Key Points
The efficacy of AMG 330 against AML cells is greatly enhanced by combining it with a STING agonist.
IFN-γ plays a key role in AMG 330–mediated cytotoxicity against AML cells and in rendering AML cells responsive to STING agonism.
Visual Abstract
T-cell–recruiting bispecific antibodies (BsAbs) are in clinical development for relapsed/refractory acute myeloid leukemia (AML). Despite promising results, early clinical trials have failed to demonstrate durable responses. We investigated whether activation of the innate immune system through stimulator of interferon (IFN) genes (STING) can enhance target cell killing by a BsAb targeting CD33 (CD33 bispecific T-cell engager molecule; AMG 330). Indeed, we show that cytotoxicity against AML mediated by AMG 330 can be greatly enhanced when combined with the STING agonist 2′,3′-cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) or diamidobenzimidazole (diABZI). We used in vitro cytotoxicity assays, immunoblotting, transcriptomic analyses, and extensive CRISPR-Cas9 knockout experiments to investigate the enhancing effect of a STING agonist on the cytotoxicity of AMG 330 against AML. Importantly, we validated our findings with primary AML cells and in a xenograft AML model. Mechanistically, in addition to direct cytotoxic effects of STING activation on AML cells, activated T cells render AML cells more susceptible to STING activation through their effector cytokines, IFN-γ and tumor necrosis factor, resulting in enhanced type I IFN production and induction of IFN-stimulated genes. This feeds back to the T cells, leading to a further increase in effector cytokines and an overall cytotoxic T-cell phenotype, contributing to the beneficial effect of cGAMP/diABZI in enhancing AMG 330–mediated lysis. We established a key role for IFN-γ in AMG 330–mediated cytotoxicity against AML cells and in rendering AML cells responsive to STING agonism. Here, we propose to improve the efficacy of CD33-targeting BsAbs by combining them with a STING agonist.
Introduction
Despite an increase in therapeutic options for acute myeloid leukemia (AML) over the past 5 years, outcomes remain poor. Allogeneic hematopoietic stem cell transplantation remains the most effective antileukemic treatment strategy,1 but it is limited to a minority of patients due to age and comorbidities. It is well recognized that T cells within a stem cell graft are the key mediators of antileukemic activity.2 The success of prophylactic, preemptive, and therapeutic donor lymphocyte infusion further serves as a proof of concept that T cells can combat AML.3 In B-cell malignancies, alternative T-cell–based immunotherapies, such as bispecific antibodies (BsAbs) and chimeric antigen receptor (CAR) T cells, have successfully translated into clinics.4,5 Although early clinical trials in myeloid malignancies demonstrated proof of concept and confirmed safety, they yielded much lower response rates than B-cell malignancies. Moreover, no long-term responses have been observed so far.6-9 The majority of early clinical trials in AML are using BsAbs that are directed against myeloid lineage antigens such as CD33, CD123, or CLL-1. AMG 330, a bispecific T-cell engager (BiTE) molecule directed against the CD33 target antigen and the CD3-epsilon subunit of the T-cell receptor, was tested in 60 patients with AML with advanced-stage disease in an early phase 1 trial.10,11
The importance of reprogramming the tumor microenvironment to enhance the effectiveness of T-cell–based immunotherapy is becoming increasingly apparent. Strategies are evolving to target innate immune system receptors to unleash natural antitumor responses. One pathway that holds great promise in this context is the cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS)–stimulator of interferon (IFN) genes (STING) pathway.12 cGAS and its downstream signaling adapter STING act as intracellular sensors for cytoplasmic DNA present during viral infection.13 Binding of cGAS to cytosolic DNA generates the second messenger 2′,3′-cyclic guanosine monophosphate–adenosine monophosphate (cGAMP), which binds to and activates STING. This triggers the phosphorylation of the transcription factor IRF3, which in turn induces an antiviral transcriptional state. Most prominently, the induced antiviral program is characterized by the release of antiviral cytokines of the type I IFN family. The secreted IFNs in turn transfer the antiviral signal to neighboring cells and immune cells. Additionally, costimulatory molecules can be induced through cGAS-STING activation.14 These, in concert with the IFNs, can potently prime and enhance adaptive immune responses. The great therapeutic potential of pharmacologically manipulating the cGAS-STING pathway for cancer immunotherapy is reflected in the numerous ongoing clinical trials with different STING agonists, mainly for the treatment of solid tumors.12
The objective of our study was to investigate whether T-cell–recruiting BsAbs against AML could be enhanced by combining them with STING agonists. This combined approach holds promise for generating more comprehensive antitumor effects.
Methods
In vitro cytotoxicity assays
Peripheral blood–derived T cells from healthy donors (HDs) were isolated using CD8/CD4 MicroBeads (Miltenyi Biotec) or EasySep Human T Cell Isolation Kit (StemCell) according to the manufacturers’ instructions. T cells were cocultured with HL-60, MOLM-13, or OCI-AML-3 cells at varying effector-to-target ratios and treated with 5 ng/mL AMG 330 or a control construct, with or without cGAMP at 40 μg/mL, unless otherwise specified. Each well contained 2 × 105 cells. After 1 to 3 days, supernatants were collected for cytokine quantification, and cells were stained with AquaLive/Dead (Invitrogen) and antibodies against CD2 and CD33. Primary AML (pAML) coculture assays were performed as described.15 AMG 330–mediated lysis of target cells was determined by flow cytometry on Cytoflex S/LX instruments (Beckman Coulter) at the iFlow Core Facility of Ludwig Maximilian University Hospital, Munich, and analyzed using FlowJo version 10.5.3 (Tree Star Inc). Lysis was calculated in reference to cell counts under the respective control conditions.
