High-risk, untreated transplant-associated thrombotic microangiopathy after HCT has a dismal outcome due to multi-organ dysfunction.
Early therapy with the C5 blocker eculizumab significantly improved outcomes in patient with high-risk TA-TMA and attenuated organ dysfunction.
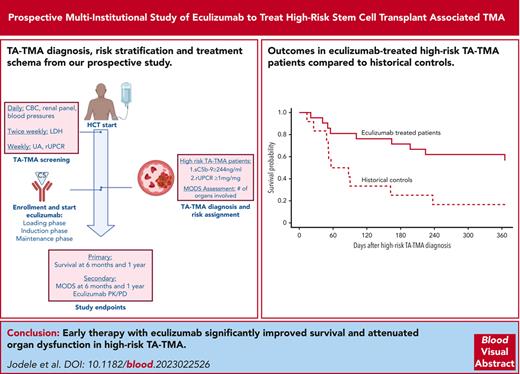
Visual Abstract
High-risk, complement mediated, untreated transplant-associated thrombotic microangiopathy (hrTMA) has dismal outcomes due to multi-organ dysfunction syndrome (MODS). The complement C5 blocker eculizumab shows promising results in hrTMA, but has not been prospectively studied in hematopoietic stem cell transplant (HCT) recipients. We performed the first multi-institutional prospective study in children and young adults to evaluate eculizumab as an early targeted intervention for hrTMA/MODS. We hypothesized that eculizumab would more than double survival in HCT recipients with hrTMA, compared to our prior study of prospectively screened, untreated hrTMAs serving as historical controls. HrTMA features (elevated terminal complement (sC5b-9) and proteinuria measured by random urine protein/creatinine ratio (≥1mg/mg)) were required for inclusion. The primary endpoint was survival at 6 six-months from hrTMA diagnosis. Secondary endpoints were cumulative incidence of MODS 6 months after hrTMA diagnosis and 1-year posttransplant survival. Eculizumab dosing included intensive loading, induction, and maintenance phases for up to 24 weeks of therapy. All 21 evaluated study subjects had MODS. Primary and secondary study endpoints were met by demonstrating survival of 71% (P < .0001) 6 months after hrTMA diagnosis and 62% 1 year after transplant. Of fifteen survivors, 11 (73%) fully recovered organ function and are well. Our study demonstrates significant improvement in survival and recovery of organ function in hrTMA using an intensified eculizumab dosing and real time biomarker monitoring. This study serves as a benchmark for planning future studies that should focus on preventative measures or targeted therapy to be initiated prior to organ injury. This trial was registered at www.clinicaltrials.gov as #NCT03518203.
Introduction
Hematopoietic stem cell transplantation (HCT)–associated thrombotic microangiopathy (TA-TMA) occurs in 20% to 30% of children and young adults undergoing transplantation, and approximately half of these patients develop multiorgan dysfunction syndrome (MODS) that is commonly lethal if not treated promptly.1-3 HCT recipients with high-risk TA-TMA (subsequently referred to as hrTMA), presenting with both high-risk disease markers (soluble terminal complement complex [sC5b-9] activation [elevated blood sC5b-9]) and nephrotic range proteinuria measured by random urine protein–to–creatine ratio (rUPCR), have dismal outcomes, typically because of MODS.4,5 TA-TMA affects the endothelium of small vessels leading to MODS, which can present as kidney failure, pulmonary hypertension with cardiac failure and lung injury, polyserositis, and bowel ischemia, sometimes with necrosis. Retrospective literature consistently reports >80% to 90% mortality with hrTMA/MODS, with most patients dying within 6 months of TA-TMA diagnosis.6-10 In our earlier prospective observational study of 100 consecutive HCT recipients screened for TA-TMA using the same diagnostic and risk stratification criteria (further referred as historical control), we reported 9% overall survival in patients with hrTMA/MODS (meeting both sC5b-9 and rUPCR criteria) who did not receive TMA-targeted therapy, with only 18% surviving by 6 months from hrTMA diagnosis.4
Recent clinical and laboratory studies of TA-TMA have provided new insights into the pathogenesis and genetic susceptibility of TA-TMA, raising awareness of this important HCT complication and allowing for the identification of novel therapeutic targets.11 Specifically, many patients with hrTMA develop MODS because of tissue injury as a consequence of endothelial damage mediated by complement activation.12-15 This knowledge has led to investigation of complement blockade using eculizumab as a targeted TA-TMA therapy.3 Eculizumab is a monoclonal antibody that specifically binds the complement protein C5, inhibiting cleavage to C5a and C5b, preventing generation of the tissue-injuring terminal complement complex C5b-9. Preliminary experience using eculizumab for hrTMA in selected academic centers showed significant improvement in survival, but this treatment needs to be studied in a systematic, prospective manner.3
We performed a prospective, multi-institutional study using eculizumab to treat hrTMA at 3 pediatric HCT centers that screen for TA-TMA. The goal of the study was to, at least, double our historical survival by early targeted intervention. The eculizumab dosing regimen was specifically designed for the HCT population using our previously published drug pharmacokinetic and pharmacodynamic (PK/PD) strategy.16 Here, we present study results demonstrating significant improvement in outcomes in patients with hrTMA treated with eculizumab as compared with patients with hrTMA from our prospective observational study who were not receiving any targeted complement-blocking therapy.
