Key Points
DNA methylation improves MRD risk group assessment in pediatric T-ALL treated by modern protocols.
DNA methylation subgroups in T-ALL are associated with distinct multiomics profiles, suggesting different routes to leukemia development.
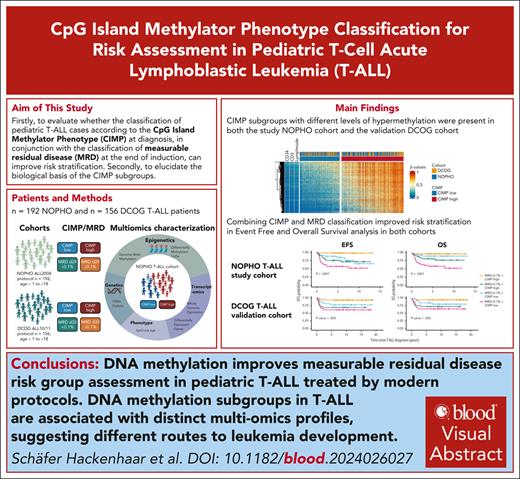
Visual Abstract
Current intensive treatment of pediatric T-cell acute lymphoblastic leukemia (T-ALL) has substantial side effects, highlighting a need for novel biomarkers to improve risk stratification. Canonical biomarkers, such as genetics and immunophenotype, are largely not used in pediatric T-ALL stratification. This study aimed to validate the prognostic relevance of DNA methylation CpG island methylator phenotype (CIMP) risk stratification in 2 pediatric T-ALL patient cohorts: the Nordic Society of Paediatric Haematology (NOPHO) ALL2008 T-ALL study cohort (n = 192) and the Dutch Childhood Oncology Group (DCOG) ALL-10/ALL-11 validation cohorts (n = 156). Both cohorts revealed that combining CIMP classification at diagnosis with measurable residual disease (MRD) at treatment day 29 (D29) or 33 (D33) significantly improved outcome prediction. The poor prognosis subgroup, characterized by CIMP low/D29 or D33 MRD ≥ 0.1%, had a cumulative incidence of relapse (pCIR5yr) of 29% and 23% and overall survival (pOS5yr) of 59.7% and 65.4%, in NOPHO and DCOG, respectively. Conversely, a good prognosis subgroup was also identified representing CIMP high/D29 or D33 MRD < 0.1% with pCIR5yr of 0% and 3.4% and pOS5yr of 98.2% and 94.8%, in NOPHO and DCOG, respectively. For NOPHO, MRD was also evaluated on D15, and the relapse prediction accuracy of CIMP/D29 MRD (0.79) and CIMP/D15 MRD (0.75) classification was comparable, indicating potential for earlier stratification. The evaluation of the biology behind the CIMP subgroups revealed associations with transcriptome profiles, genomic aberrations, and mitotic history, suggesting distinct routes for leukemia development. In conclusion, integrating MRD assessment with the novel CIMP biomarker has the potential to improve risk stratification in pediatric T-ALL and guide future therapeutic decisions.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive form of leukemia that comprises 10% to 15% of pediatric ALL. Treatment advances involving high-intensity multiagent chemotherapy have increased survival rates to >80%.1,2 However, a significant proportion of deaths occur in complete remission, and patients with relapse or primary refractory T-ALL have dismal outcomes. Therefore, diagnostic biomarkers identifying patients who could benefit from novel treatment approaches and those who could benefit from less intense treatments are urgently needed.
In the Nordic Society of Paediatric Haematology (NOPHO) ALL2008 treatment protocol, the patients with T-ALL were risk stratified based on measurable residual disease (MRD) assessment at treatment days 15 (D15), 29 (D29), and 79 (D79).3 Patients were classified as high risk when MRD was >25% on D15 or ≥0.1% on D29 or D79. However, there is no consensus on MRD cutoff values between treatment protocols, some use 0.01% or any detectable MRD to stratify the patients, and the detection method may vary.4
The clinical markers' age, white blood cell (WBC) count, and the immunophenotype-based early thymic precursor (ETP) status, along with recurrent genomic aberrations or mutations, such as NOTCH1/FBXW7, provide limited prognostic information in T-ALL.2,5-8 However, SP/1 fusions and γδ-like subtypes have been associated with dismal outcomes,9-11 and emerging next-generation sequencing panels, investigating genomic alteration of a large set of genes combined with WBC and MRD, are promising and currently being validated.11,12 Subclassification based on gene expression data has been extensively evaluated, and several heterogenous T-ALL subgroups have been identified reflecting transcriptomic profiles associated with aberrations/fusions in the BCL11B, NKX2, TAL1/2, LMO1/2, TLX1/3, HOXA, SPI1, KMT2A, and MMLT10 genes.13 Recent data suggest combining transcriptional, genomic, and clinical marker subgrouping, but clinical implementation remains.10
DNA methylation profiling has become a valuable diagnostic biomarker for subtype classification, prognosis prediction, and treatment selection in various malignancies,14,15 including adult T-ALL.16 Canonical cancer methylome aberrations are frequently linked to distinct epigenetic patterns, promoter hypermethylation and global hypomethylation, which may affect gene expression and disease phenotype. Interestingly, a recent whole-genome methylation analysis of diagnostic T-ALL samples revealed continuously increased CpG island (CGI) hypermethylation but without global loss of methylation.17
Our research team has previously identified a CGI methylator phenotype (CIMP) panel correlating with overall CGI methylation which classifies T-ALL in CIMP subgroups.18,19 Importantly, CIMP classification holds prognostic relevance in Nordic, Polish, and Japanese T-ALL cohorts19-21 and has the potential to improve MRD-based stratification.18 However, clinical implementation of CIMP classification requires a substantial validation cohort treated with modern protocols using MRD assessment during treatment.
Differences in prognosis between CIMP subgroups indicate differences in their underlying biology. CIMP subgroups overlap with some transcriptomic subgroups, that is, CIMP-low patients have increased TAL1 and BEX1 expression,22 whereas CIMP-high patients have increased expression of ANTP homeobox oncogenes TLX3, HOXA9, HOXA10, and NKX2-1.22 A similar increase in TAL1, SIX6, and TLX3 expression was also associated with distinct DNA methylation profiles that overlap with CIMP subgroups.21,23
This study aimed at validating the prognostic relevance of CIMP classification in pediatric T-ALL in combination with clinical markers (WBC count, age, sex, and central nervous system [CNS] involvement) and MRD assessment at D15 and D29 or 33 (D33). We conducted a retrospective validation using a large T-ALL patient cohort treated according to the NOPHO ALL2008 protocol, along with an external validation cohort treated with the Dutch Childhood Oncology Group (DCOG) protocols. Furthermore, a comprehensive multiomics analysis of the NOPHO cohort was performed to elucidate the biology of CIMP subgroups and their relevance for treatment outcomes.
Methods
Patient and control samples
The NOPHO study cohort comprises 252 pediatric patients with T-ALL (aged ≥1 and <18 years) diagnosed in the Nordic countries between 2008 and 2018 and treated according to the NOPHO ALL2008 protocol.3 The last follow-up was in May 2023, with a median follow-up of 6.4 (range, 0.3-14.1) years for patients without events. Except for the patients not stratified due to induction failure (IF), the final risk stratification for treatment was intermediate risk, high-risk (HR) chemotherapy, and HR stem cell treatment (HR-SCT). The patients and/or their guardians read and signed an informed consent form.
The external validation cohort comprises 156 pediatric patients with T-ALL (aged ≥1 and <18 years) treated according to the consecutive DCOG ALL-10 (November 2004 to April 2012)24 and ALL-11 (April 2012 to July 2020)25 protocols. The last follow-up of both protocols was in December 2023, with a median follow-up of 10.8 (range, 5.5-19.0) years for patients without events. Patients were stratified into standard-risk, medium-risk, or HR arms, including HR-SCT.25 Informed consent was signed by parents and patients according to the Dutch law.
Treatment protocols are described in the supplemental Methods, available on the Blood website. The study was approved by the national ethics committees and conducted in accordance with the Declaration of Helsinki.
Reference samples included cell-sorted CD34+ and CD3+ cells, a fresh frozen lymph node, and publicly available DNA methylation array data (GSE36064) from the peripheral blood leukocytes of 78 healthy children (aged 1-16 years).26
Clinical variables
MRD was monitored by T-cell receptor polymerase chain reaction (PCR) or flow cytometry if no PCR marker was available at D15, D29, and D79 in the NOPHO cohort, and at induction D33 and D79 in the DCOG validation cohort. More than 90% of the NOPHO samples and almost 100% of the DCOG samples were analyzed by PCR.27 In our study, MRD cutoffs (≥0.1% and ≥0.01%) were used as a binary variable for both cohorts. WBC at diagnosis (×109/L) and MRD were log10 transformed for continuous variables. Additional clinical data were investigated, including age, sex, and CNS involvement. Cerebrospinal fluid with leukemic blasts detectable by cytomorphology of cytospin with a WBC count ≥5 per μL (CNS3) was considered positive for CNS involvement. ETP classification was determined by flow cytometry as previously described28 in the NOPHO cohort but was missing for the DCOG cohort.
DNA methylation analysis and CIMP classification
For the NOPHO ALL2008 cohort, DNAs from all available peripheral blood or bone marrow diagnostic T-ALL samples (n = 192) stored in the NOPHO Biobank were analyzed on Illumina 450K (n = 64, GSE69954, included in a previous study,18 but with shorter follow-up time) or EPIC version (v.)1 (n = 128, GSE272021) DNA methylation arrays (Illumina, CA). For the validation DCOG cohort, 156 samples stored in the Princess Máxima biobank were analyzed using Illumina EPICv.2.0 arrays (Illumina). After extracting, normalizing, and filtering the array data, β-values were estimated for downstream analysis (supplemental Methods; supplemental Figure 1).
CIMP classification was performed using the CIMP panel consisting of 1028 to 1293 CpGs depending on methylation array version (supplemental Methods). CIMP classification was defined as CIMP high when >40% of β-values >0.4 and CIMP low when ≤40% of β-values >0.4. The CIMP methylation percentage represents the percentage of CIMP panel CpGs with a β-value >0.4.18,20
Supervised elastic net and unsupervised clustering of the most variable EPICv.1/v.2.0 CpGs were run as alternative methods attempting to improve DNA methylation–based relapse prediction (supplemental Methods).