CRISPR-Cas9 knockout generation
Ribonucleoproteins for clustered regularly interspaced short palindromic repeats-associated protein 9 (CRISPR-Cas9) knockout were produced by mixing transactivating crRNA (tracrRNA) and crisprRNA (crRNA) (100 pmol each; both IDT), annealing them at 95°C for 5 minutes, and cooling at RT for 30 minutes. The tracrRNA:crRNA pairs were incubated with 40 pmol of recombinant Streptococcus pyogenes nuclear localization signal (NLS)-Cas9 (Max Planck Institute of Biochemistry, Martinsried, Germany) for 15 minutes at RT. For IRF3, a single guide RNA (gRNA) (fused tracrRNA and crRNA) was used. A total of 5 × 105 cells were washed in phosphate-buffered saline (PBS), resuspended in 20 μL of P3 buffer (Lonza), and mixed with ribonucleoproteins. Electroporation was performed using the X-unit of a 4D nucleofector (Lonza; program EN138). After electroporation, cells were transferred to a 24-well plate in 500-μL medium. After 3 days, minimal dilution cloning was performed. Colonies were picked and deep sequenced to identify bi-allelic knockouts, as previously described,16 or screened for complete loss of protein expression by western blotting. STING-deficient primary human T cells were produced as previously described.17
In vivo studies
Eight-week-old NOD-SCID-IL2R-gamma mice (NOD.Cg-Prkdcscid Il2rgtm1WjI/SzJ) were purchased from Janvier (Le Genest Saint Isle, France). MOLM-13-LUC+-GFP+ xenograft models were established by IV injecting 1 × 106 cells into the tail vein. Seven days later, 5 × 106 T cells from HDs were injected IV in a total volume of 100-μL PBS. AMG 673 was delivered intraperitoneally, and diABZI was delivered IV (for dosing, see Figure 6). All animal experiments were approved by the local regulatory agency (Regierung von Oberbayern). Before treatment, mice were randomized according to tumor burden. Weight loss >15% after the start of experiment or decrease in general health condition (decreased mobility, general weakness, hunched posture, or ungroomed hair) are defined as human surrogate end points for survival and are later referred to as survival of mice. For in vivo imaging, mice were injected intraperitoneally with luciferin 10 minutes before imaging according to the manufacturer’s instructions (Xenolight D-Luciferin potassium salt; PerkinElmer). In Vivo Imaging System (IVIS) Lumina X5 (Perkin Elmer) was used to acquire in vivo images. The Living Image Software 4.7.2 was used for analysis (PerkinElmer).
Data visualization and statistical analysis
Data were visualized using R, Prism 9 version 9.2.0 (283; GraphPad Software Inc), and Adobe Illustrator version 25.2.3 (Adobe Inc). Statistical analyses were performed using Prism 9.
Samples were collected with written informed consent, in accordance with the Declaration of Helsinki, and with the approval of the Institutional Review Board of the Ludwig Maximilian University (Munich, Germany).
Results
The STING agonist cGAMP enhances AMG 330–mediated cytotoxicity against AML cells
To investigate the effect of STING agonism on T-cell–engaging BsAbs against AML, we used an in vitro cytotoxicity assay. Primary human T cells were cocultured with various AML cell lines at different effector-to-target ratios in the presence of the CD33-directed BiTE molecule AMG 330 or a control (herein referred to as c-BiTE).15,18 Indeed, AMG 330–mediated cytotoxicity against the AML cell lines HL-60, OCI-AML-3, and MOLM-13 was markedly increased by the addition of the STING agonist cGAMP. This was observed in AML–T-cell cocultures containing either CD8+ or CD4+ T cells (Figure 1A-D; supplemental Figure 1A-E, available on the Blood website). In addition, pAML-derived T cells were as potent as HD-derived T cells; hence, HD pan–T cells were used in all further experiments involving cocultures. In contrast, cGAMP used in conjunction with the c-BiTE did not increase death of HL-60 cells compared with the c-BiTE–only condition (Figure 1A-B). Cell death increased over time (supplemental Figure 1B), which was mirrored in an increase in intracellular caspase-3 activity in HL-60 cells exposed to cGAMP (Figure 1E). Regarding T cells in these coculture experiments, we observed an increase in the expression levels of intrinsic T-cell granzyme B (Figure 1F; supplemental Figure 1F), death receptor ligand TRAIL (Figure 1G; supplemental Figure 1G), and degranulation marker CD107a (Figure 1H; supplemental Figure 1H) through the addition of cGAMP to AMG 330–containing cocultures. The levels were comparable across Pan–, CD8+, and CD4+ T cells. There was also an increase in IFN-γ (Figure 1I) and, to a lesser extent, tumor necrosis factor (TNF; Figure 1J) in the supernatant of coculture when AMG 330 was used in combination with cGAMP. This was accompanied by T-cell expansion (Figure 1K). Previous studies had shown that an increase in AMG 330–mediated cytotoxicity against various AML cell lines was associated with higher CD33 expression levels,15 as well as the overexpression of costimulatory molecules on AML cells.19,20 However, we observed neither an increase in the expression level of the target antigen CD33 (supplemental Figure 1I) nor an upregulation of the positive costimulatory molecule CD86 upon addition of cGAMP to the cocultures (supplemental Figure 1J).
The STING agonist cGAMP enhances AMG 330–mediated cytotoxicity against AML cells. (A-B) Flow cytometric analysis of AMG 330–mediated (5 ng/mL) cytotoxicity after 72 hours against HL-60 cells in cocultures with either CD8+ (A) or CD4+ (B) human T cells (n = 4). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 40 μg/mL. (C-D) Effector-to-target cell ratio–dependent cytotoxicity after 72 hours against HL-60 cells in cocultures with either CD8+ (C) or CD4+ (D) human T cells treated as indicated (n = 3). (E) Percentage of caspase-3–positive HL-60 cells measured by intracellular staining and flow cytometry after 72 hours of coculture with human Pan–T cells treated as indicated (n = 3). (F) The percentage of granzyme B+ T cells, determined by intracellular staining and flow cytometry after 72 hours in cocultures with HL-60 cells treated as indicated (n = 3). (G) The percentage of TRAIL+ T cells, determined by flow cytometry after 72 hours in cocultures with HL-60 cells (n = 6). (H) Flow cytometric analysis of T-cell degranulation measured by staining surface CD107a after 72 hours in cocultures with HL-60 cells treated as indicated (n = 3). (I-J) Secretion of IFN-γ and TNF, determined after 72 hours by cytometric bead array (CBA) analysis, from cocultures of human T cells and HL-60 cells treated as indicated (n = 3). (K) Human T-cell proliferation expressed as fold change in CD2+ cells on day 6 of coculture with HL-60 cells in the presence of AMG 330 ± 10 μg/mL cGAMP (E:T of 1:20) normalized to c-BiTE conditions (n = 3). After 3 days, half of the medium was exchanged with fresh medium containing AMG 330 ± cGAMP. All graphs present the mean ± standard error of the mean (SEM). Statistical analysis was performed using ordinary 1-way analysis of variance (ANOVA) with the Tukey comparison or the 2-way ANOVA with Šidák correction (panels C-D). ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. E:T, effector-to-target cell ratio; ns, not significant.
