Visual Abstract
Chronic myelomonocytic leukemia (CMML) is a heterogeneous disease presenting with either myeloproliferative or myelodysplastic features. Allogeneic hematopoietic cell transplantation (allo-HCT) remains the only potentially curative option, but the inherent toxicity of this procedure makes the decision to proceed to allo-HCT challenging, particularly because patients with CMML are mostly older and comorbid. Therefore, the decision between a nonintensive treatment approach and allo-HCT represents a delicate balance, especially because prospective randomized studies are lacking and retrospective data in the literature are conflicting. International consensus on the selection of patients and the ideal timing of allo-HCT, specifically in CMML, could not be reached in international recommendations published 6 years ago. Since then, new, CMML-specific data have been published. The European Society for Blood and Marrow Transplantation (EBMT) Practice Harmonization and Guidelines (PH&G) Committee assembled a panel of experts in the field to provide the first best practice recommendations on the role of allo-HCT specifically in CMML. Recommendations were based on the results of an international survey, a comprehensive review of the literature, and expert opinions on the subject, after structured discussion and circulation of recommendations. Algorithms for patient selection, timing of allo-HCT during the course of the disease, pretransplant strategies, allo-HCT modality, as well as posttransplant management for patients with CMML were outlined. The keynote message is, that once a patient has been identified as a transplant candidate, upfront transplantation without prior disease-modifying treatment is preferred to maximize chances of reaching allo-HCT whenever possible, irrespective of bone marrow blast counts.
Introduction: current state of the art
Chronic myelomonocytic leukemia (CMML) is a hybrid or mixed myelodysplastic/myeloproliferative neoplasm characterized by a large heterogeneity of clinical features with high variability of life expectation. Median age at diagnosis ranges from 70 to 75 years. Median survival in the largest reported series is in the 2- to 3-year range1 but is <2 years in patients with higher-risk disease according to various models of prognostication specifically developed for the disease.2,3 To date, allogeneic hematopoietic cell transplantation (allo-HCT) remains the only potentially curative treatment strategy for eligible patients.4 Overall survival for patients with CMML ranges from 30% to 40% at 5 years after allo-HCT, owing to cumulative incidences of relapse of 30% to 50% and nonrelapse mortality rates of 20% to 40%.5-12 In the context of allo-HCT the absence of prospective data drags behind many uncertainties not only regarding patient selection but also possible pretransplant treatment strategies, the timing of allo-HCT, and the optimal overall transplant policy including donor selection, the choice and intensity of conditioning, graft-versus-host disease (GVHD) prophylaxis, stem cell source, and patient management in the posttransplant setting.
Given the aforementioned factors, the newly established European Society for Blood and Marrow Transplantation (EBMT) Practice Harmonization and Guidelines (PH&G) Committee included the best practice recommendation on the management of adult patients with CMML undergoing allo-HCT among the projects to be finalized during the second annual workshop, planned in Lille, France, on 25-26 September 2023. The methodology used is described in the supplemental Material, available on the Blood website.
Workshop recommendations
Patient selection for allo-HCT
Before going into further details as to which classification system, which prognostic scoring system, and which other disease- and patient-related factors define the indication(s) for, and the most appropriate timing of, allo-HCT, it is critical that patients are at all assessed for their eligibility for allo-HCT. Up to 21% of patients with myelodysplastic neoplasms (MDS) or acute myeloid leukemia (AML) in a large registry-based study were not receiving assessment or consideration for allo-HCT,6 indicating the need for heightening nontransplant specialists’ awareness in this regard. In addition, there is also a significant proportion (up to one-third) of patients failing to reach allo-HCT after the decision to transplant has been made,7-13 indicating the need for better patient selection strategies, more efficacious pretransplant treatment modalities, faster procedure to transplant, and/or increasing the rates of upfront transplantations. Careful, holistic risk assessment and patient selection is essential to recognize patient eligibility for allo-HCT on the one hand, and, on the other, to maximize patient benefit while minimizing treatment-related morbidity and mortality.
Classification of CMML
The recently published International Consensus Classification (ICC)14 and the 2022 World Health Organization15 Classification have made mostly similar adaptions in CMML (ie, elimination of CMML-0, lowering of the monocyte threshold to ≥0.5 × 109/L, and reiteration of the myelodysplastic and myeloproliferative subtype distinction), while retaining the same blast count thresholds, and can thus both be recommended. Neither classification has as yet defined CMML subtypes based on mutational signatures.
However, the threshold of blasts required for the definition of AML was lowered or eliminated when certain mutations are present. AML defined by mutations includes AML with mutated NPM1 (World Health Organization-2022 [no blast count threshold required])15 and AML with mutated bZIP CEBPA (ICC-2022 [≥10% blasts required]).14 As such, patients with CMML harboring these mutations should be considered and treated as AML.
When mutations in TP53, ASXL1, BCOR, EZH2, RUNX1, SF3B1, STAG2, U2AF1, or ZRSR2 are present, ICC-2022 proposes a new disease category MDS/AML defined by 10% to 19% blasts, that can be treated either as MDS or as AML.14 This however, does not affect patients with CMML as yet.