Methods
Study design
A prospective, single arm, multi-institution study was opened at 3 large pediatric HCT centers: Cincinnati Children’s Hospital Medical Center, Children’s Hospital of Los Angeles, and Children’s Hospital of Philadelphia. The study goal was to examine early intervention with eculizumab to treat patients with hrTMA with MODS. The study was performed under Investigational New Drug Application (IND) (number 136738) and registered at www.clinicaltrials.gov as #NCT03518203. Because we documented >80% mortality in a hrTMA group that was prospectively screened but did not receive any targeted therapy, we considered a randomized study including untreated controls not ethically feasible.4
Inclusion criteria were children and young adults of any age, of at least 5 kg of weight, undergoing allogeneic or autologous HCT, with a histologic or clinical diagnosis of hrTMA. Exclusion criteria included known hypersensitivity to the study drug, unresolved serious Neisseria meningitidis infection or progressive severe infection, and previous therapy with any complement blocker. The institutional review boards approved the protocol, and all participants provided written, informed consent, and assent, as appropriate. The study drug eculizumab was provided by Alexion Pharmaceuticals.
We hypothesized that early intervention with the complement blocker eculizumab would double survival in HCT recipients with hrTMA, as compared with our prospectively screened untreated historical controls, reported to have a survival of 18% 6 months after hrTMA diagnosis. Demographics and disease characteristics of historical controls are presented in supplemental Table 1, available on the Blood website.4 The primary end point was survival 6 months after hrTMA diagnosis. Secondary end points were (1) cumulative incidence of MODS 6 months after hrTMA diagnosis and 1-year after HCT, (2) 1-year nonrelapse mortality compared with historical controls, and (3) eculizumab PK/PD assessment in real time.
TA-TMA diagnosis, risk stratification, MODS definitions, and response assessment
Participating centers prospectively screened all HCT recipients for TA-TMA as part of their clinical care, as previously described, starting with pretransplantation baseline to at least 100 days after transplantation.1 TA-TMA was diagnosed using criteria published by Jodele et al, meeting ≥4 of 7 criteria listed in Table 1.4,5 Eligibility criteria required both hrTMA features: elevated plasma sC5b-9 more than the laboratory normal value (≥244 ng/mL) and clinically significant proteinuria measured as a rUPCR of ≥1 mg/mg (or receiving renal replacement therapy [RRT] in patients with anuria). Organ injury was assessed using the published MODS definitions by Jodele et al listed in Table 2.3,5 Intestinal TA-TMA was diagnosed by criteria proposed by El-Bietar et al, as listed in Table 2.17-19 Study participants with any clinical evidence of lower intestinal bleeding were marked as having clinically significant bleeding, and were assigned to the group of patients with bleeding for subanalysis, because we have previously reported that outcomes are inferior in patients with TA-TMA with gastrointestinal (GI) bleeding.3
In addition, we opted to assess TA-TMA–targeted therapy response. Complete response (CR) was defined as resolution of both high-risk markers (normalized sC5b-9 and rUPCR resolved to <1 mg/mg), no red blood cell and platelet transfusions for at least 14 days (Table 1), and resolution of MODS by study definitions (Table 2). Study participants were classified as having no response (NR) if they did not achieve resolution of both hrTMA markers, were receiving transfusion support, did not recover vital organ function after completing eculizumab therapy or died with active TA-TMA. Partial response was assigned to those who did not meet CR or NR criteria.
Eculizumab treatment plan and monitoring
The study treatment regimen included up to 24 weeks of eculizumab therapy consisting of loading, induction, and maintenance phases (Figure 1). Study dosing was proposed based on eculizumab PK/PD data, as previously reported.16 Intensive loading dosing was included based on prior evidence of rapid eculizumab clearance in patients who received HCT who had complement-mediated hrTMA during the first 2 weeks of complement-blocking therapy, likely due to high levels of ligand.20
Study schema. Figure displays overall study schema. Survival at 6 months from hrTMA diagnosis and at 1 year after HCT. LDH, lactate dehydrogenase; UA, urinalysis.