The STING agonist cGAMP enhances AMG 330–mediated cytotoxicity against AML cells. (A-B) Flow cytometric analysis of AMG 330–mediated (5 ng/mL) cytotoxicity after 72 hours against HL-60 cells in cocultures with either CD8+ (A) or CD4+ (B) human T cells (n = 4). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 40 μg/mL. (C-D) Effector-to-target cell ratio–dependent cytotoxicity after 72 hours against HL-60 cells in cocultures with either CD8+ (C) or CD4+ (D) human T cells treated as indicated (n = 3). (E) Percentage of caspase-3–positive HL-60 cells measured by intracellular staining and flow cytometry after 72 hours of coculture with human Pan–T cells treated as indicated (n = 3). (F) The percentage of granzyme B+ T cells, determined by intracellular staining and flow cytometry after 72 hours in cocultures with HL-60 cells treated as indicated (n = 3). (G) The percentage of TRAIL+ T cells, determined by flow cytometry after 72 hours in cocultures with HL-60 cells (n = 6). (H) Flow cytometric analysis of T-cell degranulation measured by staining surface CD107a after 72 hours in cocultures with HL-60 cells treated as indicated (n = 3). (I-J) Secretion of IFN-γ and TNF, determined after 72 hours by cytometric bead array (CBA) analysis, from cocultures of human T cells and HL-60 cells treated as indicated (n = 3). (K) Human T-cell proliferation expressed as fold change in CD2+ cells on day 6 of coculture with HL-60 cells in the presence of AMG 330 ± 10 μg/mL cGAMP (E:T of 1:20) normalized to c-BiTE conditions (n = 3). After 3 days, half of the medium was exchanged with fresh medium containing AMG 330 ± cGAMP. All graphs present the mean ± standard error of the mean (SEM). Statistical analysis was performed using ordinary 1-way analysis of variance (ANOVA) with the Tukey comparison or the 2-way ANOVA with Šidák correction (panels C-D). ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. E:T, effector-to-target cell ratio; ns, not significant.
In summary, STING agonists potently increase the efficacy of AMG 330 for killing AML cells, and various T-cell effector mechanisms are enhanced when these treatments are combined.
Transcriptional responses to IFN-α, IFN-γ, and TNF underlie the improved target cell killing in the presence of cGAMP
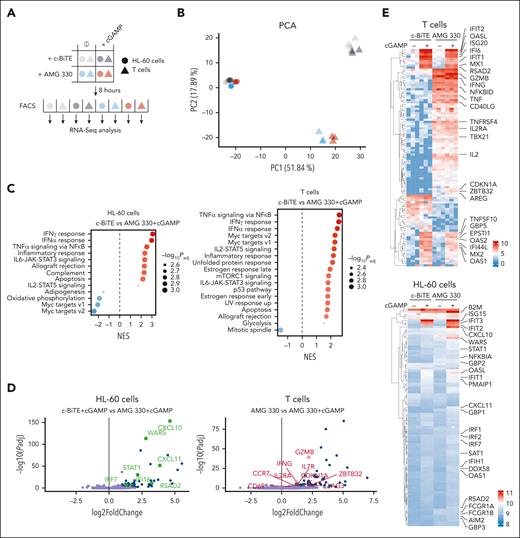
To understand how STING engagement affects AMG 330 activity, we profiled gene expression of cocultured AML cells and T cells with and without cGAMP via RNA sequencing (RNA-seq). The 4 experimental groups were c-BiTE only, AMG 330 only, c-BiTE and cGAMP, and AMG 330 and cGAMP. For each condition, HL-60 and T cells were separated via cell sorting and subjected to RNA-seq, resulting in 8 different gene expression profiles (Figure 2A). A principal component analysis revealed that cell type (HL-60 vs T cells) was the major determinant of sample separation. T-cell samples could be further clustered based on whether they were exposed to AMG 330. Exposure to cGAMP further slightly separated the T-cell samples derived from cocultures in which AMG 330 was present. Gene expression in HL-60 cells was grouped into 4 distinct but less separated groups reflecting the 4 experimental conditions from which the samples were obtained (Figure 2B). For both HL-60 and T cells, gene set enrichment analysis revealed that the transcriptional program in response to the combination treatment (AMG 330 and cGAMP) was largely determined by responses to IFN-α, IFN-γ, and TNF (Figure 2C; supplemental Figure 2A). To decipher which genes were responsible for different transcriptional states, the most highly variable genes between different treatment conditions were analyzed in both HL-60 and T cells. As expected, AMG 330 exposure, compared with conditions in which the c-BiTE was present, led to an activated T-cell phenotype with upregulation of effector molecules (IFNG, TNF, and GZMB), activation markers (IL2, IL2RA, CD40LG, and CD69), and NF-κB target genes (NFKBID and TNF; Figure 2D-E; supplemental Table 1). Conversely, HL-60 cells exhibited a signature of IFN-γ (IRF1, STAT1, GBP1, GBP2, FCGR1A/B, B2M, and AIM2) and NF-κB signaling (B2M and NFKBIA; Figure 2D-E) when exposed to AMG 330. Exposure to only cGAMP, compared with conditions without cGAMP, induced various type 1 IFN-stimulated genes (ISGs), such as genes of the IFIT and OAS gene families as well as IRF7, MX2, and RSAD2 (viperin), notable in both cell types, indicative of the cell-autonomous effects of STING activation as well as the autocrine or paracrine action of type I IFNs (Figure 2D-E). If AMG 330 and cGAMP were combined, the resulting transcriptional phenotype did not merely reflect a combination of individual treatments. Although cGAMP was the main inducer of various ISGs (eg, IFIT1, IFIT2, ISG20, OAS1, OAS2, OASL, MX1, MX2, RSAD2, TNFSF10, and IRF7) in T cells, only its combination with AMG 330 led to the upregulation of IFNG and GZMB compared with single-agent exposure (Figure 2D-E). A cluster of genes with previously reported roles in T-cell biology (CDKN1A, ZBTB32, and AREG) was exclusively upregulated in the combined treatment, supporting a distinct synergistic transcriptional response in T cells activated with AMG 330 and cGAMP (Figure 2D-E). Interestingly, ZBTB32 is proposed to repress the production of the T helper 2 cytokine interleukin-4 (IL-4),21 which is reciprocally regulated with T helper 1 cytokines, consistent with the increased levels of IFNG. In fact, with regard to HL-60 cells, responsiveness to STING activation in terms of ISG induction was increased in the presence of AMG 330, as evidenced by further upregulation of STAT1, IRF7, WARS, CXCL11, IFI16, RSAD2, and CXCL10 (Figure 2D-E). These findings shed light on the synergistic interplay between STING engagement and AMG 330 activity, which is mainly shaped by the transcriptional response to IFN-α, IFN-γ, and TNF.
