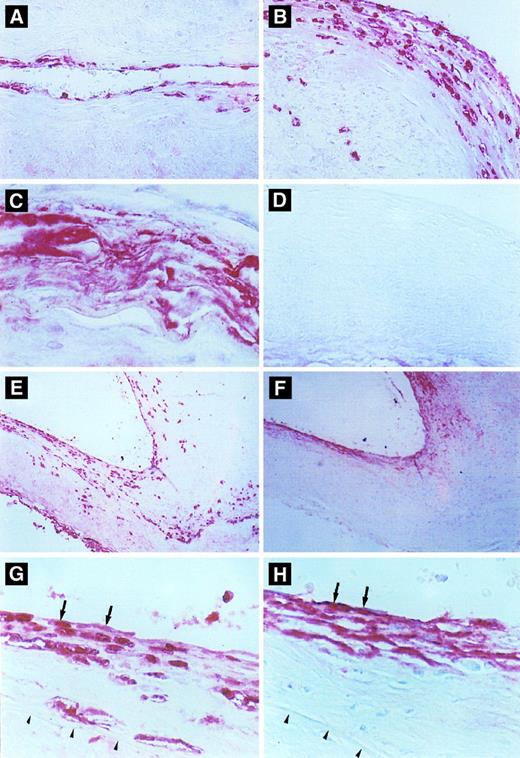
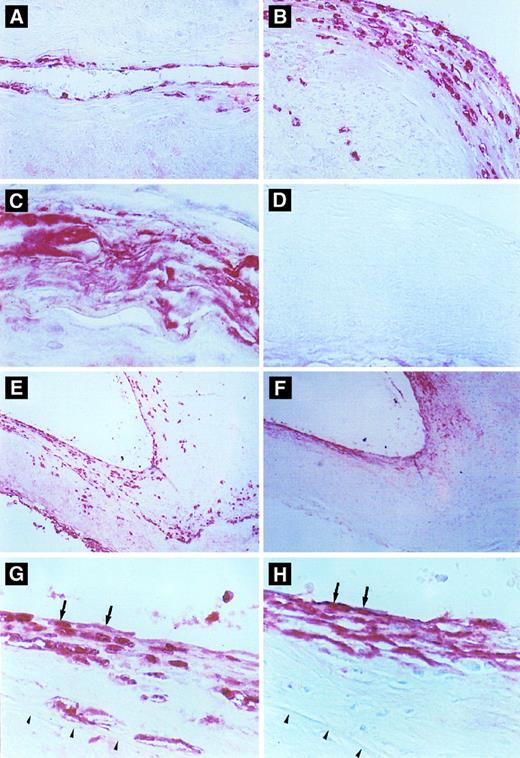
Immunohistochemical detection of defensin in cerebral vessels. (A) Cerebral vessel with mild thickening of the intima due to atherosclerotic changes. Note focal immunostaining for defensin within the cytoplasm of endothelial cells and occasional intimal cells. (B) A more severely affected vessel with extensive endothelial cell and intimal staining for defensin and staining of occasional cells within the media. (C) An advanced atherosclerotic plaque with intense, diffuse immunostaining for defensin, predominantly in extracellular locations. (D) Atherosclerotic vessel incubated with control Ig. (E and F ) Comparison between staining for defensin (E) and Lp(a) (F ) in adjacent sections of an atherosclerotic cerebral vessel. In some portions of the vessel, defensin and Lp(a) are coexpressed in the endothelium and portions of the intima (left side), whereas in other regions the distribution of the two molecules differs slightly (right side). This slide is representative of the finding that Lp(a) was found in the absence of defensin predominantly in less cellular regions of the vessel wall, whereas staining for defensin was more focal and cell-associated and reached deeper into the intimal, media, and adventitia. (G) and (H) are adjacent sections taken from the margin of an atherosclerotic plaque. The internal elastic membrane is delineated by the arrows. Staining of several endothelial cells for both defensin and Lp(a) is shown by the arrows.
Immunohistochemical detection of defensin in cerebral vessels. (A) Cerebral vessel with mild thickening of the intima due to atherosclerotic changes. Note focal immunostaining for defensin within the cytoplasm of endothelial cells and occasional intimal cells. (B) A more severely affected vessel with extensive endothelial cell and intimal staining for defensin and staining of occasional cells within the media. (C) An advanced atherosclerotic plaque with intense, diffuse immunostaining for defensin, predominantly in extracellular locations. (D) Atherosclerotic vessel incubated with control Ig. (E and F ) Comparison between staining for defensin (E) and Lp(a) (F ) in adjacent sections of an atherosclerotic cerebral vessel. In some portions of the vessel, defensin and Lp(a) are coexpressed in the endothelium and portions of the intima (left side), whereas in other regions the distribution of the two molecules differs slightly (right side). This slide is representative of the finding that Lp(a) was found in the absence of defensin predominantly in less cellular regions of the vessel wall, whereas staining for defensin was more focal and cell-associated and reached deeper into the intimal, media, and adventitia. (G) and (H) are adjacent sections taken from the margin of an atherosclerotic plaque. The internal elastic membrane is delineated by the arrows. Staining of several endothelial cells for both defensin and Lp(a) is shown by the arrows.