Study schema. Figure displays overall study schema. Survival at 6 months from hrTMA diagnosis and at 1 year after HCT. LDH, lactate dehydrogenase; UA, urinalysis.
Eculizumab loading and induction doses were weight-based: 300 mg per dose for patients with a weight of 5 to 9 kg, 600 mg per dose for those with a weight of 10 to 39 kg, and 900 mg per dose for those with a weight of ≥40 kg. Eculizumab was administered as an intravenous infusion, over 60 minutes. All patients received 5 eculizumab loading doses (every 48 hours × 2, and every 72 hours × 3). Induction doses were then given weekly × 4 with the goal of maintaining therapeutic eculizumab serum concentrations of 100 μg/mL and sustaining normal sC5b-9 (<244 ng/mL) and CH50 suppressed at <10% of the lower normal value, documenting adequate complement blockade in blood. If these goals were met, weight–based maintenance dosing was then continued every 14 days, as per the eculizumab drug label. Eculizumab drug levels, CH50, and sC5b-9 were monitored daily for the first 2 weeks; and then eculizumab drug level and CH50 were monitored before each dose and sC5b-9 was measured twice a week. Centralized drug level, CH50, and sC5b-9 testing was performed at Cincinnati Children’s Hospital Medical Center and results reported back immediately to the institutional principal investigators (PIs) via a Health Insurance Portability and Accountability Act–protected electronic laboratory portal system. The study allowed dosing interval modification if subtherapeutic eculizumab drug levels and/or inadequate complement suppression was documented. Any dose modification was discussed with the lead PI (S.J.) and the reason to modify doses was documented. Patients receiving therapeutic plasma infusion or plasma exchange received supplemental eculizumab doses, as indicated on the drug label. Early termination of eculizumab therapy was allowed if hrTMA was already resolved and additional eculizumab doses were not of benefit as deemed by the treating physician. All study participants were started on antimicrobial prophylaxis to cover N meningitidis because vaccination is known to be ineffective during the early posttransplant period. Antibiotic prophylaxis was continued until eculizumab clearance was documented (serum drug concentration of <11 μg/mL) and CH50 recovered to normal levels, demonstrating normal complement activity in the blood.3
Study participants were monitored for adverse events using the Common Terminology Criteria for Adverse Events version 5.0 through 60 days after the last eculizumab infusion. TA-TMA and MODS assessments were completed weekly by the institutional PI through 60 days after the last eculizumab infusion, then at 6 months after hrTMA diagnosis and 1 year after HCT. Study data were logged into a study-specific REDCap database. All study participants received routine supportive care according to institutional guidelines without any restrictions.
Statistical analyses
This prospective single-arm study was designed to test the null hypothesis of 18% 6-months survival from hrTMA diagnosis in patients at high risk. The level of significance was set at .05. The primary end point was survival 6 months after hrTMA diagnosis and was assessed using a 1-sided exact binomial distribution test. The sample size of 21 was determined to have a >80% power of yielding a significant P value, assuming the 6-month survival rate is 45% for patients with hrTMA who received treatment.
A priori criteria for patients to be evaluable for analysis were established before the start of the study. Children who relapsed with malignancy before 6 months on the study or received <4 doses of therapy, were considered unevaluable and were replaced, based on previous data showing that it takes 11 to 13 days to control complement activation with a therapeutic eculizumab trough level (≥100 μg/mL) in patients with hrTMA before clinical response can be anticipated.21 Of a total of 23 patients enrolled, 2 unevaluable patients were replaced to ensure 21 evaluable patients for the primary analysis. Statistical analyses for secondary end points are descriptive.
Results
Study population
In total, 23 patients were enrolled in the study. Overall, 2 cases were unevaluable per a priori criteria: 1 patient relapsed with leukemia during the study and remains alive with resolved hrTMA; another patient died from adenoviral pneumonia (autopsy proven) before receiving 4 drug doses. These 2 participants were replaced to ensure that 21 recipients of HCT with hrTMA were evaluated for primary and secondary study end points.
