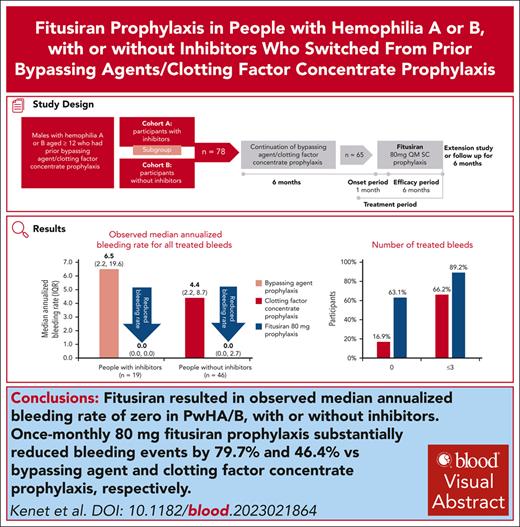
Fitusiran prophylaxis provided consistent protection against bleeding vs prior BPA/CFC prophylaxis in PwHA/B, with or without inhibitors.
Fitusiran was well tolerated, and reported adverse events were consistent with previously identified risks of fitusiran.
Visual Abstract
Fitusiran, a subcutaneous investigational small interfering RNA therapeutic, targets antithrombin to rebalance hemostasis in people with hemophilia A or B (PwHA/B), irrespective of inhibitor status. This phase 3, open-label study evaluated the efficacy and safety of fitusiran prophylaxis in males aged ≥12 years with hemophilia A or B, with or without inhibitors, who received prior bypassing agent (BPA)/clotting factor concentrate (CFC) prophylaxis. Participants continued their prior BPA/CFC prophylaxis for 6 months before switching to once-monthly 80 mg fitusiran prophylaxis for 7 months (onset and efficacy periods). Primary end point was annualized bleeding rate (ABR) in the BPA/CFC prophylaxis and fitusiran efficacy period. Secondary end points included spontaneous ABR (AsBR) and joint ABR (AjBR). Safety and tolerability were assessed. Of 80 enrolled participants, 65 (inhibitor, n = 19; noninhibitor, n = 46) were eligible for ABR analyses. Observed median ABRs were 6.5 (interquartile range [IQR], 2.2-19.6)/4.4 (IQR, 2.2-8.7) with BPA/CFC prophylaxis vs 0.0 (IQR, 0.0-0.0)/0.0 (IQR, 0.0-2.7) in the corresponding fitusiran efficacy period. Estimated mean ABRs were substantially reduced with fitusiran by 79.7% (P = .0021) and 46.4% (P = .0598) vs BPA/CFC prophylaxis, respectively. Forty-one participants (63.1%) experienced 0 treated bleeds with fitusiran vs 11 (16.9%) with BPAs/CFCs. Median AsBR and AjBR were both 2.2 with BPA/CFC prophylaxis and 0.0 in the fitusiran efficacy period. Two participants (3.0%) experienced suspected or confirmed thromboembolic events with fitusiran. Once-monthly fitusiran prophylaxis significantly reduced bleeding events vs BPA/CFC prophylaxis in PwHA/B, with or without inhibitors, and reported adverse events were generally consistent with previously identified risks of fitusiran. This trial was registered at www.ClinicalTrials.gov as #NCT03549871.
Introduction
Hemostasis results from balanced procoagulant and anticoagulant pathways that generate sufficient thrombin to control bleeding.1 Hemophilia A and hemophilia B arise from factor VIII (FVIII) or FIX deficiency, respectively, resulting in insufficient thrombin generation (TG), disrupting hemostasis.2-4 Prophylaxis, the regular administration of hemostatic agents with the goal of preventing bleeding, is recommended for hemophilia management.3 However, prophylaxis with clotting factor concentrates (CFCs) can be burdensome because of frequent IV infusions and venous access difficulties.3,5,6 CFCs may be also limited by development of inhibitory antibodies in ∼30% and ∼10% of people with hemophilia A or B (PwHA/B), respectively, which render treatment ineffective.3,7,8 Although current prophylactic options are effective, they do not satisfy the needs of all PwH9,10; options for people with inhibitors are limited,5,11,12 and despite all available therapeutics for people without inhibitors, PwH continue to experience breakthrough bleeds,1,5,13 which lead to joint damage10,14 and reduced quality of life (QoL).15,16 Efforts have focused on developing effective treatments for people with inhibitors and therapies that aim to further prevent bleeding while reducing the treatment burden for PwH.3,5,17 One such option is subcutaneous (SC) emicizumab prophylaxis, indicated for PwHA, irrespective of inhibitor status.18-20 Currently, no such SC option is available for hemophilia B.21 However, breakthrough bleeds and pain on injection may still occur with emicizumab prophylaxis, and neutralizing antidrug antibodies (ADAs) may develop.18,22-25 Novel therapeutics are needed to address the ongoing unmet needs of prophylaxis and achieve health equity among all PwH.1,3,5,17
Fitusiran is an investigational SC prophylactic small interfering RNA therapeutic designed to lower antithrombin (AT) with the goal of restoring sufficient TG to rebalance hemostasis in PwHA/B, with or without inhibitors.26-28 In previous phase 3 trials (ATLAS-A/B [NCT03417245] and ATLAS-INH [NCT03417102]), once-monthly (QM) 80 mg fitusiran prophylaxis significantly reduced annualized bleeding rate (ABR) in PwHA/B, with or without inhibitors vs on-demand treatment.29,30 This phase 3 trial evaluated the efficacy and safety of fitusiran prophylaxis vs prior bypassing agent (BPA)/CFC prophylaxis in PwHA/B, with or without inhibitors.
Methods
Study design
This phase 3, multicenter, multinational, open-label trial (ATLAS-PPX [NCT03549871]) was conducted at 35 sites in 15 countries. The trial was conducted in accordance with the protocol and ethical principles derived from international guidelines including the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice. Informed participant consent/assent was obtained before study conduct; the consent form was modified according to local regulations and requirements whenever applicable. The study protocol was approved by the independent ethics committees or institutional review boards, and an independent Data Monitoring Committee oversaw the safety and conduct of the trial and performed periodic data reviews during the trial.
Participants
Eligible participants included males aged ≥12 years with severe hemophilia A or B (FVIII [<1%] or FIX [≤2%] at screening or documented by medical record evidence), with or without inhibitors, who were receiving BPA (cohort A; people with inhibitors [Nijmegen-modified Bethesda assay ≥ 0.6 Bethesda units/mL at screening], with ≥2 BPA-treated bleeding events in the past 6 months) or CFC prophylaxis (cohort B; people without inhibitors [Nijmegen-modified Bethesda assay < 0.6 Bethesda units/mL at screening], with ≥1 CFC-treated bleeding event in the last 12 months). A subgroup of cohort A included PwHB with inhibitors who were not responding adequately to BPA prophylaxis before enrollment (historical ABR ≥ 20). Full inclusion/exclusion criteria are available in supplemental Methods, available on the Blood website.
Procedures
The trial consisted of a 6-month BPA/CFC prophylaxis period during which participants continued their prior prophylaxis regimen (supplemental Table 1). This was followed by a 1-month onset period, during which participants received the first fitusiran dose (while allowed to continue BPA/CFC prophylaxis for up to 7 days) and when fitusiran gradually reached the target pharmacodynamic effect of AT lowering; and a 6-month fitusiran efficacy period in which participants received fitusiran prophylaxis. The subgroup of cohort A (nonresponders to BPA prophylaxis) started fitusiran prophylaxis directly after the screening period. Fitusiran treatment (prophylaxis) period included both the onset and fitusiran efficacy periods. Participants initially received fitusiran 80 mg QM; after implementation of the fitusiran revised AT–based dosing regimen, 2 participants started with 50 mg once every 2 months (Q2M). The AT follow-up period lasted ∼6 months after the final fitusiran dose, until AT levels returned to ∼60% or until enrollment into an open-label extension study (ATLAS-OLE [NCT03754790]; supplemental Figure 1).
During fitusiran treatment, participants could receive on-demand BPAs/CFCs to treat breakthrough bleeds in accordance with specific breakthrough bleed management guidelines (BBMG; supplemental Table 2).