Patient-related factors
Prospective data have confirmed survival benefits for patients at higher risk for MDS aged 60 to 70 years, or ≥65 years12,16 undergoing allo-HCT. Thus, although age alone should not preclude patients from being considered as transplant eligible,17 it must nevertheless be considered. Other patient-related factors that need to be considered for identifying patients eligible for allo-HCT include: performance status (PS) assessment by the Eastern Cooperative Oncology Group or Karnofsky PS scale, HCT-specific comorbidity index,18,19 frailty assessment,20 comprehensive geriatric assessments,21,22 and/or combinations thereof.23-25
Recommendations
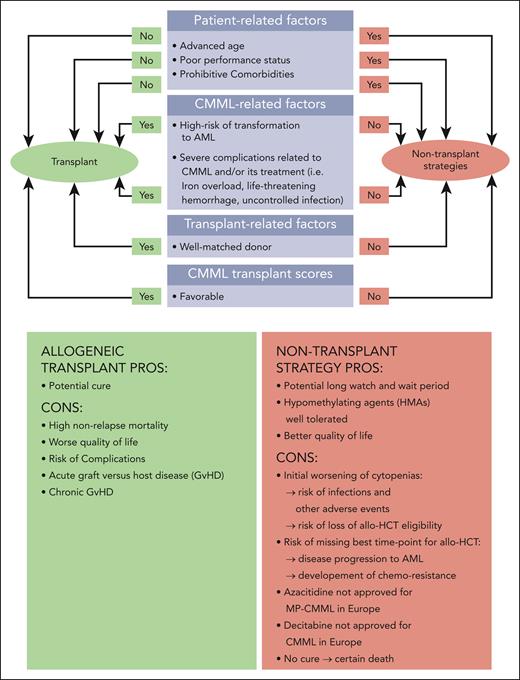
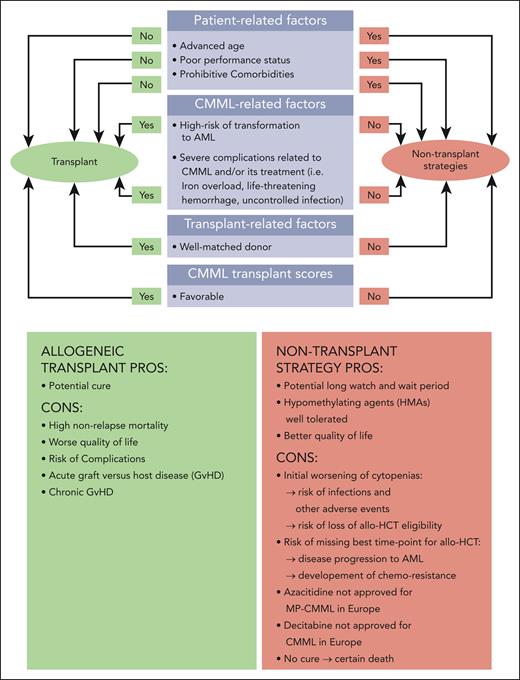
Figure 1 gives an overview of patient-, disease-, and transplant-related factors that need to be considered and carefully weighed. This panel considers the following factors to be required in patients deemed “fit for transplant” (Figure 1):
Age of ≤70 years (in select cases ≤75 years)
Eastern Cooperative Oncology Group PS of <2 or Karnofsky index of ≥70%
HCT-comorbidity index of <3
Lack of any comorbidity that the transplant specialist judges to be incompatible with intensive chemotherapy (IC), for example, as suggested by Ferrara et al.26
Timing of allo-HCT
Once the decision to proceed to allo-HCT has been made, timing is a crucial consideration, because delaying transplant may result in disease progression and/or acquisition of additional comorbidities, and/or toxicities (if the patient receives pretransplant treatment) that may preclude transplant. To date, no randomized clinical trial has addressed this important question in CMML. Expert panels could not reach a solid consensus regarding the indication for pretransplant treatment in patients candidate for allo-HCT for CMML so far,27 and 2018 European Hematology Association/European LeukemiaNet (ELN) recommendations28 were largely extrapolated from data obtained in patients with MDS.28-32
Risk stratification to identify transplant candidates
Risk stratification dictates the management of patients with CMML. Allo-HCT is the only treatment that can offer cure in this disease. Identifying those patients that will have the most benefit and the least harm from allo-HCT is thus critical. A myriad of scoring systems used in CMML have evolved from MDS-based models (International Prognostic Scoring System (IPSS),33 revised IPSS (IPSS-R),34) to CMML-specific scores predicting outcomes (Düsseldorf score,35 MD-Anderson Prognostic Score,36 modified Bournemouth score,37 CMML-specific Prognostic Scoring System [CPSS],38 and Mayo model39) or estimating time to first treatment,40 most of which have been found to be valid and with comparable performance.1,2,41-44 Updated versions of some of these scores have been published, incorporating molecular data: IPSS molecular (IPSS-M),45 Groupe Francophone des Myelodysplasies score (ASXL1),46 Mayo Molecular Model (ASXL1),47 CPSS molecular (CPSS-Mol; ASXL1, RUNX1, NRAS, and SETBP1).48 ELN and 2018 European Hematology Association guidelines recommend 5 of these scores (MD-Anderson Prognostic Score; CPSS, in case of unavailability of molecular data; Groupe Francophone des Myelodysplasies; and Mayo Molecular and CPSS-Mol when molecular data are available).28 It needs to be born in mind, that above mentioned prognostic scores were mostly devised in either untreated historic patient cohorts, and/or were not assessed for their predictive capacity on specific treatment outcomes in general, and on allo-HCT outcomes in particular.
Risk stratification has been used to identify at which time point during the course of the disease a patient should undergo transplantation. Of note, almost all data published to date used older scoring systems (IPSS, IPSS-R, or CPSS) that did not incorporate molecular data in attempts to identify the “sweet spot” at which an allo-HCT bears the most benefit for the patient while doing the least potential harm to the patient. It remains unclear whether these data can be transferred to newer molecular scores (IPSS-M and CPSS-Mol) in patients with CMML, but the IPSS-M has recently been shown to correlate with allo-HCT outcomes in MDS.49-52 These retrospective analyses indicated that allo-HCT improves outcomes for the (very) high-risk IPSS-M groups. Very recently the 1102 Study of the Blood and Marrow Transplant Clinical Trials Network was reanalyzed after inclusion of molecular data enabling restratification of patients according to IPSS-M.45 This trial identified patients with MDS with high-risk IPSS-M to be ideal candidates for early transplantation.45 In the largest cohorts of patients with CMML who had received transplantation with molecular data available (n = 31353 and n = 24054), the CPSS-Mol was shown to be significantly associated with postallogeneic disease–free survival and/or overall survival.