Transcriptional responses to IFN-α, IFN-γ, and TNF underlie the improved target cell killing in the presence of cGAMP. (A) Overview of bulk RNA sequencing approach; HL-60 and human T cells were cocultured for 8 hours in the presence of AMG 330 ± cGAMP and separated by FACS before subjecting them to RNA sequencing. c-BiTE conditions served as control. (B) Principal component analysis (PCA) applied to the RNA sequencing data collected from the 4 experimental conditions and the 2 indicated cell types (as outlined in panel A; n = 3). (C) Gene set enrichment analysis for the comparison of c-BiTE and AMG 330 + cGAMP in HL-60 and T cells. Normalized enrichment score (NES) is depicted for the indicated hallmark gene sets; the size of the dot represents the adjusted P value for each gene set. (D) Volcano plot showing the gene expression differences between cGAMP treatment and combined AMG 330 + cGAMP treatment in HL-60 cells (left) and AMG 330 treatment and combined AMG 330 + cGAMP treatment in T cells (right). Negative log10-adjusted P values (y-axis) are plotted against the log2-transformed fold changes in gene expression (x-axis). Significantly upregulated (adjusted P < .05; absolute fold change > 2) genes are shown in blue, with genes associated with an ISG response highlighted in green (left) and genes associated with T-cell activation highlighted in red (right). (E) Heat maps of the top 200 most variable genes in HL-60 and T cells clustered hierarchically across all conditions; relevant genes are highlighted. Color coding corresponds to normalized and log2-transformed read counts. FACS, fluorescence activated cell sorting; PC1, principal component 1.
Transcriptional responses to IFN-α, IFN-γ, and TNF underlie the improved target cell killing in the presence of cGAMP. (A) Overview of bulk RNA sequencing approach; HL-60 and human T cells were cocultured for 8 hours in the presence of AMG 330 ± cGAMP and separated by FACS before subjecting them to RNA sequencing. c-BiTE conditions served as control. (B) Principal component analysis (PCA) applied to the RNA sequencing data collected from the 4 experimental conditions and the 2 indicated cell types (as outlined in panel A; n = 3). (C) Gene set enrichment analysis for the comparison of c-BiTE and AMG 330 + cGAMP in HL-60 and T cells. Normalized enrichment score (NES) is depicted for the indicated hallmark gene sets; the size of the dot represents the adjusted P value for each gene set. (D) Volcano plot showing the gene expression differences between cGAMP treatment and combined AMG 330 + cGAMP treatment in HL-60 cells (left) and AMG 330 treatment and combined AMG 330 + cGAMP treatment in T cells (right). Negative log10-adjusted P values (y-axis) are plotted against the log2-transformed fold changes in gene expression (x-axis). Significantly upregulated (adjusted P < .05; absolute fold change > 2) genes are shown in blue, with genes associated with an ISG response highlighted in green (left) and genes associated with T-cell activation highlighted in red (right). (E) Heat maps of the top 200 most variable genes in HL-60 and T cells clustered hierarchically across all conditions; relevant genes are highlighted. Color coding corresponds to normalized and log2-transformed read counts. FACS, fluorescence activated cell sorting; PC1, principal component 1.
IFN-γ and TNF sensitize AML cells to STING agonism
RNA-seq analyses suggested that T-cell activation by AMG 330 modulated the responsiveness of AML cells toward STING activation, because several ISGs were more strongly induced with the combined treatment than with cGAMP alone. Indeed, an increase in type I IFN production and subsequent ISG induction was also reflected at the protein level, as corroborated by IFN-α2a (Figure 3A) and CXCL-10 (Figure 3B) concentrations in the supernatant of the coculture. To address whether T-cell effector cytokines IFN-γ and TNF are sufficient to enhance downstream ISG induction within AML cells upon STING activation, we primed HL-60 cells with IFN-γ, TNF, or their combination and subsequently treated them with cGAMP. STING activation by itself induced only low levels of CXCL-10 in the supernatant, whereas CXCL-10 induction was greatly increased when cells were primed with IFN-γ and TNF (Figure 3C). A similar picture was observed for intracellular levels of ISG proteins assessed by immunoblotting. HL-60 cells readily responded to cGAMP with phosphorylation of STING and IRF3, indicating that proximal STING signaling was operating in those cells. Nevertheless, at the protein level, cGAMP alone only weakly induced phosphorylation of the type I IFN receptor (IFNAR) signaling components STAT1 and STAT2 and minor levels of the ISG proteins OAS1 and viperin. When cGAMP was added to IFN-γ– or TNF-primed cells, IFNAR signaling and ISG induction was increased. Combining both effector cytokines with cGAMP elicited the strongest IFNAR activation and ISG induction (Figure 3C). Notably, combination of IFN-γ, TNF, and cGAMP induced cell death (supplemental Figure 3A) in HL-60 cells, indicating that IFN-γ and TNF are sufficient to render STING activation lethal. Overall, the data substantiate the hypothesis that the upregulation of ISGs in AML cells results from a synergistic effect of T-cell effector cytokines (IFN-γ and TNF) and STING activation, which facilitates the death of AML cells.
IFN-γ and TNF sensitize AML cells to STING agonism. (A-B) Levels of IFN-α2a (A) or CXCL10 (B) measured after 72 hours by CBA (IFN-α2a) or ELISA (CXCL-10), from cocultures of human T cells and HL-60 cells treated as indicated (E:T, 1:10; n = 3). (C) Levels of CXCL-10 measured after 16 hours in the supernatant of HL-60 cells treated as indicated (IFN-γ, 20 ng/mL; TNF, 0.5 ng/mL; cGAMP, 40 μg/mL). (D) Immunoblots of lysates of HL-60 cells treated for 16 hours as indicated (IFN-γ, 20 ng/mL; TNF, 20 ng/mL; cGAMP, 40 μg/mL). Means ± SEM are presented. Statistical analysis was performed using the ordinary 1-way ANOVA with the Tukey comparison or the 2-way ANOVA with the Tukey comparison (panel C). ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ELISA, enzyme-linked immunosorbent assay; ns, not significant.