The effect of defensin on the disposition of Lp(a) bound to HUVEC and SMC was then studied. Defensin stimulated the internalization of Lp(a) by SMC proportional to its effect on binding (Fig 4). In contrast, defensin had no effect on the total amount of Lp(a) degraded by SMC over 18 hours (Fig 5). Defensin had the same effect on the internalization and degradation of Lp(a) by HUVECs (not shown). Thus, the net effect of defensin was to markedly increase the total amount of cell-associated Lp(a).
Expression of defensin in human atherosclerotic vessels.The second goal of this study was to determine whether defensin is also present in the endothelium and intimal smooth muscle cells of human atherosclerotic vessels, sites where Lp(a) is localized.7 Atherosclerotic and nonatherosclerotic vessels within the circle of Willis from 28 patients were analyzed for the presence of defensin and Lp(a). Both defensin and Lp(a) were located primarily within areas of vessels involved with atherosclerosis (Fig 6). Identical results were obtained using polyclonal (Fig 6) and monoclonal antidefensin antibodies (not shown). No staining of the vessels was observed when control antibodies were used or when secondary antibody was omitted (Fig 6D).
The distribution and intensity of staining for defensin and apo(a) correlated closely with the severity of the atherosclerotic changes as reflected by both the stage and the morphology of the plaque. In areas where the vessel morphology and thickness was normal and where the endothelium was intact, little or no defensin or Lp(a) were detected, although neutrophils within the lumens of the vessels stained intensely (not shown). In normal-appearing vessel wall with minimal subendothelial thickening due to proliferation of smooth muscle cells, defensin was found focally in the cytoplasm of endothelial cells. The intensity of staining at these sites varied from speckles within the cytoplasm of an occasional endothelial cell to more intense staining of the entire cytoplasm of several endothelial cells in a row (Fig 6A and G). In areas of early plaque formation with subintimal fibrous deposition and smooth muscle proliferation, focal to diffuse staining in the subluminal region of the atheromatous plaque was seen in addition to staining of the endothelium (Fig 6A [right side] and G). Defensin was found primarily within the cytoplasm of endothelial cells as well as cells having the appearance of smooth muscle cells, although staining of an occasional macrophage or fibroblast could not be excluded. In general, the intensity of the staining correlated with the extent of the proliferative changes within these lesions (Fig 6B), although a few atherosclerotic vessels showed no staining for defensin. In plaques showing significant deposition of lipid and fibrous material, macrophages, cholesterol clefts, and significant thickening of the vessel wall, defensin was located predominantly in the endothelial and subendothelial regions, although in some regions outside the intima the appearance of defensin tended to be more diffuse (Fig 6C) consistent with the appearance of apo(a) at these sites.7 Not all the fibrinoid deposits within any particular plaque stained for apo(a) or defensin, suggesting that the antibodies did not cross-react or colocalize with fibrin (not shown). In contrast, a strong correlation was found between the intensity of staining for defensin and Lp(a) in 15 of the 28 patients studied (Tables 1 and 2), whereas in the remaining cases, expression of one or the other protein predominated. However, in almost every case, both proteins were present in the intima (Fig 6E through H). Both proteins were found in most plaques in which smooth muscle proliferation was evident (Fig 6E and F [right side]) whereas acellular, fibrous atherosclerotic plaques showed less intense staining for defensin (Fig 6E and F [left side]). Staining for defensin was also seen in the media and adventitia on occasion, whereas staining for the larger apo(a) molecule was restricted to the intima. Colocalization of Lp(a) and defensin was seen in some cells (Fig 6G and H).