Participants were to record all bleeding events and all doses of BPAs/CFCs administered during the trial in an eDiary (supplemental Methods). A treated bleeding episode was defined as any occurrence of hemorrhage requiring BPAs/CFC administration. The definitions of bleeding episode types were according to the recommendations of the International Society on Thrombosis and Hemostasis (supplemental Methods).31
Health-related QoL (HRQoL) was assessed at month –6, day 1, and month 7 in participants aged ≥17 years using the validated Haemophilia Quality of Life Questionnaire for Adults (Haem-A-QoL) instrument (supplemental Methods).32
Safety assessments included adverse events (AEs), serious AEs (SAEs), AEs of special interests (AESIs), and laboratory tests, including markers of coagulation. AESIs were predefined as alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevations >3× upper limit of normal (ULN), suspected or confirmed thrombosis, severe or serious injection site reactions, systemic injection-associated reactions, cholecystitis, and cholelithiasis (supplemental Methods).
For pharmacodynamic assessments, AT levels and peak TG were measured from blood samples and monitored, initially after 2 weeks from baseline and then monthly. ADAs to fitusiran were measured from serum blood samples using a validated enzyme-linked immunosorbent assay method (supplemental Methods).
End points
The primary end point was ABR in the BPA/CFC prophylaxis and fitusiran efficacy periods. Secondary end points included spontaneous ABR (AsBR) and joint ABR (AjBR) in the BPA/CFC prophylaxis and fitusiran efficacy periods; change in Haem-A-QoL physical health score and total score in the fitusiran treatment and BPA/CFC prophylaxis periods32; ABR in the onset and treatment periods; and annualized weight-adjusted BPA/CFC consumption.
Subgroup analysis included hemophilia type (hemophilia A or B), age category (<18 years, 18-64 years, and ≥65 years), and the number of bleeding episodes within 6 or 12 months before screening (≤10 or >10). Exploratory end points included number of treated target joint bleeds; change in AT levels and peak TG over time; and incidence and titer of ADAs (supplemental Methods).
Safety and tolerability end points included incidence, severity, seriousness, and relatedness of AEs.
Statistical analysis
Sample size determination is described in the supplemental Methods. Population analysis sets included the safety analysis sets (SASs), efficacy analysis sets (EASs), per-protocol analysis set, and COVID-19 unaffected set (supplemental Methods). The primary end point was based on bleeding episodes in the BPA/CFC prophylaxis (days –168 to –1) and the fitusiran efficacy period (days 29-190). Primary analyses were performed on EAS 1 that included all participants who received BPA/CFC prophylaxis and at least 1 dose of 80 mg QM fitusiran. Safety summaries were performed on SAS 1, which included all participants who enrolled and then received at least 1 dose of 80 mg QM fitusiran before dose resumption.
To avoid confounding the treatment effect, bleeding data from the intercurrent events (premature discontinuation, surgeries, and ATIII concentrate treatment) were excluded from the primary analysis. The number of bleeding episodes was analyzed using a repeated measures negative binomial model with fixed effect of treatment period. The logarithm number of days that each participant spent in the respective period matching the analyzed bleeding episode data was included as an offset variable to account for unequal follow-up time owing to early withdrawal or surgery. In addition, a Bayesian analysis was performed to provide the posterior probability of a clinically significant treatment effect, along with associated measures of uncertainty. Summary statistics for observed ABR, including median and interquartile range (IQR), were also calculated for each study period. The observed ABR was calculated as total number of qualifying bleeding episodes divided by total number of days in the respective period multiplied by 365.25.
Bleeding episodes in the treatment (days 1-190) and onset periods as well as AsBR and AjBR in the fitusiran efficacy and BPA/CFC prophylaxis periods were analyzed and compared using the same negative binomial model as the primary analysis. Change from baseline in Haem-A-QoL physical health score and total score (in participants aged ≥17 years) in the BPA/CFC prophylaxis and fitusiran treatment periods were analyzed using a mixed model for repeated measure analysis with robust sandwich covariance matrix, with fixed effects of study period, baseline Haem-A-QoL physical health score, and total score for each study period as covariates. Safety, pharmacodynamic, and immunogenicity results were summarized descriptively. Safety end points were analyzed for the BPA/CFC prophylaxis and the fitusiran prophylaxis periods (comprising the fitusiran treatment and AT follow-up period). All statistical analyses were performed separately for cohort A and cohort B. Pooled analyses were performed after all participants in the 2 cohorts had either finished the month 7 visit during the fitusiran treatment period or discontinued from the study.
Results
Study population
Between 25 July 2018 and 25 March 2022, a total of 99 participants were screened, and 80 were enrolled into the study (cohort A, n = 30; cohort B, n = 50). Seventy-eight participants entered the BPA/CFC prophylaxis period, and 2 participants started directly with fitusiran 80 mg QM (subgroup of cohort A; excluded from EAS 1). Of the 67 participants who completed the BPA/CFC prophylaxis period, 65 initiated fitusiran 80 mg QM and 2, excluded from this analysis, fitusiran 50 mg Q2M. Fifty-four participants completed 80 mg fitusiran treatment; 13 participants discontinued treatment (because of dosing pause, n = 9; AEs, n = 2; withdrawn consent, n = 2), but 9 remained in the study. Sixty-four participants (80.0%) completed the study (Figure 1). Efficacy and safety results are presented for EAS 1 (n = 65) and SAS 1 (n = 67), respectively. In EAS 1, a total of 50 participants had hemophilia A, and 15 had hemophilia B; 19 and 46 participants were with and without inhibitors, respectively (Table 1).
Participant disposition. ∗Among the 19 screen failures, the main reasons for screen failure were exclusion criterion of the coexisting thrombophilic disorder (7 [7.1%] of the overall participants screened; 2 [5.7%] in cohort A; and 5 [7.8%] in cohort B) or withdrawn consent (7 [7.1%] of the overall participants screened; 1 [2.9%] in cohort A; and 6 [9.4%] in cohort B). †A subgroup of cohort A included PwHB with inhibitors who were not responding adequately to BPA prophylaxis before enrollment (historical ABR, ≥ 20). ‡After sponsor initiated pause in dosing and subsequent protocol amendment. §Nine participants with fitusiran 80 mg prophylaxis who discontinued therapy remained in the study to complete study assessments and follow-up to allow for the best study integrity and interpretation. ¶One participant with fitusiran 50 mg prophylaxis who discontinued therapy remained in the study to complete study assessments and follow-up to allow for the best study integrity and interpretation.
Participant disposition. ∗Among the 19 screen failures, the main reasons for screen failure were exclusion criterion of the coexisting thrombophilic disorder (7 [7.1%] of the overall participants screened; 2 [5.7%] in cohort A; and 5 [7.8%] in cohort B) or withdrawn consent (7 [7.1%] of the overall participants screened; 1 [2.9%] in cohort A; and 6 [9.4%] in cohort B). †A subgroup of cohort A included PwHB with inhibitors who were not responding adequately to BPA prophylaxis before enrollment (historical ABR, ≥ 20). ‡After sponsor initiated pause in dosing and subsequent protocol amendment. §Nine participants with fitusiran 80 mg prophylaxis who discontinued therapy remained in the study to complete study assessments and follow-up to allow for the best study integrity and interpretation. ¶One participant with fitusiran 50 mg prophylaxis who discontinued therapy remained in the study to complete study assessments and follow-up to allow for the best study integrity and interpretation.