IFN-γ and TNF sensitize AML cells to STING agonism. (A-B) Levels of IFN-α2a (A) or CXCL10 (B) measured after 72 hours by CBA (IFN-α2a) or ELISA (CXCL-10), from cocultures of human T cells and HL-60 cells treated as indicated (E:T, 1:10; n = 3). (C) Levels of CXCL-10 measured after 16 hours in the supernatant of HL-60 cells treated as indicated (IFN-γ, 20 ng/mL; TNF, 0.5 ng/mL; cGAMP, 40 μg/mL). (D) Immunoblots of lysates of HL-60 cells treated for 16 hours as indicated (IFN-γ, 20 ng/mL; TNF, 20 ng/mL; cGAMP, 40 μg/mL). Means ± SEM are presented. Statistical analysis was performed using the ordinary 1-way ANOVA with the Tukey comparison or the 2-way ANOVA with the Tukey comparison (panel C). ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ELISA, enzyme-linked immunosorbent assay; ns, not significant.
Intrinsic STING signaling in AML cells but not in T cells is required to enhance AMG 330–mediated cytotoxicity against AML cells
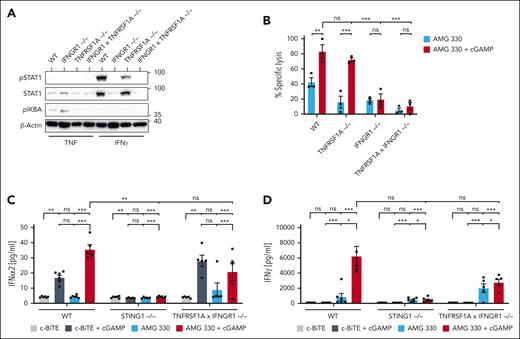
Because both T cells and target AML cells displayed a type 1 IFN signature upon exposure to cGAMP, we evaluated the contribution of intrinsic STING activation in these cell types in enhancing AMG 330–mediated cytotoxicity in the presence of cGAMP. We generated HL-60 cell lines deficient in different components of the cGAS-STING signaling cascade. Disruption of the expression of cGAS, STING, and IRF3 was confirmed by direct immunoblotting (Figure 4A), whereas knockout of IFNAR1 was confirmed by the disrupted phosphorylation of STAT1 upon exposure to IFNα2a (Figure 4B). Studying these cell lines in the coculture experiments revealed that the increase in AMG 330–mediated cytotoxicity gained with cGAMP was fully dependent on the presence of STING and IRF3 within target cells, whereas cGAS deficiency had no effect, as expected (Figure 3C). Because the death of IFNAR1-deficient target cells was still potentiated by cGAMP, we concluded that an autocrine feedback loop of type I IFNs acting on AML cells was only partially required to facilitate the effects of cGAMP (Figure 3C). Conversely, disrupting STING expression in primary human T cells in a polyclonal fashion did not affect AMG 330–dependent cytotoxicity, irrespective of the presence of cGAMP (supplemental Figure 4A). Phenotypic profiling of T cells derived from the coculture (AMG 330 + cGAMP) had shown a marked increase in T-cell effector molecules (Figures 1F-J and 2D-E). Thus, we examined whether STING signaling within the target cells had contributed to T-cell effector cytokine secretion in the coculture experiment. Studying the secretion of T-cell effector cytokine IFN-γ revealed that functional STING signaling in target cells was mainly required to boost T-cell–dependent IFN-γ production (Figure 4D). Conversely, IL-2 production was not dependent on the STING genotype of target cells (Figure 4E). Taken together, these findings suggest that the beneficial effect of cGAMP in enhancing AMG 330–mediated cytotoxicity is fully dependent on intrinsic target cell STING-IRF3 signaling, which feeds back to the T cells to induce IFN-γ secretion.
Intrinsic STING signaling in target AML cells is required for enhancing AMG 330–mediated lysis. (A) Immunoblots of monoclonal HL-60 cell lines of the indicated genotypes generated by CRISPR-Cas9 gene editing. (B) Monoclonal HL-60 cell lines of the indicated genotypes were treated with IFN-α2a, and phosphorylation of STAT1 (pSTAT1) was determined by immunoblotting. (C) Flow cytometric analysis of AMG 330–mediated (5 ng/mL) cytotoxicity after 72 hours against HL-60 cell lines of the indicated genotypes (mean of 2 independent knockout clones) in cocultures with human T cells (E:T, 1:10, n = 3). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 40 μg/mL. (D-E) Levels of IFN-γ (D) and IL-2 (E) as determined by CBA analysis of cocultures of human T cells and wild-type or STING-deficient HL-60 cells in the presence of the indicated treatments after 72 hours (n = 3). Means ± SEM are presented. Statistical analysis was performed using the 2-way ANOVA with the Tukey comparison. ns, P > .05; ∗P < .05; ∗∗∗P < .001. E:T, effector-to-target ratio; ns, not significant; WT, wild-type.
Intrinsic STING signaling in target AML cells is required for enhancing AMG 330–mediated lysis. (A) Immunoblots of monoclonal HL-60 cell lines of the indicated genotypes generated by CRISPR-Cas9 gene editing. (B) Monoclonal HL-60 cell lines of the indicated genotypes were treated with IFN-α2a, and phosphorylation of STAT1 (pSTAT1) was determined by immunoblotting. (C) Flow cytometric analysis of AMG 330–mediated (5 ng/mL) cytotoxicity after 72 hours against HL-60 cell lines of the indicated genotypes (mean of 2 independent knockout clones) in cocultures with human T cells (E:T, 1:10, n = 3). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 40 μg/mL. (D-E) Levels of IFN-γ (D) and IL-2 (E) as determined by CBA analysis of cocultures of human T cells and wild-type or STING-deficient HL-60 cells in the presence of the indicated treatments after 72 hours (n = 3). Means ± SEM are presented. Statistical analysis was performed using the 2-way ANOVA with the Tukey comparison. ns, P > .05; ∗P < .05; ∗∗∗P < .001. E:T, effector-to-target ratio; ns, not significant; WT, wild-type.