Study demographics and disease characteristics for the 21 evaluable participants are summarized in Table 3. Most of the study population were Caucasian children aged <18 years undergoing their first HCT. One-third of study participants were undergoing their second or third HCT. There was a similar distribution of malignant (n = 11) and nonmalignant diagnoses (n = 10). Five children received autologous transplantation including 4 receiving a carboplatin, etoposide, and melphalan chemotherapy regimen for treatment of neuroblastoma, a recognized high-risk regimen for endothelial injury.1,5,22 Most recipients of allogeneic HCT (68.8%) received fully matched stem cell grafts after a myeloablative preparative regimen (71.4%) and calcineurin inhibitor (CNI)–based graft-versus-host disease (GVHD) prophylaxis (75%). All study participants had normal kidney function, no proteinuria, and normal sC5b-9 before HCT.
hrTMA and MODS presentation
The median time to hrTMA diagnosis was 22 days after HCT (interquartile range [IQR], 10-56). Eleven patients met all 7 TA-TMA diagnostic criteria, 8 met 6 criteria, and2 met 5 diagnostic criteria. Both high-risk biomarkers, elevated sC5b-9 and rUPCR of >1 mg/mg, were required for inclusion, and, thus, were present in all participants. Median rUPCR at study entry was 3.4 mg/mg (IQR, 1.6-5.2). Median maximum rUPCR during study was 6.3 mg/mg (IQR, 4.8-15). Median sC5b-9 at study entry was 367ng/mL (IQR, 277-479; normal <244 ng/mL) with a median increase in sC5b-9 of 3.1 times compared with the pre-HCT baseline value (IQR, 2.4-3.8 times). Lactate dehydrogenase elevation was a median of 1.3 times (IQR, 1.2-1.8) the upper limit of normal for age. Seven patients also had a histologic diagnosis of intestinal TA-TMA. No other organs were biopsied at hrTMA diagnosis. Median ADAMTS13 activity was 69% (IQR, 60-91) at study entry. All participants experienced MODS, with a median of 4 (IQR, 3-6) organs involved. Thirteen patients (62%) required intensive care for a median of 35 days (IQR, 6-48). Median hospitalization days was 71 (IQR, 41-112).
Cystatin-C–estimated glomerular filtration rate (GFR) was performed weekly, because we have previously shown that serum creatinine is a less reliable indicator of acute kidney injury (AKI) in pediatric recipients of HCT.23-25 AKI was defined as a decline in cystatin-C GFR by 50% from pretransplant baseline. All study participants were diagnosed with AKI, with a median lowest cystatin-C GFR of 38 mL/min per 1.73 m2 (IQR, 29-55). Serum creatinine elevation at hrTMA diagnosis was a median 1.1 times the pre-HCT baseline (IQR, 1-1.8). Six patients (29%) required RRT, including continues RRT and/or hemodialysis. Severe hypertension requiring >2 medications or a continuous drug infusion were present in 81% of participants. Oxygen therapy was needed in 76% (n = 16 of 21) of patients and 43% (n = 9 of 21) required positive pressure ventilation for a median of 22 days (IQR, 9-35). Pericardial effusions occurred in 62% (n = 13 of 21) of patients with 14% (n = 3 of 21) requiring a pericardial drain for impending tamponade. One-third of the cohort had documented pulmonary hypertension. Of the 6 participants with pulmonary hypertension, 3 were treated with inhaled nitric oxide. Intestinal TA-TMA diagnosed clinically and/or tissue biopsy was present in 43% of cases (n = 9 of 21), with 38% (n = 8 of 21) having intestinal bleeding. The most common central nervous system presentation was altered mental status. One patient had a left middle cerebral artery stroke with right-sided hemiparesis diagnosed by magnetic resonance imaging angiogram. Three patients had coinciding veno-occlusive disease (VOD) and received defibrotide.
CNI and GVHD management
Twelve participants received CNIs (cyclosporine [CSA], n = 11; and tacrolimus, n = 1) and 4 had T-cell–depleted grafts for GVHD prophylaxis. CNI modification was done in 3 of 6 participants receiving RRT: CSA was stopped in 2 participants at start of RRT, and in 1 patient tacrolimus was changed to sirolimus because she had prior history of liver transplantation and required ongoing immune suppression. Another 2 patients remained on CSA while on RRT, with close drug level monitoring to keep levels at the middle of the therapeutic range. One patient was not on any CNIs at RRT initiation (T-cell–depleted graft). Other participants not requiring RRT remained on CNI prophylaxis after hrTMA diagnosis, with close monitoring of drug levels.
Acute GVHD occurred in 7 patients (38%): in 3 patients it occurred before hrTMA diagnosis, and in 4 after hrTMA diagnosis. The overall grade of GVHD was as follows: grade 4 (n = 2), grade 3 (n = 1), grade 2 (n = 2), and grade 1 (n = 2).