DISCUSSION
The interaction of Lp(a) with vascular cells may contribute to the development of atherosclerosis in some individuals. Lp(a) is found within the endothelium and intimal smooth muscle cells of involved vessels at an early stage in lesion development.7 Deposition of the lipoprotein increases as lesion development proceeds.7 The mechanism by which Lp(a) becomes localized to these sites is largely unknown. It has been reported that Lp(a) binds to cellular matrices in vitro57-60 through kringle-dependent and kringle-independent processes,59 a process that is followed by endocytosis and degradation.50,61,62 Lp(a) also binds to the fibrin found within atherosclerotic plaques.5,63-65 Yet, the available data do not provide a complete explanation for how Lp(a) is retained at sites where atherosclerosis develops.18
The results of the present study suggest one mechanism by which Lp(a) may localize and be retained in vessel walls. Leukocyte defensins bind specifically to cultured human vascular smooth muscle cells as well as to HUVECs and to fibrin.45,46 Binding of Lp(a) to SMC and HUVEC increased proportionally with the amount of defensin bound (Fig 4). The mechanism by which defensin stimulates the binding of Lp(a) is uncertain. Binding induced by defensin was not simply the result of a cationic detergent effect because no increase in Lp(a) binding was induced by a comparably charged molecule PF4. Rather, the answer may lie in part in the fact that defensin also stimulates the binding of plasminogen to cells and to fibrin,45,46 although not to the same extent as was seen with Lp(a). These interactions may result from binding of the exposed arginines on cell-associated defensin to the lysine binding sites in the kringles of Lp(a), similar to the mechanism proposed for kringle-kringle interactions among other plasma proteins.47 48 In accord with this possibility, defensin and Lp(a) were observed to form complexes in solution (Fig 2), and approximately two-thirds of the binding of Lp(a) induced by defensin was blocked by monoclonal anti-apo(a) antibodies.
Lp(a) bound to HUVECs and SMC was internalized as evidenced by the progressive loss of the ability to remove the ligand from the cells by acid elution (Fig 4). However, the additional Lp(a) bound and internalized by both cell types as a consequence of defensin was not degraded (Fig 5). For example, although defensin increased the amount of Lp(a) internalized by SMC approximately sixfold, there was essentially no increase in the amount of ligand degraded at 24 hours. The mechanism by which defensin-Lp(a) complexes are relatively protected from undergoing degradation requires further study. Defensin may shield amino acid residues most subject to degradation by lysosomal enzymes. Alternatively, defensin may alter the trafficking of defensin by preventing fusion of endosomes with lysosomal membranes or by directing the endosomes towards another pathway. Whichever mechanism is operative, the capacity of defensin to promote the binding and internalization of Lp(a) while preventing is rapid degradation may contribute to its retention at sites where atherosclerotic changes begin.
The relevance of the effects of defensin on Lp(a) binding observed in cell culture experiments is supported by the immunohistochemical analysis of human cerebral vessels involved by atherosclerosis in vivo. These studies show that defensin is present on and within the endothelial cells, in the subjacent matrix, and within intimal smooth muscle cells in involved vessels, regions in which Lp(a) is found as well. In contrast, the expression of defensin in histologically uninvolved vessels is sparse to absent. Deposition of both proteins increases as the lesions become more extensive. Defensin is also found in the deeper layers of the vessels in the absence of Lp(a), but its effect on matrix or adipocyte behavior is unknown. In more advanced lesions, the expression of defensin is more diffuse, similar to the pattern seen with apo(a).7 Also, Lp(a) may be found at some sites in the absence of defensin, suggesting either catabolism of the defensin and/or involvement of additional tissue binding sites for the lipoprotein.
The contribution of defensin to the deposition of Lp(a) in atherosclerotic lesions requires further study. For example, the sequence with which defensin and Lp(a) become deposited within the vessels and the relationship between the presence of either protein and smooth muscle cell proliferation cannot be inferred from a cross-sectional analysis of histologic sections. However, taking the results of the in vitro and in vivo studies together, we hypothesize that defensin, a small peptide, may be readily transported across endothelial barriers, permitting the molecule to gain access to the subendothelial matrix and the intimal smooth muscle cells. Defensin may be retained at these sites, affording a binding site for the larger Lp(a) particle. Our data do not exclude the possibility complexes formed in the blood between Lp(a) and defensin released from senescent or activated leukocytes bind directly to the endothelial cell surface. In either case, the cell-associated defensin-Lp(a) complexes are internalized but appear to be relatively resistant to lysosomal degradation. This may have the effect of facilitating either the transport of these complexes across the endothelium or their release into the intima as a consequence of cell activation, injury, or cell turnover. Thus, the results of the present study suggest that leukocyte defensins or related molecules may play a role in the localization of Lp(a) in the vasculature at sites where atherosclerosis develops.
Supported in part by National Institutes of Health Grants No. HL40387, HL50970, HL49517, and HL 54749 (D.B.C.) and by American Heart Association Grant No. 960105000 (A.A.H.).
Address reprint requests to Abd Al-Roof Higazi, MD, Coagulation Laboratory, 7 Founders Pavilion, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104.