Intrinsic STING signaling in target AML cells requires priming by T-cell–derived cytokines
ISG induction in AML cells upon cGAMP treatment was enhanced when these cells were primed with IFN-γ and TNF (Figure 3C). Moreover, both cytokines were elevated in T cells when STING was activated in the target cells by cGAMP (Figures 1I-J and 4D). Hence, we examined the impact of intrinsic HL-60 IFN-γ and TNF signaling in our cocultures by genetically disrupting their corresponding receptors. Immunoblotting of phospho-IκBα and phospho-STAT1 after exposure to TNF or IFN-γ confirmed that receptor knockout cells were rendered unresponsive to these cytokines (Figure 5A). Targeting TNF receptor (TNFRSF1A) resulted in a slight decrease of AMG 330–dependent cytotoxicity, but cGAMP-dependent enhancement was still observed in these cells. Deletion of the IFN-γ receptor also reduced AMG 330–dependent cytotoxicity under steady-state conditions, but it also completely abolished cGAMP-dependent enhancement of target cell killing (Figure 5B). When we analyzed cytokine production in cocultures, we observed that IFN-α2a production was fully dependent on intrinsic AML cell STING signaling, and it was slightly reduced if IFN-γ and TNF signaling was ablated in these cells (Figure 5C). More prominently, IFN-γ production was completely abolished in T cells cocultured with STING-deficient target cells and markedly reduced when TNF receptor I– and IFN-γ receptor–deficient cells were used (Figure 5D). Because the effects of IFN-γ receptor signaling rely in part on the induction of transcription factor IRF1,22 we also tested IRF1-deficient HL-60 cells. However, these studies revealed that IRF1 was only partially required for AMG 330–dependent cytotoxicity and its enhancement with cGAMP addition (supplemental Figure 5A). Aligning with previous studies proposing that IFN-γ priming sensitizes target cells to CAR T cells via an ICAM1-related mechanism,23 crucial for CAR T-cell function, especially in the context of solid tumors,24 we generated target cells deficient in ICAM1. However, ICAM1 was not required for AMG 330–mediated cytotoxicity or for the effect of STING agonism (supplemental Figure 5B-C). Together, these results demonstrate a central role for intrinsic target cell IFN-γ signaling not only in AMG 330–mediated cytotoxicity against AML cells but also in the enhancement of T-cell cytotoxicity upon cotreatment with cGAMP. As such, basal levels of IFN-γ released by activated T cells seem to be required to sensitize AML cells to STING signaling in the first instance. IFN-γ secretion from T cells is then further amplified in the presence of cGAMP. This is fully dependent on intrinsic target cell IFN-γ receptor and STING signaling. These findings highlight the intricate interdependence between these 2 pathways that underlies the improved efficacy of AMG 330–mediated killing of AML cells in the presence of a STING agonist.
Intrinsic STING signaling in target AML cells requires priming by effector T-cell–derived cytokines. (A) HL-60 cell lines of the indicated genotypes were treated with IFN-γ or TNF, and pSTAT1 or phosphorylation of IκBα (pIκBα) was assessed by immunoblotting. (B) Flow cytometric analysis of AMG 330–mediated (5 ng/mL) cytotoxicity against HL-60 cell lines of the indicated genotypes (mean of 2 independent knockout clones) in cocultures with human T cells after 72 hours (E:T, 1:10; n = 3). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 40 μg/mL. (C-D) Levels of IFN-α2a (C) and IFN-γ (D) determined after 72 hours by CBA analysis of the supernatants of cocultures of human T cells and HL-60 cells of the indicated genotype in the presence of c-BiTE or AMG 330 ± cGAMP (n = 3). Means ± SEM are presented. Statistical analysis was performed using the 2-way ANOVA with the Tukey comparison. ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. E:T, effector-to-target ratio; ns, not significant; WT, wild-type.
Intrinsic STING signaling in target AML cells requires priming by effector T-cell–derived cytokines. (A) HL-60 cell lines of the indicated genotypes were treated with IFN-γ or TNF, and pSTAT1 or phosphorylation of IκBα (pIκBα) was assessed by immunoblotting. (B) Flow cytometric analysis of AMG 330–mediated (5 ng/mL) cytotoxicity against HL-60 cell lines of the indicated genotypes (mean of 2 independent knockout clones) in cocultures with human T cells after 72 hours (E:T, 1:10; n = 3). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 40 μg/mL. (C-D) Levels of IFN-α2a (C) and IFN-γ (D) determined after 72 hours by CBA analysis of the supernatants of cocultures of human T cells and HL-60 cells of the indicated genotype in the presence of c-BiTE or AMG 330 ± cGAMP (n = 3). Means ± SEM are presented. Statistical analysis was performed using the 2-way ANOVA with the Tukey comparison. ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. E:T, effector-to-target ratio; ns, not significant; WT, wild-type.
Combination of STING agonism and CD33-directed T-cell–engaging molecules efficiently eliminates pAML cells and shows superior in vivo efficacy
Next, we set out to validate whether the combination of AMG 330 and cGAMP would also be effective against pAML cells. Using cGAMP alone, we observed dose-dependent cytotoxicity, confirming the sensitivity of pAML cells to STING agonism (Figure 6A). Therefore, we evaluated the impact of STING agonism on AMG 330–mediated cytotoxicity using lower concentrations of cGAMP (10 μg/mL) and AMG 330 (0.5 ng/mL). AMG 330–mediated cytotoxicity against 10 different pAML cells was markedly increased by the addition of the STING agonist cGAMP. We again observed a trend toward increased INF-γ and TNF levels in the supernatant of these cocultures, underscoring the relevance of our proposed mechanism also in pAML cells (Figure 6B; supplemental Figure 6A). We validated our findings with the small molecule STING agonist diABZI. AMG 330–mediated cytotoxicity against HL-60, MOLM-13, and 11 different pAML cells was markedly increased by the addition of diABZI (Figure 6C; supplemental Figure 6B-C). Using STING-deficient HL-60 cells, we confirmed the dependency on target cell–intrinsic STING signaling. In line with results using cGAMP, we again observed increased IFN-y secretion upon the combination of AMG 330 and diABZI in the presence of T cells. Notably, we observed heterogeneity between pAML samples regarding their sensitivity toward the combinatory treatment, baseline sensitivity toward STING agonism, and IFN-y secretion in cocultures. We hypothesized that the differences might be caused by varying levels of IFNGR1 expression on pAML cells; however, we found no correlation between IFNGR1 expression and cytotoxicity (supplemental Figure 6D).
Combination of STING agonism and CD33-directed T-cell–engaging molecules efficiently eliminates pAML cells and shows superior in vivo efficacy. (A) Flow cytometric analysis of cGAMP-mediated cytotoxicity after 48 hours against pAML cells (n = 4). (B) Flow cytometric analysis of AMG 330–mediated (0.5 ng/mL) cytotoxicity after 48 hours against pAML cells in cocultures with human T cells (E:T, 1:10; n = 10). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 10 μg/mL. (C) Flow cytometric analysis of AMG 330–mediated (0.5 ng/mL) cytotoxicity after 48 hours against pAML cells in cocultures with human T cells (E:T, 1:10; n = 11). The concentration of added diABZI in cocultures was 1 nM. Corresponding levels of secreted IFN-γ as determined by CBA analysis are shown. (D) Timeline and overview of the AML xenograft model. Injections are indicated with arrows (n = 3 mice per group). (E-F) Tumor burden was analyzed by bioluminescence imaging, and probability of survival is depicted after Kaplan-Meier analysis. (G) MOLM-13 tumor burden in the bone marrow of mice was analyzed by flow cytometric analysis at the time of euthanasia. Means ± SEM are presented. Statistical analysis was performed using the ordinary 1-way ANOVA with the Tukey comparison or the Kaplan-Meier analysis with the Mantel-Cox test (panel F). ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. E:T, effector-to-target ratio; i.p., intraperitoneal; ns, not significant.