All 7 patients with acute GVHD were initially treated with methylprednisolone, 2 mg/kg per day. Five participants received additional immunosuppressive agents selected by the primary treating physician based on disease course (infliximab, n = 3; alemtuzumab, n = 3, vedolizumab, n = 2; and ruxolitinib, n = 2). One study participant with overall grade 4 GVHD (liver) died without resolving GVHD. Two participants with grade 3 and 4 GVHD, respectively, resolved/improved GVHD but died with MODS. The remaining participants successfully resolved GVHD and survived. Two patients developed chronic skin GVHD, 4 and 9 months after HCT, respectively.
Eculizumab therapy
Eculizumab therapy was started at a median of 2 days (IQR, 2-4) after hrTMA diagnosis. A median of 20 doses per patient were administered (IQR, 16-27). Thirteen patients had eculizumab dosing modified because of subtherapeutic drug trough and/or inadequate complement suppression by shortening the dosing interval to maintain a therapeutic drug level. Two patients with VOD received supplemental eculizumab dosing because of plasma exchange.
Two patients stopped therapy early; 1 resolved hrTMA and returned to their home institution after 16 weeks of therapy; the second was diagnosed with diffuse alveolar damage, possibly antibiotic related, therefore all medications, including eculizumab, were removed because hrTMA had already resolved and there was no active TMA on lung biopsy after 6 weeks of eculizumab therapy.
Eculizumab was well tolerated without any adverse events attributed to the drug. Eculizumab cleared from the blood after therapy completion in a median of 28 days (IQR, 14-36) and CH50 recovered to a normal level in a median of 28 days (IQR, 19-49).
Survival in participants with hrTMA treated with eculizumab
Of 21 study participants, 15 (71%) were alive 6 months after hrTMA diagnosis. The null hypothesis, that 6-month survival after hrTMA diagnosis is 18%, was rejected in favor of the alternate hypothesis that the true survival rate is >18% in participants who receive treatment. This conclusion was based on a survival rate of 71% (P < .0001; Figure 2A). Figure 2B displays survival probability for 21 study participants with hrTMA treated with eculizumab and our historical controls with hrTMA not receiving any targeted interventions from our prospective TA-TMA screening study,4 supporting an advantage in survival with prompt therapy.
Survival probability for recipient of HCT with hrTMA. (A) Kaplan-Meier estimate of survival probability in recipients of HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) starting at hrTMA diagnosis. Survival at 6 months after hrTMA diagnosis was 71% (solid line; 95% confidence interval [CI], 55-94 [dotted lines]). (B) Kaplan-Meier estimate of survival in recipients of HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) and untreated controls with the same risk TA-TMA (n = 12) starting at hrTMA diagnosis. Survival 6 months after hrTMA diagnosis in participants treated with eculizumab was 71%, and 18% in untreated historical controls. A P value for the comparison between the historical and treated cohorts is not provided because the 2 cohorts were not accrued concurrently. (C) Kaplan-Meier estimate of survival probability in recipients with HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) starting at stem cell infusion (day 0). Survival at 1 year after HCT was 61.9% (solid line; 95% CI, 45-87 [dotted lines]).
Survival probability for recipient of HCT with hrTMA. (A) Kaplan-Meier estimate of survival probability in recipients of HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) starting at hrTMA diagnosis. Survival at 6 months after hrTMA diagnosis was 71% (solid line; 95% confidence interval [CI], 55-94 [dotted lines]). (B) Kaplan-Meier estimate of survival in recipients of HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) and untreated controls with the same risk TA-TMA (n = 12) starting at hrTMA diagnosis. Survival 6 months after hrTMA diagnosis in participants treated with eculizumab was 71%, and 18% in untreated historical controls. A P value for the comparison between the historical and treated cohorts is not provided because the 2 cohorts were not accrued concurrently. (C) Kaplan-Meier estimate of survival probability in recipients with HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) starting at stem cell infusion (day 0). Survival at 1 year after HCT was 61.9% (solid line; 95% CI, 45-87 [dotted lines]).
Our secondary aim was met by demonstrating improved 1-year post-HCT survival of 62% in the treated cohort (Figure 2C), whereas historical controls had 1-year survival of 16.7%.