RNA expression analysis
RNA sequencing was performed on 108 diagnostic NOPHO T-ALL samples (GSE272023) and reference cells (sorted CD34+ and CD3+ cells and a lymph node) using the Illumina NovaSeq 6000 system (Illumina). RNA-sequencing data were aligned and filtered for downstream analyses. RNA expression was represented as variance-stabilizing transformation counts (supplemental Methods).
Biologic and mitotic ages
CNV and fusion analyses
For the NOPHO samples, copy number variations (CNVs) were obtained from the Illumina methylation EPICv.1 array (n = 128) (supplemental Methods), then summarized based on their minimal common region,35 and used to compare their prevalence between CIMP subgroups. Previously known T-ALL fusions were analyzed in the RNAseq data (n = 108) using Arriba v.1.2.0.36
Statistical and bioinformatic analyses
Statistical and bioinformatic analyses and plots were performed with RStudio 4.3.2, and described in the supplemental Methods.
Event-free survival (EFS) was defined as the time from diagnosis until death (IF or death in first complete remission [DCR]), relapse, second malignant neoplasm (SMN), or complete remission 1 (CR) at last follow-up (censored) in NOPHO, or until death (early death or DCR), nonresponders, relapse, SMN, or CR at last follow-up (censored) in DCOG. Overall survival (OS) was defined as the time from diagnosis to death of any cause or being alive at last follow-up (censored). Five-year pEFS and pOS were estimated using the Kaplan-Meier method/log-rank test, whereas cumulative incidence of relapse (pCIR) used the Fine-Gray test to account for competing events.37 Details on the Cox models,38 including methylation and expression analyses, are provided in the supplemental Methods.
Results
Clinical characteristics of CIMP-low and CIMP-high patients in the NOPHO ALL2008 cohort
The NOPHO study T-ALL cohort comprised 252 children (aged ≥1 to <18 years) treated according to the Nordic study protocol NOPHO ALL2008. At the end of follow-up, the pCIR5yr was 10.0% and the pOS5yr was 81.6%. The outcomes were CR (n = 200), relapse (n = 29), DCR (n = 17), IF (n = 4), and SMN (n = 2) with 210 patients alive and 42 deceased (supplemental Table 1).
Methylation analysis with CIMP classification was performed in all patients with available NOPHO Biobank samples (n = 192) (supplemental Figure 1), with 76 patients classified as CIMP low and 116 as CIMP high (Table 1; Figure 1A). The DNA methylation-analyzed samples did not significantly differ from the nonanalyzed samples (n = 60) regarding D15/D29 MRD status or final risk stratification (intermediate risk and HR chemotherapy, HR-SCT, or not stratified) but had higher WBC at diagnosis (median, 109.6 vs 43.6; WBC × 109/L; P = .002), lower age (median, 7.0 vs 9.5; P = .012), and a higher proportion of males (75% vs 60%; P = .033; supplemental Table 1). The low WBC could explain the insufficient sampling for biobanking. RNA-sequenced-analyzed (n = 108) vs nonanalyzed (n = 84) samples did not differ in clinical characteristics, except for D15 MRD status (supplemental Table 2).
Demographic characteristics of the CIMP-low and CIMP-high classes in CIMP-classified pediatric patients with T-ALL in the study NOPHO ALL2008 cohort and the DCOG ALL-10/ALL-11 validation cohorts
| . | NOPHO ALL2008 T-ALL cohort (n = 192) . | . | DCOG ALL-10/ALL-11 T-ALL cohort (n = 156) . | ||||
|---|---|---|---|---|---|---|---|
| CIMP low (n = 76, 39.6%) . | CIMP high (n = 116, 60.4%) . | P value . | CIMP low (n = 60, 38.5%) . | CIMP high (n = 96, 61.5%) . | P value . | ||
| Age, y | 7 (1-17) | 7 (1-17) | .573 | Age, y | 8 (1-17) | 8 (1-17) | .335 |
| Male/female | 58 (76.3%)/18 (23.7%) | 86 (74.1%)/30 (25.9%) | .890 | Male/female | 42 (70.0%)/18 (30.0%) | 69 (71.9%)/27 (28.1%) | .857 |
| WBC × 109/L | 173.0 (1.5-983) | 67.7 (1.2-825) | <.001∗∗∗ | WBC × 109/L | 118.7 (3.8-663) | 51.4 (1.0-954) | <.001∗∗∗ |
| CNS status (CNS1-2/CNS3/NA) | 68 (89.5%)/7 (9.2%)/1 (1.3%) | 98 (84.5%)/18 (15.5%)/0 (0%) | .274 | CNS status ([CNS1-CNS2-TLPneg-TLPpos]/CNS3/NA) | 51 (85%)/2 (3.3%)/7 (11.7%) | 81 (84.4%)/9 (9.4%)/6 (6.3%) | .213 |
| ETP-ALL (non-ETP/ETP/NA) | 54 (71.1%)/3 (3.9%)/19 (25%) | 68 (58.6%)/9 (7.8%)/39 (33.6%) | .236 | — | — | — | — |
| Deceased/alive | 20 (26.3%)/56 (73.7%) | 11 (9.5%)/105 (90.5%) | .003∗∗ | Deceased/alive | 18 (30.0%)/42 (70.0%) | 13 (13.5%)/83 (86.5%) | .014∗ |
| Relapse | 18 (23.7%) | 6 (5.2%) | <.001∗∗∗ | Relapse | 13 (21.7%) | 7 (7.3%) | .013∗ |
| CR | 50 (65.8%) | 102 (87.9%) | <.001∗∗∗ | CR | 39 (65%) | 81 (84.4%) | .006∗∗ |
| DCR | 5 (6.6%) | 7 (6.0%) | 1.000 | DCR | 4 (6.7%) | 4 (4.2%) | .485 |
| IF | 1 (1.3%) | 1 (0.9%) | 1.000 | Nonresponder | 2 (3.3%) | 1 (1%) | .559 |
| SMN | 2 (2.6%) | 0 (0.0%) | .155 | SMN | 1 (1.7%) | 1 (1%) | 1.000 |
| — | — | — | — | Early death | 1 (1.7%) | 2 (2.1%) | 1.000 |
| pCIR5yr, % (95% CI) | 18% (11-28) | 5.3% (2.2-10) | <.001∗∗∗ | pCIR5yr, % (95% CI) | 25% (14-37) | 7.8% (3.4-15) | .007∗∗ |
| pEFS5yr, % (95% CI) | 72.3% (62.9-83.1) | 88.6% (82.9-94.6) | <.001∗∗∗ | pEFS5yr, % (95% CI) | 65.0% (54.0-78.3) | 84.4% (77.4-92.0) | .006∗∗ |
| pOS5yr, % (95% CI) | 74.4% (65.0-85.1) | 91.1% (86.0-96.5) | .002∗∗ | pOS5yr, % (95% CI) | 71.7% (61.1-84.0) | 86.5% (79.9-93.6) | .015∗ |
| D15MRD < 0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 10 (13.2%)/10 (13.2%)/37 (48.7%)/11 (14.5%)/7 (9.2%)/1 (1.3%) | 28 (24.1%)/18 (15.5%)/34 (29.3%)/15 (12.9%)/17 (14.7%)/4 (3.4%) | .070 | — | — | — | — |
| D29MRD < 0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 22 (28.9%)/13 (17.1%)/32 (42.1%)/3 (3.9%)/3 (3.9%)/3 (3.9%) | 38 (32.8%)/19 (16.4%)/39 (33.6%)/4 (3.4%)/0 (0%)/16 (13.8%) | .308 | D33MRD <0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 18 (30%)/9 (15%)/16 (26.7%)/6 (10%)/4 (6.7%)/7 (11.7%) | 47 (49%)/11 (11.5%)/17 (17.7%)/6 (6.3%)/3 (3.1%)/12 (12.5%) | .137 |
| D79MRD < 0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 31 (40.8%)/6 (7.9%)/1 (1.3%)/0 (0%)/0 (0%)/38 (50%) | 56 (48.3%)/7 (6%)/0 (0%)/ 0 (0%)/0 (0%)/53 (45.7%) | .349 | D79MRD <0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 39 (65%)/3 (5%)/2 (3.3%)/6 (10%)/1 (1.7%)/9 (15%) | 69 (71.9%)/8 (8.3%)/1 (1%)/0 (0%)/1 (1%)/17 (17.7%) | .019∗ |
| Final risk stratification (IR/HR/NS) | 34 (44.7%)/41 (54.0%)/1 (1.3%) | 58 (50.0%)/57 (49.1%)/1 (0.9%) | .553 | Final risk stratification (SR/MR/HR/DI) | 2 (3.3%)/39 (65%)/16 (26.7%)/3 (5%) | 15 (15.6%)/62 (64.6%)/16 (16.7%)/3 (3.1%) | .049∗ |
| Final risk stratification (IR/HR-chemotherapy/HR-SCT/IF) | 34 (44.7%)/36 (47.4%)/5 (6.6%)/1 (1.3%) | 58 (50.0%)/45 (38.8%)/12 (10.3%)/1 (0.9%) | .577 | Final risk stratification (SR/MR/HR + SCT/HR without SCT/HR with SCT intention/DI) | 2 (3.3%)/39 (65%)/16 (26.7%)/0 (0%)/0 (0%)/ 3 (5.0%) | 15 (15.6%)/62 (64.6%)/12 (12.5%)/1 (1.0%)/3 (3.1%)/3 (3.1%) | .018∗ |
| CRs final risk stratification (IR/HR) | 26 (52.0%)/24 (48.0%) | 56 (54.9%)/46 (45.1%) | .863 | CRs final risk stratification (SR/MR/HR) | 1 (2.6%)/31 (79.5%)/7 (17.9%) | 14 (17.3%)/59 (72.8%)/8 (9.9%) | .036∗ |
| . | NOPHO ALL2008 T-ALL cohort (n = 192) . | . | DCOG ALL-10/ALL-11 T-ALL cohort (n = 156) . | ||||
