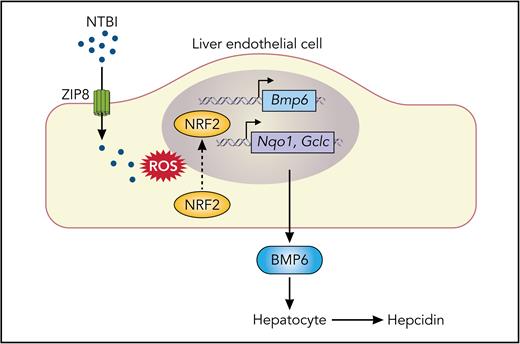
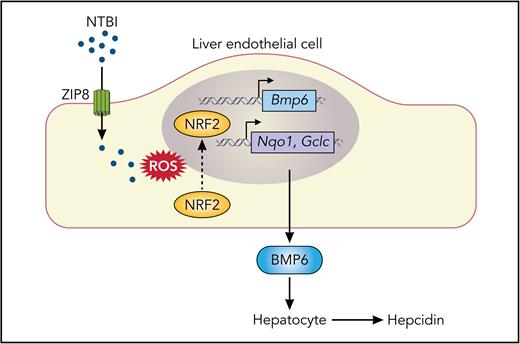
In this issue of Blood, Fisher et al1 demonstrate that metal-ion transporter ZIP8 (SLC39A8) in mouse liver endothelial cells (LECs) plays a significant, albeit modest, role in the in vivo expression of bone morphogenetic protein 6 (BMP6) in response to iron overload. This work helps to elucidate how LECs take up and sense iron to regulate body iron balance, which is a central and unanswered question in iron biology.
Research over the past 15 years has identified the essential cell types, signaling molecules, and receptors involved in conveying systemic or liver iron status to the expression of hepcidin, the chief iron-regulatory hormone. Although many molecules are involved, a seemingly direct line of communication exists between LECs and hepatocytes. For example, in response to iron loading, LECs produce and secrete BMP6, which binds to BMP receptors on hepatocytes, thereby initiating a signaling pathway that induces hepcidin transcription.2 Studies of primary mouse LEC cultures indicate that LECs can load iron and increase BMP6 expression in response to treatment with transferrin-bound iron, non-transferrin-bound iron (NTBI), or ferritin.3 A recent study using mice lacking endothelial transferrin receptor 1 (TFR1) has shown that TFR1 is dispensable for BMP6 and hepcidin expression under normal and iron-loading conditions.4 Moreover, plasma levels of NTBI, which appear in iron overload, were noted to correlate with hepatic BMP6 and hepcidin expression levels, suggesting that NTBI is the primary driver of BMP6 in response to iron loading. Although the molecular mechanism(s) by which LECs take up NTBI in vivo is unknown, at least 3 lines of evidence suggest that ZIP8, a member of the ZIP family of metal-ion transporters, may be involved: (1) ZIP8 expressed in cultured cells localizes to the cell surface, where it can mediate the uptake of NTBI, optimally at pH 7.55; (2) the abundance of ZIP8, particularly at the cell surface, increases in a dose-responsive fashion with iron loading (at least in rat hepatoma H4IIE cells)5; and (3) Slc39a8 messenger RNA (mRNA) encoding ZIP8 was recently identified via single-cell RNA sequencing analysis of primary mouse liver sinusoidal endothelial cells (LSECs) as being upregulated by dietary iron loading in vivo.4
In their study, Fisher et al rigorously examined the iron responsiveness of ZIP8 expression in LECs and generated mice lacking endothelial Slc39a8 to define its functional contribution to BMP6 regulation, hepcidin expression, and iron homeostasis. Using a suite of complementary methods to analyze primary LECs from male and female mice, they found that ZIP8 mRNA and protein levels do not increase in response to chronic or acute dietary iron loading, which increased cellular iron levels as confirmed by TFR1 or ferritin expression. To interrogate the possible involvement of LEC ZIP8 in iron-induced BMP6 regulation, control mice and mice lacking endothelial ZIP8 were fed either standard rodent chow or a high-iron diet. They observed that mice of either genotype fed the iron-loaded diet displayed higher levels of serum iron, liver iron, and hepatic BMP6 and hepcidin mRNA compared with standard diet-fed littermate control mice, indicating no major disruptions in iron homeostasis or regulation due to endothelial ZIP8 deficiency. Interestingly, however, iron-loaded male and female mice lacking endothelial ZIP8 displayed 25% to 35% lower levels of hepatic BMP6 mRNA and 20% to 30% higher liver iron concentrations than iron-loaded littermate controls. Nevertheless, hepcidin mRNA levels did not differ between genotypes when iron loaded, suggesting that hepcidin expression was inappropriately low relative to liver iron concentrations in mice deficient in endothelial ZIP8.
In an independent cohort of mice subjected to the same experimental regimen, Fisher et al isolated and analyzed LECs specifically. As with whole liver, they found similar reductions in endothelial BMP6 mRNA expression in mice lacking endothelial ZIP8, supporting their previous conclusions that endothelial cells are the predominant source of BMP6 in the liver.2 Additionally, the reduced LEC BMP6 mRNA levels were associated with reduced expression of the NRF2 target genes glutamate-cysteine ligase catalytic subunit (Gclc) and NAD(P)H dehydrogenase quinone 1 (Nqo1), which increase in response to iron loading and oxidative stress.6 It therefore seems probable that the lower expression of Nqo1 and Gclc following Slc39a8 ablation reflects lower cellular levels of pro-oxidant iron. Collectively, these observations convincingly demonstrate a role for ZIP8 in iron-dependent regulation of BMP6 in LECs (see figure). The possibility that transferrin-bound iron contributed to BMP6 expression in the absence of ZIP8 was excluded by showing that mice deficient in both ZIP8 and TFR1 displayed no alterations in hepatic BMP6 mRNA levels or other iron-related parameters when fed standard rodent chow. These data imply that alternative or redundant iron uptake mechanisms exist in LECs. ZIP8’s nearest homologue, ZIP14 (SLC39A14), which mediates NTBI uptake by hepatocytes, seems dispensable for LEC iron uptake as iron-loaded Slc39a14 knockout mice show no impairments in hepatic BMP6 or hepcidin expression.7 The iron transporter divalent metal-ion transporter 1, critical for intestinal iron uptake, also appears to be an unlikely contributor to LEC iron supply given that its levels decrease markedly in iron-loaded liver.8 Unbiased RNA-sequencing, mass spectrometry-based quantitative proteomics, and gene set enrichment analyses of iron-loaded LECs9 seem to offer promising strategies for identifying new candidate NTBI transporters (or alternative uptake mechanisms). However, as discordant results on iron-dependent BMP6 regulation have recently been reported in a different LSEC cell culture model10 vs a more general LEC population used by Fisher et al, additional studies comparing and validating the different cell culture models are warranted. Thus, the hunt for the elusive endothelial iron transporter(s) continues.
Model of the contribution of ZIP8 to iron uptake and BMP6 production by LECs during iron overload. NTBI present in iron-loaded blood plasma is taken up, at least in part, by ZIP8 at the plasma membrane of LECs. Cellular iron loading from NTBI promotes the formation of ROS, leading to translocation of NRF2 transcription factor into the nucleus, thereby activating the transcription of the Bmp6 gene and genes encoding proteins with antioxidant properties, such as Nqo1 and Gclc. BMP6 secreted by LECs acts on neighboring hepatocytes to increase the production of the iron-regulatory hormone hepcidin. The uptake of plasma transferrin-bound iron via TFR1 (not shown) is dispensable for LEC production of BMP6 during iron overload. ROS, reactive oxygen species. Professional illustration by Somersault18:24.
Model of the contribution of ZIP8 to iron uptake and BMP6 production by LECs during iron overload. NTBI present in iron-loaded blood plasma is taken up, at least in part, by ZIP8 at the plasma membrane of LECs. Cellular iron loading from NTBI promotes the formation of ROS, leading to translocation of NRF2 transcription factor into the nucleus, thereby activating the transcription of the Bmp6 gene and genes encoding proteins with antioxidant properties, such as Nqo1 and Gclc. BMP6 secreted by LECs acts on neighboring hepatocytes to increase the production of the iron-regulatory hormone hepcidin. The uptake of plasma transferrin-bound iron via TFR1 (not shown) is dispensable for LEC production of BMP6 during iron overload. ROS, reactive oxygen species. Professional illustration by Somersault18:24.
Conflict-of-interest disclosure: The author declares no competing financial interests.