Six patients died during the 6 months after hrTMA diagnosis. Contributing causes of death were GVHD (liver, n = 1), MODS/GVHD (GI/liver, n = 1), MODS/adenoviral infection (n = 2), acute respiratory failure, unclear cause (n = 1), and VOD (n = 1). Two patients died between 6 months and 1 year after HCT; the cause of death were MODS/GVHD (GI, n = 1) and MODS (n = 1). No demographic features nor pretransplant characteristics were statistically significant in survivors compared with nonsurvivors. We did not observe inferior survival in HCT recipients undergoing second and third HCTs.
hrTMA and MODS response to therapy
Therapy response by 6 months from hrTMA diagnosis was achieved in 67% (14/21) of patients: CR in 10 (48%) and partial response in 4 (19%). Seven patients (33%) had NR by study definition that required resolution of both high-risk markers (rUPCR of <1 mg/mg and normal sC5b-9). All but 1 patient who died, died with rUPCR of >1 mg/mg, and 2 nonsurvivors still had elevated sC5b-9 at death. Table 4 lists time to normalization of biomarkers in responders. All responders fully resolved hrTMA and recovered organ function at a median of 22 weeks (IQR, 13-24 weeks). Responders without bleeding resolved hrTMA/MODS at a median of 15 weeks (IQR, 12-23) whereas those with GI bleeding resolved hrTMA/MODS at a median of 23 weeks (IQR, 22-24). At therapy completion, median hemoglobin level was 9.7 g/dL (IQR, 9.1-11.3) and median platelet count 144 × 103 μL (IQR, 54-223).
Our secondary study end point was to assess cumulative incidence of MODS at 6 months after hrTMA diagnosis and at 1 year after HCT. Table 5 summarizes organ injury at study evaluation time points.
Six months after hrTMA diagnosis, 15 patients (71%) were alive of whom 4 (26.7%) had residual organ injury, including 1 patient with pulmonary failure, 2 with chronic kidney injury on maintenance dialysis, and 1 with intestinal TMA/GVHD and renal failure on dialysis.
Single-organ pulmonary failure in 1 study participant was because of biopsy-proven diffuse alveolar damage with resolved hrTMA. This patient remained on chronic ventilation during the study, but his ventilatory support need has since resolved.
Three survivors remained on dialysis 6 months after hrTMA diagnosis. The remaining 12 of 15 surviving patients (80%) recovered kidney function according to study definition. Median cystatin-C GFR was 77 mL/min per 1.73 m2 (IQR, 59-95) at the 6-month evaluation time point, and 79 mL/min per 1.73m2 (IQR, 70-95) at 1 year after HCT. All survivors had normal-for-age serum creatinine and no proteinuria, similar to their pretransplant value.
Pulmonary hypertension, cardiac dysfunction, and serositis resolved in all patients who were affected. The study participant with stroke fully recovered from neurologic symptoms. One study participant remained with intestinal TMA/GVHD and required dialysis at the 6-month evaluation time point and died 9 months after HCT. All other survivors recovered from GI symptoms.
Infections
There were no N meningitidis infections. Seven patients (33%) had total of 12 bloodstream infections during eculizumab therapy. No deaths were attributed to bloodstream infections.
All participants were screened weekly for cytomegalovirus, Epstein-Barr virus, adenovirus, and BK virus as standard of care. Twelve (57%) patients had viremias before hrTMA diagnosis, of whom 8 had infection by >1 virus. Only 1 patient developed new BK/cytomegalovirus viremia 30 days after hrTMA diagnosis. Adenoviral pneumonitis was a contributing cause of death in 2 patients (1 autopsy proven), and both had adenoviremia before hrTMA diagnosis. One patient had severe acute respiratory syndrome coronavirus 2 infection during the study, and 2 patients after completing eculizumab therapy; all recovered well.
Two patients had documented candidemia (Candida glabrata) while on treatment with eculizumab, both with intestinal GVHD on prolonged immunosuppression. No mold infections occurred.
Discussion
We performed, to our knowledge, the first prospective, multi-institutional study in children and young adults using the terminal complement blocker, eculizumab, as an early intervention for patients with hrTMA with MODS. Our study used contemporary TA-TMA diagnostic and high-risk stratification criteria by Jodele et al that are currently accepted as a standard of care by the international expert consensus published in 2023.5 The eculizumab dosing regimen for this study was derived from a large set of PK/PD data of HCT recipients previously published by our group.16 Eculizumab was well tolerated with no excess of infections, and no other drug-attributable adverse events. Our intensive eculizumab dosing regimen, combined with real-time eculizumab drug level and complement biomarker monitoring allowed to assure adequate drug dosing, especially for patients with bleeding.