Combination of STING agonism and CD33-directed T-cell–engaging molecules efficiently eliminates pAML cells and shows superior in vivo efficacy. (A) Flow cytometric analysis of cGAMP-mediated cytotoxicity after 48 hours against pAML cells (n = 4). (B) Flow cytometric analysis of AMG 330–mediated (0.5 ng/mL) cytotoxicity after 48 hours against pAML cells in cocultures with human T cells (E:T, 1:10; n = 10). Specific lysis was calculated relative to the c-BiTE condition. The concentration of added cGAMP in cocultures was 10 μg/mL. (C) Flow cytometric analysis of AMG 330–mediated (0.5 ng/mL) cytotoxicity after 48 hours against pAML cells in cocultures with human T cells (E:T, 1:10; n = 11). The concentration of added diABZI in cocultures was 1 nM. Corresponding levels of secreted IFN-γ as determined by CBA analysis are shown. (D) Timeline and overview of the AML xenograft model. Injections are indicated with arrows (n = 3 mice per group). (E-F) Tumor burden was analyzed by bioluminescence imaging, and probability of survival is depicted after Kaplan-Meier analysis. (G) MOLM-13 tumor burden in the bone marrow of mice was analyzed by flow cytometric analysis at the time of euthanasia. Means ± SEM are presented. Statistical analysis was performed using the ordinary 1-way ANOVA with the Tukey comparison or the Kaplan-Meier analysis with the Mantel-Cox test (panel F). ns, P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. E:T, effector-to-target ratio; i.p., intraperitoneal; ns, not significant.
Next, we analyzed the impact of STING agonism on other cells of the immune system by treating HD-peripheral blood mononuclear cells (PBMCs) with cGAMP/diABZI. We observed a depletion of monocytes, whereas T, B, and natural killer (NK) cells were largely unaffected (supplemental Figure 6E).
Lastly, we performed in vivo xenograft studies using a NOD-SCID-IL2R-gamma mouse model engrafted with the highly aggressive human MOLM-13 AML cell line, followed by IV injection of human T cells. For pharmacokinetic reasons, we used diABZI25 and AMG 673, which is a half-life extended version of AMG 330. Mice were treated twice weekly for 2 weeks with diABZI (IV) and received a weekly subtherapeutical dose of AMG 673, until end point criteria were reached by protocol (Figure 6D). PBS- and AMG 673–treated mice rapidly reached termination end points (Figure 6E-G). Interestingly, mice treated with diABZI alone demonstrated significantly lower tumor burden and improved overall survival than controls. Importantly, the combination of AMG 673 and diABZI demonstrated the best outcome, with complete clearance of AML cells in the bone marrow and the longest overall survival, even though mice eventually succumbed to their disease, likely because we used a subtherapeutical AMG 673 dose. Together, these data support the potential of combining STING agonism with CD33-directed T-cell–engaging molecules to combat AML.
Discussion
In this study, we identified STING agonism as a powerful catalyst of killing of AML cells by CD33-directed T-cell–engaging molecules in vitro and in vivo. However, AML cells are not a priori fully responsive to STING activation. Instead, T-cell–derived cytokines IFN-γ and TNF sensitize AML cells toward full-scale ISG induction downstream of STING agonism and tip STING signaling toward the induction of cell death. This was further highlighted by the critical role of IFN-γ receptor expression on AML cells: IFN-γ receptor–deficient cells are protected against the combination effect of AMG 330 and cGAMP. In addition, IFN-γ secretion by T cells cocultured with IFN-γ receptor–deficient AML cells was significantly lower than those cocultured with wild-type AML cells. This underscores that cross talk between T cells and target AML cells is essential for the concerted action of STING activation and T-cell–engaging molecules.
The importance of the cGAS-STING pathway for inducing endogenous antitumor responses14,26 has spurred efforts to harness the pathway for treating solid tumors. However, less is known about the activity of STING agonists in the treatment of hematological malignancies. In line with earlier reports, we observed direct and dose-dependent cytotoxicity of cGAMP/diABZI on AML cells and pAML cells.27
Here, we propose that STING activation can be used to leverage antitumor responses in the context of T-cell–redirecting therapies against AML. Although we observed that cGAMP itself induced varying levels of cell death in AML cells and reportedly only limited cell death in myeloid cells,28 the consistent additive to synergistic effect in combination with T-cell–engaging therapy underscores its enhanced efficacy as part of a combinatorial approach rather than as a standalone therapy in AML.29 A limitation of STING agonism might be the reported toxicity on T-cell survival.17,30 Although we observed an overall beneficial effect on T cells upon simultaneous engagement with STING agonists and CD33-targeting T-cell–engaging molecules, we also observed a temporary reduction of T cell counts in cocultures with AML cells. Future therapeutic strategies will need to address the therapeutic window of STING agonists when applied in conjunction with T-cell–based immunotherapy.
A critical role for IFN-γ in tumor control is well established.31 More recently, a key role for IFN-γ in the context of T-cell–based immunotherapy of solid tumors was described.32 The antitumor effects of IFN-γ were largely attributed to its ability to promote T-cell recruitment.23,32,33 Disruption of IFN-γ receptor signaling is a mechanism by which cancer cells can become resistant to T-cell–dependent immunotherapies.34 In line with these observations, Xu et al showed in a murine breast cancer model that the benefit of STING activation in decreasing tumor growth through CAR T cells depends on IFN-γ,35 whereas another study found a critical role for IL-18 in a similar scenario.36
In our study, IFN-γ, in concert with TNF, sensitizes target AML cells to STING activation. Although proximal signaling in AML cells was independent of T-cell–derived cytokines, downstream effects such as the secretion of type I IFNs and their autocrine action with subsequent ISG induction were increased in the presence of AMG 330 and T cells. A critical role for IFN-γ and TNF was established by disrupting the expression of their corresponding receptors in the target cells, which decreased the secretion of type I IFNs and rendered cells resistant to AMG 330–mediated cytotoxicity. Recombinant IFN-γ and TNF could mimic the effects of AMG 330–redirected T cells on STING activation and boosted the production of ISGs. The combination of IFN-γ and TNF with STING activation could even induce cell death by itself. This raises the question of how IFN-γ and TNF affect STING and IFNAR1 signaling. It was recently reported that NF-κB can enhance STING signaling by inhibiting the activation-dependent degradation of STING.37 However, in our study, activated STING was degraded irrespective of the addition of TNF.