Although transplantation of early-stage lower-risk disease offers the lowest rate of nonrelapse mortality, it exposes patients who might have had a long period without disease progression to immediate morbidity and mortality. Because life expectancy of patients with CMML with lower-risk disease is >5 years,48 it is generally accepted that the risks of allo-HCT outweigh the potential benefits. Markov models applied to patients with MDS indicate that allo-HCT should be delayed in patients with lower-risk MDS (according to IPSS, WPSS [WHO-based prognostic scoring system], or R-IPSS) until disease progression to a higher risk category, whereas HCT should be immediately offered to patients with higher-risk disease.29-32 In corroboration thereof, retrospective studies exclusively analyzing patients with CMML have confirmed the absence of significant survival benefit of allo-HCT for patients with CMML with lower-risk disease as categorized by the CPSS.4,55 Although allo-HCT provides modest survival benefit as compared with all other treatment modalities including IC for patients after having transformed from CMML to AML,4,56 most evidence underscores the importance of early transplantation before transformation to AML.57-59 The latter coincides with disease progression and an increased potential for the acquisition of contraindications for transplantation. It also needs to be kept in mind, that although high-risk features are indications for allo-HCT in CMML, they also adversely affect allo-HCT outcomes.5,55,60-63
This panel acknowledges the recent developments in, and increasing importance of, molecular data. The CPSS-Mol (Table 1) has been shown to outperform the CPSS in all patients with CMML for both overall survival and cumulative incidence of AML evolution (Table 2).48 The panel acknowledges that specific data for the use of the CPSS-Mol in patient selection and timing decisions for allo-HCT are lacking. However, very recently, data generated in 8326 MDS cases with semi-Markov multistate decision models were presented, showing clinical relevance and statistically significant superiority of using the IPSS-M over the IPSS-R regarding the optimal timing of allo-HCT in the transplant decision making process.64 Different from other molecularly integrated CMML-specific prognostic models, only considering nonsense/frameshift ASXL1 mutations as independent adverse factors,44,45 the CPSS-Mol relies on a more comprehensive genetic risk score. In light of these data, and after much internal discussion, the panel ultimately decided to recommend the use of the CPSS-Mol, wherever possible, to stratify patient risk and to identify the optimal timing of allo-HCT in patients with CMML. Taking the possibility for posttransplant interventions into account, it may be important to dissect those disease features that affect cumulative incidences or relapse from those that can portend higher nonrelapse mortality (see also “Risk-stratification to predict post-HCT outcomes”).
Risk-stratification to predict post-HCT outcomes
Although both CPSS risk stratifications53,61 have been significantly associated with posttransplant relapse and/or overall survival, risk stratification by CPSS5,55 or CPSS-Mol5 alone has limitations in predicting posttransplant outcomes.55 Dynamic use of prognostic scores (IPSS-R,65 CPSS, or CPSS-Mol61,62) at the time of transplant (rather than at initial diagnosis) may be more relevant for the prediction of allo-HCT outcome, but this remains to be validated. Of note, aberrations of several genes (eg, TP53), although infrequent in CMML, have also been acknowledged as adverse molecular predictors of outcome, but are not captured by current molecularly integrated prognostic models. These are discussed in “Cytogenetics and gene mutations.”
Once the decision to proceed to transplant has been made, it is necessary to differentiate the use of prognostic scores for the identification of transplant eligibility on the one hand, and, on the other hand, for the prediction of posttransplant outcomes. For the former, we propose the use of the CPSS-Mol, as discussed earlier. For the latter, transplantation-specific scores to predict transplant outcomes have been developed for patients with CMML54,66 or validated in patients with CMML,67 and outperform scores typically used in the nontransplant setting. The CMML transplant score,54 which incorporates both molecular (ASXL1 and NRAS mutations) and clinical information (bone marrow blasts and increasing comorbidity index), was prognostic in patients specifically undergoing transplantation and may facilitate personalized counseling. In particular, the CMML-specific transplant score was designed and validated in a cohort of 240 patients with CMML undergoing allo-HCT. Five risk groups were identified with 5-year survival rates ranging from 81% to 19%, and nonrelapse mortality rates ranging from 5% to 51% for an increasing transplant score.54 The score retained performance after validation, and predictions were significant and superior to existing scores incorporating molecular data (including the CPSS-Mol) designed in the nontransplant setting.54 In addition, the endothelial activation and stress index score might help to predict nonrelapse mortality,67 whereas a more recent score has been criticized because of inclusion of GVHD as a risk factor without adjusting for inherent statistical bias.66 The inclusion of donor type and source as well as conditioning intensity might further refine transplant-specific prognostic scores.
Recommendations
Choice of transplant candidates and timing of allo-HCT
The panel recommends the use of the CPSS-Mol together with additional patient- and disease-related risk factors to identify transplant candidates, and for the optimal timing of allo-HCT during the course of the disease.
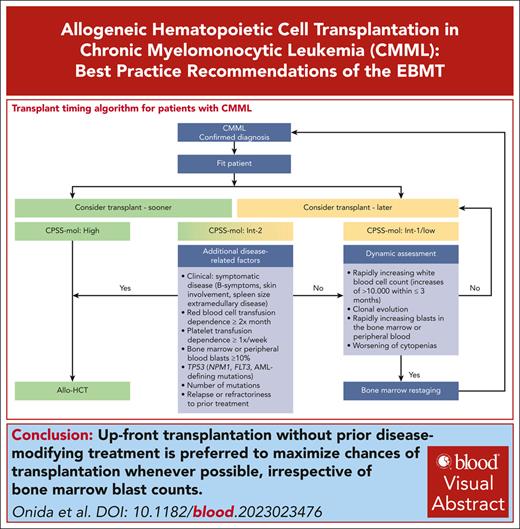
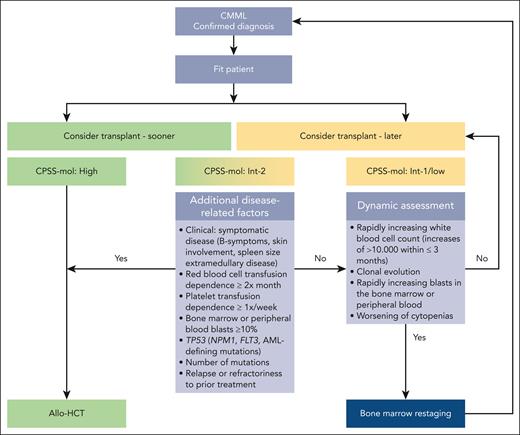
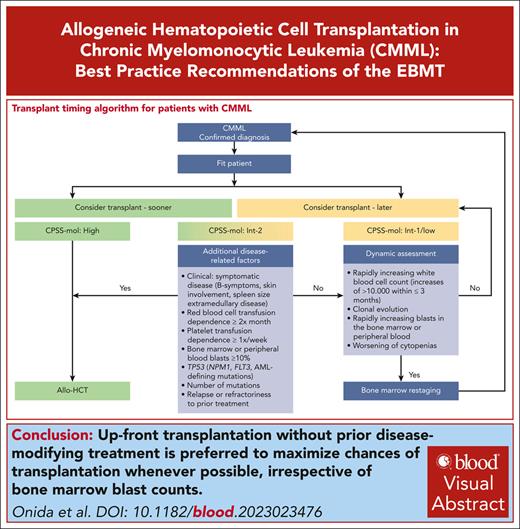
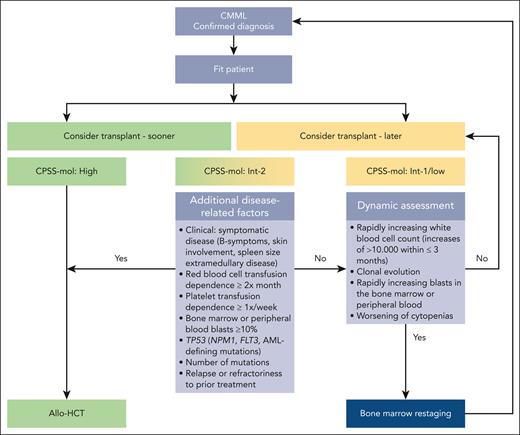
Patients with high-risk CPSS-Mol disease have a median overall survival of 17 to 18 months and a cumulative incidence of transformation to AML at 48 months of 48% to 52% (Table 2).48 The panel recommends that they should proceed to transplant as soon as possible (Figure 2), preferably without disease-modifying treatment to maximize chances of reaching allo-HCT (for the discussion on how the recommendation for upfront transplant was reached please see “Role of pretransplant therapy”).