Our study goal was to improve (to double) survival in HCT recipients with hrTMA, by initiating early targeted intervention with eculizumab as compared with historical controls from our prior study of prospectively screened hrTMA cases not receiving any targeted therapy with complement blockers. Our primary and secondary study end points were met because we achieved 71% survival in the eculizumab-treated cohort, 6 months after hrTMA diagnosis and 62% survival at 1 year after HCT, whereas survival in an our prospectively screened historical cohort using the same diagnostic biomarkers and risk stratification but not receiving any TA-TMA–targeted complement-blocking therapy was 18% and 16.7%, at 6 months and 1 year, respectively.4 Posttherapy MODS resolution was very encouraging but not complete, with >70% of patients having organ function recovery. This is clinically significant, taking into consideration that a median of 4 organ systems were damaged in participants with hrTMA. The significant reversal of MODS in most patients was, likely or at least in part, because of prompt control of overactive complement using an intensive eculizumab dosing regimen derived specifically for HCT recipients with hrTMA. There were expected challenges in controlling complement activation in patients with intestinal TA-TMA and bleeding, having previously demonstrated the need for more personalized eculizumab dose adjustments in such cases.16 Patients with intestinal TA-TMA and clinical bleeding benefited from full 24 weeks of complement-blocking therapy, whereas in patients without bleeding, the total therapy course may likely be shortened to ∼12 to 15 weeks in those with disease response confirmed by laboratory biomarkers and clinical assessment.
Although recognizing the improved outcomes achieved in this study, it is also clear that morbidity and mortality remain significant and that there is an important opportunity for further improvement in treatments. Although we uniformly and prospectively screened for high-risk TA-TMA markers, organ dysfunction was already identified at time of study enrollment, suggesting that less-stringent hrTMA features and/or even earlier intervention would likely have led to even better outcomes. We previously demonstrated that elevated sC5b-9 alone is an indicator of reduced survival and is associated with a nearly threefold higher risk of death in multivariate analyses (P = .01), and that sustained sC5b-9 elevation for >2 weeks is associated with an increased risk of MODS and mortality.2 This again supports our prior observations that delay in complement-blocking therapy significantly affects outcomes, making randomized placebo studies not feasible and unethical in such patients who are at high risk.
Another important area for consideration is that a proportion of patients, mostly those with severe GI bleed, did not have prompt response to C5 inhibition despite aggressive therapy. This suggests that a specific dosing regimen is required for this group of patients. Additional complement inhibition strategies and/or novel complement or other therapeutic targets would also be of value. We have previously identified interferon activation as an early event in initiation of hrTMA that likely contributes to subsequent complement activation. Interferon inhibition may need to be examined along with other druggable targets for hrTMA.26,27
CNI prophylaxis was continued after hrTMA diagnosis in most of our study participants while assuring steady CSA drug levels in the middle of the therapeutic range, because patients were at high-risk for GVHD or had active GVHD. In agreement with this practice, a recent large study by Matsui et al examined CNI use in TA-TMA and demonstrated that maintaining a moderate CNI drug level resulted in significantly superior outcomes as compared with the strategy replacing CNIs with steroids.28
We are still learning about the long-term impact of TA-TMA on organ function in HCT recipients. In this study, survivors not requiring dialysis had full recovery of kidney function and absence of proteinuria at 6 months after hrTMA diagnosis and at 1 year after HCT. This is a promising observation in patients who received treatment, because we previously demonstrated that children with less severe TA-TMA who were not considered for complement-blocking therapy still exhibited high degree of proteinuria (rUPCR >1 mg/mg) 1 year after transplantion.2 Moreover, kidney and cardiac injury are key causes of mortality in HCT survivors, therefore having unresolved long-term proteinuria may increase the risk of kidney dysfunction later in life.29,30 Detailed organ function assessment, especially renal, cardiac, endocrine, should be included as part of survivorship clinic monitoring in patients with TA-TMA.
In summary, this prospective study using contemporary diagnostic, risk stratification, and therapeutic approaches marks an important milestone in our knowledge using targeted complement-blocking therapy for hrTMA with MODS, demonstrating significant improvement in survival and attenuation of organ injury with early intervention. Although our knowledge using complement blockade for hrTMA increased exponentially in the past decade, there are no drugs approved for a TA-TMA indication. To date, eculizumab is the only complement blocker with extensive PK/PD experience in hrTMA. This includes clinically available biomarkers and drug level monitoring to assess adequate complement blockade and therapy response using personalized dosing algorithms. These precision dosing tools guide both therapy intensity and therapy duration, minimize the risk of undertreating or overtreating and facilitate the economical use of the drug16 We have also previously shown that multiple complement pathways can be activated in TA-TMA,26 thus, using a terminal complement blocker like eculizumab as a first-line therapy makes clinical sense.