|---|---|---|---|---|---|---|---|
| CIMP low (n = 76, 39.6%) . | CIMP high (n = 116, 60.4%) . | P value . | CIMP low (n = 60, 38.5%) . | CIMP high (n = 96, 61.5%) . | P value . | ||
| Age, y | 7 (1-17) | 7 (1-17) | .573 | Age, y | 8 (1-17) | 8 (1-17) | .335 |
| Male/female | 58 (76.3%)/18 (23.7%) | 86 (74.1%)/30 (25.9%) | .890 | Male/female | 42 (70.0%)/18 (30.0%) | 69 (71.9%)/27 (28.1%) | .857 |
| WBC × 109/L | 173.0 (1.5-983) | 67.7 (1.2-825) | <.001∗∗∗ | WBC × 109/L | 118.7 (3.8-663) | 51.4 (1.0-954) | <.001∗∗∗ |
| CNS status (CNS1-2/CNS3/NA) | 68 (89.5%)/7 (9.2%)/1 (1.3%) | 98 (84.5%)/18 (15.5%)/0 (0%) | .274 | CNS status ([CNS1-CNS2-TLPneg-TLPpos]/CNS3/NA) | 51 (85%)/2 (3.3%)/7 (11.7%) | 81 (84.4%)/9 (9.4%)/6 (6.3%) | .213 |
| ETP-ALL (non-ETP/ETP/NA) | 54 (71.1%)/3 (3.9%)/19 (25%) | 68 (58.6%)/9 (7.8%)/39 (33.6%) | .236 | — | — | — | — |
| Deceased/alive | 20 (26.3%)/56 (73.7%) | 11 (9.5%)/105 (90.5%) | .003∗∗ | Deceased/alive | 18 (30.0%)/42 (70.0%) | 13 (13.5%)/83 (86.5%) | .014∗ |
| Relapse | 18 (23.7%) | 6 (5.2%) | <.001∗∗∗ | Relapse | 13 (21.7%) | 7 (7.3%) | .013∗ |
| CR | 50 (65.8%) | 102 (87.9%) | <.001∗∗∗ | CR | 39 (65%) | 81 (84.4%) | .006∗∗ |
| DCR | 5 (6.6%) | 7 (6.0%) | 1.000 | DCR | 4 (6.7%) | 4 (4.2%) | .485 |
| IF | 1 (1.3%) | 1 (0.9%) | 1.000 | Nonresponder | 2 (3.3%) | 1 (1%) | .559 |
| SMN | 2 (2.6%) | 0 (0.0%) | .155 | SMN | 1 (1.7%) | 1 (1%) | 1.000 |
| — | — | — | — | Early death | 1 (1.7%) | 2 (2.1%) | 1.000 |
| pCIR5yr, % (95% CI) | 18% (11-28) | 5.3% (2.2-10) | <.001∗∗∗ | pCIR5yr, % (95% CI) | 25% (14-37) | 7.8% (3.4-15) | .007∗∗ |
| pEFS5yr, % (95% CI) | 72.3% (62.9-83.1) | 88.6% (82.9-94.6) | <.001∗∗∗ | pEFS5yr, % (95% CI) | 65.0% (54.0-78.3) | 84.4% (77.4-92.0) | .006∗∗ |
| pOS5yr, % (95% CI) | 74.4% (65.0-85.1) | 91.1% (86.0-96.5) | .002∗∗ | pOS5yr, % (95% CI) | 71.7% (61.1-84.0) | 86.5% (79.9-93.6) | .015∗ |
| D15MRD < 0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 10 (13.2%)/10 (13.2%)/37 (48.7%)/11 (14.5%)/7 (9.2%)/1 (1.3%) | 28 (24.1%)/18 (15.5%)/34 (29.3%)/15 (12.9%)/17 (14.7%)/4 (3.4%) | .070 | — | — | — | — |
| D29MRD < 0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 22 (28.9%)/13 (17.1%)/32 (42.1%)/3 (3.9%)/3 (3.9%)/3 (3.9%) | 38 (32.8%)/19 (16.4%)/39 (33.6%)/4 (3.4%)/0 (0%)/16 (13.8%) | .308 | D33MRD <0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 18 (30%)/9 (15%)/16 (26.7%)/6 (10%)/4 (6.7%)/7 (11.7%) | 47 (49%)/11 (11.5%)/17 (17.7%)/6 (6.3%)/3 (3.1%)/12 (12.5%) | .137 |
| D79MRD < 0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 31 (40.8%)/6 (7.9%)/1 (1.3%)/0 (0%)/0 (0%)/38 (50%) | 56 (48.3%)/7 (6%)/0 (0%)/ 0 (0%)/0 (0%)/53 (45.7%) | .349 | D79MRD <0.01/0.01-0.1/0.1-5/5-25/≥25/NA | 39 (65%)/3 (5%)/2 (3.3%)/6 (10%)/1 (1.7%)/9 (15%) | 69 (71.9%)/8 (8.3%)/1 (1%)/0 (0%)/1 (1%)/17 (17.7%) | .019∗ |
| Final risk stratification (IR/HR/NS) | 34 (44.7%)/41 (54.0%)/1 (1.3%) | 58 (50.0%)/57 (49.1%)/1 (0.9%) | .553 | Final risk stratification (SR/MR/HR/DI) | 2 (3.3%)/39 (65%)/16 (26.7%)/3 (5%) | 15 (15.6%)/62 (64.6%)/16 (16.7%)/3 (3.1%) | .049∗ |
| Final risk stratification (IR/HR-chemotherapy/HR-SCT/IF) | 34 (44.7%)/36 (47.4%)/5 (6.6%)/1 (1.3%) | 58 (50.0%)/45 (38.8%)/12 (10.3%)/1 (0.9%) | .577 | Final risk stratification (SR/MR/HR + SCT/HR without SCT/HR with SCT intention/DI) | 2 (3.3%)/39 (65%)/16 (26.7%)/0 (0%)/0 (0%)/ 3 (5.0%) | 15 (15.6%)/62 (64.6%)/12 (12.5%)/1 (1.0%)/3 (3.1%)/3 (3.1%) | .018∗ |
| CRs final risk stratification (IR/HR) | 26 (52.0%)/24 (48.0%) | 56 (54.9%)/46 (45.1%) | .863 | CRs final risk stratification (SR/MR/HR) | 1 (2.6%)/31 (79.5%)/7 (17.9%) | 14 (17.3%)/59 (72.8%)/8 (9.9%) | .036∗ |
Categorical variables are presented as number (%) and compared by the Fisher exact test. Continuous variables are presented as median (min-max) and compared by the Mann-Whitney U test. Five-year cumulative incidence of release (pCIR5yr) probability was estimated accounting for competing events by the Fine-Gray test, and EFS (pEFS5yr) and OS (pOS5yr) are presented as probabilities (95% CI) and compared by the log-rank test.
CI, confidence interval; DI, death in induction; NS, not stratified due to induction failure, thus not included in the Fisher exact test; NA, not available; TLP, traumatic lumbar puncture. ∗P < .05, ∗∗P < .01, ∗∗∗ P < .001.
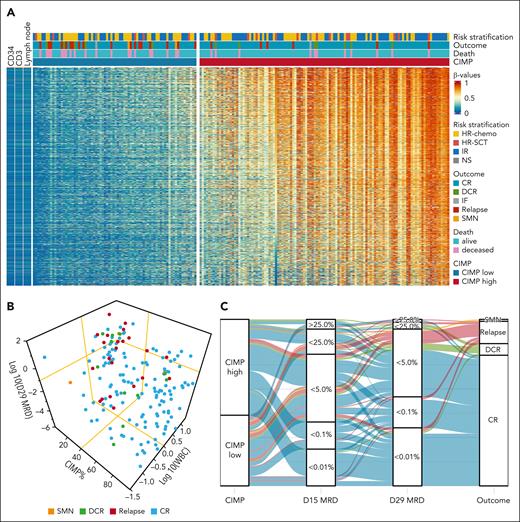
CIMP, MRD, WBC, and outcome in T-ALL in the study of NOPHO ALL2008 cohort. (A) Heat map of the 1091 CIMP panel CpGs in n = 192 NOPHO ALL2008 T-ALL samples at diagnosis, classified as CIMP low (n = 76) and CIMP high (n = 116) in CIMP% order. Healthy sorted CD34+ and CD3+ T cells and lymph node samples are revealed for comparison. (B) Distribution of the outcomes in 172 patients in relation to D29 MRD, WBC, and CIMP methylation %. MRD is log10 transformed, and WBC is zero scaled at 50 and log10 transformed. Yellow lines represent MRD 0.1%, WBC 50, and CIMP 40% cutoffs. (C) Alluvial plot depicting the timeline of patients from CIMP subgroups at diagnosis to the D15 MRD and D29 MRD until outcomes. In panels B and C, only the 172 patients with complete CIMP, WBC, and MRD data are illustrated. NS, not stratified due to IF.
CIMP, MRD, WBC, and outcome in T-ALL in the study of NOPHO ALL2008 cohort. (A) Heat map of the 1091 CIMP panel CpGs in n = 192 NOPHO ALL2008 T-ALL samples at diagnosis, classified as CIMP low (n = 76) and CIMP high (n = 116) in CIMP% order. Healthy sorted CD34+ and CD3+ T cells and lymph node samples are revealed for comparison. (B) Distribution of the outcomes in 172 patients in relation to D29 MRD, WBC, and CIMP methylation %. MRD is log10 transformed, and WBC is zero scaled at 50 and log10 transformed. Yellow lines represent MRD 0.1%, WBC 50, and CIMP 40% cutoffs. (C) Alluvial plot depicting the timeline of patients from CIMP subgroups at diagnosis to the D15 MRD and D29 MRD until outcomes. In panels B and C, only the 172 patients with complete CIMP, WBC, and MRD data are illustrated. NS, not stratified due to IF.
CIMP-low patients had significantly higher WBC (173.0 vs 67.7 WBC × 109/L; P < .001) compared with CIMP-high patients (Table 1; Figure 1B). Other clinical variables did not differ significantly: age (P = .573), sex (P = .890), CNS3 involvement (P = .274), D15/D29/D79 MRD status (P = .070/0.308/0.349), and final risk stratification (P = .553). ETP status did not differ between the CIMP subgroups (Table 1), but 30.2% of CIMP-classified patients had missing ETP data (supplemental Table 1). CIMP-low patients had a significantly higher proportion of relapses (23.7% vs 5.2%; P < .001) and death (26.3% vs 9.5%; P = .003) and a lower fraction in continuous CR (65.8% vs 87.9%; P < .001) compared with the CIMP-high subgroup (Table 1; Figure 1A). A high proportion of the relapses grouped together and had lower CIMP methylation percentage at diagnosis, higher WBC, and D29 MRD ≥ 0.1% (Figure 1B). In addition, all 7 relapses in patients with D29 MRD < 0.1% had CIMP-low methylation pattern, including 2 children who developed SMN (Figures 1C and 3A; Table 1). CR patients had no difference in risk stratification between the CIMP subgroups (P = .863; Table 1).