Patients with intermediate-2 risk CPSS-Mol disease have a median overall survival of 30 to 37 months and a cumulative incidence of transformation to AML at 48 months of 21% to 24% (Table 2).48 The panel recommends that patients with ≥1 additional risk factor should proceed to upfront transplant preferably without disease-modifying treatment to maximize chances of reaching allo-HCT (Figure 2). Such risk factors include extramedullary involvement, (hyper)leukocytosis, iron overload, splenomegaly, as well as adverse cytogenetics and/or gene mutations. Further details on additional disease-related risk factors are discussed in “Additional disease-related risk factors” and “Pretransplant management of disease symptoms.”
Patients with intermediate-2 risk CPSS-Mol disease without additional risk factors: a watch and wait strategy with dynamic assessment should be followed whenever possible. Nontransplant treatment strategies, if deemed necessary, should be discussed with the transplant center. Dynamic reassessment every 3 months, or sooner in case of suspected disease progression, should occur. In the presence of rapidly increasing white blood cell count (WBC; increases of >10 000/μL within ≤3 months) in the absence of signs of active inflammation and or infection, rapidly increasing peripheral blood or bone marrow blasts, and/or worsening of cytopenias, we recommend accurate serial restaging of disease and/or response status, which not only includes bone marrow workups (including cytology, histology, flow cytometry, conventional cytogenetics, fluorescence in situ hybridization, and next generation sequencing)68 but also increasingly relies on analyses of the peripheral blood.69,70 Reclassification and renewed calculation of CPSS-Mol risk should ensue (Figure 2).
Patients with low and intermediate-1 CPSS-Mol disease have a median overall survival of 64 to 68 months and a cumulative incidence of transformation to AML at 48 months of 3% to 8% (Table 2).48 The panel recommends that these patients should be managed with nontransplant approaches. Allo-HCT should be deferred until progression to higher-risk disease and/or the occurrence of ≥1 additional risk factor. To this end, dynamic reassessment should be performed as stated earlier (Figure 2).
Prediction of posttransplant outcomes
Based on aforementioned data, the panel recommends the use of the CMML transplant score54 to predict posttransplant outcomes for patients that have been previously identified as transplant candidates with the CPSS-Mol.
Additional disease-related risk factors
As mentioned above, the presence or emergence of additional risk factors should result in upfront transplantation whenever possible in patients with CPSS-Mol intermediate-2 risk disease, and should result in dynamic reassessment in patients with CPSS-Mol intermediate-1 and low-risk disease (Figure 2; “Choice of transplant candidates and timing of allo-HCT”).
Clinical symptoms
General symptoms (such as drenching night sweats, unintended weight loss, and unexplained fever) are acknowledged signs of higher disease activity and potentially pending/imminent disease progression in many cancer types and may be early signs of transformation to AML. Bone marrow fibrosis adversely affects survival and correlates with higher relapse rates and delayed engraftment in patients with MDS and CMML.72-79 Extramedullary disease with involvement of the skin,80,81 pericardium,82,83 pleura,84-86 kidney,87,88 and other sites89 has been associated with disease acceleration or transformation.90,91 Transfusion dependence, high transfusion burden, hyperleukocytosis,5,92 splenomegaly, and iron overload (which may be confounded by prolonged anemia and complications resulting therefrom) are acknowledged adverse risk factors in CMML and are discussed in “Pretransplant management of disease symptoms.”
Cytogenetics and gene mutations
The genetic landscape and its prognostic relevance have been explored in CMML. Cytogenetic risk stratifications and somatic mutations guide prognosis.5,47,48,93-100 High-risk aberrations are considered an indication for transplant. Not only the type, but potentially also the mutational burden (ie, the total number of mutational abnormalities and their variant allele frequency) may be relevant to prognosis. High overall mutation burden (≥10 mutations), and ≥4 mutated epigenetic regulatory genes have been linked to increased risk of relapse after transplant in patients with CMML.101 However, the spectrum of molecular aberrations in CMML seems to be more restricted than in MDS or AML, and the clinical heterogeneity of the disease is thought to exceed genetic heterogeneity.102 As such, the impact of somatic aberrations may be less straightforward than in MDS or AML.
Although several mutations have already been incorporated into molecular scores (ASXL1, RUNX1, NRAS, and SETBP1),48 their prognostic impact in the transplant setting remains less clear. For example, mutations typically associated with altered risk in the nontransplant setting could not predict post-HCT survival in several studies: ASXL1,5,57,101RUNX1,5,101SRSF2,57 and SETBP1,101 with conflicting results for TET2.5,57,101 In contrast, abnormal karyotype or adverse cytogenetics had an adverse impact on outcomes of patients with CMML who underwent transplants in most,57,63,101,103 but not all5 studies. However, a recent multicenter cohort identified ASXL1 and NRAS as potential strong molecular predictors of posttransplant outcome.54DNMT3A and JAK2 mutations had adverse impacts on posttransplant outcomes of patients with CMML, and the CPSS-Mol was shown to be significantly associated with postallogeneic overall and disease-free survival.53 Presence of mutated NPM1 should result in the diagnosis of AML as per new classifications (see “Classification of CMML”). Presence of FLT3 aberrations in patients with CMML is rare and data is inconclusive regarding prognostic relevance in this disease.104
Presence of TP53 mutations, although very rare in CMML (∼1%-2%),105 had strong multivariate adjusted adverse associations with posttransplant overall survival in patients with MDS106 and CMML.53 Very recently, a prospective clinical trial allocated patients with MDS with high-risk genetics to receive either allo-HCT or non-HCT treatment based on donor availability, demonstrated unequivocally, that allo-HCT after reduced intensity conditioning mediates long-term survival for patients bearing TP53-mutations, as compared to patients with mutated TP53 with non-HCT treatment, and this remained independent of TP53 allelic state and variant allele frequency.45 Thus, although the absolute survival benefit remains modest, presence of TP53 mutations alone should not preclude a patient from consideration for allo-HCT based on TP-53 status alone. The prognostic significance of reducing or clearing the burden of mutations associated with adverse outcomes before allo-HCT needs to be further elucidated, because data on molecular clearance of TP53 mutations with hypomethylating agents (HMAs; azacitidine or decitabine) before allo-HCT in patients with higher-risk MDS are conflicting.45,107 The evolving field of (molecular) prognostic factors in CMML will continue to play a critical role in identifying those patients for whom allo-HCT portends the highest benefits.
Pretransplant management of disease symptoms
Pretransplant management of (hyper)leukocytosis
A recent meta-analysis and several reviews argue against the routine use of leukapheresis for cytoreduction in hyperleukocytotic AML (ie, >100 000 WBCs/μL).108-110 There is no formal demonstration that control of leukocytosis (in the absence of symptoms resulting therefrom), or so-called “blood count cosmetics” has any impact on disease outcome. Neither is there a consensus regarding a target WBC or monocyte count. Hydroxyurea is recommended by the National Comprehensive Cancer Network111 and the ELN112 for cytoreduction before IC in AML. Supportive treatment with allopurinol for the prophylaxis/therapy of tumor lysis syndrome, as well as the transfusion of blood products for the management of disseminated intravascular coagulation, should be given if needed.
Pretransplant management of iron overload
Although high transfusion burden, iron overload, and iron toxicity are known to have an adverse effect on allo-HCT outcomes among patients with MDS (and CMML),113-125 it remains unresolved whether and when iron chelation treatment should be initiated in transplant eligible patients. Elevated serum ferritin levels in the pretransplant setting usually result from red blood cell transfusion, and in some of these patients, the presence of hemostatic iron regulator (HFE) gene mutations may accelerate transfusion-induced iron overload.126 Non–transferrin bound iron and labile plasma iron are toxic in both the pretransplant and the posttransplant setting, and may be elevated because of macrophage iron recycling from transfused red blood cells and iron-release from dying red blood cells during and after myeloablative chemotherapy. Early posttransplant iron toxicity can impair engraftment, delay recovery of anemia, increase the risk of infections because of iron capability to support microorganism growth and to compromise immune cell functions, increase the risk of veno-occlusive disease and/or renal failure, and may aggravate both acute and chronic GVHD. Late posttransplant iron toxicity can result in typical end-organ damage due to accumulation of iron deposits in the heart, liver, pancreas, and other vital organs (as reviewed elsewhere123).
Because retrospective studies in the allo-HCT setting are limited, and prospective studies are lacking, it currently remains unclear whether pretransplant, peritransplant, and/or posttransplant iron chelation therapy is most beneficial for patients. Phlebotomy and deferoxamine are not indicated in CMML because of obvious reasons, and deferiprone may result in agranulocytosis. Hence, deferasirox remains the only viable option for patients with CMML.
Several prospective and retrospective studies indicate that treatment with deferasirox in transplant recipients is feasible and associated with improved engraftment, hematologic recovery, and potentially also longer relapse-free and/or overall survival.127-132 A prospective noninterventional study of 222 patients with MDS or CMML indicates that iron reduction therapy (with either iron chelation therapies or phlebotomies) started within 6 months after allo-HCT resulted in significant improvement of relapse-free survival.116
Pretransplant management of splenomegaly
Although splenomegaly is frequently observed in CMML, it is often limited and manageable without specific treatment in CMML. However, some patients do present with massive splenomegaly. In these cases, the need for splenectomy, splenic irradiation, or other means of reducing spleen size before transplant remains debated. Splenomegaly before transplant is a well-recognized risk factor known to adversely affect transplant outcomes in patients with MDS/ myeloproliferative neoplasm,133 and is also associated with delayed neutrophil and platelet engraftment as well as higher nonrelapse mortality. In patients with myelofibrosis, splenectomy was shown to improve neutrophil and platelet recovery (but did not result in longer overall survival) when compared with patients who received either splenic irradiation or no treatment for splenomegaly.134-136 Patients without splenectomy have delayed hematopoietic recovery, but spleen size does recede eventually after transplant in cases of adequate donor engraftment.137 Thus, perioperative morbidity (43%) and mortality (13%) rates of splenectomy in patients with CMML138 need to be weighed carefully. Splenic irradiation before transplant may be considered as an alternative139 but can be associated with severe and protracted pancytopenia. Therefore, if performed, splenic irradiation should possibly be used as an adjunct to conditioning so that ensuing pancytopenia may be rescued by donor engraftment.140JAK2 inhibitors may offer an alternative approach.141-146
Recommendations
All transplant-eligible patients with CMML should be considered for iron chelation therapy pretransplant, peritransplant, and posttransplant when serum ferritin levels exceed 1000 μg/L; secondary causes of hyperferritinemia have been excluded; and in the absence of contraindications (eg, elevated renal function parameters). In patients receiving upfront transplantations, iron reduction therapy is preferred in the posttransplant setting to avoid potential additional pretransplant toxicity.
In the absence of clinical sequelae, hydroxyurea-based cytoreduction is only recommended ≤6 weeks before transplant. We suggest an empiric target of <10 000 WBCs/μL, based on experience, rather than evidence.
For patients with massively enlarged spleen (ie, >20 cm below the costal margin), splenectomy, splenic irradiation, or reduction of spleen size with JAK inhibitors is recommended. A unified coordinated approach needs to be orchestrated between the treating physician and the transplant center.
Role of pretransplant therapy
Role of debulking strategies in CMML
It remains unclear whether debulking, that is, reduction of disease burden as typically measured by reduction of bone marrow blasts to <2%,54 <5% to 10%, and/or complete remission (CR) status is advantageous for allo-HCT outcomes in patients with MDS7,8,10,27,30,147-164 or CMML.8,27,59,101,165-170 It remains unknown and unexplored in both MDS and CMML, whether patients who achieved CR without minimal residual disease (MRD) negativity, might benefit from bridging treatment before allo-HCT.171 Reducing disease burden before transplant may also be more relevant in the reduced-intensity conditioning setting.152
Prospective data on the optimal pretransplant strategy for patients with CMML identified as allo-HCT candidates are lacking. Whereas the role of allo-HCT in CMML is established, the sequence of pretransplant treatment, or whether to treat the patient before allo-HCT at all, is not. Several retrospective analyses compared pretransplant treatment with HMAs vs AML induction-type IC in patients with MDS or CMML, showing either no difference,57,62,155-157,170 or an advantage with HMAs for all patients,5,10,113,150,162,172-174 or in subgroups with higher-risk disease,157 >5% bone marrow blast count at diagnosis,155 or older patients.175 Retrospective analyses comparing HMAs vs best supportive care12,113,149,176-178 before transplant in MDS could not observe a clear beneficial (or adverse) effect for HMAs. Similarly, neither an improvement in relapse-free or overall survival, nor an additional risk of nonrelapse mortality was shown in patients treated with decitabine10,155,163,177,179 or azacitidine in a pretransplant setting in patients with MDS (as compared with either best supportive care or IC) in single retrospective studies,10,12,149,150,155-157,162,172,173,176 a prospective phase 2 clinical trial,8 as well as a meta-analysis published in 2019175 collating data from 6 retrospective studies.150,155-157,172,176 It must be kept in mind, that most (but not all, eg, Kroger et al,7 Voso et al,8 Lindholm et al,9 Yahng et al,10 and Nakamura et al12) of these studies captured patients who did not proceed to transplant, which is a relevant caveat, because it remains obscure how many of them failed to proceed to transplant because of disease progression or pretransplant treatment-related mortality. With regard to patients with CMML, a recent phase 3 trial remains the only evidence of higher rates of death without progression or transformation for decitabine vs hydroxyurea, albeit these data need to be interpreted with caution because most deaths occurred after study exit.13
Randomization at the start of pretransplant therapy and the inclusion of a best-supportive-care arm would be needed to identify whether any disease-modifying treatment is required before allo-HCT.
Although prospective randomized data are lacking, the acceptable toxicity of HMAs combined with their potential for cytoreduction and disease stabilization (which may provide time for patients to reach transplant) led several experts to recommend HMAs as pretransplant treatment for patients with MDS5,8,28,71,162,175,176,180-182 or CMML.174 However, many transplant specialists, including the American Society for Transplantation and Cellular Therapy Committee on Practice Guidelines,183 as well as this panel, consider the evidence (with regard to CMML) not to be clear enough to support this conclusion. Thus, the use of HMAs in the pretransplant setting remains controversial.
One of the main concerns of this panel regarding pretransplant treatment is that up to 13% to 36% of patients with MDS who started HMAs and for whom a transplant was intended could not proceed to transplant because of disease progression, drug-related adverse events, or new comorbidities.7-12 Similarly, in patients with CMML, death without progression or transformation was significantly higher with decitabine,13 underlining that the primary goal should be to bring eligible patients to transplant in a good general condition and that achieving CR before transplant may be of subordinate importance. Thus, pre-HCT debulking strategies (be it with HMAs or IC) may be a double-edged sword,184 potentially resulting in worsening cytopenias, increased transfusion dependence with ensuing complications such as iron overload or alloimmunization,185 and/or infections that may preclude proceeding to transplant. Hence, this panel recommends that, once a patient has been identified as an allo-HCT candidate, upfront transplantation without prior disease-modifying treatment is preferred, in order to maximize chances of reaching allo-HCT whenever possible, irrespective of mere bone marrow blast counts of 10% to 19%. However, in cases of aggressive disease with kinetics indicating rapid disease progression and/or severe clinical symptoms requiring immediate alleviation, bridging therapy with HMA (possibly in combination with off-label use of venetoclax) may be considered, as long as this does not postpone, or reduce the patients’ chances of, receiving curative treatment and should ideally be studied within clinical trials.
Optimal choice of pretransplant treatment in CMML
A large retrospective analysis demonstrated multivariable-adjusted overall survival and time to next treatment to be significantly longer with the use of HMAs as compared with IC in patients with higher-risk CMML (n = 949).71 We consider the existing evidence to be strong enough to no longer support an indication for conventional IC in any setting in this disease. Patients with newly diagnosed high-risk/secondary AML had significantly longer posttransplant survival and lower early mortality when treated with CPX-351 as opposed to IC with “7 + 3.”186 Data on the CPX-351 (liposomal formulation of cytarabine and daunoribicin) for higher-risk CMML and AML secondary to CMML are available for a handful of patients only from small phase 1/2 clinical trials performed in both the first-line187 and second-line settings,188 indicating that the drug may be safe, and it allowed for bridging to allo-HCT in selected (1 of 6187) patients with CMML. Five of 5 patients with CMML receiving CPX-351 as first-line treatment responded,187 whereas only 1 of 6 patients with CMML receiving the drug after HMA failure achieved a response and could proceed to allo-HCT.188 Given the very small numbers of patients with CMML treated with CPX-351, the efficacy of the drug needs to be further studied in this disease. Novel debulking strategies are needed. Azacitidine plus venetoclax by itself189-193 or possibly in the future as a backbone for potential triplet combinations incorporating newer substances (eg, CD123 targeting with tagraxofusp or flotetuzumab) may well be the way forward. Data from early phase clinical trials evaluating azacytidine with venetoclax in patients with MDS in the first-line194 or relapsed refractory setting195 are starting to emerge, as are data on the use of the combination as bridging to allo-HCT in patients with high-risk MDS and AML.196-198 Clinical trials incorporating venetoclax in conditioning regimen (eg, clinicaltrials.gov identifier: NCT05005299, NCT05823714, NCT05807932, and NCT03613532) or as bridging (eg, clinicaltrials.gov identifier: NCT04476199 and NCT04904237) to allo-HCT in patients with MDS and AML are currently underway, and patients with CMML can be included in some of them (eg, clinicaltrials.gov identifier: NCT05807932 and NCT03613532). Because venetoclax in combination with HMA leads to much higher rates of CR (without necessarily translating into longer overall survival),199 which are achieved more rapidly than with HMA alone, data emerging in patients with CMML who proceeded to allo-HCT will have to be reviewed carefully and may possibly result in a future alteration of this panel’s current recommendation. We acknowledge, and perhaps anticipate, that venetoclax with an HMA may be an ideal bridging therapy for patients with CMML (and probably also MDS or AML) with high blast counts and/or proliferative disease and/or other signs of rapid disease kinetics.
Optimal timing of allo-HCT in patients who are pretreated
Both retrospective92,200 and prospective178 data indicate that it is significantly better to proceed to allo-HCT while patients are responding to HMAs rather than to wait for treatment failure.165,200 The primary goal may be to bring patients eligible for allo-HCT in a good general condition, or to render patients who were initially transplant ineligible into a transplant-eligible state, whereas achieving CR before transplant may be of subordinate importance. As outcome after HMA-failure is mostly dismal (<6 months), patients should receive transplantation after achieving the best possible response. In patients with CMML treated with azacytidine the median time to first and best response is 4 (interquartile range, 2-5) and 5 (interquartile range, 3-7) cycles, respectively.70,201,202 Approximately onethird of patients experience further deepening of response after first response, with the median time from first response to best response being 3 to 4 cycles.201,203 Best outcomes in the HMA-relapsed/refractory setting were observed for patients with MDS able to receive allo-HCT,204,205 which explains why this procedure should be offered when possible.
Recommendations
All patients should be included within clinical trials whenever available and possible.
Once a patient has been identified to be a transplant candidate, we support an upfront transplantation as soon as a suitable donor is available, without any disease-modifying pretreatment for all patients with CMML, whenever possible, regardless of the bone marrow blast count. Timely referral to a transplant center is essential.
In the rare cases in which pretransplant treatment is unavoidable (eg, no matching donor available), we recommend the use of HMAs, and no longer see any indication for the use of IC in patients with CMML.71
Upfront transplantation without prior treatment is preferred and recommended whenever possible. In cases in which front-line treatment (most often with HMAs) may have been commenced (because of immediate need of treatment of severe clinical symptoms and/or aggressive disease with kinetics indicating rapid disease progression), allo-HCT should be performed after establishing the best possible response status, which is achieved after ≤7 cycles of HMAs in 75% of patients with CMML,70,201,202 provided the patient remains transplant eligible. The patient should not continue the treatment until loss of response, or when disease relapse or progression occur. To this end, we recommend close monitoring and performing of a bone marrow evaluation as soon as response is suspected from amelioration of peripheral blood values, for example, every 2 cycles, with the aim of not subjecting the patient to unnecessary delays in the transplant, and not to lengthen the period at risk for losing, and, most importantly, to avoid loss of, transplant eligibility.
If relapse after any treatment has already occurred, allo-HCT should nevertheless always be considered for eligible patients, because this remains the best option for these patients.
Value-based discussions between treating physicians, transplant centers, and patients as to the appropriateness of the procedure are merited in those instances, where the expected benefit from allo-HCT remains modest, eg, when mutations associated with adverse risk, complex or monosomal karyotype are present and any of the following factors are additionally present: age >70 years, comorbidities, and/or other variables adversely influencing prognosis and transplant outcomes (eg, iron overload, bone marrow fibrosis, therapy-related disease). Possibly, HMAs might be of use (as bridging strategy or instead of allo-HCT) for selected patients aged >60 years,175 with TP53 mutations and/or with complex or monosomal karyotoypes.45,206
Donor selection
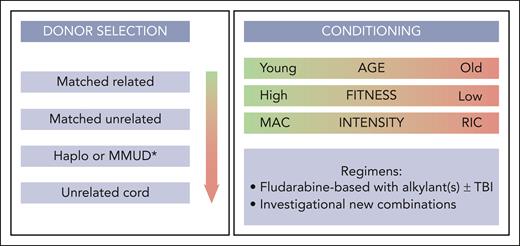
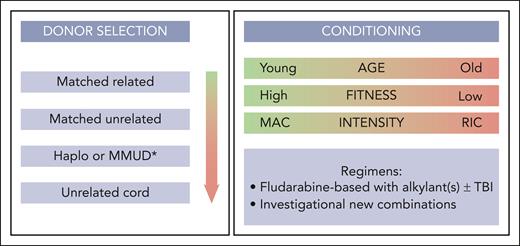
Potential stem cell donors include standard donors (such as HLA-matched siblings and matched unrelated donors), alternative stem cell donors including haploidentical or mismatched unrelated donors, and (less frequently) unrelated umbilical cord donors. Selection of stem cell donors for patients with CMML has improved markedly during the last 2 decades, similar to what has been observed for allo-HCT in other indications. Several studies found differences in absolute survival and relapse rates between HLA-identical sibling and matched unrelated donors, whereas some showed no significant difference after multivariable adjustment.53,54,165,207 The expert panel agreed to recommend as standard donors (starting with highest preference): HLA-identical siblings, followed by matched unrelated donors (Figure 3). Recent studies in the MDS setting found higher disease-free survival and lower relapse for allo-HCT with younger matched unrelated donors compared with older HLA-identical siblings.208 Another large EBMT study found an independent effect of cytomegalovirus serostatus of donors (although this study included patients mostly from the preletermovir era).207 Therefore, the expert panel agreed to take age and cytomegalovirus status of donors into account when balancing the risk for nonrelapse mortality vs relapse during the donor selection process, and to follow the previously formulated donor suitability criteria.209
Transplant modalities. ∗According to center preference. MAC, myeloablative conditioning; MMUD, mismatched unrelated donor; RIC, reduced intensity conditioning; TBI, total body irradiation.
Transplant modalities. ∗According to center preference. MAC, myeloablative conditioning; MMUD, mismatched unrelated donor; RIC, reduced intensity conditioning; TBI, total body irradiation.
Alternative donor transplants may be considered for patients with higher-risk disease and fit patients, for whom no matched sibling or unrelated donor can be identified within a reasonable search period. Unrelated cord blood transplants showed very poor results in CMML,103 and should therefore be carefully used, in case no other suitable donor is available.
Recommendations
Currently, there are no systematic comparative studies between haploidentical transplants and mismatched unrelated donors, and the panel agreed to use either of them, considering access, timing, and other suitability criteria. For haploidentical donor allo-HCT, based on previous reports in other diseases,210 posttransplant cyclophosphamide may be the preferred platform.
Stem cell source
Limited data are available on transplant outcomes in CMML according to stem cell source. The panel agreed that peripheral blood is the recommended hematopoietic stem cell source for HLA matched sibling and unrelated donor transplants. No data exist on the preferred source for haploidentical transplants. Higher doses of CD34+ cells, if possible, might be targeted for unrelated donor transplants.211 However, because of lack of data, a preferred stem cell dose cannot be recommended for any transplant modality.
Conditioning intensity
Choosing the right conditioning intensity and regimen is a cornerstone of allo-HCT, balancing the risk of increased nonrelapse mortality when choosing more intensive treatment compared with increased risk for relapse when choosing less intensive regimens. The expert panel defined the various preparatory intensities according to the classification used by several previous studies (mostly in the setting of MDS).212 Most retrospective studies in patients with CMML and MDS report equivocal outcome after commonly used myeloablative or reduced intensity conditioning regimens.52,165,213,214 Therefore, the panel agreed that there is currently no superiority of 1 intensity over another. However, it can be extrapolated from clinical practice that posttransplant relapse in CMML appears to be more frequent than in MDS (with the largest retrospective series reporting relapse rates in the 27%-to-52% range57,62 and ∼80% of patients experience relapse within the first year from allo-HCT). Thus, if patients are fit enough to undergo more intensive treatment, myeloablative conditioning should be preferred (Figure 3).
If myeloablative conditioning is not feasible, combination of fludarabine and busulfan appeared to be associated with best outcomes across diseases in MDS and myeloproliferative neoplasm and can therefore also be considered in CMML, with or without total-body irradiation.214-217 Recent increased adoption of treosulfan-based regimens within a reduced-toxicity approach showed promising results in other indication.166,218 However, there is no direct evidence to favor a particular regimen over another. There is currently no evidence for the association of disease and mutation burden or the threshold of MRD with the choice for conditioning intensity or regimen.
Recommendations
The expert panel recommended not to adopt pretreatment decisions based on intensity of planned conditioning but to focus on posttransplant strategies, including chimerism and/or MRD monitoring, as well as to prevent relapse (see “Posttransplant management” in supplemental pp2-5).
Acknowledgments
The European Society for Blood and Marrow Transplantation (EBMT) is a not-for-profit medical and scientific organization. The EBMT performed administrative and legal management, as well as funding acquisition. No pharmaceutical company and no other funding source were involved in any way or had a role in the study design, data collection, data analysis, data interpretation, or writing of the report. No medical writer or editor was involved.
Authorship
Contribution: F.O., N.G., Y.C., G.K., M.R., A.S., I.Y.-A., and L.P. were responsible for literature research, and interpretation of data, conceived and designed the manuscript topics to be covered as well as the recommendations to be made, and conception of figures; F.O. was responsible for writing of the “Introduction” and “Unanswered questions”; L.P. was responsible for writing sections “Patient selection for allo-HCT,” “Timing of allo-HCT,” “Pretransplant management of disease symptoms,” and “Role of pretransplant therapy” and design of figures and tables; N.G. was responsible for writing “Donor selection,” “Stem cell source,” “Conditioning intensity,” and the supplemental Data; I.Y.-A. was responsible for writing of the abstract; F.O., R.G., I.S.-O., and I.Y.-A. were responsible for defining the EBMT Practice Harmonization and Guidelines according to which this manuscript was written; all authors played an important role in interpreting results, revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and F.O., N.G., Y.C., G.K., M.R., A.S., I.Y.-A., and L.P. accept final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: F.O. received honoraria from Takeda, MEDAC Pharma, and Kyowa Kirin; and received travel support from Takeda, Jazz Pharma, and Kyowa Kirin. Y.C. received honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, Incyte, Jazz, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, Servier, and Takeda; and received travel support from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, Incyte, Jazz, MSD, Novartis, Pfizer, Roche, and Sanofi. G.K. received research support from Bristol Myers Squibb/Celgene, Amgen, AbbVie, Medac, and Eurocept; received honoraria from Novartis, MSD, Pfizer, Amgen, Gilead, Bristol Myers Squibb/Celgene, AbbVie, Biotest, Takeda, and Eurocept; and received travel support from Medac, Gilead, and Neovii. M.R. received research support from AbbVie, Astex, Medac, Neovii, and Novartis; and received travel support from Medac and Jazz. A.S. received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Incyte/Genesis, Gilead, Sanofy, and Servier; and received travel support from AbbVie, Bristol Myers Squibb, Roche, Servier, and Takeda. R.I. received research support from AbbVie; received honoraria from AbbVie, Bristol Myers Squibb/Celgene, CTI Biopharma, Gilead, Jazz, Novartis, and Servier; and received travel support from AbbVie and Gilead/Kite. M.J. received honoraria from AbbVie, Bristol Myers Squibb/Celgene, Novartis, JAZZ Pharmaceuticals, and Pfizer; and received travel support from AbbVie, Bristol Myers Squibb/Celgene, and Pfizer. U.P. received research support from Bristol Myers Squibb, Curis, Jazz, Amgen, Bergenbio, Janssen, and Novartis; and received honoraria from Bristol Myers Squibb, Curis, Jazz, Amgen, Bergenbio, Janssen, and Novartis. V.S. received honoraria from AbbVie, Bristol Myers Squibb/Celgene, CTI Biopharma, Curis, Geron, Gilead, Novartis, Otsuka Pharmaceutical Co, Servier, and Syros; and received travel support from Janssen. G.S. received research funding from Celgene; received honoraria from AbbVie, Bristol Myers Squibb/Celgene, ExCellThera, Novartis, Roche, and Takeda; and received travel support from BeiGene and Celgene. C.S. received research support from Janssen, Novartis, and Takeda; received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Novartis, Roche, Sanofi, and Takeda; and received travel support from GlaxoSmithKline, Janssen, and Sanofi. E.S. received research support from Fondation Amgen; and received honoraria from Blueprint and Novartis. P.V. received research support from AOP Orphan and Pfizer; and received honoraria from Blueprint, Bristol Myers Squibb/Celgene, Cogent, Incyte, Novartis, Pfizer, Servier, and Stemline. R.G. received honoraria from Biotest, Pfizer, Medac, and Magenta. I.Y.-A. received honoraria from Bristol Myers Squibb, Novartis, Janssen, Kite/Gilead, and Biotest. L.P. received honoraria from AbbVie, Bristol Myers Squibb/Celgene, Novartis, and Otsuka Pharmaceutical Co; and received travel support from AbbVie, AstraZeneca, BeiGene, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Lisa Pleyer, 3rd Medical Department, Paracelsus Medical University Hospital, Müllnerhauptstr 48, 5020 Salzburg, Austria; email: dr.lisa.pleyer@gmail.com.
References
Author notes
The online version of this article contains a data supplement.