Our study emphasizes the need for prospective screening, less stringent high-risk criteria to facilitate early disease control, timely availability of targeted therapy, and multidisciplinary collaboration in caring for such complex cases of those receiving HCT high-risk TA-TMA with MODS. Further studies should focus on predictive biomarkers in which preventative measures or targeted therapy can be initiated before organ injury.
Acknowledgments
The authors thank their data and safety monitoring board members Thomas Hofstra, Jens Goebel, and Joseph Palumbo; their coordinators and regulatory team Stephanie Edwards, Monica Trapp, Celeste Dourson, Stacie McLean, Sarah Casello, Molly Mead, and Stephanie Hoffman; the Cincinnati Children’s Hospital Medical Center Bone Marrow Tissue Repository and the REDCap team for outstanding technical assistance; and their institutional support for making this study possible during the COVID-19 pandemic.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award number R01HD093773 (principal investigator, S.J.). Alexion Pharmaceuticals provided the drug eculizumab at no charge for all study participants, provided partial financial support for setting up the REDCap database, and reviewed the manuscript but did not influence the content of the protocol or the decision to publish study data or the content of the manuscript.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: S.J. and S.M.D. designed the study, enrolled study participants, collected and analyzed data, and wrote manuscript; C.E.D., A.S., and B.L.L. provided vital contributions in study planning and multidisciplinary team integration in prospective TA-TMA screening, and collected and analyzed clinical data; A.T.-C. monitored eculizumab administration and summarized pharmacology data; A.L. performed statistical analyses, and prepared data tables and figures; K.M. performed eculizumab PK/PD testing; and P.A.-H. and J.F. opened the study at respective institutions as site PIs, enrolled study participants, collected data, and edited manuscript.
Conflict-of-interest disclosure: S.J. and B.L.L. are coinventors on US patent number US 10,815,296 B2. S.J. is a lead PI for the National Institute of Health–funded multi-institutional study investigating TA-TMA (R01HD093773), and received honoraria for lectures and consultancy from Omeros, Sobi, and Alexion Pharmaceuticals. S.M.D. has received research support from Alexion Pharmaceuticals, and consultants with AlloVir. C.E.D. has received honoraria from Omeros. A.S. received honoraria from Sobi. A.T.-C. received honoraria from Jazz Pharmaceuticals. K.M. and S.J. are listed as inventors in pending patent applications. The remaining authors declare no competing financial interests.
Correspondence: Sonata Jodele, Division of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, 45229 OH; email: sonata.jodele@cchmc.org.
References
Author notes
Data sharing will be in accordance with the National Institutes of Health policy. Data are available from the corresponding author (sonata.jodele@cchmc.org) on request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Survival probability for recipient of HCT with hrTMA. (A) Kaplan-Meier estimate of survival probability in recipients of HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) starting at hrTMA diagnosis. Survival at 6 months after hrTMA diagnosis was 71% (solid line; 95% confidence interval [CI], 55-94 [dotted lines]). (B) Kaplan-Meier estimate of survival in recipients of HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) and untreated controls with the same risk TA-TMA (n = 12) starting at hrTMA diagnosis. Survival 6 months after hrTMA diagnosis in participants treated with eculizumab was 71%, and 18% in untreated historical controls. A P value for the comparison between the historical and treated cohorts is not provided because the 2 cohorts were not accrued concurrently. (C) Kaplan-Meier estimate of survival probability in recipients with HCT with hrTMA treated with the terminal complement blocker eculizumab (n = 21) starting at stem cell infusion (day 0). Survival at 1 year after HCT was 61.9% (solid line; 95% CI, 45-87 [dotted lines]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/12/10.1182_blood.2023022526/2/m_blood_bld-2023-022526-gr2.jpeg?Expires=1763990961&Signature=J8SgTU41Be9KqwXaSvF80fR5aUmRdMP7FCffS6QuhA9qousfqo7-Mlg22JouBJFwbBJKM0D0rc4x7yN4buKZuPaCnSQ9xj3zle6ffWME5ZFZSNBDoWwPsyhe9~87uVJfsAQyYWG7WGkZIEzY8ViIGPE-tLRHPJEeZ5FKCcUWMinoRcYbQ-xY2PhmSR23S0vU9oieqQptksycq~6jCAOISNUQ80cYNAzEAS1Rd8DP-TkuZqQFWK2HHF9CGmG8gW-9v13NZfVplN~lhpfimdwKL5wA4InSGdXAvO6jP21WC6Tcp-zkXSdbz9-QTOFRxukUTWSwFitvPlULuNMPQFRKwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