CIMP, D29/D33 MRD (0.1%), and survival in the study of NOPHO ALL2008 and validation DCOG ALL-10/ALL-11 cohorts. (A-D) pEFS and (E-H) pOS probabilities in pediatric patients with T-ALL stratified by CIMP and MRD. In the NOPHO cohort, samples were grouped by CIMP (n = 116 CIMP high and n = 76 CIMP low), at D29 MRD (<0.1%, n = 128 and ≥0.1%, n = 101), or CIMP and D29 MRD combined subgroups (CIMP low/D29 MRD ≥ 0.1%, n = 38; CIMP low/D29 MRD < 0.1%, n = 35; CIMP high/D29 MRD ≥ 0.1%, n = 42; and CIMP high/D29 MRD < 0.1%, n = 57). In the validation DCOG cohort, CIMP and D33 MRD were combined (CIMP low/D33 MRD ≥ 0.1%, n = 26; CIMP low/D33 MRD < 0.1%, n = 27; CIMP high/D33 MRD ≥ 0.1%, n = 26; and CIMP high/D33 MRD < 0.1%, n = 58).
CIMP, D29/D33 MRD (0.1%), and survival in the study of NOPHO ALL2008 and validation DCOG ALL-10/ALL-11 cohorts. (A-D) pEFS and (E-H) pOS probabilities in pediatric patients with T-ALL stratified by CIMP and MRD. In the NOPHO cohort, samples were grouped by CIMP (n = 116 CIMP high and n = 76 CIMP low), at D29 MRD (<0.1%, n = 128 and ≥0.1%, n = 101), or CIMP and D29 MRD combined subgroups (CIMP low/D29 MRD ≥ 0.1%, n = 38; CIMP low/D29 MRD < 0.1%, n = 35; CIMP high/D29 MRD ≥ 0.1%, n = 42; and CIMP high/D29 MRD < 0.1%, n = 57). In the validation DCOG cohort, CIMP and D33 MRD were combined (CIMP low/D33 MRD ≥ 0.1%, n = 26; CIMP low/D33 MRD < 0.1%, n = 27; CIMP high/D33 MRD ≥ 0.1%, n = 26; and CIMP high/D33 MRD < 0.1%, n = 58).
CIMP, MRD 0.1%, and relapse in the study of NOPHO ALL2008 and validation DCOG ALL-10/ALL-11 cohorts. Proportion of outcomes in 172 NOPHO (A) and 137 DCOG (D) pediatric patients with T-ALL; outcomes were relapse (n = 23/n = 18), DCR (n = 12/n = 8), SMN (n = 2/n = 1), and nonresponders (n = NA/n = 1) with CR (n = 135/n = 109) as reference, respectively. Samples were stratified by CIMP and MRD at D29/D33 MRD combined subgroups and (B,E) corresponding risk of relapse tables. Forest plots reveal the accuracy (estimated as [true positives + true negatives]/total) of D15 MRD and D29/D33 MRD above 0.1/0.01% cutoffs and CIMP-low status, alone or in combination, for differentiating relapse from CR in (C) the NOPHO and (F) the DCOG cohorts. NA, not available.
CIMP, MRD 0.1%, and relapse in the study of NOPHO ALL2008 and validation DCOG ALL-10/ALL-11 cohorts. Proportion of outcomes in 172 NOPHO (A) and 137 DCOG (D) pediatric patients with T-ALL; outcomes were relapse (n = 23/n = 18), DCR (n = 12/n = 8), SMN (n = 2/n = 1), and nonresponders (n = NA/n = 1) with CR (n = 135/n = 109) as reference, respectively. Samples were stratified by CIMP and MRD at D29/D33 MRD combined subgroups and (B,E) corresponding risk of relapse tables. Forest plots reveal the accuracy (estimated as [true positives + true negatives]/total) of D15 MRD and D29/D33 MRD above 0.1/0.01% cutoffs and CIMP-low status, alone or in combination, for differentiating relapse from CR in (C) the NOPHO and (F) the DCOG cohorts. NA, not available.
CIMP classification can be used together with MRD assessment to predict prognosis
CIMP classification was evaluated in relation to outcome in the NOPHO study cohort. The CIMP-low subgroup (n = 76) had pEFS5yr/pOS5yr of 72.3%/74.4% and pCIR5yr of 18.0%, whereas the CIMP-high subgroup (n = 116) had higher pEFS5yr/pOS5yr of 88.6%/91.1% and a lower pCIR5yr of 5.3% (P < .001; Figure 2A,E; Table 1; supplemental Figure 3A). Univariable Cox models revealed CIMP as the most fit variable to predict the risk of relapse, that is, highest hazard ratio and lowest Akaike information criterion (Table 2). All late relapses (>5 years postdiagnosis, n = 4) had a CIMP-low profile at diagnosis (Figure 3A).
Cause-specific Cox proportional hazard regression models using CIMP subgroups and D29 (NOPHO)/33 MRD (DCOG) to predict the risk of relapse (accounting for competing events) and death in pediatric patients with T-ALL
| . | Risk of relapse . | Risk of death . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOPHO (n = 172, n = 23 relapses) . | DCOG (n = 137, n = 18 relapses)† . | NOPHO (n = 172, n = 28 deaths) . | DCOG (n = 137, n = 24 deaths)† . | |||||||||
| Hazard ratio (95% CI) . | P value . | AIC . | Hazard ratio (95% CI) . | P value . | AIC . | Hazard ratio (95% CI) . | P value . | AIC . | Hazard ratio (95% CI) . | P value . | AIC . | |
| Univariable analysis | ||||||||||||
| CIMP low | 5.27 (1.95-14.2) | .001∗∗ | 213.5 | 3.43 (1.29-9.15) | .014∗ | 167.9 | 3.06 (1.38-6.77) | .006∗∗ | 272.6 | 2.31 (1.03-5.20) | .044∗ | 229.4 |
| D29/33 MRD ≥ 0.1% | 3.10 (1.27-7.53) | .013∗ | 220.3 | 2.55 (1.00-6.46) | .049∗ | 170.6 | 7.84 (2.72-22.6) | <.001∗∗∗ | 259.3 | 3.89 (1.66-9.10) | .002∗∗ | 223.0 |
| log10(WBC) | 1.69 (1.02-2.82) | .044∗ | 222.4 | 1.78 (0.72-4.38) | .212 | 162.5 | 1.19 (0.80-1.79) | .386 | 280.2 | 1.43 (0.90-2.28) | .131 | 220.5 |
| CNS3 | 1.13 (0.38-3.33) | .827 | 227.1 | 1.70 (0.39-7.44) | .481 | 161.9 | 0.67 (0.20-2.22) | .509 | 280.5 | 1.85 (0.55-6.27) | .321 | 210.1 |
| Male sex | 1.38 (0.47-4.05) | .560 | 226.7 | 0.70 (0.26-1.88) | .483 | 174.0 | 0.50 (0.23-1.09) | .081 | 278.1 | 0.57 (0.25-1.30) | .183 | 231.9 |
| Age | 0.56 (0.34-0.92) | .022∗ | 221.0 | 1.99 (1.21-3.25) | .006∗∗ | 166.4 | 1.12 (0.77-1.63) | .552 | 280.6 | 1.09 (0.73-1.64) | .669 | 233.4 |
| Multivariate analysis | ||||||||||||
| CIMP low | 4.92 (1.82-13.28) | .002∗∗ | 209.9 | 2.98 (1.10-8.09) | .032∗ | 167.7 | 2.72 (1.23-6.03) | .013∗ | 254.7 | 1.83 (0.80-4.19) | .150 | 222.9 |
| D29/33 MRD ≥ 0.1% | 2.80 (1.15-6.84) | .023∗ | 2.05 (0.79-5.30) | .138 | 7.32 (2.53-21.13) | <.001∗∗∗ | 3.47 (1.46-8.23) | .005∗∗ | ||||
| Multivariate analysis | ||||||||||||
| CIMP low | 4.06 (1.46-11.3) | .007∗∗ | 208.1 | 2.53 (0.90-7.07) | .078 | 156.0 | 2.71 (1.20-6.12) | .017∗ | 258.1 | 1.65 (0.70-3.88) | .254 | 216.4 |
| D29/33 MRD ≥ 0.1% | 2.58 (1.05-6.37) | .039∗ | 1.94 (0.74-5.07) | .176 | 7.40 (2.56-21.3) | <.001∗∗∗ | 3.33 (1.40-7.94) | .006∗∗ | ||||
| Age | 0.65 (0.41-1.01) | .055 | 1.81 (1.12-2.92) | .016∗ | 1.14 (0.80-1.64) | .473 | 1.06 (0.71-1.59) | .781 | ||||
| log10(WBC) | 1.30 (0.76-2.20) | .337 | 1.34 (0.70-2.55) | .378 | 1.07 (0.74-1.55) | .723 | 1.26 (0.78-2.05) | .345 | ||||
| CIMP-MRD interaction analysis | ||||||||||||
| D29/33 MRD < 0.1%/CIMP high | Ref. | Ref. | Ref. | Ref. | ||||||||
| D29/33 MRD < 0.1%/CIMP low | 13.21 (2.42-245) | .001∗∗ | 6.89 (1.39-34.13) | .018∗ | 3.30 (0.64-23.8) | .152 | 3.63 (0.87-15.18) | .078 | ||||
| D29/33 MRD ≥ 0.1%/CIMP high | 9.11 (1.56-172) | .012∗ | 5.68 (1.04-31.03) | .045∗ | 6.63 (1.71-43.5) | .005∗∗ | 6.22 (1.61-24.05) | .008∗∗ | ||||
| D29/33 MRD ≥ 0.1%/CIMP low | 22.76 (4.48-415) | <.001∗∗∗ | 8.53 (1.72-42.29) | .009∗∗ | 15.48 (4.43-97.7) | <.001∗∗∗ | 8.01 (2.17-29.61) | .002∗∗ | ||||
| . | Risk of relapse . | Risk of death . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOPHO (n = 172, n = 23 relapses) . | DCOG (n = 137, n = 18 relapses)† . | NOPHO (n = 172, n = 28 deaths) . | DCOG (n = 137, n = 24 deaths)† . | |||||||||
| Hazard ratio (95% CI) . | P value . | AIC . | Hazard ratio (95% CI) . | P value . | AIC . | Hazard ratio (95% CI) . | P value . | AIC . | Hazard ratio (95% CI) . | P value . | AIC . | |
| Univariable analysis | ||||||||||||
| CIMP low | 5.27 (1.95-14.2) | .001∗∗ | 213.5 | 3.43 (1.29-9.15) | .014∗ | 167.9 | 3.06 (1.38-6.77) | .006∗∗ | 272.6 | 2.31 (1.03-5.20) | .044∗ | 229.4 |
| D29/33 MRD ≥ 0.1% | 3.10 (1.27-7.53) | .013∗ | 220.3 | 2.55 (1.00-6.46) | .049∗ | 170.6 | 7.84 (2.72-22.6) | <.001∗∗∗ | 259.3 | 3.89 (1.66-9.10) | .002∗∗ | 223.0 |
| log10(WBC) | 1.69 (1.02-2.82) | .044∗ | 222.4 | 1.78 (0.72-4.38) | .212 | 162.5 | 1.19 (0.80-1.79) | .386 | 280.2 | 1.43 (0.90-2.28) | .131 | 220.5 |
| CNS3 | 1.13 (0.38-3.33) | .827 | 227.1 | 1.70 (0.39-7.44) | .481 | 161.9 | 0.67 (0.20-2.22) | .509 | 280.5 | 1.85 (0.55-6.27) | .321 | 210.1 |
| Male sex | 1.38 (0.47-4.05) | .560 | 226.7 | 0.70 (0.26-1.88) | .483 | 174.0 | 0.50 (0.23-1.09) | .081 | 278.1 | 0.57 (0.25-1.30) | .183 | 231.9 |
| Age | 0.56 (0.34-0.92) | .022∗ | 221.0 | 1.99 (1.21-3.25) | .006∗∗ | 166.4 | 1.12 (0.77-1.63) | .552 | 280.6 | 1.09 (0.73-1.64) | .669 | 233.4 |
| Multivariate analysis | ||||||||||||
| CIMP low | 4.92 (1.82-13.28) | .002∗∗ | 209.9 | 2.98 (1.10-8.09) | .032∗ | 167.7 | 2.72 (1.23-6.03) | .013∗ | 254.7 | 1.83 (0.80-4.19) | .150 | 222.9 |
| D29/33 MRD ≥ 0.1% | 2.80 (1.15-6.84) | .023∗ | 2.05 (0.79-5.30) | .138 | 7.32 (2.53-21.13) | <.001∗∗∗ | 3.47 (1.46-8.23) | .005∗∗ | ||||
| Multivariate analysis | ||||||||||||
| CIMP low | 4.06 (1.46-11.3) | .007∗∗ | 208.1 | 2.53 (0.90-7.07) | .078 | 156.0 | 2.71 (1.20-6.12) | .017∗ | 258.1 | 1.65 (0.70-3.88) | .254 | 216.4 |
| D29/33 MRD ≥ 0.1% | 2.58 (1.05-6.37) | .039∗ | 1.94 (0.74-5.07) | .176 | 7.40 (2.56-21.3) | <.001∗∗∗ | 3.33 (1.40-7.94) | .006∗∗ | ||||
| Age | 0.65 (0.41-1.01) | .055 | 1.81 (1.12-2.92) | .016∗ | 1.14 (0.80-1.64) | .473 | 1.06 (0.71-1.59) | .781 | ||||
| log10(WBC) | 1.30 (0.76-2.20) | .337 | 1.34 (0.70-2.55) | .378 | 1.07 (0.74-1.55) | .723 | 1.26 (0.78-2.05) | .345 | ||||
| CIMP-MRD interaction analysis | ||||||||||||
| D29/33 MRD < 0.1%/CIMP high | Ref. | Ref. | Ref. | Ref. | ||||||||
| D29/33 MRD < 0.1%/CIMP low | 13.21 (2.42-245) | .001∗∗ | 6.89 (1.39-34.13) | .018∗ | 3.30 (0.64-23.8) | .152 | 3.63 (0.87-15.18) | .078 | ||||
| D29/33 MRD ≥ 0.1%/CIMP high | 9.11 (1.56-172) | .012∗ | 5.68 (1.04-31.03) | .045∗ | 6.63 (1.71-43.5) | .005∗∗ | 6.22 (1.61-24.05) | .008∗∗ | ||||
| D29/33 MRD ≥ 0.1%/CIMP low | 22.76 (4.48-415) | <.001∗∗∗ | 8.53 (1.72-42.29) | .009∗∗ | 15.48 (4.43-97.7) | <.001∗∗∗ | 8.01 (2.17-29.61) | .002∗∗ | ||||
AIC, Akaike information criterion, in which the fittest model reveals the lowest value and vice versa; CI, confidence interval; CNS3, central nervous system 3 involvement status; Ref., reference group.
∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Due to WBC count missings, DCOG models including WBC have n = 134 with 17 relapses and 23 deaths.
Patients with D29 MRD ≥ 0.1% (n = 101) had a poorer prognosis, with pEFS5yr/pOS5yr of 69.0%/72.5% and pCIR5yr of 19.0%, compared with patients with D29 MRD < 0.1% (n = 128), with pEFS5yr/pOS5yr of 91.2%/92.8% and pCIR5yr of 4.0% (P = .006; Figure 2B,F; supplemental Figure 3B).
Most importantly, CIMP classification at diagnosis combined with D29 MRD identified a poor prognosis subgroup (CIMP low/D29 MRD ≥ 0.1%, n = 38) with pEFS5yr of 57.7%, pOS5yr of 59.7% (Figure 2C,G), and pCIR5yr of 29.0% (P < .001; Figure 3A,B; supplemental Figure 3D), but also a very favorable outcome subgroup (CIMP high/D29 MRD < 0.1%, n = 57) with pEFS5yr of 98.2%, pOS5yr of 98.2% (Figure 2C,G), and pCIR5yr of 0% (Figure 3A,B; supplemental Figure 3D). Penalized Cox models with interaction between CIMP and MRD revealed that both CIMP-low combined subgroups were highly associated with increased risk of relapse (CIMP low/D29 MRD ≥ 0.1%; hazard ratio, 22.8; P < .001; and CIMP low/D29 MRD < 0.1%; hazard ratio, 13.2; P = .001), whereas both D29 MRD ≥ 0.1% combined subgroups were highly associated with increased risk of death (MRD ≥ 0.1%/CIMP low; hazard ratio, 15.5; P < .001; and MRD ≥ 0.1%/CIMP high; hazard ratio, 6.6; P = .005; Table 2).
The accuracy of CIMP and MRD individually and combined as markers to discriminate relapse from CR was evaluated with the highest accuracy of 0.79 when combining CIMP low and D29 MRD ≥ 0.1% (Figure 3C). Univariable Cox models revealed that both CIMP-low status and D29 MRD ≥ 0.1% were associated with an increased risk of relapse (hazard ratio, 5.27; P = .001; and hazard ratio, 3.10; P = .013, respectively) and death (hazard ratio, 3.06; P = .006; and hazard ratio, 7.84; P < .001, respectively; Table 2). Multivariable Cox model analysis revealed that both CIMP low and D29 MRD ≥ 0.1% were independent prognostic markers of relapse (hazard ratio, 4.92; P = .002 and hazard ratio, 2.80; P = .023, respectively) and death (hazard ratio, 2.72; P = .013 and hazard ratio, 7.32; P < .001, respectively) in the NOPHO cohort. When including all the significant variables from the univariable analysis (CIMP status, D29 MRD, WBC, and age) in the model, both CIMP low and D29 MRD ≥ 0.1% remained independent prognostic markers for relapse risk (hazard ratio, 4.06; P = .007; and hazard ratio, 2.58; P = .039, respectively) and death (hazard ratio, 2.71; P = .017; and hazard ratio, 7.40; P < .001, respectively; Table 2).
In addition, D29 MRD 0.01% cutoff was not significantly associated with relapse in a univariable Cox model (P = .069; supplemental Table 3) but significantly associated with pCIR alone or combined with CIMP (P = .040 and P < .001; supplemental Figure 3C,E) and when adjusted for CIMP, WBC, and age in a multivariable Cox model (hazard ratio, 2.58; P = .039), whereas it was significantly associated with increased risk of death in both univariable (hazard ratio, 3.39; P = .024) and multivariable (hazard ratio, 3.13; P = .035) models (supplemental Table 3).
Similar results were found when combining CIMP and D15 MRD 0.1/0.01% (Cox models in supplemental Table 3 and pCIR/pOS in supplemental Figure 5). The accuracy of discriminating relapses from CR was lower for D15 MRD ≥ 0.1% (0.52) than for D29 MRD ≥ 0.1% (0.63). However, combining CIMP low/D15 MRD ≥ 0.1% increased the accuracy to 0.75, which is almost as strong as the accuracy of CIMP low/D29 MRD ≥ 0.1% of 0.79 (Figure 3C).
External validation of CIMP/MRD classification in the DCOG ALL-10/11 cohort
The pediatric DCOG ALL-10/11 validation cohort (n = 156) had similar demographics as the NOPHO study cohort, including similar proportions of CIMP low (39.6% vs 38.5%) and CIMP high (60.4% vs 61.5%) subgroups and a higher WBC in CIMP low compared with CIMP high (118.7 vs 51.4; WBC × 109/L; P < .001; Table 1; supplemental Table 4). The poorer prognosis of the D33 MRD ≥ 0.1% (n = 85, pCIR5yr = 19.0%) compared with D33 MRD < 0.1% (n = 52, pCIR5yr = 9.4%; P = .041) was confirmed, along with a poorer prognosis of CIMP-low patients (n = 60, pCIR5yr = 23.0%) compared with CIMP-high patients (n = 96, pCIR5yr = 7.1%; P = .007; Table 1; supplemental Figure 4A-B).
The combination of CIMP and D33 MRD confirmed the good prognosis for CIMP high/D33 MRD < 0.1% subgroup (n = 58, pEFS5yr = 93.1%, pOS5yr = 94.8%, and pCIR5yr = 3.4%) and poor prognosis for CIMP low/D33 MRD ≥ 0.1% subgroup (n = 26, pEFS5yr = 61.5%, pOS5yr = 65.4%, and pCIR5yr = 23.0%; Figure 2D,H; Figure 3D,E; supplemental Figure 4D).