By exposing AML cells to IFN-γ, we observed a marked increase in STAT1 levels. STAT1 is not only critical for signaling downstream of the IFN-γ receptor but also for IFNAR. As such, it is conceivable that the induction of STAT1 by IFN-γ increases the responsiveness of the cells toward type I IFNs, facilitating the induction of ISGs, similar to what was observed in melanoma cells.38
Although our study integrated the important cross talk between AML and T cells, it did not address the impact of STING agonists on other cells of the innate immune system and their influence on T-cell–based immunotherapy. Potential benefits of STING agonists include priming or remodeling the immune-exhausted tumor microenvironment. Moreover, STING agonists may contribute to immunomodulation through STING-induced monocyte cell death, potentially decreasing immune-related adverse events. However, a major concern remains that STING agonists may still aggravate cytokine release syndrome and possibly immune effector cell-associated neurotoxicity syndrome or immune effector cell-associated hematotoxicity by further activating the innate myeloid compartment. Our proof-of-principle in vivo study suggested the potential of our combinatory approach in a limited number of animals. Future studies should address the efficacy and safety of combining STING agonists with CD33-targeting T-cell engagers in immune-competent humanized mice. Priming with a STING agonist, followed by the application of a T-cell engager, might be a strategy to ensure safety and at the same time increase efficacy. Because we observed heterogeneity in the responsiveness of pAML toward the combinatory treatment, it will also be crucial to decipher which patients are more likely to respond.
In summary, we propose the combination of CD33-targeting T-cell engagers with STING agonism for the treatment of AML and establish a critical role for IFN-γ signaling in AML immunotherapy.
Acknowledgments
The authors acknowledge the iFlow Core Facility of the University Hospital Munich (INST 409/225-1 FUGG) for assistance with generating flow cytometry data.
This work was supported by the Bundesministerium für Bildung und Forschung in the framework of the Cluster4Future program (Cluster for Nucleic Acid Therapeutics Munich; Project ID, 03ZU1201AA) and the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) TRR 338 (project number 452881907; V.H., S.K., and M.S.), the DFG research grant 451580403 (M.S.), and the Else Kröner-Fresenius-Stiftung (Immunonkologie und lokale Intervention; S.K. and A.V.H.). S.K. is further supported by Bavarian Cancer Research Center, the DFG (KO5055-2-1 and KO5055/3-1), the international doctoral program “i-Target: immunotargeting of cancer” (funded by the Elite Network of Bavaria), Marie Sklodowska-Curie Training Network for Optimizing Adoptive T Cell Therapy of Cancer (funded by the Horizon 2020 program of the European Union; grant 955575), German Cancer Aid (AvantCAR.de), the Wilhelm Sander-Stiftung, Ernst Jung Stiftung, Institutional Strategy LMUexcellent of LMU Munich (within the framework of the German Excellence Initiative), the Go-Bio-Initiative, the m4-Award of the Bavarian Ministry for Economical Affairs, Bundesministerium für Bildung und Forschung (European Research Council; Starting Grant 756017, Proof of Concept Grant 101100460, and Consolidator Grant 101124203), Fritz Bender Foundation, Deutsche José Carreras Leukämie-Stiftung, Hector Foundation, Bavarian Research Foundation (BAYCELLATOR), and the Bruno and Helene Jöster Foundation (360° CAR).
Authorship
Contribution: V.H., M.S., R.K., A.L., and D.N. designed the study and supervised the project; A.L., D.N., V.H., and M.S. wrote the manuscript; A.L., D.N., N.K., T.X., I.P., R.E., N.P., and V.B. designed and performed the experiments and analyzed/interpreted the data; G.K. conducted the RNA sequencing bioinformatic analysis; A.V.H. and S.K. helped in designing and performed the in vivo studies; J.d.G. and T.C. were involved in synthesizing cGAMP; and all authors contributed to the preparation of the manuscript and approved the submitted version.
Conflict-of-interest disclosure: M.S. receives industry research support from Amgen, Bristol Myers Squibb (BMS)/Celgene, Gilead/Kite, Johnson & Johnson, Miltenyi Biotec, Novartis, Roche, Seattle Genetics, and Takeda; and serves as a consultant/advisor for AbbVie, Crossbow, Debiopharm, Gilead/Kite, Interius, Johnson & Johnson, Molecular Partners, Novartis, and Otsuka; and serves on the speakers’ bureau at Amgen, BMS/Celgene, Gilead/Kite, Miltenyi Biotec, Novartis, Roche, and Takeda. R.K. is employed by Amgen; and holds stock ownership in Amgen. V.B. has received research funding from Gilead/Kite and Miltenyi Biotec; educational grants from BMS, Novartis, Takeda, and Roche; served as a consultant/advisor for Amgen, Gilead/Kite, Novartis, Pfizer, and Priothera; and serves on the speakers’ bureau for Novartis and Pfizer. S.K. has received honoraria from Cymab, Plectonic, TCR2 Inc, Miltenyi Biotec, Galapagos, Novartis, BMS, and GlaxoSmithKline; is an inventor of several patents in the field of immuno-oncology; received license fees from TCR2 Inc and Carina Biotech; and received research support from TCR2 Inc, Tabby Therapeutics, CatalYm GmbH, Plectonic GmbH, and Arcus Biosciences for work unrelated to the manuscript. The remaining authors declare no competing financial interests.
Correspondence: Veit Hornung, Gene Center and Department of Biochemistry, Ludwig Maximilian University of Munich, Feodor-Lynen-Str 25, 81377 Munich, Germany; email: hornung@genzentrum.lmu.de; and Marion Subklewe, Laboratory for Translational Cancer Immunology, Gene Center, Ludwig Maximilian University of Munich, Feodor-Lynen-Str 25, 81377 Munich, Germany; email: subklewe@genzentrum.lmu.de.
References
Author notes
A.L., D.N., V.H., and M.S. contributed equally to this work.
The RNA sequencing data have been deposited in the Gene Expression Omnibus database (accession number GSE253398).
The data sets generated and/or analyzed during this study are available on reasonable request from the corresponding authors, Veit Hornung (hornung@genzentrum.lmu.de) and Marion Subklewe (subklewe@genzentrum.lmu.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.