Demographics and baseline characteristics (EAS 1)
| Characteristic . | Participants with inhibitors (n = 19) . | Participants without inhibitors (n = 46) . | All (N = 65) . |
|---|---|---|---|
| Age at baseline, y | |||
| Mean (SD) | 27.8 (17.1) | 23.5 (7.3) | 24.8 (11.2) |
| Median (IQR) | 23 (15.0-30.0) | 23 (17.0-29.0) | 23 (16.0-29.0) |
| Age group at baseline, n (%) | |||
| 12-17 y | 7 (36.8) | 12 (26.1) | 19 (29.2) |
| 18-64 y | 11 (57.9) | 34 (73.9) | 45 (69.2) |
| ≥65 y | 1 (5.3) | 0 | 1 (1.5) |
| Sex, male, n (%) | 19 (100) | 46 (100) | 65 (100) |
| Hemophilia type, n (%) | |||
| Hemophilia A | 14 (73.7) | 36 (78.3) | 50 (76.9) |
| Hemophilia B | 5 (26.3) | 10 (21.7) | 15 (23.1) |
| Weight, mean (SD), kg | 68.2 (16.2) | 71.7 (15.3) | 70.7 (15.5) |
| BMI, mean (SD), kg/m2 | 23.7 (4.6) | 23.6 (4.1) | 23.6 (4.2) |
| Race, n (%) | |||
| White | 15 (78.9) | 27 (58.7) | 42 (64.6) |
| Asian | 3 (15.8) | 17 (37.0) | 20 (30.8) |
| Black or African American | 1 (5.3) | 0 | 1 (1.5) |
| Other | 0 | 2 (4.3) | 2 (3.1) |
| Region, n (%) | |||
| North America | 0 | 0 | 0 |
| Europe | 15 (78.9) | 28 (60.9) | 43 (66.2) |
| Asia | 3 (15.8) | 17 (37.0) | 20 (30.8) |
| Other | 1 (5.3) | 1 (2.2) | 2 (3.1) |
| Number of bleeding episodes in the last 6 months before screening, mean (SD)∗ | 4.6 (2.6) | NR | NR |
| Number of bleeding episodes in the last 12 months before screening, mean (SD)∗ | NR | 4.0 (5.2) | NR |
| Inhibitor type, n (%) | |||
| Highest historical inhibitor titer result† | |||
| <5 BU/mL | 1 (5.3) | N/A | 1 (1.5) |
| ≥5 BU/mL | 18 (94.7) | N/A | 18 (27.7) |
| Number of target joints‡, median (IQR) | 0.0 (0.0-1.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) |
| Characteristic . | Participants with inhibitors (n = 19) . | Participants without inhibitors (n = 46) . | All (N = 65) . |
|---|---|---|---|
| Age at baseline, y | |||
| Mean (SD) | 27.8 (17.1) | 23.5 (7.3) | 24.8 (11.2) |
| Median (IQR) | 23 (15.0-30.0) | 23 (17.0-29.0) | 23 (16.0-29.0) |
| Age group at baseline, n (%) | |||
| 12-17 y | 7 (36.8) | 12 (26.1) | 19 (29.2) |
| 18-64 y | 11 (57.9) | 34 (73.9) | 45 (69.2) |
| ≥65 y | 1 (5.3) | 0 | 1 (1.5) |
| Sex, male, n (%) | 19 (100) | 46 (100) | 65 (100) |
| Hemophilia type, n (%) | |||
| Hemophilia A | 14 (73.7) | 36 (78.3) | 50 (76.9) |
| Hemophilia B | 5 (26.3) | 10 (21.7) | 15 (23.1) |
| Weight, mean (SD), kg | 68.2 (16.2) | 71.7 (15.3) | 70.7 (15.5) |
| BMI, mean (SD), kg/m2 | 23.7 (4.6) | 23.6 (4.1) | 23.6 (4.2) |
| Race, n (%) | |||
| White | 15 (78.9) | 27 (58.7) | 42 (64.6) |
| Asian | 3 (15.8) | 17 (37.0) | 20 (30.8) |
| Black or African American | 1 (5.3) | 0 | 1 (1.5) |
| Other | 0 | 2 (4.3) | 2 (3.1) |
| Region, n (%) | |||
| North America | 0 | 0 | 0 |
| Europe | 15 (78.9) | 28 (60.9) | 43 (66.2) |
| Asia | 3 (15.8) | 17 (37.0) | 20 (30.8) |
| Other | 1 (5.3) | 1 (2.2) | 2 (3.1) |
| Number of bleeding episodes in the last 6 months before screening, mean (SD)∗ | 4.6 (2.6) | NR | NR |
| Number of bleeding episodes in the last 12 months before screening, mean (SD)∗ | NR | 4.0 (5.2) | NR |
| Inhibitor type, n (%) | |||
| Highest historical inhibitor titer result† | |||
| <5 BU/mL | 1 (5.3) | N/A | 1 (1.5) |
| ≥5 BU/mL | 18 (94.7) | N/A | 18 (27.7) |
| Number of target joints‡, median (IQR) | 0.0 (0.0-1.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) |
EAS 1 includes all participants who received BPA/CFC prophylaxis and at least 1 dose of 80 mg fitusiran before dose resumption (after the sponsor initiated pause in dosing).
BMI, body mass index; BU, Bethesda unit; N/A, not applicable; NR, not reported.
Number of bleeding episodes in the last 6 months was only reported for participants with inhibitors and in the last 12 months only for participants without inhibitors.
Bethesda assay.
A target joint is defined as a joint where 3 or more spontaneous bleeding episodes in a single joint within a consecutive 6-month period has occurred; where there have been ≤2 bleeding episodes in the joint within a consecutive 12-month period, the joint is no longer considered a target joint.
Efficacy
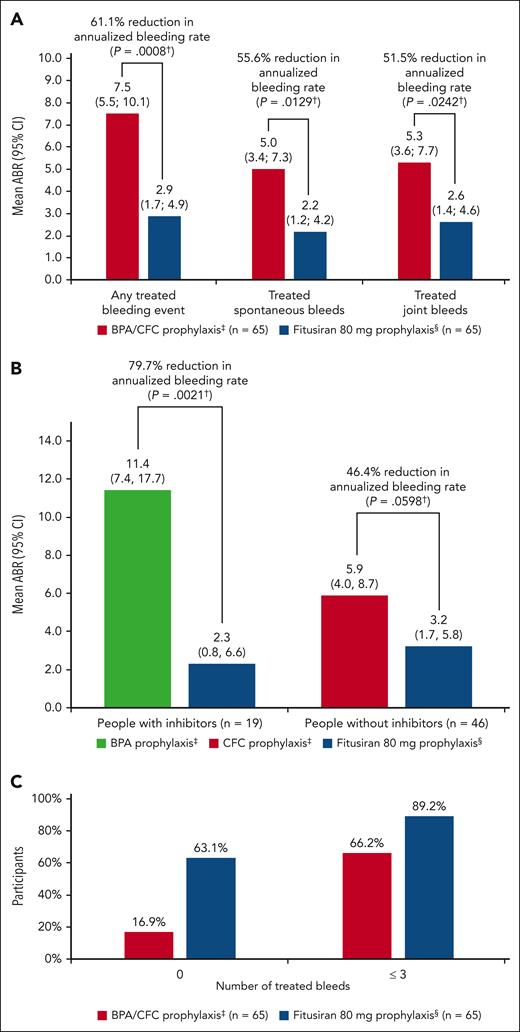
Observed median ABR was 0.0 (IQR, 0.0-2.3) in the fitusiran efficacy and 4.4 (IQR, 2.2-10.9) in the BPA/CFC prophylaxis period. Estimated mean ABR was 2.9 (95% confidence interval [CI], 1.7-4.9) in the fitusiran efficacy period, which was significantly lower than the estimated ABR of 7.5 (95% CI, 5.5-10.1) in the BPA/CFC prophylaxis period, corresponding to a significant 61.1% reduction in bleeding rate (95% CI, 32.5-77.6; P = .0008). With BPA/CFC prophylaxis, 11 of 65 participants (16.9%) had 0 treated bleeds, and 43 of 65 participants (66.2%) had ≤3 treated bleeds vs 41 of 65 (63.1%) and 58 of 65 participants (89.2%) in the fitusiran efficacy period, respectively. In people with inhibitors, median ABR was 6.5 (IQR, 2.2-19.6) in the BPA prophylaxis and 0.0 (IQR, 0.0-0.0) in the fitusiran efficacy period; in people without inhibitors, median ABR was 4.4 (IQR, 2.2-8.7) in the CFC prophylaxis and 0.0 (IQR, 0.0-2.7) in the fitusiran efficacy period (Figure 2; Table 2).
Bleeding events in the fitusiran efficacy and BPA/CFC prophylaxis period (EAS 1). (A) ABRs for treated bleeds (estimated by negative binomial model). (B) ABRs for treated bleeds by inhibitor status (estimated by negative binomial model). (C) Number of treated bleeds. ∗Includes all participants who received BPA/CFC prophylaxis and at least 1 dose of 80 mg fitusiran before dose resumption (after the sponsor initiated pause in dosing). †P value from a negative binomial regression model with study period (fitusiran efficacy period or BPA/CFC prophylaxis period) as a fixed effect and a robust sandwich covariance matrix constructed to account for the within subject dependence, the logarithm of the duration (in years) that each participant spends in each study period matching the bleeding episode data being analyzed as an offset variable (P value vs null hypothesis of ratio = 1). ‡The BPA/CFC prophylaxis period was defined as starting on day –168 to day –1 or the last day of bleeding follow-up, whichever was the earliest. §Fitusiran efficacy period was defined as starting on day 29 after the first dose of fitusiran up to day 197 or the last day of bleeding follow-up, whichever was the earliest.
Bleeding events in the fitusiran efficacy and BPA/CFC prophylaxis period (EAS 1). (A) ABRs for treated bleeds (estimated by negative binomial model). (B) ABRs for treated bleeds by inhibitor status (estimated by negative binomial model). (C) Number of treated bleeds. ∗Includes all participants who received BPA/CFC prophylaxis and at least 1 dose of 80 mg fitusiran before dose resumption (after the sponsor initiated pause in dosing). †P value from a negative binomial regression model with study period (fitusiran efficacy period or BPA/CFC prophylaxis period) as a fixed effect and a robust sandwich covariance matrix constructed to account for the within subject dependence, the logarithm of the duration (in years) that each participant spends in each study period matching the bleeding episode data being analyzed as an offset variable (P value vs null hypothesis of ratio = 1). ‡The BPA/CFC prophylaxis period was defined as starting on day –168 to day –1 or the last day of bleeding follow-up, whichever was the earliest. §Fitusiran efficacy period was defined as starting on day 29 after the first dose of fitusiran up to day 197 or the last day of bleeding follow-up, whichever was the earliest.
Bleeding events in the fitusiran and BPA/CFC prophylaxis period (EAS 1)
Event . | Inhibitor . | Noninhibitor . | Overall . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BPA PPX (n = 19) . | Fitusiran 80 mg PPX (n = 19) . | P value∗ . | CFC PPX (n = 46) . | Fitusiran 80 mg PPX (n = 46) . | P value∗ . | BPA/CFC PPX (n = 65) . | Fitusiran 80 mg PPX (n = 65) . | P value∗ . | |
| Any treated bleeding event | |||||||||
| Estimated ABR (95% CI) | 11.4 (7.4-17.7) | 2.3 (0.8-6.6) | 5.9 (4.0-8.7) | 3.2 (1.7-5.8) | 7.5 (5.5-10.1) | 2.9 (1.7-4.9) | |||
| ABR reduction, % | 79.7 | .0021 | 46.4 | .0598 | 61.1 | .0008 | |||
| Median ABR (IQR) | 6.5 (2.2; 19.6) | 0.0 (0.0; 0.0) | 4.4 (2.2; 8.7) | 0.0 (0.0; 2.7) | 4.4 (2.2; 10.9) | 0.0 (0.0; 2.3) | |||
| Participants with 0 treated bleeds, n (%) | 1 (5.3) | 15 (78.9) | 10 (21.7) | 26 (56.5) | 11 (16.9) | 41 (63.1) | |||
| Treated spontaneous bleeds | |||||||||
| Estimated AsBR (95% CI) | 7.2 (4.4-11.8) | 1.9 (0.7-5.7) | 4.1 (2.4-7.0) | 2.4 (1.1-5.1) | 5.0 (3.4-7.3) | 2.2 (1.2-4.2) | |||
| AsBR reduction, % | 73.2 | .0100 | 42.3 | .1842 | 55.6 | .0129 | |||
| Median AsBR (IQR) | 4.4 (2.2-15.2) | 0.0 (0.0-0.0) | 2.2 (0.0; 4.4) | 0.0 (0.0-2.3) | 2.2 (0.0-6.5) | 0.0 (0.0-2.3) | |||
| Participants with 0 spontaneous treated bleeds, n (%) | 3 (15.8) | 15 (78.9) | 20 (43.5) | 31 (67.4) | 23 (35.4) | 46 (70.8) | |||
| Treated joint bleeds | |||||||||
| Estimated AjBR (95% CI) | 7.9 (4.7, 13.0) | 2.1 (0.7, 5.8) | 4.2 (2.5, 7.1) | 2.8 (1.4, 5.5) | 5.3 (3.6, 7.7) | 2.6 (1.4, 4.6) | |||
| AjBR reduction, % | 73.6 | .0207 | 34.4 | .2681 | 51.5 | .0242 | |||
| Median AjBR (IQR) | 4.4 (0.0-15.2) | 0.0 (0.0-0.0) | 2.2 (0.0-4.4) | 0.0 (0.0-2.3) | 2.2 (0.0-6.5) | 0.0 (0.0-2.3) | |||
| Participants with 0 joint treated bleeds, n (%) | 5 (26.3) | 15 (78.9) | 17 (37.0) | 29 (63.0) | 22 (33.8) | 44 (67.7) | |||
Event . | Inhibitor . | Noninhibitor . | Overall . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BPA PPX (n = 19) . | Fitusiran 80 mg PPX (n = 19) . | P value∗ . | CFC PPX (n = 46) . | Fitusiran 80 mg PPX (n = 46) . | P value∗ . | BPA/CFC PPX (n = 65) . | Fitusiran 80 mg PPX (n = 65) . | P value∗ . | |
| Any treated bleeding event | |||||||||
| Estimated ABR (95% CI) | 11.4 (7.4-17.7) | 2.3 (0.8-6.6) | 5.9 (4.0-8.7) | 3.2 (1.7-5.8) | 7.5 (5.5-10.1) | 2.9 (1.7-4.9) | |||
| ABR reduction, % | 79.7 | .0021 | 46.4 | .0598 | 61.1 | .0008 | |||
| Median ABR (IQR) | 6.5 (2.2; 19.6) | 0.0 (0.0; 0.0) | 4.4 (2.2; 8.7) | 0.0 (0.0; 2.7) | 4.4 (2.2; 10.9) | 0.0 (0.0; 2.3) | |||
| Participants with 0 treated bleeds, n (%) | 1 (5.3) | 15 (78.9) | 10 (21.7) | 26 (56.5) | 11 (16.9) | 41 (63.1) | |||
| Treated spontaneous bleeds | |||||||||
| Estimated AsBR (95% CI) | 7.2 (4.4-11.8) | 1.9 (0.7-5.7) | 4.1 (2.4-7.0) | 2.4 (1.1-5.1) | 5.0 (3.4-7.3) | 2.2 (1.2-4.2) | |||
| AsBR reduction, % | 73.2 | .0100 | 42.3 | .1842 | 55.6 | .0129 | |||
| Median AsBR (IQR) | 4.4 (2.2-15.2) | 0.0 (0.0-0.0) | 2.2 (0.0; 4.4) | 0.0 (0.0-2.3) | 2.2 (0.0-6.5) | 0.0 (0.0-2.3) | |||
| Participants with 0 spontaneous treated bleeds, n (%) | 3 (15.8) | 15 (78.9) | 20 (43.5) | 31 (67.4) | 23 (35.4) | 46 (70.8) | |||
| Treated joint bleeds | |||||||||
| Estimated AjBR (95% CI) | 7.9 (4.7, 13.0) | 2.1 (0.7, 5.8) | 4.2 (2.5, 7.1) | 2.8 (1.4, 5.5) | 5.3 (3.6, 7.7) | 2.6 (1.4, 4.6) | |||
| AjBR reduction, % | 73.6 | .0207 | 34.4 | .2681 | 51.5 | .0242 | |||
| Median AjBR (IQR) | 4.4 (0.0-15.2) | 0.0 (0.0-0.0) | 2.2 (0.0-4.4) | 0.0 (0.0-2.3) | 2.2 (0.0-6.5) | 0.0 (0.0-2.3) | |||
| Participants with 0 joint treated bleeds, n (%) | 5 (26.3) | 15 (78.9) | 17 (37.0) | 29 (63.0) | 22 (33.8) | 44 (67.7) | |||
Fitusiran efficacy period (fitusiran prophylaxis) was defined as starting on day 29 after the first dose of fitusiran up to day 190 or the last day of bleeding follow-up, whichever was the earliest. The BPA/CFC prophylaxis period was defined as starting on day –168 to day –1 or the last day of bleeding follow-up, whichever was the earliest. EAS 1 includes all participants who received BPA/CFC prophylaxis and at least 1 dose of 80 mg fitusiran before dose resumption (after the sponsor initiated pause in dosing).
The analysis is based on an on-treatment strategy, which included all treated bleeding events in the fitusiran efficacy period and the BPA/CFC prophylaxis period and excluded any bleeding events in the period of intercurrent events.
PPX, prophylaxis.
P value from a negative binomial regression model with study period (fitusiran efficacy period or BPA/CFC prophylaxis period) as a fixed effect and a robust sandwich covariance matrix constructed to account for the within subject dependence, the logarithm of the duration (in years) that each participant spends in each study period matching the bleeding episode data being analyzed as an offset variable (P value vs null hypothesis of ratio = 1).