Multivariable Cox models of the DCOG cohort including categorized CIMP and D33 MRD ≥ 0.1% status revealed that only CIMP low remained significantly associated with relapse risk (CIMP hazard ratio, 2.98; P = .032; and D33 MRD ≥ 0.1% hazard ratio, 2.05; P = .138), whereas only D33 MRD ≥ 0.1% remained significantly associated with risk of death (CIMP hazard ratio, 1.83; P = .150; and D33 MRD ≥ 0.1% hazard ratio, 3.47; P = .005; Table 2). The multivariable analysis incorporating the 4 variables (CIMP status, MRD, WBC, and age) revealed the highest hazard ratio (hazard ratio, 2.53) for CIMP-low status but did not reach significance (P = .078).
Cox models with interaction between CIMP and D33 MRD validated that the CIMP low/D33 MRD ≥ 0.1% subgroup had the highest risk of relapse (hazard ratio, 8.53; P = .009) and death (hazard ratio, 8.01; P = .002; Table 2).
The accuracy of CIMP low combined with D29/D33 MRD ≥ 0.1% was similar in the cohorts (0.79 in NOPHO and 0.78 in DCOG) (Figure 3C,F). A high accuracy was also found using MRD 0.01% as the threshold (0.75 in NOPHO and 0.76 in DCOG; Figure 3C,F; supplemental Figures 3E and 4E).
We used supervised elastic net and unsupervised clustering of the most variable CpGs as alternative strategies for DNA methylation–based relapse prediction, using NOPHO-EPICv.1 and DCOG-EPICv.2.0 analyzed samples as discovery and validation sets, respectively. Although relapse prediction was enhanced in the discovery set, it did not surpass the CIMP classifier within the validation set, and the unsupervised clustering strategy strongly overlapped with CIMP subgroups (supplemental Figure 2A-C).
Genome-wide changes in T-ALL are associated with CIMP subgroups in the NOPHO cohort
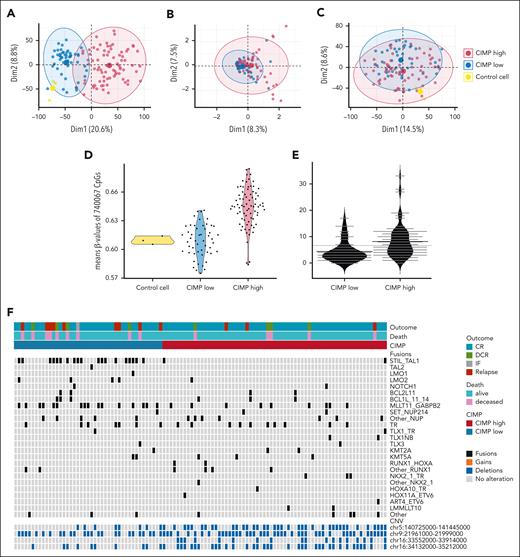
Unsupervised principal component analysis of genome-wide DNA methylation data (Figure 4A) revealed that T-ALL samples' first dimension of variance overlapped with CIMP subgroups. CIMP-high T-ALLs had higher mean β-values of all CpGs (n = 128 samples, 740 067 EPICv.1 array CpGs) compared with CIMP-low T-ALLs and reference cells, where CIMP-low variance and mean methylation were more similar to the reference cells (Figure 4A,D). In contrast to methylation, genome-wide CNV and whole-genome RNA expression variance were less associated with CIMP (Figure 4B,C). CIMP high presented a higher number of CNVs (Figure 4E), with a significantly higher number of deletions than CIMP low in the chr5:140725000-141445000, chr16:33552000-33914000, and chr16:34132000-35212000 chromosome regions and recurrent alterations in the CDKN2A/B gene locus within the chr9:21961000-21999000 region (Figure 4F; supplemental Table 5). Among previously described fusions in T-ALL, STIL::TAL1 (44.2% vs 3.1%) and MLLT11::GABPB2 (37.2% vs 13.8%) were more frequently found in CIMP-low compared with CIMP-high T-ALLs (Figure 4F). STIL::TAL1 fusions were not significantly associated with relapse (pCIR5yr 19.0% vs 5.8%; P = .200) but with death (pOS5yr 71.4% vs 90.4%; P = .012; supplemental Figure 6A-B). No association of MLLT11::GABPB2 fusions with outcome was found (supplemental Figure 6C-D).
Genome-wide multiomics biology phenotype of CIMP subgroups in the NOPHO ALL2008 cohort. Unsupervised principal component analysis of (A) genome-wide DNA methylation (740 067 CpGs, n = 128 EPICv.1-analyzed T-ALL), (B) genomic alterations (294 unique CNVs, n = 128 T-ALL), and (C) whole-genome RNA expression (19 043 protein-coding genes, n = 108 T-ALL). (D) Average β-values of the array CpGs (740 067 CpGs, n = 128 EPIC-analyzed T-ALL) separated into CIMP subgroups and controls. (E) Average number of CNVs in CIMP-low (n = 51) vs CIMP-high (n = 77) T-ALL. (F) Common fusions in T-ALL and a relevant CNV in T-ALL (chr9) and 3 significantly different CNVs between CIMP low and CIMP high in 108 T-ALLs. The control cells in panels A, C, and D consisted of CD34+, CD3+, and lymph node, respectively.
Genome-wide multiomics biology phenotype of CIMP subgroups in the NOPHO ALL2008 cohort. Unsupervised principal component analysis of (A) genome-wide DNA methylation (740 067 CpGs, n = 128 EPICv.1-analyzed T-ALL), (B) genomic alterations (294 unique CNVs, n = 128 T-ALL), and (C) whole-genome RNA expression (19 043 protein-coding genes, n = 108 T-ALL). (D) Average β-values of the array CpGs (740 067 CpGs, n = 128 EPIC-analyzed T-ALL) separated into CIMP subgroups and controls. (E) Average number of CNVs in CIMP-low (n = 51) vs CIMP-high (n = 77) T-ALL. (F) Common fusions in T-ALL and a relevant CNV in T-ALL (chr9) and 3 significantly different CNVs between CIMP low and CIMP high in 108 T-ALLs. The control cells in panels A, C, and D consisted of CD34+, CD3+, and lymph node, respectively.
Methylome phenotype of CIMP subgroups is associated with nonexpressed genes
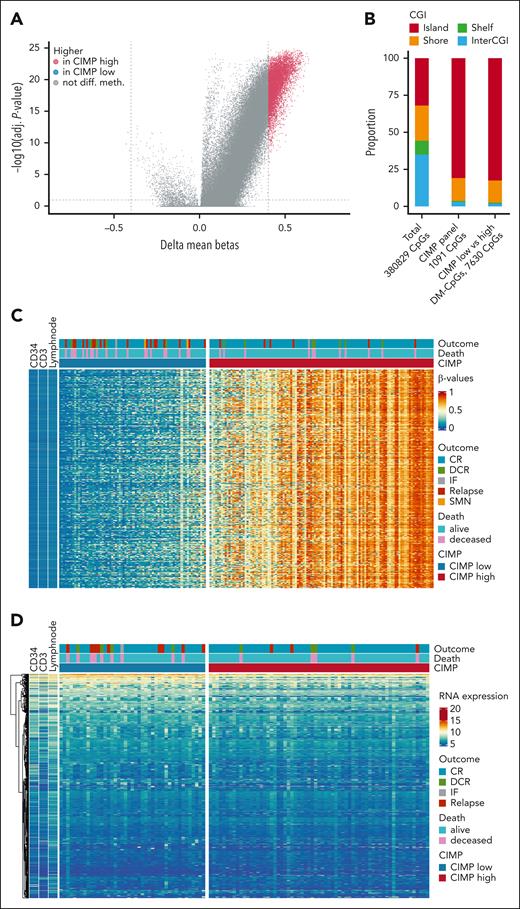
Increasing CIMP methylation percentage correlated with a gradual increase in overall methylation pattern for all T-ALLs. Genome-wide differentially methylated CpGs (DM-CpGs, delta-β > 0.4) analysis of the 380 829 overlapping EPICv.1 and 450K array CpGs found 7627 CpGs with higher mean β-values in CIMP high and only 3 CpGs with higher mean β-values in CIMP low (Figure 5A,C). These CpGs were unmethylated in reference cells, regardless of the cell maturation stage (Figure 5C). Similar to CIMP-associated CpGs, the DM-CpGs were enriched in CGI genomic regions (Figure 5B). The genes associated with the DM-CpGs (n = 1046 unique genes) revealed, in general, no or low RNA expression (Figure 5D).
Methylome phenotype of the CIMP-low and CIMP-high subgroups in the NOPHO ALL2008 cohort. (A) Differentially methylated CpGs between CIMP-low and CIMP-high leukemias (n = 192 450K+EPICv.1 array-analyzed samples). β-values from EPICv.1 arrays (n = 128 T-ALL and 743 434 CpGs) were merged with 450K arrays (n = 64 T-ALL, 410 369 CpGs), whereafter 380 829 CpGs remained for differential methylation analysis. Of 380 829 CpGs, 3 CpGs had higher mean β-values in CIMP low and 7627 CpGs had higher mean β-values in CIMP high. (B) CGI distribution of the merged array data, the CIMP panel, and the DM-CpGs. (C) Heat map of the β-values of the 7630 DM-CpGs between CIMP subgroups in 192 T-ALL and 3 reference cells. (D) Gene expression (in VST counts) of the corresponding 1046 unique genes annotated to the DM-CpGs in 108 T-ALL and 3 reference cells. Reference cells in panels C and D are CD34+, CD3+, and lymph node. VST, variance-stabilizing transformation.
Methylome phenotype of the CIMP-low and CIMP-high subgroups in the NOPHO ALL2008 cohort. (A) Differentially methylated CpGs between CIMP-low and CIMP-high leukemias (n = 192 450K+EPICv.1 array-analyzed samples). β-values from EPICv.1 arrays (n = 128 T-ALL and 743 434 CpGs) were merged with 450K arrays (n = 64 T-ALL, 410 369 CpGs), whereafter 380 829 CpGs remained for differential methylation analysis. Of 380 829 CpGs, 3 CpGs had higher mean β-values in CIMP low and 7627 CpGs had higher mean β-values in CIMP high. (B) CGI distribution of the merged array data, the CIMP panel, and the DM-CpGs. (C) Heat map of the β-values of the 7630 DM-CpGs between CIMP subgroups in 192 T-ALL and 3 reference cells. (D) Gene expression (in VST counts) of the corresponding 1046 unique genes annotated to the DM-CpGs in 108 T-ALL and 3 reference cells. Reference cells in panels C and D are CD34+, CD3+, and lymph node. VST, variance-stabilizing transformation.
Gene expression associated with CIMP subgroups
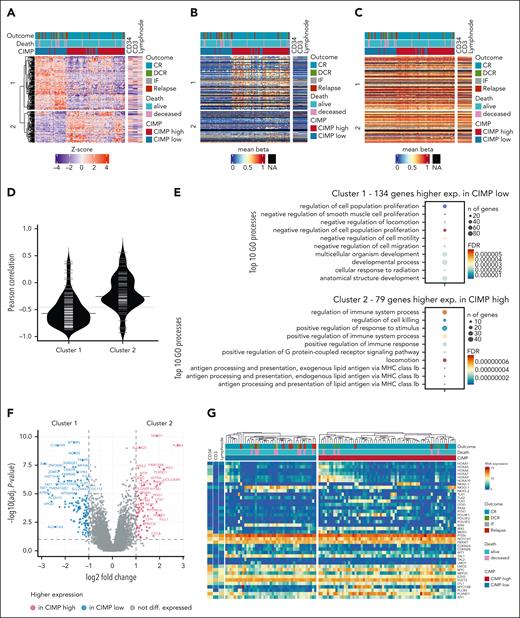
Differential gene expression analysis between CIMP subgroups revealed higher expression of 134 genes in CIMP low T-ALLs (cluster 1) and 79 genes in CIMP high T-ALLs (cluster 2), in a hierarchical cluster heat map. Cluster 1 genes had a significantly stronger (P < .01) negative correlation between expression and mean promoter DNA methylation (R = −0.57) compared with cluster 2 genes (R = −0.26). Both clusters had similar high mean gene body DNA methylation (Figure 6A-D; supplemental Table 6).
Transcriptome phenotype of CIMP-low and CIMP-high subgroups in the NOPHO ALL2008 cohort. (A) Heat map revealing the relative RNA expression of n = 213 DEG between CIMP-low (n = 43) and CIMP-high (n = 65) T-ALL samples; genes hierarchical clustering highlights a cluster 1 with higher expression in CIMP low (134 genes) and a cluster 2 with higher expression in CIMP high (79 genes). (B,C) Heat maps presenting the mean β-values of CpGs located on the promoter region (B) or on the gene body (C) of the DEG visualized in the same gene and sample order as in panel A. (D) Pearson correlation between clusters 1 and 2 gene RNA expression and their mean promoter region DNA methylation. (E) GO analysis of DEGs with higher expression in CIMP low (n = 134 genes, cluster 1) and CIMP high (n = 79 genes, cluster 2), respectively. (F) Volcano plot with highlighted n = 213 DEG in n = 108 T-ALL. (G) RNA expression (in VST counts) heat map using hierarchical clustering of 108 T-ALL samples, revealing 40 selected genes relevant in T-ALL biology or differentially expressed between CIMP-low and CIMP-high subgroups. Sorted CD34+ and CD3+ T cells and lymph node are illustrated as normal cell types in the heat maps. DEG, differentially expressed gene; GO, gene ontology.
Transcriptome phenotype of CIMP-low and CIMP-high subgroups in the NOPHO ALL2008 cohort. (A) Heat map revealing the relative RNA expression of n = 213 DEG between CIMP-low (n = 43) and CIMP-high (n = 65) T-ALL samples; genes hierarchical clustering highlights a cluster 1 with higher expression in CIMP low (134 genes) and a cluster 2 with higher expression in CIMP high (79 genes). (B,C) Heat maps presenting the mean β-values of CpGs located on the promoter region (B) or on the gene body (C) of the DEG visualized in the same gene and sample order as in panel A. (D) Pearson correlation between clusters 1 and 2 gene RNA expression and their mean promoter region DNA methylation. (E) GO analysis of DEGs with higher expression in CIMP low (n = 134 genes, cluster 1) and CIMP high (n = 79 genes, cluster 2), respectively. (F) Volcano plot with highlighted n = 213 DEG in n = 108 T-ALL. (G) RNA expression (in VST counts) heat map using hierarchical clustering of 108 T-ALL samples, revealing 40 selected genes relevant in T-ALL biology or differentially expressed between CIMP-low and CIMP-high subgroups. Sorted CD34+ and CD3+ T cells and lymph node are illustrated as normal cell types in the heat maps. DEG, differentially expressed gene; GO, gene ontology.
Genes with significant gene expression level changes for CIMP low included MTURN, C14orf39, WD35, TRIM59, and SIX6, whereas CIMP high included TRIM72, PLCB4, SLC6A20, FAM124B, NEIL2, and IRX3 genes (Figure 6F). Pathway enrichment analysis revealed that CIMP-low expressed genes were enriched in cell proliferation and cell migration regulation pathways, whereas CIMP-high expressed genes were enriched in immune system regulation pathways (Figure 6E).
Regarding the expression of known genes in T-ALL biology, we confirmed their association with CIMP subgroups. SIX6, TAL1, and NKX3-1 had higher expression in CIMP-low T-ALLs, whereas HOX and POU homeobox genes, TLX3, IRX3, and PLXND1, had higher expression in CIMP-high T-ALLs (Figure 6G). The expression of most of these genes was either not or only weakly associated with mean promoter region DNA methylation; however, MYO18B (R = −0.92) and SPI1 (R = −0.60) expression was inversely strongly associated with mean promoter DNA methylation (supplemental Figure 7; supplemental Table 7).
CIMP percentage was evaluated against the RNA expression of DNA methylation regulators, but no strong correlation was found for DNMT1, TET1, TET3, EZH2, SUZ12, IDH1, PHF6, and DNMT3A. However, a weak positive correlation was found in DNMT3B (rho = 0.31; P < .001) and UHRF1 (rho = 0.24; P = .013) and a week negative correlation was found for TET2 (rho = −0.19; P = .048), MECP2 (rho = −0.34; P < .001), and IDH2 (rho = −0.31; P = .001; Figure 7A-B; supplemental Figure 8).
CIMP percentage association with DNA methyltransferase gene expression, chronological and cellular age in the NOPHO ALL2008 cohort. Correlation of CIMP methylation percentage and (A) DNA methyltransferase 3 alpha (DNMT3A) and (B) beta (DNMT3B) gene expression (in VST counts) in 108 T-ALL patient samples and 3 control cells (CD34+, CD3+, and lymph node). Correlation of CIMP methylation percentage and (C) chronological age of peripheral blood leukocytes in healthy children (n = 78, aged 1-16 years) and diagnostic T-ALL patient samples (n = 192). (D) Horvath biological age and (E) EpiTOC mitotic clock. Spearman correlation rho and P values were estimated among T-ALL samples only, not including healthy samples. CIMP percentage is defined as the percentage of CIMP panel CpGs with a β-value >0.4.
CIMP percentage association with DNA methyltransferase gene expression, chronological and cellular age in the NOPHO ALL2008 cohort. Correlation of CIMP methylation percentage and (A) DNA methyltransferase 3 alpha (DNMT3A) and (B) beta (DNMT3B) gene expression (in VST counts) in 108 T-ALL patient samples and 3 control cells (CD34+, CD3+, and lymph node). Correlation of CIMP methylation percentage and (C) chronological age of peripheral blood leukocytes in healthy children (n = 78, aged 1-16 years) and diagnostic T-ALL patient samples (n = 192). (D) Horvath biological age and (E) EpiTOC mitotic clock. Spearman correlation rho and P values were estimated among T-ALL samples only, not including healthy samples. CIMP percentage is defined as the percentage of CIMP panel CpGs with a β-value >0.4.
CIMP is associated with the biological and mitotic age of leukemic blasts
CIMP percentage was evaluated in relation to chronological age, epigenetic age clocks, and markers of proliferative history. In peripheral blood leukocytes from healthy children (n = 78, aged 1-16 years), the CIMP percentage was close to 0% independent of the individuals’ chronological age (Figure 7C). Likewise, the CIMP percentage was not associated with the pediatric T-ALL (n = 192) patients’ chronological age (rho = 0.12; P = .113; Figure 7C).
The potential longer replicative history of CIMP high cells was supported by the strong positive correlation of CIMP percentage and predicted Horvath biological age (rho = 0.85; P < .001; Figure 7D), EpiTOC mitotic clock (rho = 0.94; P < .001; Figure 7E), and other known biological and mitotic clocks (supplemental Table 8).
Discussion
Treatment protocols for T-ALL have improved over the decades. However, resistant and relapsed T-ALL remain challenging and biomarkers at diagnosis in addition to MRD after induction therapy are needed.3,39
We have previously found a prognostic relevance of DNA methylation classification in the NOPHO ALL1992/2000 T-ALL cohort.19 The CIMP classifier has been validated in independent cohorts, but all these had less effective regimens than the modern protocols, with limited cohort sizes and/or missing MRD data.18,20,21 This study confirmed that CIMP classification holds prognostic value in 2 large pediatric T-ALL cohorts treated with different protocols: the NOPHO ALL2008 protocol with 252 patients and the DCOG protocol with 156 patients, both including MRD for risk stratification. We successfully validated the novel epigenetic CIMP biomarker at diagnosis in combination with D15 and D29/D33 MRD assessment.
The evaluation of CIMP and MRD along with clinical phenotypes, including age, sex, CNS involvement, and WBC at diagnosis confirmed the prognostic relevance of CIMP as a biomarker for predicting relapse and survival in pediatric T-ALL in both cohorts.
Although both CIMP and D29/D33 MRD were independent prognostic markers for relapse, the combination of CIMP status at diagnosis and D29/D33 MRD status further improved the prognosis prediction. This combined assessment identified a poor prognosis subgroup (CIMP low and D29/D33 MRD ≥ 0.1%) and a very favorable outcome subgroup (CIMP high and D29/D33 MRD < 0.1%). The poor prognosis subgroup included 22% of the NOPHO patients and 19% of the DCOG patients with pCIR5yr of 29.0% and pOS5yr of 59.7% in the NOPHO patients and pCIR5yr of 23% and pOS5yr of 65.4% in the DCOG patients. The very favorable prognosis subgroup included 33% of the NOPHO patients and 42% of the DCOG patients, with pCIR5yr of 0% and pOS5yr of 98.2% in the NOPHO patients and pCIR5yr of 3.4% and pOS5yr of 94.8% in the DCOG patients.
These robust data suggest that incorporating CIMP classification in clinical diagnostics could identify patients who may benefit from intensive or novel treatments due to poor prognosis and those who could benefit from treatment de-escalation. De-intensification is currently being evaluated in childhood ALL in the European ALLTogether1 protocol (ClinicalTrials.gov identifier: NCT04307576), to spare the patients from long-term side effects. We also found that assessing CIMP status at diagnosis alongside D15 MRD status can provide earlier risk stratification, allowing for even earlier treatment adjustments.
The CIMP panel consists of more than a thousand CpG sites from the Illumina Methylation Beadarrays, classifying each sample into CIMP-low or CIMP-high subgroups. Although the CIMP methylation percentage is gradually increasing without a distinct separation of the CIMP subgroups, the previously set threshold for CIMP classification was here validated in these larger NOPHO and DCOG cohorts, as highly relevant for risk stratification.
Alternative supervised and unsupervised methylation profiling strategies failed to surpass CIMP prediction in this study and others.20,21 In adult T-ALL, unsupervised clustering identified similar poorer outcome in T-ALLs with lower mean DNA methylation, but they also identified a poor outcome subgroup with higher mean DNA methylation, which remains to be confirmed in pediatric cohorts.16
Methylation arrays are widely used for DNA methylation research but have also been standardized for diagnosing CNS tumors according to the Heidelberg classifier.40 In clinical diagnostics, samples from different origins, such as T-ALL and CNS tumors, could be analyzed on the same array platform, allowing fast clinical integration with the use of separate classification algorithms. This workflow makes arrays a cost- and time-effective technology for standardizing CIMP classification in clinical settings. Alternative methods for DNA methylation classification, such as sequencing-based approaches, also exist but require validation.
The molecular differences between CIMP subgroups suggest different routes for leukemia development, but underlying biology is still largely unknown. We used a multiomics approach to further decipher the molecular phenotype of CIMP subgroups. Methylation analysis of >700 000 CpGs highlighted extreme DNA methylation differences where gradual genome-wide hypermethylation strongly correlated with CIMP methylation percentage. This hypermethylation was also observed in a recent whole-genome methylation sequencing study of T-ALL.17 A possible hypothesis for the hypermethylated profile is dysregulation of DNA methylation-regulating enzymes, including DNA methyltransferases and/or demethylating TET enzymes,17,41,42 but we did not find evidence for dysregulation associated with CIMP subgroups.
No significant differences in ETP status between the CIMP subgroups were found. However, additional studies are needed for validation and improved understanding of T-cell maturation stages within the CIMP subgroups.
CIMP methylation percentage was strongly associated with predicted biological and mitotic ages, suggesting that the gradual hypermethylation could reflect the leukemic cells’ history of mitosis. This would argue for stronger drivers in the CIMP-low samples, which reveal a methylation profile more similar to nonmalignant hematopoietic cells and have a younger predicted biological and mitotic age. Of specific notice, also observed in other malignancies,14,41,43 the top 7630 hypermethylated CpGs in T-ALL were located in already silenced genes, and the consequence of the promoter-associated hypermethylation remains unclear.17,44 In line with this, whole-genome transcriptome principal component analysis could not discriminate the CIMP subgroups. However, a cluster of lower expressed genes in the CIMP-high group revealed promoter hypermethylation, suggesting methylation regulation of these genes. In contrast, the cluster of lower expressed genes in the CIMP-low group revealed promoter hypomethylation regardless of gene expression levels, strengthening that CIMP subgroups are biologically different. Moreover, differential expression analysis revealed several previously known genes in T-ALL biology to be associated with the CIMP subgroups,22 including overexpression of SIX6 and NKX3-1 in the CIMP-low subgroup and overexpression of TLX3, IRX3, and PLXND1 in the CIMP-high subgroup. The methylation pattern of these genes suggests that they are not directly regulated by promoter methylation. In contrast, MYO18B and SPI1 expression, known to be regulated by DNA methylation,21,45 was inversely associated with mean promoter DNA methylation, and functional studies are needed to further evaluate these findings in relation to T-ALL biology.
The most pronounced fusion was the overrepresentation of STIL::TAL almost exclusively found in the CIMP-low subgroup. Despite CIMP low being associated with poor prognosis, the STIL::TAL fusions were only significant for OS in our analysis but not relapse.
The NOPHO samples were analyzed for CNVs by extracting information from the methylation array, allowing a low-resolution identification of genomic aberrations. Overall, more genomic aberrations were observed in the CIMP-high subgroup, potentially supporting the theory of a longer proliferative history. The deletion of the commonly observed genomic alterations in the CDKN2A/B gene locus (chr9:21961000-21999000) was distributed evenly in both CIMP subgroups, whereas the CIMP-high group had a significantly higher number of deletions in the chr5:140725000-141445000, chr16:33552000-33914000, and chr16:34132000-35212000 chromosome regions. No significant gains were observed between the subgroups.
A limitation of this study was that not all samples were available for the multiomics analysis due to missing material; still, this remains a large cohort of patients with T-ALL. Another limitation is the DNA methylation array bias toward gene-promoter–associated CpGs and its denser coverage of cancer-associated CpGs. Furthermore, array analysis relies on bisulfite-converted DNA and cannot distinguish between 5-methyl cytosine and 5-hydroxycytocine. This study also lacks comparison with recent genomic predictors of outcome.9-12 Future studies combining CIMP, MRD, next-generation sequencing-based classification, and transcriptomics are needed to evaluate further improvement in prognostic prediction.
Nevertheless, the study has several strengths, including the large, well-characterized Nordic and DCOG T-ALL cohorts treated according to modern treatment protocols, comprehensive MRD evaluations (D15/29 for NOPHO and D33 for DCOG) with a median follow-up time of >6 years and >10 years, respectively. Together, these cohorts provide robust validation of our findings. The combination of CIMP classification at diagnosis and MRD evaluation at D29/D33 is a promising molecular biomarker for clinical implementation in pediatric T-ALL risk stratification.
Conclusions
CIMP classification at diagnosis provides a strong novel biomarker that, in combination with MRD evaluation of treatment response, can improve contemporary risk assessment in pediatric T-ALL. A future prospective trial could permit to evaluate whether patients would benefit from this new risk stratification strategy, which can potentially be implemented earlier with D15 MRD. These advancements hold promise for improving patient outcomes and tailoring therapeutic approaches.
Acknowledgments
The authors thank Sanna Siitonen for contribution to the early thymic precursor data review. RNA sequencing was performed by the SNP&SEQ Technology Platform in Uppsala. The facility is part of the National Genomics Infrastructure Sweden and Science for Life Laboratory supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. The methylation analysis was performed at the Epigenetic platform in Umeå supported by the Medical Faculty at Umeå University (FS.2.1.6.1813-22 and FS.1.3.2.2339-22).
This study was supported by grants from The Swedish Childhood Cancer Foundation (PR2021-0049), the Swedish Cancer Society (20 1053 Pj), the Lion's Cancer Research Foundation (AMP24-1152), Umeå University, the Cancer Research Foundation in Northern Sweden, the Kempe Foundation (JCK2155), and the Medical Faculty of Umeå University (FS.2.1.6.338-20). Financial support was provided through a regional agreement between Umeå University and Västerbottens County Council on cooperation in the fields of medicine, odontology, and health.
Authorship
Contribution: F.S.H., N.R., and S.D. conceived and designed the study; S.D. and M. Hultdin. supervised the study; P.O. performed the laboratory experiments; F.S.H., N.R., M.L., and S.D. analyzed the data and performed the statistical analysis; U.N.N., M. Heyman, F.v.L., and M. Hultdin were responsible for the clinical evaluation and registry; H.V.M. and H.O.M. were responsible for the NOPHO MRD analysis and registry; J.K., K.S., T.F., Ó.J., and J.A. were national NOPHO representatives; M. Hagleitner and F.v.L. were responsible for the DCOG cohort and registry; F.S.H., N.R., and S.D. wrote the first draft of the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sofie Degerman, Medical Biosciences, Umeå University, NUS, Blg 6M, 2nd floor M21, SE-90185 Umeå, Sweden; email: sofie.degerman@umu.se.
References
Author notes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![CIMP, MRD 0.1%, and relapse in the study of NOPHO ALL2008 and validation DCOG ALL-10/ALL-11 cohorts. Proportion of outcomes in 172 NOPHO (A) and 137 DCOG (D) pediatric patients with T-ALL; outcomes were relapse (n = 23/n = 18), DCR (n = 12/n = 8), SMN (n = 2/n = 1), and nonresponders (n = NA/n = 1) with CR (n = 135/n = 109) as reference, respectively. Samples were stratified by CIMP and MRD at D29/D33 MRD combined subgroups and (B,E) corresponding risk of relapse tables. Forest plots reveal the accuracy (estimated as [true positives + true negatives]/total) of D15 MRD and D29/D33 MRD above 0.1/0.01% cutoffs and CIMP-low status, alone or in combination, for differentiating relapse from CR in (C) the NOPHO and (F) the DCOG cohorts. NA, not available.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/19/10.1182_blood.2024026027/1/m_blood_bld-2024-026027-gr3.jpeg?Expires=1767721853&Signature=Y1g0e4ALzWAf36HIk0rZICQQj~n2hUExZC91wM0ujDsuaErfIuudg1Y88DRq4TOkZZtsJ-Rxflv6OOgk8aalcwk690RQ4rfLCx5aJA5s5hbZ7aMs8U2snQNKTVNH8TUN7pHcFAfjWN7bA4BSi3i7k2tG68cjxgWHrlyn7YFS777yp3hMFVYZFJKHcx4MfTszoe9ArbZnZpxwx3qVYyxc4RMvbHuIjsUPvtQ7T9pf9D96VhYV0I-ZR5QIU2QxraHLwVY6FobNSKnFRUZFp2Tz9FvqKyyH1Qz8hsAlP5PUvTpUTfZSz1~UAD1jf16rwFX4HPK8pt7xLzyT4XtoJnLI8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal