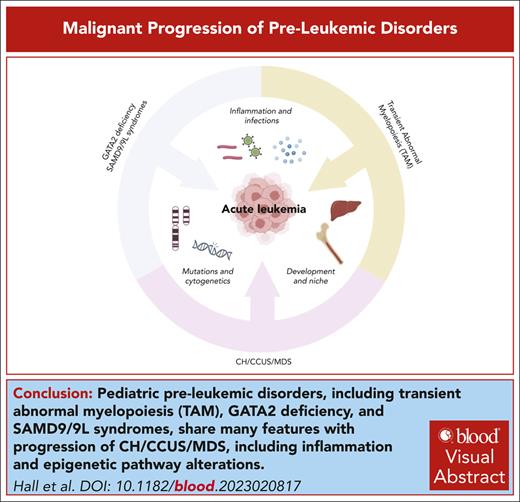
Visual Abstract
The spectrum of myeloid disorders ranges from aplastic bone marrow failure characterized by an empty bone marrow completely lacking in hematopoiesis to acute myeloid leukemia in which the marrow space is replaced by undifferentiated leukemic blasts. Recent advances in the capacity to sequence bulk tumor population as well as at a single-cell level has provided significant insight into the stepwise process of transformation to acute myeloid leukemia. Using models of progression in the context of germ line predisposition (trisomy 21, GATA2 deficiency, and SAMD9/9L syndrome), premalignant states (clonal hematopoiesis and clonal cytopenia of unknown significance), and myelodysplastic syndrome, we review the mechanisms of progression focusing on the hierarchy of clonal mutation and potential roles of transcription factor alterations, splicing factor mutations, and the bone marrow environment in progression to acute myeloid leukemia. Despite major advances in our understanding, preventing the progression of these disorders or treating them at the acute leukemia phase remains a major area of unmet medical need.
Introduction
Several myeloid disorders have a high propensity to evolve to acute myeloid leukemia (AML). For example, GATA2 deficiency, SAMD9/9L syndromes, and transient abnormal myelopoiesis (TAM) are prevalent in young children and are caused by germ line mutations in GATA2 and SAMD9/9L or acquired mutations in GATA1, respectively. By contrast, myelodysplastic syndrome (MDS) primarily affects adults, with the highest incidence in older individuals. However, MDS often evolves from clonal hematopoiesis (CH) or clonal cytopenia of unknown significance (CCUS), in which the associated mutations can occur decades before diagnosis of MDS. Here, we review the current understanding of the drivers of malignant progression of these disorders and then compare and contrast these events.
Founder mutations
GATA2 deficiency and SAMD9/9L syndromes
These syndromes fall under the 2022 World Health Organization (WHO) classification of “myeloid neoplasms with germ line predisposition and organ dysfunction,” a subtype of myeloid neoplasms associated with germ line predisposition (CEBPA/DDX41/RUNX1 disorders, among others).1 These syndromes are the most prevalent predisposing conditions in children and adolescents with primary MDS, with GATA2 deficiency associated with up to 15% of advanced MDS cases and SAMD9/9L syndromes accounting for 8% to 17% of primary MDS cases in children, depending on the study cohort.2-5 GATA2 is a transcription factor that is indispensable for hematopoietic stem cell (HSC) generation and maintenance.6 The first germ line GATA2 mutations described predisposing to MDS/AML were reported in 2011, when 2 heterozygous missense mutations proposed to abrogate GATA2 DNA binding ability were identified as inherited mutations driving familial MDS/AML.7 At the same time, germ line GATA2 mutations were identified as drivers of 3 other autosomal dominant immunodeficiency disorders: MonoMAC syndrome,8 Emberger syndrome,9 and dendritic cell, monocyte, B and natural killer lymphoid (DCML) deficiency.10 These immunodeficiencies and familial MDS/AML arising from heterozygous germ line mutations in GATA2 were termed GATA2 deficiency. Meanwhile, SAMD9 and its paralog SAMD9L, sterile alpha motif domain (SAMD) containing proteins that are responsive to interferon (IFN), have been shown to play a role in numerous processes, including antiviral response and inflammation.3 In 2016, heterozygous missense gain-of-function (GOF) SAMD9 mutations were found to be associated with the development of MIRAGE (myelodysplasia, infections, restriction of growth, adrenal hypoplasia, genital phenotypes and enteropathy) syndrome, whereas missense GOF SAMD9L mutations were found to contribute to a syndrome of hypocellular bone marrow, pancytopenia, and progressive neurological phenotype termed ataxia pancytopenia.11-14 Subsequently, several cohorts of pediatric patients with MDS were found to have germ line missense mutations in SAMD9 and/or SAMD9L.4,15-17
Leukemia in children with DS
Children with Down syndrome (DS) are at a 150-fold elevated risk of myeloid leukemia in DS (ML-DS), a subtype of acute megakaryoblastic leukemia, compared with children without DS.18 In addition, infants with DS are uniquely susceptible to a preleukemia known as TAM.19 TAM has its origins in the fetal liver and is caused by the acquisition of mutations in GATA1, a gene that is required for proper development of erythroid cells and megakaryocytes (Table 1).20GATA1 mutations are associated with nearly every case of TAM, and the combination of trisomy 21 and a GATA1 mutation is sufficient to drive this disease.21,22 Although TAM is lethal in 10% to 15% of cases, it undergoes spontaneous remission within months in the majority of cases. However, TAM blasts persist at low frequency and with acquisition of additional driver mutations, evolve to ML-DS in 10% to 20% of cases.19 Seminal work by Vyas and Roberts has shown that GATA1 mutations are detectable in nearly 30% of newborns with DS, even in cases without obvious clinical manifestations.23 This represents a remarkable incidence of specific mutations in a population and underscores the link between GATA1 and trisomy 21. Although the reason for this tight association remains murky, factors may include the presence of a progenitor cell that is unique to DS and highly susceptible to responding to dysregulated GATA1 or microenvironmental factors in the setting of DS.
CH, CCUS, and MDS
CH, including CH of indeterminate potential (CHIP), is defined by the presence of a hematologic neoplasm associated mutation in an otherwise healthy individual without cytopenia that can be readily detected (conventionally defined as a variant allele frequency ≥2%).24 By contrast, the presence of MDS-associated somatic variants in the context of unexplained cytopenia with morphologic dysplasia or increased bone marrow blasts is referred to as CCUS.25 Although the list of mutations associated with CCUS as well as age-associated CH is long, the most frequent mutations are seen in epigenetic regulators (eg, DNMT3A, TET2, and ASXL1), RNA splicing factors (eg, SF3B1 and SRSF2), TP53, and PPM1D.24,26 CHIP and CCUS are associated with increased risk of progression to overt myeloid malignancies, including AML. The risk of progression is dependent on the specific mutations as well as clone size, defined by variant allele frequency27; CCUS has a very high rate of progression within 5 years, whereas CH may take decades to progress, if at all.28 Of note, CH can also be seen in the absence of known driver mutations in a phenomenon referred to as “neutral drift.”24
MDS represents a heterogeneous group of hematologic disorders presenting with peripheral cytopenia, single or multilineage dysplasia, and an increased risk of transformation to AML.1,29 Although the competing International Consensus Classification and WHO classifications use slightly different terminology, both recognize CCUS, MDS, and AML as continuum with increasing severity of cytopenias, morphologic dysplasia, and blast count suggestive of disease progression. Overall, almost 30% of patients diagnosed with MDS will progress to AML, and 18% to 20% of all AML cases are diagnosed in patients with antecedent history of MDS. AML progressing from MDS has worse clinical outcome than de novo AML. Interestingly a subset of patients diagnosed with de novo AML without antecedent MDS share the same cytogenetic and molecular abnormalities as patients progressing from MDS and have a similar adverse outcome. These are classified as AML with myelodysplasia-related gene mutations and AML with myelodysplasia-related cytogenetic abnormalities in the International Consensus Classification and myelodysplasia-related AML (AML-MR) in the WHO classification.1,29
Molecular features of progression
GATA2 deficiency and SAMD9/9L syndromes to MDS/AML
Progression of GATA2 deficiency to MDS/AML most likely results from the acquisition of cytogenetic abnormalities followed by somatic driver mutations (Table 2). In a cohort of 508 pediatric patients with MDS, Wlodarski et al found germ line GATA2 mutations in 37% of all patients with monosomy 7, with this number climbing to 72% in adolescence.2 Donadieu et al observed a 35% incidence of whole or partial loss of chromosome 7 in a cohort of 66 patients with GATA2 deficiency.30 Other cohorts have also identified a high prevalence of trisomy 8 among patients with an underlying GATA2 deficiency,31,32 and der(1;7)(q10;p10) resulting in trisomy 1q and deletion of 7q was significantly enriched in a cohort of 25 patients.33 Although several recurrent somatic mutations have been described in cases with GATA2 deficiency–driven myeloid malignancies, the most prevalent mutations that may cooperate with monosomy 7 to drive progression are ASXL1, STAG2, and SETBP1.15,32,34-37 A recent study by Largeaud et al compared the karyotype and somatic genetic alterations in 78 patients with GATA2 deficiency with a series of 500 adult patients with sporadic AML to define mutational differences.38STAG2, ASXL1, and SETBP1 were the most common mutations in patients with GATA2 deficiency. By correlating cytogenetic and somatic mutation information with bone marrow smears staged for leukemic progression, Largeaud et al confirmed enrichment of monosomy 7 and SETBP1, RAS pathway, and ASXL1 mutations along with progression (Figure 1). Of note, RUNX1 mutations were also reported in this study. Interestingly, STAG2 was the only mutation significantly enriched during early MDS and did not seem to contribute to progression, suggesting that it may provide a selective advantage to GATA2 mutant clones but are not sufficient to drive transformation. Knockout (KO) of Stag2 has been shown to enhance GATA binding motifs in mouse hematopoietic stem and progenitor cells (HSPCs).39,40 This implies a compensatory mechanism in which STAG2 loss drives upregulation of GATA2 targets in the context of GATA2 deficiency, allowing for improved fitness in nonleukemic clones. Pairing new models of specific GATA2 missense mutations with current models of chromosome 7 loss will be beneficial in studying disease progression.41,42
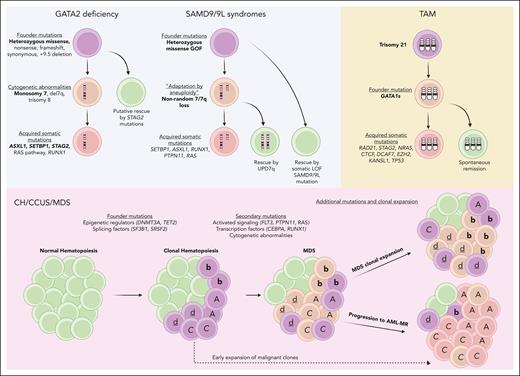
Molecular mechanisms of disease progression in pediatric and adult myeloid neoplasms. Leukemic progression of GATA2 deficiency and SAMD9/9L syndromes (top left, blue) are characterized by a high association with monosomy 7. STAG2 mutations are thought to have the ability to rescue progression of GATA2 deficiency.38 Nonrandom loss of chromosome 7 in SAMD9/9L syndromes can be rescued by UPD7q. Somatic LOF SAMD9/9L mutations have also been shown to have ameliorating effects on disease progression. TAM development (top right, yellow) is characterized by trisomy 21 followed by GATA1 mutations. Further acquired somatic mutations in cohesin complex genes and others are thought to drive progression to the leukemic phase. Progression of CH/CCUS/MDS to AML-MR (bottom, red) is driven by a mixture of genetically related but distinct clones (depicted here as A, b, c, d) in a nonlinear fashion termed “parallel evolution.”43 Figure created with Biorender.com.
Molecular mechanisms of disease progression in pediatric and adult myeloid neoplasms. Leukemic progression of GATA2 deficiency and SAMD9/9L syndromes (top left, blue) are characterized by a high association with monosomy 7. STAG2 mutations are thought to have the ability to rescue progression of GATA2 deficiency.38 Nonrandom loss of chromosome 7 in SAMD9/9L syndromes can be rescued by UPD7q. Somatic LOF SAMD9/9L mutations have also been shown to have ameliorating effects on disease progression. TAM development (top right, yellow) is characterized by trisomy 21 followed by GATA1 mutations. Further acquired somatic mutations in cohesin complex genes and others are thought to drive progression to the leukemic phase. Progression of CH/CCUS/MDS to AML-MR (bottom, red) is driven by a mixture of genetically related but distinct clones (depicted here as A, b, c, d) in a nonlinear fashion termed “parallel evolution.”43 Figure created with Biorender.com.
Malignant progression in SAMD9/9L syndromes is also highly associated with monosomy 7 (Table 3) due to nonrandom loss of chromosome 7 or 7q after acquisition of a heterozygous germ line SAMD9/9L mutation, termed “adaptation by aneuploidy.”3 Further progression is characterized by somatic mutations in genes typically associated with monosomy 7 and GATA2 deficiency progression, including ASXL1, SETBP1, RUNX1, and KRAS.4,5,15,16,44 Interestingly, “adaptation by aneuploidy” or the SAMD9/9L missense mutation can be corrected via uniparental disomy of 7q or the somatic acquisition of loss-of-function (LOF) SAMD9/9L mutations in cis (Figure 1).3,5 Although it is assumed that the nonrandom loss of chromosome 7/7q is an adaptation to a growth restriction inherent in SAMD9/9L mutant cells, the precise mechanism is still unclear. However, Klco et al recently recapitulated this phenomenon in a Samd9l GOF mouse model,45 and further studies are needed to identify drivers of clonal expansion.
TAM to ML-DS
The first comprehensive analysis of the genetics of TAM and ML-DS was provided by Ogawa et al.22 Whole-exome sequencing of TAM and ML-DS samples identified GATA1 mutations in all samples and 8 genes that were recurrently mutated in the acute leukemia: RAD21, STAG2, NRAS, CTCF, DCAF7, EZH2, KANSL1, and TP53 (Table 1). Targeted deep sequencing of these genes and functionally related genes in additional ML-DS samples revealed that the cohesin complex, including RAD21, STAG2, SMC3, and SMC1A, as well as the associated factor NIPBL, was mutated in 53% of the ML-DS cases but not often altered in TAM. Recurrent mutations in the insulator protein gene CTCF were detected in 20% of the ML-DS cases, whereas 45% and 47% contained mutations in epigenetic regulators and signaling pathway genes, respectively. A subsequent investigation by Klusmann et al confirmed that alterations in cohesin are the most common secondary events in ML-DS.21 A new class of mutations in the CSF2Rβ gene, which encodes the common β-chain of the interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor cytokine receptors, were detected in nearly 5% of cases. Together these and other reports implicate changes in genes that control signaling, epigenetics, and chromatin architecture as drivers of malignant progression. The observation that >50% of TAM cases that progress to ML-DS have mutations in cohesin suggest that there is a special relationship between trisomy 21 or GATA1 mutations with chromatin architecture. As reported in prior studies, cohesin mutations alter chromatin accessibility at ERG, RUNX, and GATA motifs40,46,47; therefore, we speculate that altered expression of the chromosome 21 genes ERG, ETS2, and RUNX1 and/or aberrant GATA1 function underlie this heightened association.
CH, CCUS, and MDS to AML-MR
MDS and AML-MR share common cytogenetic abnormalities that include a complex karyotype (frequently but not invariably associated with TP53 mutations), loss of 5q, monosomy 7 or deletion 7q, deletion 11q, loss of 12p, monosomy 13 or deletion 13q, loss of 17p, or idic(X)(q13). In addition, massive parallel or next-generation sequencing has identified somatic mutations that converge on 6 major cellular pathways: splicing, transcription factors, the cohesin complex, epigenetic modifiers, activated signaling genes, and TP53.48 Of these, ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2, and RUNX1 are highly predictive of secondary AML and allow for a clinical diagnosis of AML-MR even in patients without prior history of MDS.49-54
The prevalence of specific mutations during clinical progression from CCUS to AML provides insights into genetic progression. Epigenetic regulators and TP53 appear to be consistently mutated in CH, MDS, and AML,55,56 and splicing factors are mutated early in the development of MDS.57,58 Meanwhile, mutations in signaling pathways (FLT3, PTPN11, and RAS) and transcription factors (CEBPA and RUNX1) tend to be enriched in secondary AML.50,51,58-63 Comparison of mutational status of paired MDS/AML-MR largely confirmed this mutational order of progression. These data derived from bulk sequencing of tumor sample can be reconciled with several recent studies performed at single-cell level that suggest MDS and AML-MR are composed of a mixture of genetically related but distinct clones, and the expansion of subclones is a hallmark of leukemic progression.43,62,64-66 This suggests a model of nonlinear, “parallel evolution” (Figure 1),43 and although mutations may be acquired stochastically, there appears to be selective advantage for a specific combination of genetic events indicated by the presence of a combination of mutations at higher or lower frequencies than expected by chance.
Mechanisms of progression
Cell of origin and the microenvironment
GATA2 and SAMD9/9L syndromes
The contributions of a specific cell type or developmental stage to the propagation of a founder clone in GATA2 deficiency and SAMD9/9L syndromes is not well understood. Given that these syndromes arise from germ line mutations, it is possible that founder clones are selected as early as definitive HSC generation in the aorta gonad mesonephros region (AGM). Mouse models of Gata2 loss have identified GATA2 as required for HSC generation, because Gata2−/− mice die of anemia by embryonic day 10.5.6 Although Gata2+/− heterozygous mice show reduced numbers of HSCs generated in the AGM, they survive to adulthood with relatively unaffected hematopoiesis during homeostasis.6Gata2+/− mice show reduced repopulating ability in competitive transplants as well as defective hematopoiesis after stress insult throughout development, suggesting both functional and qualitative defects in HSC stemness under haploinsufficiency.67-69 A recent mouse model of one of the most recurring GATA2 missense mutations, R398W, displayed a DCML phenotype and reductions in HSPC subtypes in adult bone marrow, but the functional consequences of this mutation on HSPCs are unknown.42 However, no mouse models of GATA2 deficiency display a myeloid neoplasm. One possibility is that GATA2 germ line haploinsufficiency contributes to future leukemic development by reducing the clonal complexity of the existing hematopoietic system (Figure 2). Gata2+/− mice have reduced numbers of AGM-derived HSCs, which contribute to lifelong hematopoiesis.6 This lack of clonal heterogeneity may increase the likelihood of a mutant clone expanding to drive leukemic progression. Studies using endogenous cell-labeling strategies in conjunction with Gata2 mutations would be beneficial to test this hypothesis.
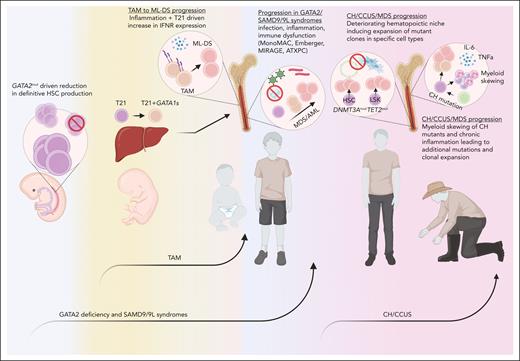
Extrinsic factors contributing to leukemic progression. Germ line mutations in GATA2 or SAMD9/9L may contribute to a reduction in the clonal complexity of the hematopoietic system, providing the impetus for rapid expansion of mutant clones derived from an inflamed, oft-challenged, and hypocellular adolescence bone marrow microenvironment (blue). TAM originates in the fetal liver after the acquisition of GATA1 mutations in trisomy 21 progenitors and migrates to the pediatric bone marrow, in which IFN hyperactivity and inflammation may drive the acquisition of further leukemic mutations (yellow). CH/CCUS/MDS progression is influenced by an aging hematopoietic niche, chronic inflammation, and myeloid skewing of mutant clones (red). ATXPC, ataxia pancytopenia. Figure created with Biorender.com.
Extrinsic factors contributing to leukemic progression. Germ line mutations in GATA2 or SAMD9/9L may contribute to a reduction in the clonal complexity of the hematopoietic system, providing the impetus for rapid expansion of mutant clones derived from an inflamed, oft-challenged, and hypocellular adolescence bone marrow microenvironment (blue). TAM originates in the fetal liver after the acquisition of GATA1 mutations in trisomy 21 progenitors and migrates to the pediatric bone marrow, in which IFN hyperactivity and inflammation may drive the acquisition of further leukemic mutations (yellow). CH/CCUS/MDS progression is influenced by an aging hematopoietic niche, chronic inflammation, and myeloid skewing of mutant clones (red). ATXPC, ataxia pancytopenia. Figure created with Biorender.com.
Given that SAMD9 is not present in mice, mouse models of SAMD9/9L use either KO or GOF mutations in Samd9l.45,70,71Samd9l is dispensable for hematopoiesis in mice, because Samd9l−/− and Samd9l+/− mice survive to adulthood and even display enhanced HSC function.70 Interestingly, Samd9l KO and haploinsufficient mice develop myeloid disease resembling monosomy 7/del 7q in humans.70 Two models of Samd9l heterozygous GOF mutations display reduced repopulating potential after transplantation,45,71 whereas 1 model also displays myeloid lineage skewing, bone marrow hypocellularity, and nonrandom chromosome 6 loss, which is the site of murine Samd9l.45 Although the specific contributions of SAMD9L heterozygous GOF mutations at the earliest stages of hematopoietic development are not known, current models suggest these mutations induce a lack of clonal complexity, similar to GATA2 deficiency. Given the prevalence of GATA2- and SAMD9/9L-driven MDS in older children and young adults,72 it is likely that mutant clonal expansion does not occur in embryonic sites of hematopoiesis such as fetal liver but rather in the adolescent bone marrow after further extrinsic and genetic selection.
TAM
TAM has its origins in utero, with GATA1 mutations occurring during fetal development as early as 21 weeks of gestation (Figure 2).73 Multiple studies have shown that the malignant effects are driven by mutations at this stage. For example, GATA1 mutations were found to affect megakaryocyte expansion only when introduced into fetal liver HSPCs74; megakaryopoiesis in GATA1 mutant cord blood and adult peripheral blood–mobilized HSPC cultures was largely unaffected. Furthermore, leukemic GATA1 mutations could drive a TAM phenotype only within trisomic fetal liver cells and specifically long-term HSCs.75 By contrast, progression to acute leukemia was observed upon acquisition of a STAG2 mutation in multiple GATA1 mutant HSPC populations. Similar to the Klusmann study, introducing GATA1 mutations in adult HSPCs failed to lead to transformation even with STAG2 mutations, and a contributor to this fetal long-term HSC requirement may be a differential dependence on insulin-like growth factor signaling.76 Of note, STAG2 and other mutations associated with transformation to ML-DS generally occur within the bone marrow after TAM resolves. These observations suggest that trisomy 21 is predominantly associated with TAM formation specifically in the fetal liver but does not directly contribute to leukemic progression. However, a recent study from Rowe et al using an isogenic induced pluripotent stem cell-hematopoietic progenitor system suggests that trisomy 21 induces high levels of genome instability and stress, contributing to an increased risk of somatic mutations associated with leukemic progression.77
CH, CCUS, and MDS
Emerging data suggest that CH-associated mutations arise as an adaptive response to progressively deteriorating hematopoietic niche associated with a depleting pool of HSCs; the acquisition of these mutations may result in a pool of stem cells with a self-renewal advantage (Figure 2).78 Furthermore, the mutations appear to be acquired within distinct stem cell compartments with differential impact on downstream differentiation and the bone marrow niche. For example, DNMT3A mutations result in altered DNA methylation of HSC regulatory elements and indefinite in vivo expansion.79 By contrast, TET2 mutations result in expansion of Lineage(neg) Sca1+ c-Kit+.80,81 Even though both mutations skew hematopoiesis to a granulocytic compartment, alterations in the composition of the stem cell pool may, in part, explain the differences in the combination of mutations, the latency, and the type of myeloid malignancy at the time of progression.
Inflammation and other extrinsic factors
GATA2 and SAMD9/9L syndromes
The role of inflammation and/or immune dysregulation in leukemic progression of GATA2 deficiency and SAMD9/9L syndromes is still not fully elucidated, primarily due to the lack of disease models. However, insights from disease pathogenesis in patients combined with the few models that exist provide hypothetical frameworks for progression. GATA2 deficiency is primarily a disease of immune dysregulation, with patients experiencing a range of syndromes including MonoMAC syndrome,8,82 Emberger syndrome,9,83 DCML deficiency,10,84 defects in natural killer cell maturation,85 hypocellular bone marrow mimicking aplastic anemia,86 B-cell lymphopenia,87 and chronic neutropenia.88 Up to 70% of patients suffer from severe infections including human papillomavirus infection, Epstein-Barr virus, cytomegalovirus, and nontuberculosis mycobacteria, and immune dysregulation can increase the risk of autoimmune and inflammatory diseases.89 In a cohort of patients with GATA2 deficiency, elevated serum levels of several inflammatory cytokines, particularly Flt3L, was shown to be associated with disease progression.10 Single-cell RNA sequencing of patients with GATA2 deficiency also identified a reduction in expression of genes associated with immune response compared with healthy controls, further suggesting immune dysregulation.90Gata2+/− mice were also found to have reduced inflammatory response to lipopolysaccharide and impaired bacterial clearance compared with wild-type mice.91 Together, these observations suggest a model in which GATA2 mutant–driven immune dysregulation leads to a hypocellular and oft-challenged bone marrow environment that selects for leukemic clones (Figure 2). More studies with existing and new mouse models, as well as patient cell–derived models, will be crucial to confirm this hypothesis.
SAMD9/9L mutations are also associated with immune dysfunction (MIRAGE and ataxia pancytopenia syndromes),11-14 and SAMD9/9L are IFN-responsive genes important in innate immunity against viral infections.92-94 A recent study with a mice harboring a heterozygous Samd9l mutation provided important insights into the role of inflammation on leukemic progression45; the authors observed a type I IFN response in Samd9l mutant cells that resulted in increased apoptosis, reduced engraftment, decreased lymphocyte counts, and increased myeloid hyperplasia. SAMD9L HSPCs also displayed transforming growth factor (TGF)-β hyperactivity in response to type I IFN treatment, which has been shown to reduce the HSC pool by inducing apoptosis or quiescence.95 According to this model, SAMD9/9L mutant proteins may be responding to inflammatory responses to affect the clonal complexity of the founder population (Figure 2). This would be distinct from the GATA2 deficiency model proposed above, in which the cellular landscape induced by GATA2 mutations is predicted to provide a milieu for inflammation/infection-induced clonal selection. However, more studies involving SAMD9/9L mutant models are needed.
TAM/ML-DS
DS is considered an interferonopathy, because chronic IFN hyperactivity is a feature of DS, potentially due to the amplification of 4 IFN receptors on chromosome 21.96 IFNs have been shown to have conflicting roles in tumor development. For instance, type I and type II IFNs promoted survival of chronic lymphocytic leukemia cells via STAT3-mediated apoptotic protection,97 whereas treatment of chronic myelogenous leukemia with IFN-α was a major therapeutic option and induced a clear cytogenetic and antitumor immune response.98 For individuals with DS, IFN hyperactivity has been shown to be associated with a proinflammatory phenotype, depletion of B cells, increased cytotoxic T cells, and elevated risk of autoimmunity.99 In addition to chronic IFN hyperactivity, several studies have observed increased levels of circulating proinflammatory cytokines in individuals with DS, including IL-2, IL-6, IL-10, IL-1ra, and tumor necrosis factor (TNF)-α.100 IL-6 has recently been implicated as a driver of AML progression; its levels are markedly elevated in MDS, and it is required for malignant progression in mouse models.101 In addition, TNF-α has been implicated in leukemic transformation via several mechanisms including alterations in the tumor microenvironment and the promotion of tumor cell proliferation.102 Persistent TNF-α activity was also reported to drive hematopoietic regeneration and contribute to hematologic malignancies including MDS and AML.103 Therefore, a DS-driven proinflammatory microenvironment may contribute to leukemic progression in TAM; further studies are needed to confirm this association (Figure 2).
CH, CCUS, and MDS
Real world experience suggests that HSCs carrying CH/CCUS/MDS type mutations are characterized by inefficient expansion ex vivo as well as poor engraftment in xenograft models.104 This discordance between observed clonal dominance in the innate host (patients) vs xenografts has drawn increasing attention to cell-extrinsic factors that are not replicated either in the ex vivo cultures or in the xenograft models. In this context, the role inflammation in altering the hematopoietic niche as well as the HSC is an exciting area of investigation that provides insight into the role of systemic inflammation in hematologic malignancies. Elevated levels of circulating cytokines such as IL-6 and TNF-α induce changes in the bone marrow microenvironment that favor clonal dominance of HSCs over the unmutated progenitors.105 Furthermore, mouse data suggest that specific mutations may be selected to overcome inflammatory pathway rewired by specific cytokines. For example, elevated IL-6 levels appear to be associated with TET2 mutations and elevated TNF-α with DNMT3A mutations.106 Acquisition of CH mutations results in skewing of the hematopoietic progenitor pool to a myeloid predominant hematopoiesis as well as generation of progeny that further exacerbate the inflammation within the bone marrow niche triggering a self-propagating cycle of inflammation that results in acquisition of additional mutations that continue to provide survival advantage to the mutated hematopoietic cells (Figure 2).107 Furthermore, it would appear that not only do CH type mutations change the inflammatory milieu of the bone marrow niche, but they also result in generation of defective effector immune cells that allow the mutated stem cells to escape immune surveillance further contributing to progression.108
Comparing and contrasting the mechanisms of progression
Reviewing the mechanisms of progression among these pediatric/adolescent and adult disorders reveals important similarities and differences that can inform overarching questions surrounding leukemic progression at large. First, the developmental time point at which leukemic progression begins, even within the same hematopoietic organ, may contribute on both intrinsic and extrinsic levels. Indeed, the bone marrow microenvironment and intrinsic nature of bone marrow HSPCs change during early life,109 suggesting that there may be specific “fetal-like,” “adult-like,” or transitional HSPCs that are preferentially selected as leukemic clones in the young bone marrow of predisposition disorders such as GATA2 deficiency and SAMD9/9L syndrome (Figure 3). This appears to be clear in the case of TAM vs GATA2 deficiency and SAMD9/9L syndromes, in which the TAM cell of origin is decidedly “fetal-like,” arising from fetal liver progenitors that preferentially evolve into a megakaryocyte-biased AML in the pediatric bone marrow. Meanwhile, extrinsic forces arising from the underlying effects of germ line mutations in GATA2 and SAMD9/9L may contribute to leukemic progression in a similar manner to CH/CCUS/MDS. One proposed model of progression in GATA2 deficiency is that an accelerated hypocellular bone marrow environment and frequent infections/inflammation selects for monosomy 7 clones within the adolescent bone marrow. It may be that the early hypocellular environment mimics an aged or hyperdamaged environment, because monosomy 7 in adults has been shown to be correlated with advanced age and therapy-related neoplasms.110 Germ line mutations in DDX41 predispose to MDS/AML, but these patients do not normally present with cytogenetic abnormalities or MDS/AML until late in life.72 It will be critical for investigators to be fastidious in the selection of stage-specific cells to model disease progression in murine studies.
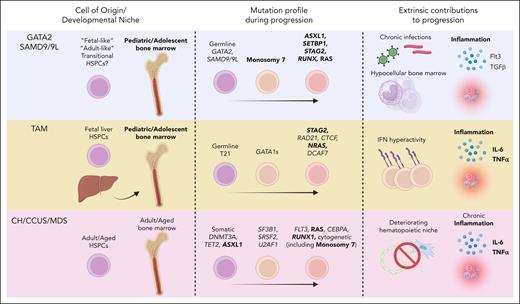
Comparing and contrasting mechanisms of leukemic progression in pediatric and adult disease. Similarities (bold) and differences in cell of origin/developmental niche, mutation profiles, and extrinsic factors during disease progression between GATA2 deficiency/SAMD9/9L syndromes, TAM, and CH/CCUS/MDS. Figure created with Biorender.com.
Comparing and contrasting mechanisms of leukemic progression in pediatric and adult disease. Similarities (bold) and differences in cell of origin/developmental niche, mutation profiles, and extrinsic factors during disease progression between GATA2 deficiency/SAMD9/9L syndromes, TAM, and CH/CCUS/MDS. Figure created with Biorender.com.
Secondly, it is interesting that splicing factor mutations are rarely seen in pediatric disorders but are prevalent and early mutations in AML deriving from CH/CCUS (Figure 3). An explanation could be that a fully functional spliceosome is critical for rapid hematopoietic development in fetal and neonatal stages, which select against splicing factor mutants early in life. Perhaps once fully established, the hematopoietic system can still be sustained with a low level of splicing factor mutant clones, later progressing to leukemia after the acquisition of cooperating mutations. It also remains unclear why monosomy 7 is highly prevalent in GATA2 deficiency and SAMD9/9L syndromes. Haploinsufficiency of several candidate 7q genes has been proposed; prominent candidates include CUX1, EZH2, and MLL3.111,112 A recent comprehensive study of >600 samples of -7/del7q myeloid neoplasm revealed 27 potentially synthetic lethal target genes and 26 differentially expressed genes that likely contribute to neoplasm with this chromosomal aberration.113 Further studies with advanced models are needed.
Finally, the similarities between the inflammatory state of pediatric/adult bone marrow may inform disease progression. Chronic inflammation and “inflammageing,” the acceleration of proinflammatory markers with age, are now thought to be bona fide contributors to AML progression in adults. Persistent inflammation and aging exhaust normal HSCs via excessive proliferation to replenish immune cells, leading to an acceleration in the selective advantage of mutant clones.114 Although not exhibiting age-related chronic inflammation, the pediatric syndromes discussed here may be either mimicking a chronic inflammatory state or exhibiting chronic inflammation due to persistent infections and immune dysfunction (Figure 3). As discussed above, IFN hyperactivity is a feature of DS and is associated with an increased risk of autoimmunity.99 GATA2 deficiency and SAMD9/9L are tightly linked with syndromes of immune dysfunction and persistent infections, and there is evidence that the GATA2 deficient hematopoietic system already exhibits reduced clonal complexity.3 IL-6 and TNF-α are the most commonly overproduced cytokines in patients with hematologic malignancies, and both have either been directly implicated in the progression of leukemia in concert with specific CH/CCUS mutations or proven to be upregulated in patients with DS.106,107 Interestingly, elevated FLT3L was associated with disease progression in patients with GATA2 deficiency, and hyperactivity of the TGF-β pathway was shown in a Samd9l mouse model, suggesting that distinct inflammatory cues may be necessary for progression in these disorders.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (grant R35CA253096), the Samuel Waxman Cancer Research Foundation, and St. Jude/American Lebanese Syrian Associated Charities. T.H. is supported by a fellowship from the Leukemia and Lymphoma Society.
Authorship
Contribution: T.H., S.G., and J.D.C. wrote the manuscript.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict-of-interest disclosure: J.D.C. is a member of the scientific advisory board of Alethiomics; is a consultant for Cellarity; and receives research funding from Syndax. S.G. has served as a consultant for Jazz Pharmaceuticals. T.H. declares no competing financial interests.
Correspondence: John D. Crispino, St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS341, Memphis, TN 38105; email: john.crispino@stjude.org.