ABRs in the onset and treatment periods demonstrated similar results to the primary end point (supplemental Results). Median AsBR was 2.2 (IQR, 0.0-6.5) and 0.0 (IQR, 0.0-2.3) in the BPA/CFC prophylaxis and fitusiran efficacy period, respectively. Median AjBR was 2.2 (IQR, 0.0-6.5) and 0.0 (IQR, 0.0-2.3) in the BPA/CFC prophylaxis and fitusiran efficacy period, respectively.
In participants with ≥1 target joint identified at baseline, 5 of 14 participants (35.7%) in the BPA/CFC period had 0 treated target joint bleeds vs 10 of 14 (71.4%) in the fitusiran efficacy period.
Subgroup analyses demonstrated a consistent effect in favor of fitusiran prophylaxis vs BPA/CFC prophylaxis (supplemental Table 3). Sensitivity analyses of the per-protocol analysis set and the COVID-19 unaffected set were consistent with the primary analysis (supplemental Table 4). There was no notable impact on the primary analysis because of the COVID-19 pandemic.
Annualized weight-adjusted consumption of BPA/CFC
Annualized mean weight-adjusted consumption, total number of injections, and mean total weight-adjusted dose per bleed of CFCs/BPAs were markedly reduced in the fitusiran treatment vs BPA/CFC prophylaxis periods (Table 3). In the fitusiran treatment period, 21 of 22 (95.5%) and 60 of 61 bleeds (98.4%) in participants with and without inhibitors, respectively, were treated in compliance with the single- and repeat-dose BBMG (supplemental Table 5).
Consumption of BPA/CFC for treatment of breakthrough bleeds (EAS 1)
| Event . | Inhibitor . | Noninhibitor . | ||||||
|---|---|---|---|---|---|---|---|---|
| BPA prophylaxis (n = 19) . | Fitusiran 80 mg prophylaxis (n = 19) . | CFC prophylaxis (n = 46) . | Fitusiran 80 mg prophylaxis (n = 46) . | |||||
| aPCC (U/kg) . | rFVIIa (μg/kg) . | aPCC (U/kg) . | rFVIIa (μg/kg) . | FVIII (IU/kg)∗ . | FIX (IU/kg)† . | FVIII (IU/kg)∗ . | FIX (IU/kg)† . | |
| Mean annualized weigh-adjusted consumption, unit (SD) | 2353.0 (4317.0) | 7468.7 (5756.6) | 25.6 (82.6) | 236.4 (327.0) | 294.5 (366.6) | 288.6 (402.9) | 60.7 (148.3) | 17.8 (56.1) |
| Mean total weight-adjusted dose per bleed, unit (SD) | 199.8 (366.1) | 709.9 (1163.8) | 34.1 (16.1) | 38.2 (17.0) | 45.3 (41.8) | 73.6 (54.7) | 13.4 (5.5) | 26.2 (0.0) |
| Total number of injections, n | 260 | 159 | 7 | 19 | 159 | 30 | 56 | 3 |
| Total number of treated bleeds, n | 77 | 24 | 5 | 13 | 108 | 18 | 51 | 3 |
| Event . | Inhibitor . | Noninhibitor . | ||||||
|---|---|---|---|---|---|---|---|---|
| BPA prophylaxis (n = 19) . | Fitusiran 80 mg prophylaxis (n = 19) . | CFC prophylaxis (n = 46) . | Fitusiran 80 mg prophylaxis (n = 46) . | |||||
| aPCC (U/kg) . | rFVIIa (μg/kg) . | aPCC (U/kg) . | rFVIIa (μg/kg) . | FVIII (IU/kg)∗ . | FIX (IU/kg)† . | FVIII (IU/kg)∗ . | FIX (IU/kg)† . | |
| Mean annualized weigh-adjusted consumption, unit (SD) | 2353.0 (4317.0) | 7468.7 (5756.6) | 25.6 (82.6) | 236.4 (327.0) | 294.5 (366.6) | 288.6 (402.9) | 60.7 (148.3) | 17.8 (56.1) |
| Mean total weight-adjusted dose per bleed, unit (SD) | 199.8 (366.1) | 709.9 (1163.8) | 34.1 (16.1) | 38.2 (17.0) | 45.3 (41.8) | 73.6 (54.7) | 13.4 (5.5) | 26.2 (0.0) |
| Total number of injections, n | 260 | 159 | 7 | 19 | 159 | 30 | 56 | 3 |
| Total number of treated bleeds, n | 77 | 24 | 5 | 13 | 108 | 18 | 51 | 3 |
EAS 1 includes all participants who received BPA/CFC prophylaxis and at least 1 dose of 80 mg fitusiran before dose resumption (after the sponsor initiated pause in dosing).
aPCC, prothrombin complex concentrate; EHL, extended half-life; rFVIIa, recombinant activated factor VII; SHL, standard half-life.
SHL products.
Because of the very limited number of participants with FIX EHL product consumption for treatment of breakthrough bleeds (only 1 participant received FIX EHL), further distinction between SHL and EHL product consumption for breakthrough bleeds treatment was not feasible in this study.
HRQoL
There was an improvement in the Haem-A-QoL transformed physical health score from month –6 with fitusiran (–9.6; 95% CI, –15.4 to –3.8) vs BPA/CFC prophylaxis (–6.0; 95% CI, –10.2 to –1.8), with a least squares (LS) mean difference of –3.6 (95% CI, –10.5 to 3.3; P = .3008). For the Haem-A-QoL transformed total score relative to month –6, there was a significant improvement in HRQoL with fitusiran (–7.6; 95% CI, –10.3 to –5.0) vs BPA/CFC prophylaxis (–3.1; 95% CI, –5.6 to –0.6), with an LS mean difference of –4.6 (95% CI, –7.6 to –1.5; P < .01; supplemental Figure 2).
Safety
Overall, 22 of 65 participants (33.8%) with BPA/CFC prophylaxis and 48 of 67 (71.6%) with fitusiran prophylaxis experienced ≥1 AE. The most common AEs with fitusiran prophylaxis (>5% of participants) are summarized in Table 4. With BPA/CFC prophylaxis, 5 SAEs were reported in 5 of 65 participants (7.7%); 13 SAEs were reported in 9 of 67 participants (13.4%) with fitusiran prophylaxis (3 SAEs in 3 of 67 participants were assessed by the investigator as related to fitusiran). With fitusiran prophylaxis, 2 of 67 participants (3.0%) experienced AEs that resulted in fitusiran discontinuation and study withdrawal (cerebrovascular accident and abdominal discomfort classified by the investigator as not related to fitusiran in a participant with reported gastroesophageal reflux disease). There were no AEs leading to death.
Summary of AEs (SAS 1)
| Event, n (%) . | BPA/CFC prophylaxis (n = 65) . | Fitusiran 80 mg prophylaxis (n = 67∗) . |
|---|---|---|
| Participants with any AE | 22 (33.8) | 48 (71.6) |
| Most common AEs in fitusiran group (in >5% of participants) | ||
| ALT increased | 1 (1.5) | 18 (26.9) |
| Nasopharyngitis | 1 (1.5) | 8 (11.9) |
| Upper respiratory tract infection | 4 (6.2) | 6 (9.0) |
| Arthralgia | 4 (6.2) | 5 (7.5) |
| Cholelithiasis | 0 | 5 (7.5) |
| Fibrin D-dimer increased | 0 | 5 (7.5) |
| Injection site pain | 0 | 5 (7.5) |
| AST increased | 1 (1.5) | 4 (6.0) |
| Cholecystitis | 0 | 4 (6.0) |
| Cough | 0 | 4 (6.0) |
| Participants with any SAE | 5 (7.7) | 9 (13.4) |
| SAEs assessed as related to fitusiran† | N/A | 3 (4.5) |
| Cerebrovascular accident | N/A | 1 (1.5) |
| Acute pancreatitis | N/A | 1 (1.5) |
| Cholelithiasis | N/A | 1 (1.5) |
| Most common SAEs‡ | ||
| Hemophilic arthropathy§ | 2 (3.1) | 2 (3.0) |
| Participants with any AESI | 2 (3.1) | 22 (32.8) |
| Any suspected or confirmed thromboembolic events‖ | 0 | 2 (3.0) |
| Any ALT or AST elevations >3× ULN¶ | 2 (3.1) | 17 (25.4) |
| Cholecystitis# | 0 | 5 (7.5) |
| Cholelithiasis | 0 | 5 (7.5) |
| Participants with any AE leading to fitusiran discontinuation∗∗ | N/A | 2 (3.0) |
| Participants with any AE leading to study withdrawal∗∗ | 0 | 2 (3.0) |
| Participants with any AE leading to death | 0 | 0 |
| Event, n (%) . | BPA/CFC prophylaxis (n = 65) . | Fitusiran 80 mg prophylaxis (n = 67∗) . |
|---|---|---|
| Participants with any AE | 22 (33.8) | 48 (71.6) |
| Most common AEs in fitusiran group (in >5% of participants) | ||
| ALT increased | 1 (1.5) | 18 (26.9) |
| Nasopharyngitis | 1 (1.5) | 8 (11.9) |
| Upper respiratory tract infection | 4 (6.2) | 6 (9.0) |
| Arthralgia | 4 (6.2) | 5 (7.5) |
| Cholelithiasis | 0 | 5 (7.5) |
| Fibrin D-dimer increased | 0 | 5 (7.5) |
| Injection site pain | 0 | 5 (7.5) |
| AST increased | 1 (1.5) | 4 (6.0) |
| Cholecystitis | 0 | 4 (6.0) |
| Cough | 0 | 4 (6.0) |
| Participants with any SAE | 5 (7.7) | 9 (13.4) |
| SAEs assessed as related to fitusiran† | N/A | 3 (4.5) |
| Cerebrovascular accident | N/A | 1 (1.5) |
| Acute pancreatitis | N/A | 1 (1.5) |
| Cholelithiasis | N/A | 1 (1.5) |
| Most common SAEs‡ | ||
| Hemophilic arthropathy§ | 2 (3.1) | 2 (3.0) |
| Participants with any AESI | 2 (3.1) | 22 (32.8) |
| Any suspected or confirmed thromboembolic events‖ | 0 | 2 (3.0) |
| Any ALT or AST elevations >3× ULN¶ | 2 (3.1) | 17 (25.4) |
| Cholecystitis# | 0 | 5 (7.5) |
| Cholelithiasis | 0 | 5 (7.5) |
| Participants with any AE leading to fitusiran discontinuation∗∗ | N/A | 2 (3.0) |
| Participants with any AE leading to study withdrawal∗∗ | 0 | 2 (3.0) |
| Participants with any AE leading to death | 0 | 0 |
N/A, not applicable.
Includes all participants who enrolled and then received at least 1 dose of fitusiran before dose resumption (after the sponsor initiated pause in dosing).
The participant with cerebrovascular accident recovered from the event with sequelae 28 days after the onset. This event resulted in fitusiran discontinuation and study withdrawal. Acute pancreatitis and cholelithiasis were assessed as serious because the need for hospitalization; both of these events were resolved by the end of the study.
In the BPA/CFC prophylaxis period, additional SAEs included gastroenteritis, hemarthrosis, and muscle hemorrhage (1 [1.5%] participant each). In the fitusiran prophylaxis period, additional SAEs included vascular device infection, biliary neoplasm, cerebrovascular accident, asthma late onset, acute pancreatitis, cholelithiasis, Stevens-Johnson syndrome (occurred 62 days after the last dose of fitusiran prophylaxis; the event was due to an allergic reaction to concomitant medications and was assessed by the investigator as not related to fitusiran prophylaxis), C-reactive protein increased, fall, femur fracture, and central venous catheter removal (1 [1.5%] participant each).
Reported as worsening of hemophilic arthropathy. Both participants with events of worsening of hemophilic arthropathy during fitusiran prophylaxis had the history of hemophilic arthropathy ongoing before the study entry. Because of traumatic joint bleeds, these events occurred, and participants recovered from these events within 2-3 days of onset. None of the events were assessed by the investigator as related to fitusiran prophylaxis.
Includes AEs of cerebrovascular accident and thrombosis (suspected thrombosis on papilla of left eye). The participant with cerebrovascular accident had a history of right lower extremity deep vein thrombosis not known by the investigator at the time of enrollment (exclusion criterion).
Laboratory abnormalities consistent with Hy’s law were identified in 1 of these participants at the end of study visit. Additional doses of fitusiran were not administered and all abnormalities resolved.
Includes one AE of chronic cholecystitis.
Includes AEs of cerebrovascular accident and abdominal discomfort.
AESI of “any suspected or confirmed thromboembolic events” were reported in 2 of 67 participants (3.0%) receiving fitusiran prophylaxis. These events were cerebrovascular accident (CVA) and thrombosis (suspected thrombosis on papilla of left eye; onset reported 87 days after final fitusiran dose). The participant with CVA was a 37-year-old male with a history of right lower extremity deep vein thrombosis not known by the investigator at the time of enrollment (exclusion criterion), and several risk factors including diabetes and possible hypertension and hyperlipidemia around the time of the event. This participant experienced complete left-sided weakness and then likely developed right middle cerebral artery territory infarct hemorrhagic with transformation and subsequent subarachnoid bleed. On 2 consecutive days before the CVA onset, the participant was treated with recombinant FVIII for a spontaneous bleed. The AT values range before the event onset was 9% to 13%. The participant recovered from the event with sequelae 28 days after the onset. The CVA was classified by the investigator as serious and possibly related to fitusiran, resulting in fitusiran discontinuation and study withdrawal. The participant with thrombosis was a 56-year-old male, with papilledema of both eyes 10 days before the event onset. He experienced pain in eye with the thrombosis onset. No bleeds were reported immediately before the thrombosis, and the participant did not use CFCs or BPAs. It was suspected that there was obstruction in the capillary vessels in the eyes. The investigator considered it possible that the observed papilledema was secondary to a thrombotic event in a capillary that supplies the optic nerve. A brain magnetic resonance imaging revealed inflammatory changes in the maxillary sinus and normal orbital structures; magnetic resonance venography was unremarkable. The AT values range before the thrombosis onset was 9% to 11%. The thrombosis on papilla was classified as nonserious and assessed by the investigator as possibly related to fitusiran. The participant was in the follow-up period when the event occurred, and therefore, no action with fitusiran was applicable. There were no AESIs of “suspected or confirmed thromboembolic events” reported with BPA/CFC prophylaxis.
AESIs of “any ALT/AST elevations >3× ULN” were reported in 17 of 67 participants (25.4%) receiving fitusiran prophylaxis. The participants had no medical history relevant to the events, and their cases varied and included positive antibodies to hepatitis A and C, Epstein-Barr virus, and human herpesvirus, among others, and concomitant use of hepatoxic medications could not be ruled out. The highest mean of ALT/AST values was 4.8 (standard deviation [SD], 1.3) × ULN; their mean duration was 41.1 (SD, 36.9) days. The majority of ALT and AST elevations occurred within 90 and 135 days of fitusiran initiation, respectively. All events were classified by the investigator as nonserious and the majority as mild to moderate in severity. The majority (14/20) of liver enzyme elevations were reported by the investigator as recovered or resolved within 2 to 3 months of onset. AESIs of increased ALT resulted in interruption of fitusiran in 6 of 67 participants (9.0%), all of whom resumed fitusiran prophylaxis thereafter; 1 participant had recurring ALT elevation after fitusiran interruption. Nonsymptomatic laboratory abnormalities consistent with Hy’s law33 were identified in 1 participant during the last study visit, with resolution after the final fitusiran dose. Additional doses were not administered, and all abnormalities were resolved by the following month. AESIs of “any ALT/AST elevations >3× ULN” were reported in 2 of 65 participants (3.1%) with BPA/CFC prophylaxis.
AESIs of “cholecystitis” and/or “cholelithiasis” were reported in 8 participants receiving fitusiran prophylaxis (cholecystitis and cholelithiasis reported concomitantly, n = 2; acalculous cholecystitis, n = 3; cholelithiasis, n = 3; chronic cholecystitis, n = 1). Four participants with cholecystitis and/or cholelithiasis had ALT/AST elevations >3× ULN. In cases of concomitant cholecystitis/cholelithiasis, biliary sludge was noted with associated biliary wall thickening. None of the participants had significant risk factors commonly associated with cholelithiasis/cholecystitis. Hepatic steatosis was noted in 5 cases (2 of which were related to concomitant cholelithiasis/cholecystitis), with 1 additional case of a biliary polyp, but no association was made with cholelithiasis/cholecystitis or transaminase elevations. These events were not reported with BPA/CFC prophylaxis.
There were no AESIs of severe or serious injection site reactions or systemic injection-associated reactions with fitusiran prophylaxis. Clinical laboratory assessments of coagulation markers showed a trend toward increased D-dimer and prothrombin fragments 1 + 2 values and decreased fibrinogen values with fitusiran vs BPA/CFC prophylaxis (supplemental Figure 3).
Pharmacodynamics and immunogenicity
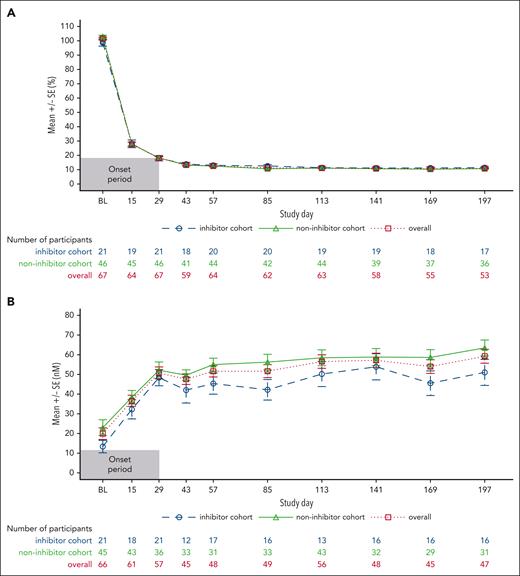
With fitusiran prophylaxis, mean AT activity level was 101.6% (SD, 8.6) at baseline and 18.2% (SD, 7.5) at the end of onset period; mean AT levels were maintained at 10.6% to 13.4% from day 43 onward. On day 29, there was a mean decrease of 83.4% (SD, 11.5) from the baseline in AT levels, maintained at 88.3% to 90.6% from day 43 onward. Mean peak thrombin values of 50.7 nM were reported on day 29 and maintained at 47.6 nM to 62.6 nM from day 43 onward. AT levels and TG results were similar in participants with and without inhibitors (Figure 3).
Pharmacodynamic outcomes. (A) Mean change in AT levels from baseline. (B) Mean peak TG. Measurements after fitusiran discontinuation +28 days were excluded. Measurements from start date of heparin, AT concentrate, and FXa inhibitor to the final date on which those products were administered plus 5 half-lives of that specific product were excluded. Measurements in the period of missing at least 2 consecutive fitusiran doses were excluded. Only central laboratory assessments were taken into account. SE, standard error.
Pharmacodynamic outcomes. (A) Mean change in AT levels from baseline. (B) Mean peak TG. Measurements after fitusiran discontinuation +28 days were excluded. Measurements from start date of heparin, AT concentrate, and FXa inhibitor to the final date on which those products were administered plus 5 half-lives of that specific product were excluded. Measurements in the period of missing at least 2 consecutive fitusiran doses were excluded. Only central laboratory assessments were taken into account. SE, standard error.
Overall, 2 of 67 participants (3%) who received fitusiran had 1 confirmed ADA-positive sample each at month 1, both with a titer of 50 (equivalent to the minimum required dilution of the assay); however, these had no effect on AT reduction in these participants. No participant had confirmed ADA samples at baseline.
Discussion
QM 80 mg SC fitusiran prophylaxis resulted in a median ABR of 0 and 63.1% of participants with 0 bleeds during the fitusiran efficacy period, demonstrating that fitusiran prophylaxis provided significant and consistent protection against bleeding in PwHA/B, with or without inhibitors who switched from prior BPA/CFC prophylaxis. Primary efficacy end point results were supported by subgroup and sensitivity analyses and clinically meaningful reductions in spontaneous and joint bleeding events. These outcomes confirm and extend the findings from previous fitusiran studies27,29,30,34,35 and suggest fitusiran may provide an effective, SC, prophylactic option in PwHA/B, irrespective of inhibitor status.
Currently, management of people with inhibitors may be complex, because a response to immune tolerance induction (ITI) or BPAs may not be optimal, which can result in frequent hospitalizations, high treatment burden, morbidity, and costs.3,11,12 Fitusiran may provide an additional prophylactic option for all PwH. In this study, fitusiran prophylaxis significantly reduced the mean ABR by 79.7% in people with inhibitors vs BPA prophylaxis. Fitusiran may also improve prophylaxis outcomes for people without inhibitors receiving CFC prophylaxis because the 46.4% mean ABR reduction in the fitusiran vs CFC prophylaxis period was clinically meaningful. The difference in reduction rates may be due to a higher variability in efficacy of BPAs vs CFCs.11
Prevention of spontaneous and joint bleeds is a reliable indicator of a significant level of protection and efficient prophylaxis in severe hemophilia. Joint bleeds can lead to joint damage, which ultimately results in arthropathy, one of the leading causes of morbidity in hemophilia.36 QoL of PwH is considerably impaired, mainly because of pain and disability associated with joint bleeds and resulting complications.14,16,36-38 Current prophylactic regimens are insufficient to completely protect joints over time, despite early prophylaxis.10,14 Fitusiran prophylaxis resulted in significantly lower rates of spontaneous and joint bleeds, as well as reduction in target joint bleeds vs BPA/CFC prophylaxis; this led to meaningful improvements in HRQoL as measured by Haem-A-QoL scores, fewer IV infusions, and lower consumption of BPAs/CFCs. These results suggest fitusiran prophylaxis may provide significant protection against bleeding while reducing the overall treatment and disease burden. Despite advances in prophylactic treatments, some PwH still experience bleeds because the current therapeutic landscape does not cater to everyone or satisfy all medical needs of people requiring consistent protection or different management approaches.5,10,39 Fitusiran might help address unmet needs of achieving consistent bleed protection, less burdensome prophylaxis with a convenient dosing regimen, and improved QoL in PwH.
Fitusiran prophylaxis was well tolerated, and reported AEs were generally consistent with previously identified risks of fitusiran, including increased liver transaminases, thrombosis, cholecystitis, and symptomatic cholelithiasis.27,29,30,34 Transaminase elevations were defined as AESI because of liver-targeted delivery; these have been also reported with approved small interfering RNA therapies for conditions other than hemophilia.40,41 Participants with transaminase elevations and cholelithiasis/cholecystitis had no relevant risk factors, and their cases varied. The underlying pathophysiology of the development of transaminase elevations, cholecystitis, and cholelithiasis is unknown; these events remain under investigation in ongoing trials. Risk mitigation strategies for hepatotoxicity included transaminase monitoring, guidelines for withholding and permanent discontinuation of fitusiran, and restricted alcohol consumption. Thrombotic events with the original dose regimen (80 mg QM) have been reported in 6 participants in completed phase 2 and 3 studies, including this study.42 In previous completed phase 3 fitusiran trials, thrombotic events were reported in 2 participants with inhibitors (ATLAS-INH30) and in none of the participants without inhibitors (ATLAS-A/B29). In this study, thrombotic events were reported in 2 participants with fitusiran prophylaxis. These events were CVA (the participant should have been excluded as per exclusion criteria) and thrombosis on the papilla of left eye, both events assessed as possibly related to fitusiran. In both participants, AT levels were low before the onset of events. The participant with the CVA had a history of thrombosis risk factors and was treated with recombinant FVIII just before the event onset. Thrombosis was associated with papilledema of both eyes and suspicion of obstruction in the capillary vessels in the eyes.
Thrombosis is a potential AE of clinical interest for therapies aiming to restore TG; thrombotic events have been reported with emicizumab, BPAs, CFCs, concizumab, and investigational antitissue factor pathway inhibitor therapies.3,43-47 Initial risk mitigation for thrombosis included screening and exclusion of participants with thrombophilia or certain thrombotic risk factors, and the provision of BBMG to enable treatment of bleeds during fitusiran prophylaxis. Compliance to BBMG demonstrated their effectiveness in managing episodic bleeds. After the voluntary pause in dosing initiated by the sponsor in response to reports of nonfatal vascular events in a separate fitusiran trial, AT levels were evaluated as a potential modifiable target for risk mitigation, and additional risk mitigation measures including a revised AT–based dosing regimen have been implemented. The revised dosing regimen was designed to target AT activity levels of 15% to 35%, beginning with 50 mg Q2M and escalating or deescalating dose/frequency based on individual participant response, as determined by measurements of AT activity levels. Because the study progress at the time, only 2 participants started with 50 mg Q2M of fitusiran; however, both participants discontinued fitusiran prematurely owing to AT levels <15% and withdrawal of consent. All reported AESIs in the current study occurred with the original dose regimen (80 mg QM). Additional risk mitigation measures are currently being evaluated in ongoing phase 3 trials (ATLAS-OLE [NCT03754790]; ATLAS-NEO [NCT05662319]).48 Observations of increased coagulation markers, D-dimers, and prothrombin fragments 1 + 2 during fitusiran vs BPA/CFC prophylaxis may be secondary to prophylaxis with procoagulant therapies and, generally, are not associated with reported clinical outcomes.
The pharmacokinetic profile of fitusiran has been presented.27,34 Pharmacodynamic analysis confirmed that fitusiran achieved sustained reductions in AT levels and increased peak TG by day 29 (onset period) and during the efficacy period, consistent with its pharmacokinetics.27,28,34 TG values associated with lowering in AT levels >75% from baseline were close to the lower range of normal peak TG previously observed in healthy volunteers (64-210 nM).27,49 Together, these sustained pharmacodynamic effects and a low ABR suggest fitusiran has the potential to rebalance hemostasis and improve bleeding phenotype in PwH.
The open-label nature of this study could be a limitation because of potential bias, particularly in patient-reported outcomes; however, this design was consistent with previous fitusiran trials and justified, because hemophilia is a rare disease with a wealth of data regarding expected bleeding outcomes with BPA/CFC prophylaxis. The sample size was based on clinical considerations and was expected to provide reasonable precision around the bleeding rate with fitusiran vs BPA/CFC prophylaxis. Given a low ABR with CFC prophylaxis and a relatively small number of participants among subgroups, the study was not powered to show statistically significant differences among subpopulations. A lack of participants with prior emicizumab prophylaxis could be considered a limitation; however, at the time of this study, emicizumab was an investigational therapy.
In conclusion, QM SC fitusiran prophylaxis resulted in a significant reduction in ABR vs BPA/CFC prophylaxis with a median ABR of 0 in PwHA/B, with or without inhibitors, resulting in a meaningful improvement in HRQoL. Fitusiran was well tolerated, and reported AEs were generally consistent with previously identified risks of fitusiran; the currently evaluated fitusiran revised AT–based dosing regimen aims to further enhance its benefit-risk profile. Fitusiran prophylaxis may provide effective and consistent bleeding protection reducing overall treatment and disease burden; therefore, it has the potential to address the unmet needs of PwHA/B, with or without inhibitors, with a favorable benefit-risk profile.
Acknowledgments
The authors thank all participants, their families, and the hospital staff who were involved in the study. The authors acknowledge Sajida Iqbal (Sanofi, Cambridge, MA) for her expertise and advice for interpreting data related to antidrug antibodies in this study; Marion Afonso (Sanofi, Chilly-Mazarin, France) for her interpretation of patient-reported outcomes data; Stacey Poloskey (Sanofi, Cambridge, MA) for her input on safety-related data; and Pratik Bhagunde (Sanofi, Bridgewater, NJ) for his expertise. Medical writing and editorial support for the development of the manuscript, under the direction of the authors, were provided by Zofia Zgrundo and Sherriden Beard of Ashfield MedComms, an Inizio company, and funded by Sanofi in accordance with good publication practice guidelines.
This study was sponsored by Sanofi.
Authorship
Contribution: All authors contributed substantially to the conception and design of the trial, data acquisition, and data analysis; and all authors revised the manuscript critically for important intellectual content and approved the final submitted version.
Conflict-of-interest disclosure: B.N. has been a study investigator for Sobi, Biogen/Bioverativ, CSL, Bayer, and Sanofi; and received honoraria from Sobi (honoraria donated to the Irish Haemophilia Society). B.Z. has acted in advisory board and/or provided consultancy for Pfizer, Shire, Novo Nordisk, Roche, Sobi, Bayer, and BioMarin. B.A. has received honoraria and has acted in advisory board and/or provided consultancy for Pfizer, Takeda, Novo Nordisk, Roche, Sobi, Bayer, and BioMarin Pharmaceutical. P.K. has received honoraria as a member of an advisory board and/or speaker from BioMarin, Novo Nordisk, Roche, Takeda, AbbVie, and CSL Behring. T.M. has received honoraria from Bayer, Takeda/Shire, Novo Nordisk, Bioverative/Sanofi, CSL Behring, and Chugai. F.P. has received honoraria from Roche, Sanofi, Sobi, and Takeda; and is a member of advisory boards of Roche, Sanofi, Sobi, and Takeda. G.K. consults for Alnylam, Bayer, BioMarin Pharmaceutical, CSL, Novo Nordisk, Opko Biologics, Pfizer, Takeda, Roche, Sanofi, and uniQure; receives research funding from Alnylam, Bayer, BPL, Opko Biologics, Pfizer, Roche, and Takeda; has been involved with speaker bureaus for Bayer, Pfizer, CSL, Shire, Novo Nordisk, and Roche; and has a membership on an entity’s board of directors or advisory committees for Alnylam, Bayer, BioMarin Pharmaceutical, CSL, Novo Nordisk, Opko Biologics, Pfizer, Takeda, Roche, Sanofi, and uniQure. C.N. received honoraria/consultancy fees from BioMarin, Novo Nordisk, Roche, Sanofi, Sobi, Takeda, Pfizer, Spark, and Bayer; grants from Novo Nordisk, Roche, Sanofi, and Sobi; and has a membership on an entity’s board of directors or advisory committees for BioMarin, Novo Nordisk, Roche, Sanofi, Takeda, Pfizer, Spark, Bayer, CSL Behring, and uniQure. G.Y. has received grants from Genentech/Roche, Grifols, and Takeda; and speaking and consultancy fees from ApcinteX, BioMarin, CSL Behring, Genentech/Roche, Grifols, Hema Biologics/LFB, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda. J.C. and B.K. are current employees of Sanofi. C.S. is a current employee and stockholder in Sanofi. F.S. is a current employee in Sanofi and equity holder in Sanofi. S.A. is an employee and equity holder in Sanofi; and a member of the WEST advisory committee. B.M. was an employee and equity holder in Sanofi at the time of the study; also has divested equity in Sanofi in the past 24 months; and is an employee of Editas Medicine. K.K. received honoraria from Pfizer, Bayer, Takeda, Roche, and Novo Nordisk; has attended speaker bureaus for Pfizer, Bayer, Takeda, Roche, and Novo Nordisk; and has a membership on an entity's board of directors or advisory committees for Pfizer, Bayer, Takeda, Roche, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Gili Kenet, The National Hemophilia Centre, Amalia Biron Thrombosis Research Institute, Sheba Medical Centre, Tel Hashomer, Tel Aviv University, Tel Aviv 52621, Israel; email: gili.kenet@sheba.health.gov.il.
References
Author notes
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Participant disposition. ∗Among the 19 screen failures, the main reasons for screen failure were exclusion criterion of the coexisting thrombophilic disorder (7 [7.1%] of the overall participants screened; 2 [5.7%] in cohort A; and 5 [7.8%] in cohort B) or withdrawn consent (7 [7.1%] of the overall participants screened; 1 [2.9%] in cohort A; and 6 [9.4%] in cohort B). †A subgroup of cohort A included PwHB with inhibitors who were not responding adequately to BPA prophylaxis before enrollment (historical ABR, ≥ 20). ‡After sponsor initiated pause in dosing and subsequent protocol amendment. §Nine participants with fitusiran 80 mg prophylaxis who discontinued therapy remained in the study to complete study assessments and follow-up to allow for the best study integrity and interpretation. ¶One participant with fitusiran 50 mg prophylaxis who discontinued therapy remained in the study to complete study assessments and follow-up to allow for the best study integrity and interpretation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/22/10.1182_blood.2023021864/2/m_blood_bld-2023-021864-gr1.jpeg?Expires=1769080017&Signature=07ByW-NpShE-2FIzd6JvFAQDbEy3ywAuht~1i6A3N0lpCeg8DtcDedOkOaaakSMVv3G~uaCaxAd63W2QhZ-ddv5wktqNlFOKUGsZazOvo9LdmZlS0ykYojCsvFt1KbFoKCSi3QCVVk1KOU6Anwgt564c96JPDFo1Kret-uOIaBIz3vK~hQ39Nro03F1-rWH3RSHMSGHZHRsxZ1LRao5tPhn-UaSr6WoEVWffM2VlGLOx3z~KtX1DRnCcQuU2jvPTsmleAeQdVEgH-Aeeol96s0bGzRC~bBrEATEblD22gFjbkHgeJXu6hZfhgasNoi6MgGAnW7XDZiqvxQbdBF3vBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal